Abstract
More volatile organic carbon is lost from plants as isoprene than any other molecule. This flux of carbon to the atmosphere affects atmospheric chemistry and can serve as a substrate for ozone production in polluted air. Isoprene synthesis may help leaves cope with heatflecks and active oxygen species. Isoprene synthase, an enzyme related to monoterpene synthases, converts dimethylallyl diphosphate derived from the methylerythritol 4-phosphate pathway to isoprene. We used dideuterated deoxyxylulose (DOX-d2) to study the regulation of the isoprene biosynthetic pathway. Exogenous DOX-d2 displaced endogenous sources of carbon for isoprene synthesis without increasing the overall rate of isoprene synthesis. However, at higher concentrations, DOX-d2 completely suppressed isoprene synthesis from endogenous sources and increased the overall rate of isoprene synthesis. We interpret these results to indicate strong feedback control of deoxyxylulose-5-phosphate synthase. We related the emission of labeled isoprene to the concentration of labeled dimethylallyl diphosphate in order to estimate the in situ Km of isoprene synthase. The results confirm that isoprene synthase has a Km 10- to 100-fold higher for its allylic diphosphate substrate than related monoterpene synthases for geranyl diphosphate.
Isoprene is emitted from many plants, including mosses, ferns, gymnosperms, and angiosperms (Kesselmeier and Staudt, 1999; Sharkey and Yeh, 2001). Isoprene emission from plants is one of the most important sources of volatile organic compounds released to the atmosphere (Fehsenfeld et al., 1992; Guenther et al., 1995, 2000; Fuentes et al., 2000). Isoprene emission has been hypothesized to provide leaves protection from heat damage (Sharkey and Singsaas, 1995), especially during short heatflecks (Sharkey et al., 2001), and to protect against ozone and other active oxygen species (Loreto and Velikova, 2001; Loreto et al., 2001; Affek and Yakir, 2002). Isoprene is made in chloroplasts from dimethylallyl diphosphate (DMAPP) by isoprene synthase (Silver and Fall, 1991, 1995; Schnitzler et al., 1996), an enzyme related to monoterpene synthases (Miller et al., 2001). Monoterpene synthases have a high affinity for their substrate, geranyl diphosphate (Km in the micromolar range), but isoprene synthases examined to date have a much lower affinity (Kms in the millimolar range; Wildermuth and Fall, 1996; Lehning et al., 1999).
Isoprene is made mostly from carbon previously fixed by photosynthesis (Delwiche and Sharkey, 1993; Karl et al., 2002; Affek and Yakir, 2003). A low but significant amount (10%–30%) of the carbon in isoprene is not rapidly labeled when 13CO2 is fed to leaves, about the same proportion of carbon in phosphoglyceric acid that is not quickly labeled (Atkins and Canvin, 1971). Current hypotheses are that this slowly labeling pool is pyruvate imported into the chloroplast to be joined with glyceraldehyde 3-phosphate (GAP) in the first step of the methyl erythritol 4-phosphate (MEP) pathway, which supplies carbon for isoprene synthesis (Kreuzwieser et al., 2002; Rosenstiel et al., 2003).
The flux of carbon through the MEP pathway required for isoprene synthesis far exceeds that required for other purposes, such as carotenoid synthesis (Sharkey et al., 1991). This raises the following questions: (1) How is the MEP pathway regulated to provide carbon required for isoprene synthesis, and (2) is isoprene emission limited by MEP pathway capacity or by isoprene synthase?
The first intermediate of the MEP pathway is deoxyxylulose 5-phosphate (DXP). The nonphosphorylated, free deoxyxylulose obtained according to Jux and Boland (1999) can be fed to leaves and is rapidly incorporated into the products of the MEP pathway (Arigoni et al., 1997; Schwender et al., 1997; Sagner et al., 1998). Isoprene derived from dideuterated deoxyxylulose (DOX-d2) can be detected and differentiated from unlabeled isoprene originating from endogenous sources by laser photoacoustics (Dahnke et al., 2000; Kühnemann et al., 2002). We used this technique to study the biochemical regulation of isoprene emission (Wolfertz et al., 2003). We found that feeding DOX stimulated the rate of isoprene emission only when leaves became starved for carbon. We also found that a large and variable proportion of the total DMAPP in leaves is located outside the chloroplast and is, thus, not available for isoprene emission.
