Abstract
Maize plants (Zea mays) attacked by caterpillars release a mixture of odorous compounds that attract parasitic wasps, natural enemies of the herbivores. We assessed the genetic variability of these induced volatile emissions among 31 maize inbred lines representing a broad range of genetic diversity used by breeders in Europe and North America. Odors were collected from young plants that had been induced by injecting them with caterpillar regurgitant. Significant variation among lines was found for all 23 volatile compounds included in the analysis: the lines differed enormously in the total amount of volatiles emitted and showed highly variable odor profiles distinctive of each genotype. Principal component analysis performed on the relative quantities of particular compounds within the blend revealed clusters of highly correlated volatiles, which may share common metabolic pathways. European and American lines belonging to established heterotic groups were loosely separated from each other, with the most clear-cut difference in the typical release of (E)-β-caryophyllene by European lines. There was no correlation between the distances among the lines based on their odor profiles and their respective genetic distances previously assessed by neutral RFLP markers. This most comprehensive study to date on intraspecific variation in induced odor emission by maize plants provides a further example of the remarkably high genetic diversity conserved within this important crop plant. A better understanding of the genetic control of induced odor emissions may help in the development of maize varieties particularly attractive to parasitoids and other biological control agents and perhaps more repellent for herbivores.
The release of odorous compounds by plants in response to herbivore attack and the subsequent use of these odorous signals by natural enemies to locate the herbivores is a widespread phenomenon observed in various tritrophic systems, e.g. predatory mites and spider mites on Lima bean (Dicke and Sabelis, 1988), parasitic wasps and lepidopteran caterpillars on cabbage (Mattiacci et al., 1994) and cotton (Loughrin et al., 1995), and anthocorid predators and psyllids on pear trees (Scutareanu et al., 1997). We are currently studying this kind of interaction in a system that comprises maize (Zea mays), folivorous caterpillars in the genus Spodoptera, and the associated endoparasitic wasps (Turlings et al., 1990). Odor release by maize plants is a rapid systemic response (Turlings and Tumlinson, 1992) induced by elicitors present in the oral secretions of the herbivores, such as volicitin, a compound isolated from regurgitant of Spodoptera exigua Hübner caterpillars (Alborn et al., 1997; Turlings et al., 2000). Plants belonging to various species and families differ in their odor profiles (Turlings et al., 1993b). Nonetheless, the odor patterns of different plant taxa exhibit considerable overlap, and certain compounds like the terpenoids linalool, (3E)-4,8-dimethyl-1,3,7-nonatriene, and (E)-β-ocimene seem to be quite common or ubiquitous components of herbivore-induced odor emissions (Dicke, 1994; Paré and Tumlinson, 1999). Varietal or genotypic differences in herbivore-induced volatile emission have been recorded for several plant species as well (Takabayashi et al., 1991; Loughrin et al., 1995; Halitschke et al., 2000). Studies on different maize varieties revealed intraspecific variation both in quantity and quality of the odors released (Turlings et al., 1998b; Gouinguené et al., 2001). However, in all cases only a restricted range of genotypes was considered. Here, we present the results of a more comprehensive study on the genetic variability of herbivore-induced volatile emission within maize cultivated in temperate regions. The examined inbred lines represent a large spectrum of genetic diversity, and their genetic relatedness has already been established by means of molecular markers (Burstin et al., 1994; Dubreuil et al., 1996). The objectives of this investigation were (1) to assess the amount of genetic variability for herbivore-induced volatile emission present among maize inbred lines, (2) to study the pattern of correlations between the various volatile compounds, and (3) to screen for genotypes that could be used either to identify genes involved in quantitative variation of induced odor release or to elucidate the ecological role played by these chemical signals in the tritrophic system. Such knowledge may eventually help us develop methods to enhance the attractiveness of herbivore-injured maize plants to beneficial arthropods.
RESULTS
Identity of Volatiles
Of the volatiles detected in the odor samples from maize plants injected with caterpillar regurgitant, the 23 most prominent compounds were selected for quantification. The majority of these compounds were derived from the isoprenoid pathway: 3 monoterpenoids, 2 homoterpenes, and 12 sesquiterpenoids. The remaining compounds were four acetate esters and two nitrogen-containing aromatics. Along with the 23 selected compounds, gas chromatography-mass spectroscopy analysis revealed the presence, mostly in minute amounts, of green leaf odors ((E)-2-hexenal, (Z)-3-hexen-1-ol), additional monoterpenes (limonene, cis-ocimene), esters (neryl acetate), aromatics (methyl salicylate, methyl eugenol (MS)), cis-jasmone (MS), and at least 15 additional sesquiterpenes, e.g. α-copaene, various bisabolenes (MS), and cadinenes (MS).
Variation in Total Emission
The 31 maize inbred lines varied largely in the mean total amount of volatiles emitted, i.e. the sum of the 23 compounds chosen for analysis. There was an about 70-fold difference between the two extreme lines, W401 and F1852 (Table I). The total quantity of odors emitted was not related to the geographical origin of the lines. The line effect was highly significant in the ANOVA (Table I), and with a broad-sense heritability of H2 = 0.84, most of the variation proved to be genetic (Table II). The repeatability was r2 = 0.97, which indicates that the residual variation was extremely well controlled with our experimental design. The differences in total emission observed with plants damaged by caterpillars (Table III) were somewhat less pronounced, only 20-fold between the two extreme lines F7 and F476, but the values obtained with both induction methods were correlated, albeit the relationship was not linear (Spearman rank correlation coefficient ρ = 0.76; P = 0.01; n = 12). Lines with high constitutive odor production tended also to release higher amounts of induced odors (ρ = 0.59; P = 0.05; n = 12). Undamaged plants of line F476 emitted the same or higher total quantities of volatiles than attacked plants of some other lines (e.g. F7).
Table I.
Total amount of volatiles, i.e. the sum of 23 selected odorous compounds (see Table II), emitted by seedlings of 31 maize inbred lines and the variety Delprim (laboratory standard) in response to injection of caterpillar regurgitant
| Line | Class (Cluster) | Amount
|
|
|---|---|---|---|
| Mean ± se | N | ||
| μg h−1 g−1 shoot dry weight | |||
| W401 | EAD (7) | 0.7 ± 0.2 a | 6 |
| F7 | EUF (10) | 0.9 ± 0.2 ab | 9 |
| MIS3a | LSC (5) | 1.9 ± 0.5 abc | 6 |
| F113 | EAD (7) | 2.9 ± 0.2 bcd | 6 |
| F752 | EUF (12) | 3.4 ± 0.9 bcd | 6 |
| MIS4ab | M13 (3) | 5.0 ± 0.7 cdef | 6 |
| F584 | RYD (9) | 5.7 ± 1.6 cdef | 5 |
| F284 | M13 (3) | 6.0 ± 2.5 cde | 6 |
| F292 | EAD (7) | 8.8 ± 2.1 defg | 6 |
| F591 | M13 (3) | 10.3 ± 1.1 defgh | 5 |
| CM174 | RYD (9) | 11.4 ± 3.7 defg | 6 |
| Co158 | M13 (3) | 11.4 ± 1.6 defghi | 6 |
| F618 | RYD (9) | 11.9 ± 2.6 defgh | 6 |
| F608 | M13 (3) | 12.3 ± 2.6 defgh | 6 |
| F670 | M13 (1) | 15.6 ± 4.6 efghij | 6 |
| A188 | M13 (3) | 15.8 ± 3.7 efghij | 6 |
| F252 | EAD (6) | 20.8 ± 3.7 fghij | 6 |
| F268 | EUF (11) | 21.0 ± 5.9 efghij | 6 |
| RYD2a | RYD (9) | 21.4 ± 3.4 ghij | 5 |
| F283 | EUF (10) | 23.4 ± 5.3 ghij | 5 |
| Ia5pop | 24.6 ± 7.3 fghij | 6 | |
| F2 | EUF (11) | 27.7 ± 3.9 ghij | 6 |
| F7001 | EUF (10) | 28.8 ± 8.2 ghij | 5 |
| Co255b | EUF (10) | 29.5 ± 5.4 ghij | 6 |
| A654 | M13 (1) | 38.6 ± 5.8 hij | 5 |
| C6 | 39.2 ± 3.4 hij | 6 | |
| Delprim | 42.5 ± 10.8 hij | 6 | |
| Io3a | M13 (2) | 44.9 ± 4.1 ij | 6 |
| Du101 | EUF (10) | 48.0 ± 4.9 j | 6 |
| F476 | RYD (9) | 50.2 ± 4.1 j | 6 |
| Io4a | M13 (2) | 51.1 ± 8.9 j | 6 |
| F1852 | M13 (1) | 54.2 ± 7.3 j | 6 |
Lines with the same letter are not significantly different at the 1% level according to the Bonferroni/Dunn posthoc test (ANOVA with ln-transformed values: F = 30.3; degrees of freedom = 31; P < 0.0001). Classes and clusters defined according to a dendrogram obtained by cluster analysis of RFLP markers (Dubreuil et al., 1996): EAD, Early Dent; EUF, European Flint; LSC, Lancaster Sure Crop; M13, Minnesota 13 complex; RYD, Reid Yellow Dent.