DOX-d2 was used to investigate properties of isoprene synthase, especially the affinity of isoprene synthase for its substrate DMAPP. In addition, we studied the regulation of the MEP pathway and its ability to supply a regulated amount of DMAPP for isoprene synthesis during and after heatflecks.
RESULTS
Effect of Feeding DOX-d2
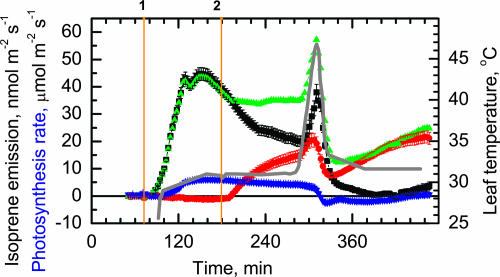
Feeding DOX-d2 (2.94 mm) caused the emitted isoprene to become labeled with deuterium (Fig. 1). The first deuterium-labeled isoprene was emitted approximately 10 to 15 min after the onset of feeding, and the degree of labeling increased continuously over the next 2 h until a rather constant level was reached (Fig. 1). When the DOX-d2 solution was replaced with water, the degree of deuterium labeling of isoprene decreased after about 20 min and was further reduced to low levels in about 2 h (Fig. 1). The addition of DOX-d2 did not significantly affect the total (labeled plus unlabeled) rate of isoprene emission. The deuterium-labeled isoprene displaced unlabeled isoprene, but the total emission rate of isoprene remained fairly constant even as the source of precursors was changed from primarily endogenous (unlabeled) to primarily exogenous (deuterium labeled) and back again. Photosynthesis was largely unaffected by administration of 2.94 mm DOX-d2.
Figure 1.
Time course of isoprene emission and photosynthesis rate of a leaf fed with DOX-d2. The light was turned on at point 1 (1,000 μmol m−2 s−1), and DOX-d2 (2.94 mm) was added to the water fed to the petiole of the leaf at point 2. At point 3, the solution feeding the petiole was replaced with water. Green triangles represent total isoprene emission, black squares represent isoprene not labeled with deuterium, red circles represent deuterium-labeled isoprene, and blue inverted triangles represent photosynthetic rate. The solid gray line represents leaf temperature.
In most experiments, feeding 2.94 mm DOX-d2 resulted in half or more of the emitted isoprene to be labeled. The average proportion after 90 min of feeding was 56 ± 5% (average ± se, n = 8). The total isoprene emission rate (labeled plus unlabeled) 90 min after feeding was 108 ± 11% (average ± se, n = 8) of the rate observed before feeding.
At higher concentration (36.25 mm) DOX-d2, isoprene became completely labeled within 45 min (Fig. 2). In addition, the total rate of isoprene emission increased while photosynthesis was decreased. The relatively high se of the isoprene emission rates reflects leaf-to-leaf variation. The rate of isoprene emission from DOX-d2 was 35% ± 10% (average ± se, n = 3) more than the total isoprene emission before feeding, and the increase occurred in all three trials. The inhibition of photosynthesis was always less than the inhibition of unlabeled isoprene (Fig. 3).
Figure 2.
Isoprene emission and CO2 assimilation before and 45 min after feeding DOX-d2 (36.25 mm). The white bar is emission of unlabeled isoprene, the black bar is deuterated isoprene, and the gray bar is CO2 assimilation (right scale). Values are means ± se, n = 3.
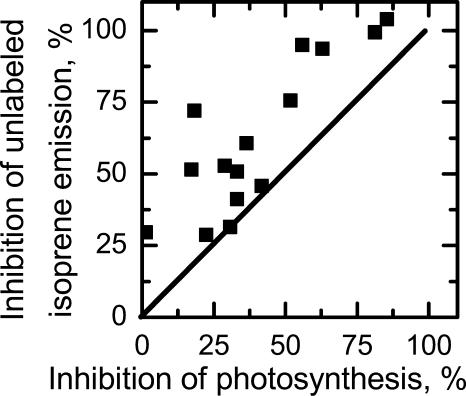
Figure 3.
Inhibition of unlabeled isoprene emission versus inhibition of photosynthesis (CO2 uptake). A 1:1 line is shown. All points lie above this line, indicating greater inhibition of isoprene emission than photosynthesis in all cases. The data are drawn from all experiments and include feedings of three different DOX feedings.
Chloroplast Levels of DMAPP-d2
We hypothesize that DOX-d2 is converted to DMAPP-d2 inside the chloroplast and that no DMAPP-d2 derived from DOX-d2 occurs in the cytosol. Attempts to feed DMAPP-d2 were unsuccessful, as expected. This confirms that the C5-diphosphates do not cross membranes to reach the chloroplasts in sufficient amounts to be detected by isoprene emission. In addition, we note that one of the xylulose kinases in the Arabidopsis genome is predicted to have a transit sequence. Therefore, all DMAPP-d2 derived from the exogenous precursor should be in the chloroplast, although the cytosol has substantial amounts of DMAPP resulting from the MVA pathway. By measuring the DMAPP-d2 we could relate isoprene-d2 emission to its substrate independent of the cytosolic DMAPP.
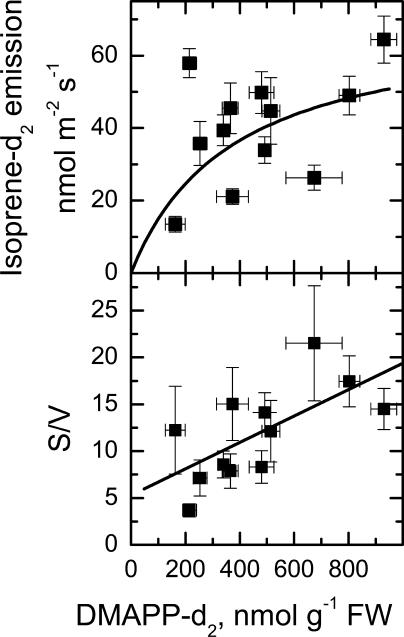
The rate of emission of isoprene-d2 was correlated with the amount of DMAPP-d2 measured in the leaf (Fig. 4). Using a Hanes-Wolff plot, we calculated a Km of 139 nmol g−1 fresh weight and Vmax of 71 nmol m−2 s−1. To relate the Km to a concentration, the plastid volume of Eucalyptus globulus was assumed to be about 15% of the leaf fresh weight (i.e. 0.15 mL/g fresh weight) and the leaves about 90% liquid volume (based on data from Winter et al. [1993, 1994] for spinach and barley), then the Km is calculated to be 0.97 mm.
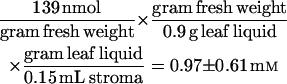 |
Figure 4.
Isoprene-d2 emission versus DMAPP-d2 concentration in the leaf. The fitted line in the top section assumed Michalis-Menten kinetics. The bottom section is a Hanes-Woolf plot (S/V is substrate concentration/velocity of the reaction) that gave an estimated Vmax of 71 nmol m−2 s−1 and Km of 375 nmol g−1 fresh weight. The line had an R2 of 0.66.
If the Eucalyptus leaves had a higher dry weight to fresh weight ratio compared with spinach and barley, then the Km would be higher, thus this calculation is conservative and likely underestimates the Km.
Effect of Heating
Leaves emitting roughly equal amounts of labeled and unlabeled isoprene at 30°C were heated to leaf temperatures between 45°C and 50°C. In Figure 5A, a representative example with the rate and extent of heating is shown. Initially, the total isoprene emission rate increased substantially, but the majority of the increased emission was derived from endogenous sources (i.e. was unlabeled; Fig. 5). Photosynthesis was eventually inhibited by the heat treatment and did not recover immediately after returning the leaf temperature to 30°C. Following the heat treatment, the total rate of isoprene emission was severely depressed. DOX-d2-dependent isoprene emission decreased immediately after the heatfleck but recovered beyond prestress levels within 2 h, while the isoprene derived from endogenous sources decreased to zero after the heatfleck and did not significantly increase after photosynthesis began to recover 2 h later (Fig. 5). For the experiment shown in Figure 5 (one of three), endogenous isoprene production was 19.8 nmol m−2 s−1 and isoprene-d2 production was 15.9 nmol m−2 s−1 at 30°C prior to the heatfleck (time 291 min).