Private lines, names changed.
Lines derived from crosses between lines tracing back to two different heterotic groups.
Table II.
Broad-sense heritabilities H2 of absolute and relative amounts of odorous compounds emitted
| Absolute Quantities
|
Relative Quantities
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Compound | Identification | PL | Pr | Pm | σ2 | H2 | CV | PL | Pr | Pm | Pz | σ2 | H2 | CV |
| 1 | a β-Myrcene | Rt/MS | § | § | 1.08 | 0.90 | 13.2 | § | † | § | § | 0.88 | 0.91 | 10.9 | |
| b (E)-β-Ocimene | Rt/MS | § | § | 1.19 | 0.84 | 16.3 | § | § | § | 0.84 | 0.85 | 12.8 | |||
| c Linalool | Rt/MS | § | * | § | 3.48 | 0.89 | 10.8 | § | § | 1.46 | 0.88 | 7.5 | |||
| 2 | d (Z)-3-Hexen-1-yl acetate | Rt/MS | § | * | § | 0.99 | 0.64 | 20.1 | § | * | § | § | 0.79 | 0.61 | 19.1 |
| e Benzyl acetate | Rt/MS | § | § | 1.20 | 0.65 | 49.3 | § | § | 0.72 | 0.62 | 40.9 | ||||
| f Phenethyl acetate | Rt/MS | § | * | § | 4.77 | 0.88 | 23.6 | § | § | § | 3.36 | 0.91 | 16.9 | ||
| g Geranyl acetate | Rt/MS | § | † | § | 3.30 | 0.83 | 26.4 | § | * | § | 2.35 | 0.88 | 18.6 | ||
| 3 | h (3E)-4,8-Dimethyl-1,3,7-nonatriene | Rt/MS | § | * | § | 3.34 | 0.90 | 9.6 | § | * | * | § | 1.09 | 0.91 | 5.2 |
| i (3E,7E)-4,8,12-Trimethyl-1,3,7,11-tridecatetraene | Rt/MS | § | § | § | 1.42 | 0.90 | 9.1 | § | § | § | § | 0.30 | 0.79 | 6.4 | |
| 4 | j 1-H-indole | Rt/MS | § | † | § | 3.67 | 0.76 | 19.5 | § | ‡ | § | 0.97 | 0.57 | 15.5 | |
| k Methyl anthranilate | Rt/MS | § | † | § | 1.39 | 0.66 | 35.8 | § | § | § | 0.51 | 0.50 | 30.4 | ||
| 5 | l Cyclosisosativene | MS | § | § | § | 1.58 | 0.75 | 73.5 | § | § | ‡ | 1.46 | 0.74 | 73.5 | |
| m α-Ylangene | MS | § | § | * | 1.61 | 0.73 | 77.2 | § | § | 1.56 | 0.72 | 77.5 | |||
| n Unknowna | MS | § | § | § | 1.08 | 0.85 | 35.6 | § | § | § | 0.58 | 0.80 | 30.3 | ||
| o (E)-β-Caryophyllene | Rt/MS | § | † | § | 5.65 | 0.91 | 20.3 | § | ‡ | § | 4.52 | 0.93 | 16.3 | ||
| p (E)-α-Bergamotene | Rt/MS | § | † | § | 3.73 | 0.85 | 18.9 | § | † | § | 2.13 | 0.88 | 12.6 | ||
| q (E)-β-Farnesene | Rt/MS | § | ‡ | § | 3.30 | 0.85 | 14.5 | § | § | * | § | 1.33 | 0.89 | 7.8 | |
| r Germacrene-D | MS | § | § | § | 1.96 | 0.89 | 23.8 | § | § | § | 1.49 | 0.89 | 20.4 | ||
| s Unknownb | MS | § | § | † | 1.40 | 0.78 | 38.2 | § | § | § | 0.95 | 0.76 | 33.4 | ||
| t (E,E)-α-Farnesene | Rt/MS | § | † | 2.12 | 0.82 | 33.4 | § | * | † | 2.19 | 0.83 | 32.6 | |||
| u β-Bisabolene | Rt/MS | § | * | 1.52 | 0.68 | 47.0 | § | 1.26 | 0.64 | 46.9 | |||||
| v Unknownc | MS | § | § | § | 2.53 | 0.92 | 18.3 | § | § | § | 1.51 | 0.94 | 12.2 | ||
| w (E)-Nerolidol | Rt/MS | § | § | § | 2.26 | 0.80 | 25.0 | § | § | § | 0.97 | 0.77 | 17.5 | ||
| Total (sum of all compounds) | § | * | § | 1.51 | 0.84 | 6.6 | |||||||||
Values were calculated from the results of an ANCOVA with ln-transformed amounts (in ng/h) as dependent variable, line (L) and replicate (r) as independent variables, and shoot dry weight (m) and total emission (z; for relative amounts) as covariates. Abbreviations for probability levels: *, <0.05; †, <0.01; ‡, <0.001; §, <0.0001; σ2, genetic variance; CV, coefficient of variation; Rt, retention time; MS, mass spectrum; 1, monoterpenoids; 2, esters (acetates); 3, homoterpenes; 4, N-aromatics; 5, sesquiterpenoids.
MS similar to that of α-zingiberene, in some cases (e.g. Delprim, F2) coelution of another sesquiterpene, most probably β-elemene (MS).
MS similar to that of α-zingiberene.
MS similar to that of β-sesquiphellandrene, in several cases (e.g. Du101, F476) coelution of another sesquiterpene, most probably δ-cadinene (MS).
Table III.