Figure 5.
Time course of isoprene emission and photosynthesis rate of a leaf fed with DOX-d2. The light was turned on at point 1 (1,000 μmol m−2 s−1), and DOX-d2 was added to the water (2.94 mm) fed to the petiole of the leaf at point 2. Symbols are described in Figure 1.
DISCUSSION
Pathways of Isoprene Synthesis
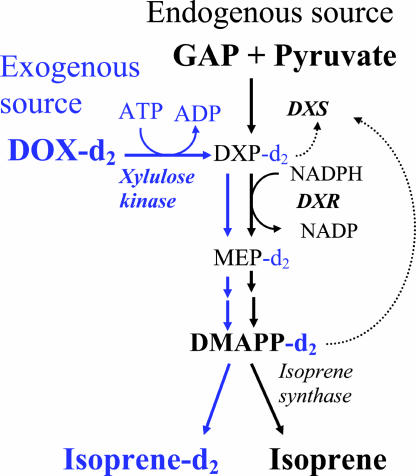
Isoprene can be made from endogenous carbon sources, especially from the Calvin cycle (Sharkey and Yeh, 2001) or from exogenous DOX (Arigoni et al., 1997; Schwender et al., 1997; Fig. 6). The pathway for incorporating exogenous DOX into plastid isoprenoids has a surprisingly high capacity. Rates of isoprene-d2 emission as high as 60 nmol m−2 s−1 were observed. Because the label in DOX-d2 appeared in isoprene rather rapidly and faded out with a similar kinetics after consumption, it is likely that labeled isoprene emission is indicative of the rate of phosphorylation of DOX. One may speculate that plants have an endogenous capacity to phosphorylate DOX since the latter may act as a transport molecule in the plant. The phosphorylation is assumed to be catalyzed by xylulose kinase, and there are two genes for this enzyme in Arabidopsis. In Escherichia coli this enzyme confers the ability for the bacterium to utilize the sugar lyxose, an epimer of Xyl (Sanchez et al., 1994).
Figure 6.
Pathway for isoprene synthesis from either endogenous sources (GAP + pyruvate) or exogenous DOX-d2. Dotted lines indicate hypothesized feedback loops. DXR, DXP reductoisomerase.
High concentrations of DOX (>3 mm) caused an inhibition of photosynthesis. This could be related to imbalance in phosphate inside the chloroplast as the DOX became phosphorylated since low levels of phosphate can occur when phosphorylation substrates are fed to leaves (Sharkey and Vanderveer, 1989). Since each DMAPP sequesters two phosphates, this can reduce the phosphate available for ATP synthesis and in so doing limit the rate of photosynthesis (Sharkey, 1990). The decline in photosynthesis was associated with a clear decrease in the synthesis of unlabeled isoprene, even though total rate of isoprene synthesis was increased. This shows that the ability of leaves to convert DXP to isoprene was unaffected by DOX feeding, even though photosynthesis was. In all experiments, the extent of the inhibition of the photosynthesis was less than that of the production of unlabeled isoprene. In previous work we saw that only when the photosynthetic Calvin cycle was limited by removing both CO2 and O2 from the airstream flowing over a leaf was isoprene emission limited by carbon availability (Loreto and Sharkey, 1993). This indicates that the reduction of unlabeled isoprene in response to feeding DOX was not the result of the effects of DOX on photosynthesis.