Odor emission (in μg h−1 g−1 shoot dry weight) of intact maize plants (und) and plants damaged by caterpillar feeding (dam)
| Line
|
F7
|
F752
|
F7001
|
F618
|
W401
|
F113
|
A188
|
F2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicates
|
7
|
7
|
6
|
6
|
7
|
7
|
7
|
7
|
7
|
7
|
6
|
6
|
7
|
7
|
7
|
7
|
| Compound/Treatment | und | dam | und | dam | und | dam | und | dam | und | dam | und | dam | und | dam | und | dam |
| a Myrcene | 0.03 | 0.05 | − | + | − | + | + | 0.03 | 0.02 | 0.03 | − | 0.02 | 0.04 | 0.05 | − | + |
| b Ocimene | + | + | + | + | 0.02 | 0.02 | + | 0.02 | 0.02 | 0.04 | + | + | + | + | + | 0.02 |
| c Linalool | 0.16 | 0.34 | + | 0.04 | + | 0.03 | 0.02 | 0.17 | 0.16 | 0.24 | 0.04 | 0.15 | 0.31 | 0.45 | + | 1.17 |
| d Hexenyl acetate | 0.08 | 0.15 | + | 0.09 | 0.15 | 0.26 | 0.02 | 0.17 | 0.13 | 0.36 | 0.04 | 0.23 | 0.07 | 0.45 | 0.01 | 0.17 |
| e Benzyl acetate | + | 0.01 | + | + | − | + | + | + | + | 0.06 | − | 0.02 | + | 0.06 | + | + |
| f Phenethyl acetate | + | + | − | + | − | + | − | 0.11 | + | 0.26 | − | 0.43 | + | 0.64 | − | 0.02 |
| g Geranyl acetate | + | 0.01 | − | − | + | 0.01 | − | 0.01 | − | + | + | 0.10 | + | 0.05 | − | + |
| h Dimethyl nonatriene | + | 0.15 | 0.02 | 0.21 | + | 0.16 | + | 0.13 | + | 0.02 | + | + | 0.07 | 0.76 | + | 0.47 |
| i Trimethyl tridecatetraene | 0.02 | 0.11 | 0.01 | 0.06 | 0.01 | 0.03 | 0.05 | 0.17 | 0.01 | 0.02 | + | + | 0.04 | 0.51 | 0.02 | 0.80 |
| j 1-H-indole | − | + | 0.32 | 0.24 | − | + | 0.14 | 0.36 | + | 0.05 | + | 0.61 | + | 0.02 | + | 0.14 |
| k Methyl anthranilate | + | + | − | + | + | + | − | 0.01 | + | + | − | − | + | + | − | 0.09 |
| l Cyclosisosativene | − | − | − | − | − | − | − | − | + | 0.01 | − | − | − | − | + | 0.02 |
| m α-Ylangene | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + | + |
| n Unknown | − | + | − | 0.02 | + | + | − | + | − | + | − | + | − | 0.01 | − | + |
| o Caryophyllene | 0.02 | 0.11 | 0.03 | 0.13 | + | 0.01 | + | 0.03 | 0.01 | 0.03 | − | 0.04 | + | 0.02 | 0.05 | 1.18 |
| p Bergamotene | + | 0.01 | − | 0.37 | + | 0.73 | + | 0.26 | + | 0.28 | − | 0.15 | + | 0.02 | + | 0.29 |
| q β-Farnesene | 0.02 | 0.05 | + | 0.36 | + | 0.82 | + | 0.94 | + | 1.03 | + | 0.77 | 0.01 | 0.03 | + | 0.58 |
| r Germacrene-D | − | − | 0.18 | 0.36 | 0.01 | 0.03 | + | 0.01 | + | 0.01 | − | + | + | 0.02 | + | 0.02 |
| s Unknown | + | + | + | + | + | 0.03 | + | 0.02 | + | 0.02 | + | + | − | − | + | 0.03 |
| t α-Farnesene | + | 0.02 | − | − | − | − | − | − | − | − | − | − | − | − | 0.11 | 0.34 |
| u β-Bisabolene | − | − | + | 0.02 | − | + | − | − | − | − | − | − | − | − | − | − |
| v Unknown | + | + | − | 0.06 | + | 0.14 | + | 0.03 | + | 0.01 | − | − | + | 0.02 | + | 0.06 |
| w Nerolidol | − | − | + | 0.01 | − | − | − | + | − | − | + | + | − | + | + | 0.04 |
| Total emission | 0.35 | 1.07 | 0.59 | 2.00 | 0.24 | 2.32 | 0.26 | 2.52 | 0.42 | 2.52 | 0.11 | 2.54 | 0.58 | 3.20 | 0.24 | 5.45 |
| Line
|
F1852
|
Delprim
|
Du101
|
F476
|
Factor of Increase of Emission by Caterpillar-Damaged Plants versus Intact Plants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicates
|
7
|
7
|
6
|
12
|
6
|
6
|
7
|
7
|
|
|
|
|
|
|
| Compound/Treatment | und | dam | und | dam | und | dam | und | dam | Median | Mean | sd | Min | Max | n |
| a Myrcene | 0.02 | 0.07 | 0.09 | 0.17 | 0.02 | 0.08 | 0.13 | 0.25 | 2 | 3 | 3 | 1.3 | 9 | 9 |
| b Ocimene | 0.02 | 0.06 | + | 0.03 | + | 0.02 | 0.02 | 0.02 | 3 | 5 | 4 | 1.0 | 13 | 8 |
| c Linalool | 0.17 | 1.01 | 1.19 | 2.30 | 0.50 | 4.08 | 1.76 | 5.80 | 5 | 23 | 55 | 1.5 | 195 | 12 |
| d Hexenyl acetate | − | − | 0.07 | 0.29 | + | 0.49 | 0.15 | 1.05 | 7 | 12 | 19 | 1.7 | 67 | 11 |
| e Benzyl acetate | − | − | + | 0.04 | + | 0.02 | 0.02 | 0.23 | 10 | 15 | 19 | 1.6 | 54 | 6 |
| f Henethyl acetate | − | − | + | 0.52 | − | 0.91 | + | 0.54 | 1,147 | 1,459 | 1,210 | 436 | 3,105 | 4 |
| g Geranyl acetate | − | − | + | 0.53 | − | − | 0.02 | 1.25 | 45 | 40 | 34 | 4.1 | 72 | 6 |
| h Dimethyl nonatriene | 0.03 | 0.47 | 0.01 | 0.91 | 0.02 | 1.29 | 0.09 | 1.93 | 61 | 84 | 106 | 9.2 | 362 | 11 |
| i Trimethyl tridecatetraene | 0.02 | 0.34 | 0.02 | 0.21 | 0.04 | 0.91 | 0.07 | 0.99 | 13 | 13 | 11 | 1.7 | 38 | 11 |
| j 1-H-indole | + | 0.20 | + | 0.57 | + | 4.32 | 0.23 | 2.48 | 107 | 262 | 545 | 0.7 | 1,794 | 10 |
| k Methyl anthranilate | 0.01 | 0.02 | + | + | + | 0.15 | 0.01 | 0.03 | 2 | 17 | 25 | 2.2 | 46 | 3 |
| l Cyclosisosativene | 0.09 | 0.17 | − | − | − | − | 0.03 | 0.05 | 3 | 4 | 4 | 1.6 | 11 | 4 |
| m α-Ylangene | 0.07 | 0.14 | − | − | − | − | 0.03 | 0.06 | 2 | 2 | 0 | 1.8 | 1.8 | 2 |
| n Unknown | + | 0.02 | − | 0.01 | − | + | + | 0.03 | 8 | 8 | 4 | 5.1 | 10 | 2 |
| o Caryophyllene | 0.04 | 0.35 | 0.02 | 2.40 | 0.02 | 3.26 | 0.16 | 2.29 | 7 | 29 | 47 | 1.3 | 133 | 11 |
| p Bergamotene | 0.01 | 1.20 | − | 1.69 | − | 0.72 | 0.01 | 0.89 | 71 | 65 | 49 | 1.4 | 125 | 8 |
| q β-Farnesene | 0.02 | 2.30 | 0.01 | 3.00 | + | 3.18 | 0.07 | 1.77 | 112 | 422 | 759 | 2.8 | 2,210 | 12 |
| r Germacrene-D | 0.03 | 0.10 | − | + | 0.08 | 0.28 | 0.08 | 0.35 | 3 | 5 | 5 | 1.8 | 18 | 9 |
| s Unknown | + | 0.04 | − | 0.03 | − | + | + | 0.03 | 17 | 45 | 54 | 2.8 | 126 | 6 |
| t α-Farnesene | − | − | − | − | − | − | − | − | 3 | 3 | 0 | 3.1 | 3.2 | 2 |
| u β-Bisabolene | + | 0.06 | + | 0.09 | + | 0.01 | − | 0.01 | 12 | 21 | 25 | 4.0 | 57 | 4 |
| v Unknown | 0.02 | 0.25 | + | 0.22 | − | + | 0.02 | 0.21 | 11 | 29 | 53 | 2.7 | 159 | 8 |
| w Nerolidol | + | 0.01 | + | 0.02 | + | 0.03 | 0.04 | 0.09 | 5 | 18 | 28 | 2.6 | 74 | 6 |
| Total emission | 0.60 | 6.85 | 1.46 | 13.03 | 0.71 | 19.79 | 3.09 | 20.67 | 9 | 12 | 8 | 3.1 | 28 | 12 |
Significant differences (P < 0.05; Mann-Whitney U-test) between the treatments are indicated by bold letters. +, Trace amounts (<0.01 μg h−1 g−1); −, Below detection threshold. Increase factors were calculated for all cases where volatile amounts of damaged plants were above 0.01 μg h−1 g−1 and amounts of undamaged plants above detection threshold.
Variation in the Odor Profiles
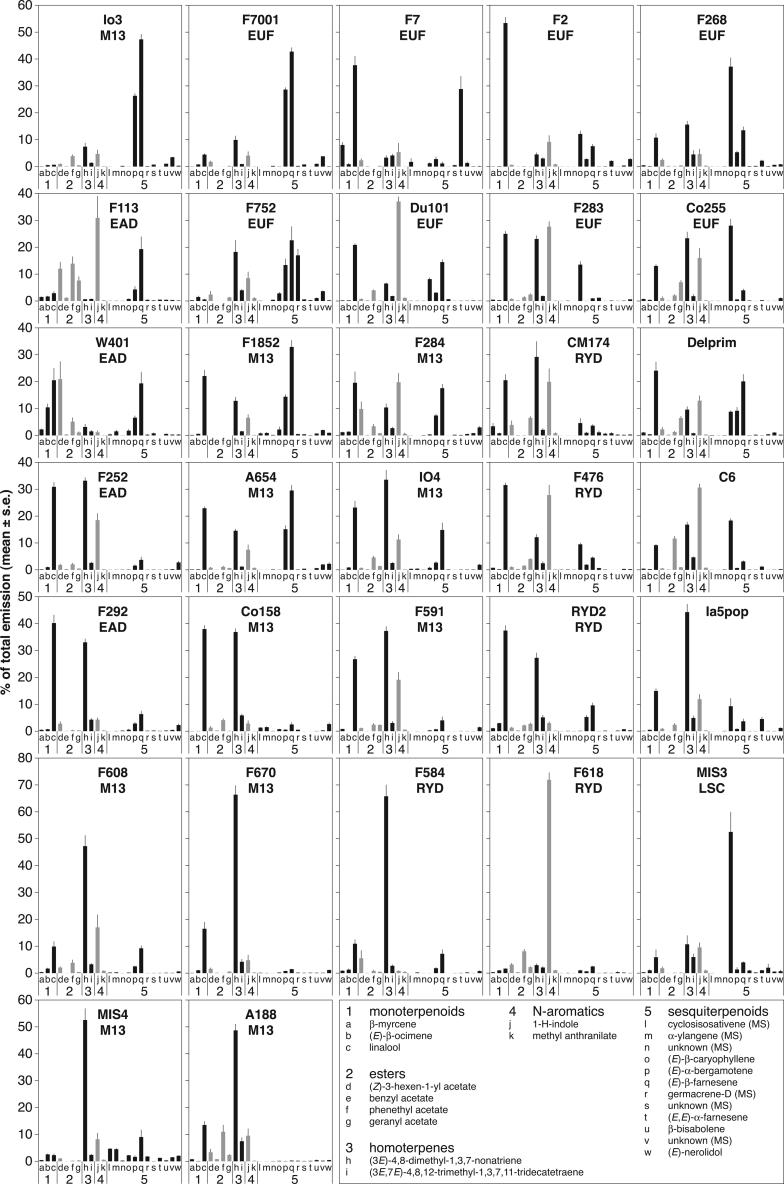
In addition to the pronounced quantitative variation, we found substantial qualitative variation among the lines in the relative proportion of particular compounds within the odor blend (Fig. 1). Some lines were characterized by very low amounts or the complete absence of whole compound groups. Line F1852 was deficient in the four esters, including the green leaf volatile (Z)-3-hexen-1-yl acetate, which was found in all the other lines. Lines F113 and W401 released only trace amounts of the two homoterpenes (3E)-4,8-dimethyl-1,3,7-nonatriene and (3E,7E)-4,8,12-trimethyl-1,3,7,11-decatetraene. By contrast, these homoterpenes were the dominant components in the odor blends of line A188, which was in turn characterized by extremely low levels of sesquiterpenes. Another highly variable trait was the ratio of (E)-β-caryophyllene to the two other quantitatively important sesquiterpenes, trans-α-bergamotene and (E)-β-farnesene. While some lines that produced high quantities of trans-α-bergamotene and (E)-β-farnesene almost completely lacked (E)-β-caryophyllene (e.g. A654, F584, F7001, and Io3), this compound was the prominent sesquiterpene in other lines (e.g. MIS3, F268, F283, and Co255). Two sesquiterpenes, eluting almost simultaneously and tentatively identified from their mass spectra as cycloisosativene and α-ylangene, were only detected in significant amounts in four lines belonging to the Minnesota 13 complex (MIS4, Co158, Io4, and F1852). A further sesquiterpene, tentatively identified as germacrene-D (MS), was produced in moderate quantities by some lines, notably by F752, where it was one of the dominant compounds of the odor blend. In six lines, one single compound made up more than 50% of total emission: linalool in F2; (3E)-4,8-dimethyl-1,3,7-nonatriene in MIS4, F584, and F670; indole in F618; and (E)-β-caryophyllene in MIS3.
Figure 1.
Odor profiles of 31 maize inbred lines and of the hybrid Delprim. The amount of each compound is expressed in percent of the total emission, i.e. the sum of all 23 compounds.
Odor profiles recorded from plants damaged by caterpillar feeding (Table III) proved very similar to those of plants induced by injection (Fig. 1). In all but three lines (F618, F2, and F7), the log-transformed mean amounts (in μg h−1 g−1 dry weight) for the 23 compounds were more strongly correlated between the same line induced with the two different methods (mean correlation coefficient ± sd = 0.84 ± 0.18; n = 12) than between different lines induced with the same (0.50 ± 0.25; n = 132) or with a different method (0.46 ± 0.26; n = 132). The only conspicuous deviations were observed in the lines F618 and F2: indole and linalool, respectively, were no longer as dominant in the odor bouquets as with the injection method.
In general, caterpillar damage caused an increase in the release of all 23 volatile compounds, but some of these were set free in non-negligible amounts by intact plants as well, and their levels did not increase as dramatically as those of the other compounds upon plant injury (Table III). The most notable of these already constitutively emitted compounds were the monoterpenes β-myrcene, β-ocimene, linalool, and the sesquiterpenes cycloisosativene (MS), α-ylangene (MS), germacrene-D (MS), and α-farnesene. Linalool is the most frequently occurring constitutive compound, present in moderate to high amounts in all lines in which it makes up an important part of the induced odor profile, with the interesting exception of line F7, in which it increases much steeper in response to herbivore injury.
For all the 23 included compounds, we found a highly significant effect of the line on both the absolute and the relative amounts (Table II). Absolute quantities of particular compounds and total emission were correlated for almost all individual compounds, except for three minor ones, which were either not expressed or expressed in very small quantities, namely bisabolene and cycloisosativene/α-ylangene, the two also constitutively occurring compounds. Shoot dry weight as a measure of plant size and physiological state generally influenced the absolute quantities, but its effect was generally no more significant on relative amounts. Hence, the physiological state of the plants mostly affected the total amount of volatile emission but not the odor profile.
For almost all compounds, there was a minor but significant block effect. Repeatability was remarkably high for all compounds, ranging from 0.91 to 0.98 for absolute amounts and from 0.85 to 0.99 for relative amounts. Hence, our experimental design allowed us to characterize the inbred lines by their odor profiles with a very good confidence. Accordingly, the residual coefficients of variation (CVs) ranged between 10% and 50%. The two sesquiterpenes with a residual CV higher than 70% were detected in significant amounts in four lines only. So their high CV values are due to very low average quantities in the whole genotype set. Like for the total volatile emission, most of the phenotypic variation in the release of individual compounds is due to genetic differences among the lines; values for broad-sense heritability ranged from 0.50 to 0.94. They were similar for absolute and relative amounts, except for 4,8,12-trimethyl-1,3,7,11-tridecatetraene and the two aromatic compounds 1-H-indole and methyl anthranilate.
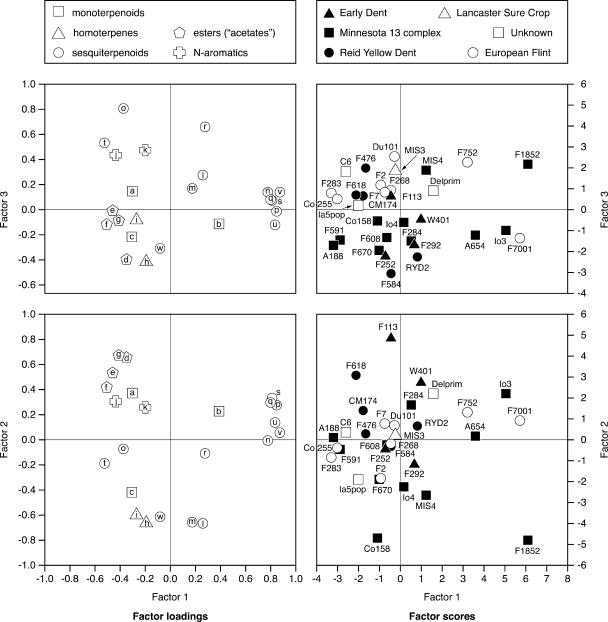
Multivariate Analyses
The first axis of a principal component analysis (PCA) on least square means of absolute amounts accounted for 40% of the total variation. It was positively correlated with the quantity of all compounds and hence separated the lines according to total volatile emission. Therefore, we focused rather on the proportions of the compounds in the blend, the chemical signature, as this characteristic may better distinguish between lines from different geographic origins. Plots of the factor loadings from a PCA on relative amounts show clusters of compounds whose proportions in the blend were correlated: six sesquiterpenes, including trans-α-bergamotene, (E)-β-farnesene, and β-bisabolene, form an aggregation separated from the remaining sesquiterpenes, germacrene-D (MS), and two pairs of associated compounds, namely (E)-β-caryophyllene/(E,E)-α-farnesene and cycloisosativene (MS)/α-ylangene (MS; Fig. 2). Interestingly, the first group consists of fully inducible compounds, while the latter, with the exception of (E)-β-caryophyllene, are also produced constitutively by many lines. The two homoterpenes along with the two alcohols nerolidol and linalool, the acetate esters, and the two nitrogen-containing aromatics, respectively, represent other examples of chemically related compounds that grouped relatively close together.
Figure 2.
Factor loadings and factor scores representing the association among the 31 maize inbred lines as revealed by PCA on relative amounts of odors emitted (least square means obtained by ANCOVA; see Table II). The first three axes account for 26.6%, 18.4%, and 10.8% of the total variation, respectively.
The first principal component axis (27% of the variation) is highly positively correlated with the proportions of the above-mentioned six sesquiterpenes, and the second axis (18% of the variation) is positively correlated to acetate esters and negatively correlated to homoterpenes and the two sesquiterpenes cycloisosativene (MS)/α-ylangene (MS). Lines from different origins were not clearly separated along these two axes. Only axis 3 (11% of the variation), which is highly positively correlated to (E)-β-caryophyllene and to a lesser degree to germacrene-D (MS) and (E,E)-α-farnesene, splits the European Flint lines from the American Dent lines of the Minnesota 13 complex (Fig. 2). The two apparent outliers F7001 and F1852 are actually of mixed origin. European Flint lines were characterized by on average higher release rates of (E)-β-caryophyllene as compared to Minnesota 13 complex and Early Dent lines.
Relationship with Neutral Variation
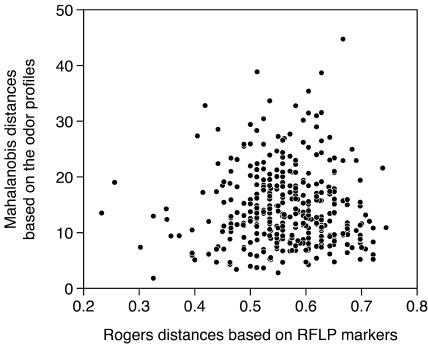
The distances among the lines according to the volatile profiles and the corresponding genetic distances based on RFLP markers were not in agreement (r = −0.009): closely related lines exhibit similar volatile profiles, while genetically distant lines may release qualitatively either similar or very distinct odor blends (Fig. 3).
Figure 3.
Comparison of genetic distances among the maize inbred lines based on RFLP markers (Rogers distances obtained from Dubreuil et al., 1996) and the corresponding Mahalanobis distances based on the odor profiles (least square means obtained by ANCOVA; see Table II).
DISCUSSION
Our study revealed an impressive variability among the lines in the absolute quantity of odors emitted as well as in the qualitative composition of the blend, both characters that are to a large degree genetically determined. Maternal effects could not be estimated by this experiment but are expected to have had little impact on the differences between the lines because they were obtained by sexual reproduction (controlled selfing) and seeds were produced the same year in the same location. The extent of variation observed in this study by far surpasses what was recorded in previous studies with European maize hybrids (Gouinguené et al., 2001) and Mexican germlines (Fritzsche-Hoballah et al., 2002). This may be due to the wider range of origins but also due to the fact that inbred lines carry only one allele of the genes encoding for the enzymes that are involved in the biosynthesis of the odor compounds, which could result in greater polarization of the volatile blends between plants. As suggested by the scarce information available, polymorphism in the odor profiles observed among teosintes, i.e. wild Zea species and subspecies, seems to be comparable or less pronounced than within cultivated maize (Gouinguené et al., 2001). Clearly, maize has retained a high degree of genetic variability during the process of domestication (Wang et al., 1999), of which our findings provide another example.
Injection of caterpillar regurgitate proved a powerful method to induce the emission of considerable amounts of volatiles. With few exceptions, the characteristic odor pattern of each line observed with the injection method was remarkably similar to that obtained by exposing plants to caterpillar damage for 1 d. A perfect match cannot be expected because the proportion of a specific compound in the blend depends to some degree on the overall strength of the elicitation, as different compounds show different dose-response curves to elicitors (T. Degen, unpublished data). If the induction is only weak, for example, already constitutively present compounds are expected to be more dominant in the odor blend. Differences in the time course of induction among the compounds (Turlings et al., 1998b) may further contribute to divergence of odor profiles obtained with different induction methods.
While PCA based on RFLP markers showed a clear-cut separation between European flint lines and U.S. dent lines (Dubreuil et al., 1996), our PCA data on induced odors revealed only a comparatively loose association among lines belonging to the same classified heterotic group. Nevertheless, European flint lines form a relatively homogeneous group, most notably distinguished by a high proportion of (E)-β-caryophyllene in the odor bouquet. The outlying Yugoslavian line F7001 with very low levels of (E)-β-caryophyllene has U.S. pop corn and Iowa Stiff Stalk Synthetic parents in its pedigree. It also showed a diverging protein content (Burstin et al., 1994). The same applies to the other aberrant line, F1852, which is classified with the dent line of the Minnesota 13 complex according to RFLP markers (Dubreuil et al., 1996) but in fact is derived from Eastern European flint germ plasm.
Strong correlations among the variable compounds as illustrated by the clusters for the factor loadings of the PCA may mostly reflect shared biosynthetic pathways of the volatiles concerned. For example, common genetic factors, terpene synthases, may be involved in the formation of the terpene alcohols linalool, (E)-nerolidol, and geranyllinalool from the respective universal mono-, sesqui-, and diterpene precursors geranyl-, farnesyl-, and geranylgeranyldiphosphates. The two homoterpenes (3E)-4,8-dimethyl-1,3,7-nonatriene and (3E,7E)-4,8,12-trimethyl-1,3,7,11-decatetraene are oxidative degradation products of (E)-nerolidol and geranyllinalool, respectively (Boland et al., 1999). This may explain the relatively close association of linalool and (E)-nerolidol with the homoterpenes. However, a recently demonstrated and characterized (E)-nerolidol synthase from maize did not seem to be capable of converting geranyldiphosphate to linalool in detectable amount (Degenhardt and Gershenzon, 2000). The acetate esters represent another interesting cluster of compounds with correlated proportions in the blend. Apart from the shared acetyl group, the moieties of these compounds are of different biosynthetic origin: (Z)-3-hexenyl acetate is a product of the lipoxygenase pathway leading to green leaf volatiles, benzyl and phenethyl acetate are aromatics, and geranyl acetate originates from the isoprenoid pathway. We may speculate that a common acetyl-transferring enzyme participates in the formation of these four compounds.
Quantitative protein data were reported to give a picture of relationships between lines clearly different from that obtained by RFLP markers (Burstin et al., 1994). Since the quantity of volatiles emitted can be expected to reflect among others variation in the amounts of enzymes, it is not surprising that we recorded analogous results, i.e. no obvious correspondence of distances based on odor profiles with those based on monogenic molecular markers, whose variation is to a large extent selectively and phenotypically neutral.
For some tritrophic systems, recent studies pointed at an intriguing degree of sophistication in the communication between plants and the third trophic level, inasmuch as the odor blends released by the plants may provide specific information on the identity of the attacking herbivore and, hence, on its suitability for the prey-seeking carnivores (De Moraes et al., 1998; Du et al., 1998). By contrast, our extensive experience with chemical analyses of induced maize volatiles indicates that differences in the induced odor blends between different genotypes attacked by the same herbivore exceed the differences between plants belonging to the same genotype but fed upon by different herbivores (Turlings et al., 1993a, 1998a, 1998b; Gouinguené et al., 2001, 2003). It appears that the enormous intraspecific variability may compromise the reliability of herbivore-specific signals across a wide range of plant genotypes, at least for naive wasps. By relying on their ability to learn and associate successful foraging and egg-laying experiences with the encountered odor pattern (Turlings et al., 1993b; Vet et al., 1995), female parasitoids may in part overcome this problem.
One of the major unsolved problems to be addressed in future studies is the identification of the behaviorally active components in herbivore-induced odor blends and of optimal combinations of such semiochemicals for attraction of predators and parasitoids (Dicke and van Loon, 2000). The highly variable maize inbred lines screened here lend themselves for further behavioral studies with parasitoids and to verify whether parasitism rates of caterpillars under field conditions differ in response to the variable amounts of induced volatiles released by their host plants. Several studies have demonstrated the importance of herbivore-induced odor emissions outside the laboratory (Scutareanu et al., 1997; De Moraes et al., 1998; Thaler, 1999; Bernasconi Ockroy et al., 2001; Kessler and Baldwin, 2001). These findings and the observed genetic variability in induced maize volatiles open the possibility to exploit the phenomenon of herbivore-induced volatile emissions in biological control of caterpillars by breeding varieties with enhanced attractiveness to the natural enemies (Bottrell et al., 1998). Such an approach may also be applicable to increase the repellent effect of induced volatiles to herbivores (De Moraes et al., 2001). It should, however, be considered that the observed differences in odor emissions for plants of different geographic origin may imply that insects from different regions have adapted differently to optimize their use of plant-provided volatile signals.
MATERIAL AND METHODS
Plant Sources
Thirty-one maize (Zea mays) inbred lines were chosen from the collection of the Station de Génétique Végétale, Ferme du Moulon, INRA Gif-sur-Yvette, France, in order to cover as much of the genetic diversity as possible from lines used by breeders in Europe and North America. In a previous RFLP study (Dubreuil et al., 1996), 116 maize inbred lines were assigned on the basis of their molecular genetic distances to classes that roughly corresponded to 5 well-known heterotic groups in maize, named after the geographical origin of the lines with known pedigree. The lines included in this study (Table I) were selected from clusters within each of these heterotic groups to represent the original set of 116 lines. They were tested together with the commercial hybrid Delprim, which was already well studied with respect to odor emission and served as a reference in an earlier investigation (Gouinguené et al., 2001). The inbred lines have undergone more than 10 generations of controlled selfing, so that they can be considered as completely homozygous. No heterozygosity was found at any of the 43 RFLP loci (data not shown; P. Dubreuil, unpublished data). All the seed were produced in 1995 at a single location near Toulouse, France.
Plant Rearing
Seeds were kept in petri dishes lined with moist filter paper until germination and then transferred individually into plastic pots (volume 360 mL) filled with a mixture of regular potting soil and vermiculite. The seedlings were grown in a climate chamber under the following conditions: 23°C, 60% relative humidity, and 40,000 lm/m2 with a photoperiod of 16-h-light/8-h-dark and light phase starting at 1 am. They were used for the experiments about 10 d after germination, when they had developed on average four leaves and the fifth leaf started to show (mean shoot dry weight ± sd = 0.26 ± 0.08 g).
Plant Treatments
Regurgitant for odor induction was collected from third- to fifth-instar larvae of Spodoptera littoralis Boisd. (Turlings et al., 1993a), which before had been fed with maize leaves for at least 1 d. All samples were taken from the same stock solution stored at −76°C. Odor emission by the plants was induced by injecting twice 10 μL of undiluted regurgitant with a 10-μl syringe into the base of the stem, about 2 to 5 mm above the first lateral roots. The needle was inserted in such a way as to avoid perforating the stem on the opposite side of the injection and thereby avoiding leaking of regurgitant as much as possible. Injections were done about 9 h after the onset of the light period. Afterward, the plants were kept in the laboratory under three fluorescent tubes (Standard F 36 W/133-T8; Sylvania, Erlangen, Germany) at 26 ± 1°C, 35 ± 5% relative humidity, and 10,000 lm/m2. The induction period, i.e. the interval between the injection and the start of the odor collections, lasted 5 to 5.5 h. To verify whether a more natural induction method leads to comparable results, plants of 12 lines representing the extremes in total emission and/or profile were subjected to caterpillar attack. To this end, 20 second- or third-instar larvae of S. littoralis were allowed to feed on the plants for a duration of 22 h, starting 5 h before the onset of the dark period. They were prevented from escaping by covering the plants with a cellophane bag and then removed 1 h before the start of the odor collection. This experiment also included undamaged plants to assess constitutive odor emission. Shoot dry weight was determined after the odor collections and introduced as a factor in the statistical analyses in order to account for differences in size at the time of the odor collections due to variable growth rates of the plants.
Odor Collection
The seedlings were cut off at the stem base with a razor blade and placed with the severed end in a small glass vial filled with water and sealed with cotton wool, before being introduced into a previously described (Turlings et al., 1991) closed push-and-pull odor collection system. Compressed air purified over a charcoal filter and humidified in a gas dispersion tube entered five parallel collection chambers consisting of Pyrex glass tubes (approximately 30 cm long, 6.5 cm in diameter) assembled from two detachable sections connected by ground glass joints. Air was blown into each glass cylinder and pulled out by a vacuum pump at a rate of 0.6 L/min. Volatile compounds carried by the air flow were adsorbed at the outlet of the collection chamber on a trap consisting of a glass tube (4 mm i.d., length 8 cm) packed with 25 mg Super-Q polymer (80-100 mesh; Alltech, Deerfield, IL; Heath and Manukian, 1992). During the collection, which lasted 2 h, the plants were illuminated by eight fluorescent tubes (Osram [Munich] L 20 W/25 S; 9,000 ± 1,000 lm/m2), and the temperature measured outside the glass chambers was 31 ± 1°C. Afterward, the Super-Q filters were extracted with 150 μL of dichloromethane (Lichrosolv; Merck, Darmstadt, Germany), and 200 ng of n-octane and n-nonyl acetate (Sigma, St. Louis) in 10 μL of dichloromethane were added to the samples as internal standards. The solutions were stored at −76°C until analysis.
Chemical Analysis: Identification and Quantification
Chemical analyses were performed with a Hewlett-Packard HP 6890 series gas chromatograph (Palo Alto, CA) equipped with an automated on-column injection system and a flame ionization detector. A 3-μL aliquot of each sample was injected into an apolar EC-1 capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness; Alltech Associates) preceded by a deactivated retention gap (10 m, 0.25 mm i.d.; Connex; Agilent, Palo Alto, CA) and a deactivated pre-column (30 cm, 0.53 mm ID, Connex). Helium at 24 cm/s was used as carrier gas. Following injection, column temperature was maintained at 50°C for 3 min, raised to 230°C at 8°C/min, and held at 230°C for 9.5 min. The detected volatiles were quantified based on comparison of their peak area with those of the internal standards and identified by comparison of retention times with those observed in previous analyses (Turlings et al., 1998b). To confirm these identities, we analyzed one sample per line using a gas chromatograph (Agilent 6890 Series GC system G1530A), with the same kind of apolar column (HP-1) and an identical temperature program, coupled to a mass spectrometer operated in electron impact mode (Agilent 5973 Network Mass Selective Detector; transfer line 230°C, source 230°C, quadrupole 150°C, ionization potential 70 eV, scan range 50–400 atomic mass units). Volatiles were identified by comparison of their mass spectra with those of the Wiley275 and NIST98 libraries and by comparison of their retention times with those of authentic standards. The few compounds for which no pure standards were available to determine retention times are marked with (MS) in the text, and their identification should be considered tentative. In certain lines, mass spectra obtained for some sesquiterpenes hinted at coelution with additional compounds, mostly other sesquiterpenes. When present in trace amounts only, the identity of the compounds could not always be confirmed by gas chromatography-mass spectrometry.
Experimental Design
The 32 different genotypes, 31 inbred lines, and the commercial hybrid Delprim were analyzed in a randomized block design. Each block consisted of one plant of each genotype raised together in the growth chamber. The collection system allowed the simultaneous testing of five individual plants a day, which corresponded to one plot. Accordingly, seven plots (days) were necessary for the analysis of one block of the entire genotype set along with a blank, i.e. an empty glass cylinder containing a vial and moist cotton. Within each block, the plants and the blank were randomly assigned to the seven plots, and the experiment was repeated six times. A few missing data from plants that did not grow were replaced by blanks in the odor collection system. A total of 189 plants and 9 blanks were analyzed. For the second experiment with only 12 lines, 2 units with 5 collection chambers were available, and the lines were tested in 3 groups, each block including 4 plants belonging to different lines induced by caterpillar feeding, the 4 corresponding intact plants, a blank, and a Delprim hybrid as a reference. No significant differences in total odor emission were detected among induced Delprim plants belonging to the three different groups. The treatments, i.e. herbivore-damaged versus intact plants, were replicated six or seven times for each line.
Statistical Analysis
Fifty prominent peaks in the chromatogram were integrated. Peaks for which no significant differences were found between the system blanks and the plant extracts were considered impurities and discarded from the analysis. For each compound, the odor quantities exhibited log-normal distributions. Consequently, for each selected compound k, the raw data 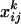 (ng/h) were transformed according to
(ng/h) were transformed according to 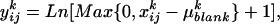 where
where 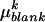 is the average quantity of compound k found in the blank, i indices the inbred line, and j the repetition. Among the remaining substances, only those for which the average log-transformed amount (in ng h−1 g−1 dry weight) exceeded a value of 2 in at least one inbred line were retained. In quantity, the 23 thus selected compounds (Table II) typically accounted for approximately 98% of the constituents of plant origin. Highly volatile alcohols and aldehydes derived from the lipoxygenase pathway (green leaf volatiles) and immediately set free by the plants upon mechanical damage were only very sporadically recorded in substantial amounts and were not included. Due to variable growth rates among the lines, there were some differences in plant size at the time of the odor collections. To eliminate the influence of size and physiological state on volatile emission, shoot dry weight was introduced as a covariate in the statistical analysis. So, ANCOVAs were performed on the data using the following model
is the average quantity of compound k found in the blank, i indices the inbred line, and j the repetition. Among the remaining substances, only those for which the average log-transformed amount (in ng h−1 g−1 dry weight) exceeded a value of 2 in at least one inbred line were retained. In quantity, the 23 thus selected compounds (Table II) typically accounted for approximately 98% of the constituents of plant origin. Highly volatile alcohols and aldehydes derived from the lipoxygenase pathway (green leaf volatiles) and immediately set free by the plants upon mechanical damage were only very sporadically recorded in substantial amounts and were not included. Due to variable growth rates among the lines, there were some differences in plant size at the time of the odor collections. To eliminate the influence of size and physiological state on volatile emission, shoot dry weight was introduced as a covariate in the statistical analysis. So, ANCOVAs were performed on the data using the following model 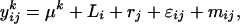 where Li is the random line effect, rj (j = 1..n, n = 6) the repetition,
where Li is the random line effect, rj (j = 1..n, n = 6) the repetition, 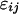 the residual variation, and
the residual variation, and 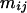 the shoot dry weight of the plant. We estimated the residual variance
the shoot dry weight of the plant. We estimated the residual variance 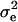 and the genetic variance among the inbred lines
and the genetic variance among the inbred lines 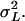 As the inbred lines are completely homozygous and can be considered as clones,
As the inbred lines are completely homozygous and can be considered as clones, 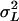 estimates the total genetic variance of the line collection, and the broad-sense heritability can be calculated as
estimates the total genetic variance of the line collection, and the broad-sense heritability can be calculated as 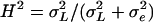 (Lynch and Walsh, 1998). We also computed the following parameters: the residual coefficient of variation for compound
(Lynch and Walsh, 1998). We also computed the following parameters: the residual coefficient of variation for compound 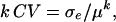 and the repeatability
and the repeatability 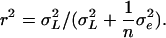 The repeatability measures how precisely the amount of volatile compound emitted by one inbred line is estimated in this experiment. The broad-sense heritability measures the part of the phenotypic variation that is due to genetic differences between the inbred lines. The analyses were also performed on relative quantities of the odors in the blend by introducing the total amount of volatile emission (ln-transformed sum of the quantities of all 23 compounds; zij) as covariate:
The repeatability measures how precisely the amount of volatile compound emitted by one inbred line is estimated in this experiment. The broad-sense heritability measures the part of the phenotypic variation that is due to genetic differences between the inbred lines. The analyses were also performed on relative quantities of the odors in the blend by introducing the total amount of volatile emission (ln-transformed sum of the quantities of all 23 compounds; zij) as covariate: 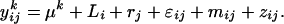 This was done to check whether significant differences among lines for emission of a given compound were not only due to genetic differences for the total amount of volatile emission. Least square means computed for each inbred line by using this model were used to perform a PCA on the relative volatile amounts and to calculate Mahalanobis distances between the lines. For each pair of inbred lines, the Mahalanobis distances were compared to the molecular Rogers' distances based on 39 to 43 RFLP markers, which were available from an earlier study (Dubreuil et al., 1996), except for lines Ia5pop and C6.
This was done to check whether significant differences among lines for emission of a given compound were not only due to genetic differences for the total amount of volatile emission. Least square means computed for each inbred line by using this model were used to perform a PCA on the relative volatile amounts and to calculate Mahalanobis distances between the lines. For each pair of inbred lines, the Mahalanobis distances were compared to the molecular Rogers' distances based on 39 to 43 RFLP markers, which were available from an earlier study (Dubreuil et al., 1996), except for lines Ia5pop and C6.
Acknowledgments
Caterpillars and eggs of S. littoralis were supplied by Novartis (Syngenta) Insect Control, Basel, and by Josette Chaufaux, INRA, Domaine de la Minière, Guyancourt, France. We thank Marisa Mamede and Christian Malosse for assistance with the experiments and the gas chromatography analyses, respectively.
This work was supported by the Swiss National Science Foundation (grant no. 823A–053427), by the Roche Research Foundation, and by the Institut National d'Agriculture Paris-Grignon (INA P-G).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.039891.
References
- Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276: 945–949 [Google Scholar]
- Bernasconi Ockroy ML, Turlings TCJ, Edwards PJ, Fritzsche-Hoballah ME, Ambrosetti L, Bassetti P, Dorn S (2001) Response of natural populations of predators and parasitoids to artificially induced volatile emissions in maize plants (Zea mays L.). Agric For Entomol 3: 201–209 [Google Scholar]
- Boland W, Koch T, Krumm T, Piel J, Jux A (1999) Induced biosynthesis of insect semiochemicals in plants. In DJ Chadwick, JA Goode, eds, Insect-Plant Interactions and Induced Plant Defence, Novartis Foundation Symposium 223, October 13–15, 1998, London. John Wiley & Sons, Chichester, UK, pp 110–131 [DOI] [PubMed]
- Bottrell DG, Barbosa P, Gould F (1998) Manipulating natural enemies by plant variety selection and modification: a realistic strategy? Annu Rev Entomol 43: 347–367 [DOI] [PubMed] [Google Scholar]
- Burstin J, de Vienne D, Dubreuil P, Damerval C (1994) Molecular markers and protein quantities as genetic descriptors in maize. I. Genetic diversity among 21 inbred lines. Theor Appl Genet 89: 943–950 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570–573 [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410: 577–580 [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Gershenzon J (2000) Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210: 815–822 [DOI] [PubMed] [Google Scholar]
- Dicke M (1994) Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J Plant Physiol 143: 465–472 [Google Scholar]
- Dicke M, Sabelis MW (1988) How plants obtain predatory mites as bodyguards. Neth J Zool 38: 148–165 [Google Scholar]
- Dicke M, van Loon JJA (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97: 237–249 [Google Scholar]
- Du Y, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24: 1355–1368 [Google Scholar]
- Dubreuil P, Dufour P, Krejci E, Causse M, de Vienne D, Gallais A, Charcosset A (1996) Organization of RFLP diversity among inbred lines of maize representing the most significant heterotic groups. Crop Sci 36: 790–799 [Google Scholar]
- Fritzsche-Hoballah ME, Tamò C, Turlings TCJ (2002) Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? J Chem Ecol 28: 951–968 [DOI] [PubMed] [Google Scholar]
- Gouinguené S, Alborn H, Turlings TCJ (2003) Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J Chem Ecol 29: 145–162 [DOI] [PubMed] [Google Scholar]
- Gouinguené S, Degen T, Turlings TCJ (2001) Variability in herbivore-induced odour emissions among maize cultivars and their wild ancestors (teosinte). Chemoecology 11: 9–16 [Google Scholar]
- Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT (2000) Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124: 408–417 [DOI] [PubMed] [Google Scholar]
- Heath RR, Manukian A (1992) Development and evaluation of systems to collect volatile semiochemicals from insects and plants using charcoal-infused medium for air purification. J Chem Ecol 18: 1209–1226 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Tumlinson JH (1995) Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol 21: 1217–1227 [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B (1998) Genetics and Analysis of Quantitative Traits. Sinauer Associates, Sunderland, MA
- Mattiacci L, Dicke M, Posthumus MA (1994) Induction of parasitoid attracting synomone in Brussel sprouts plants by feeding of Pieris brassicae larvae: role of mechanical damage and herbivore elicitor. J Chem Ecol 20: 2229–2247 [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–331 [PMC free article] [PubMed] [Google Scholar]
- Scutareanu P, Drukker B, Bruin J, Posthumus MA, Sabelis MW (1997) Volatiles from Psylla-infested pear trees and their possible involvement in attraction of anthocorid predators. J Chem Ecol 23: 2241–2260 [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2: 1–6 [Google Scholar]
- Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399: 686–688 [Google Scholar]
- Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH (2000) Volicitin, an elicitor of maize volatiles in oral secretions of Spodoptera exigua: isolation and bioactivity. J Chem Ecol 26: 189–202 [Google Scholar]
- Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S (1998. a) The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control 11: 122–129 [Google Scholar]
- Turlings TCJ, Lengwiler UB, Bernasconi ML, Wechsler D (1998. b) Timing of induced volatile emissions in maize seedlings. Planta 207: 146–152 [Google Scholar]
- Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH (1993. a) An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol 19: 411–425 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH (1992) Systemic release of chemical signals by herbivore-injured corn. Proc Natl Acad Sci USA 89: 8399–8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE (1991) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol 17: 2235–2251 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Wäckers FL, Vet LEM, Lewis WJ, Tumlinson JH (1993. b) Learning of host-finding cues by hymenopterous parasitoids. In DR Papaj, AC Lewis, eds, Insect Learning. Ecology and Evolutionary Perspectives. Chapman & Hall, New York, pp 51–78
- Vet LEM, Lewis WJ, Cardé RT (1995) Parasitoid foraging and learning. In RT Cardé, WJ Bell, eds, Chemical Ecology of Insects. Chapman & Hall, New York, pp 65–101
- Wang R-L, Stec A, Hey J, Lukens L, Doebley J (1999) The limits of selection during maize domestication. Nature 398: 236–239 [DOI] [PubMed] [Google Scholar]