Kinetic Parameters of Isoprene Synthase
To determine the in vivo Km for isoprene synthesis, we assayed DMAPP-d2 and assumed that this entire compound was in the plastid compartment. A significant amount of the total DMAPP in leaves is outside of chloroplasts (Rosenstiel et al., 2002; Wolfertz et al., 2003) and, hence, whole-leaf measurements of DMAPP do not give reliable information on the amount of DMAPP that is available to isoprene synthase in the plastid. There is evidence for DOX-d2 contributing to sesquiterpene synthesis (Piel et al., 1998) but little evidence that the diphosphates can readily cross the chloroplast envelope. Other cases of cross-talk between the isoprenoid pathways normally involve long time periods (Laule et al., 2003). The very large fluxes of carbon needed for isoprene synthesis, as much as 100 times the MEP pathway flux needed to make other isoprenoids in plastids (Sharkey et al., 1991), and the nearly complete elimination of isoprene synthesis in fosmidomycin-fed leaves (Zeidler et al., 1998; Loreto and Velikova, 2001; Sharkey et al., 2001) indicate that DMAPP cannot cross into the chloroplast at rates that are significant for isoprene synthesis in the species tested so far (Phragmites australis, Platanus x acerifolia, Populus nigra, Pureria montana, and Quercus rubra). Thus, we assumed that all DMAPP-d2 was in the chloroplasts. Loreto et al. (2004) measured the 13C-labeled pool of DMAPP in a similar way to distinguish the plastid DMAPP (quickly labeled when plants were fed 13CO2) and cytosolic DMAPP.
The estimated Km was 0.97 mm and may have been higher. This is an indirect measure that may have more uncertainty than in vitro measurements, but this estimate is important because it reflects the enzyme kinetics in situ. We did not measure the Km directly in this work, but our estimated in vivo Km corresponds very well with the high in vitro estimates of the Km of isoprene synthase (Silver and Fall, 1995; Schnitzler et al., 1996; Wildermuth and Fall, 1998; Lehning et al., 1999; Miller et al., 2001). Related monoterpene synthases have Kms for their substrate, geranyl diphosphate, between 10 and 100 μm (Fischbach et al., 2000) and sometimes as low as 2.6 μm (Alonso and Croteau, 1991). The results presented here show that in situ the Km for the allylic diphosphate substrate is much higher for isoprene synthase than for the related monoterpene synthases.
Regulation of the MEP Pathway
Using DOX-d2 we were able to examine the regulation of the early steps of the isoprene biosynthetic pathway. When 2.9 (e.g. Fig. 1) or 7.5 mm (data not shown) DOX-d2 was fed, the exogenous DXP displaced the endogenous supply, but the overall rate of isoprene emission remained constant and presumably so did the concentrations of DMAPP and DXP in the plastid. This could be achieved by a negative feedback loop from DXP, DMAPP, or other intermediates of the pathway modulating the activity of DXP synthase (DXS) to keep the supply of DMAPP constant. If the regulation of the rate of DMAPP synthesis would be localized downstream of DXP, we would not expect exogenous DXP (from administration of DOX-d2) to displace the endogenous source of DMAPP precursors. The fact that the overall rate of isoprene emission changed very little until the endogenous isoprene was reduced nearly to zero indicates that the feedback loop is very effective. Very high levels of DOX-d2 can overcome the regulation.
When leaves were heated, isoprene synthase, known to be highly temperature dependent (Monson et al., 1992; Lehning et al., 1999), increased in activity. This would relieve the feedback on DXS, and so substantially more carbon was diverted to isoprene through DXS. Heat also likely increased the activity of xylulose kinase and may have increased the rate of arrival of DOX-d2 through effects on transpiration. However, these effects were much smaller than the relief of feedback inhibition on DXS, and so heat stimulated production of unlabeled isoprene more than of labeled isoprene. However, following the heating, leaves had a net negative carbon balance and, presumably, GAP and pyruvate were in limited supply. Labeled isoprene was emitted at about the rate it was before the heatfleck, showing that the regulation of isoprene emission following a heatfleck depends on availability of substrate rather than changes in isoprene synthase activity. Even heat stress severe enough to eliminate photosynthesis does not appear to affect the capacity to convert DOX to isoprene. Draining pools of intermediates at temperatures above 35°C may account for the reduction in isoprene emission seen when leaves are above 35°C for more than 15 min (Singsaas and Sharkey, 1998).
The hypothesized regulation of the MEP pathway in response to the various treatments is summarized in Table I. Assuming a high gain feedback loop, we make the following interpretations. When 2.94 mm DOX-d2 is fed to leaves, the rate of arrival and conversion to DXP is less than the rate of use of DXP, and DXS is perfectly regulated to compensate for the exogenous carbon source, keeping the DXP and DMAPP concentrations constant (and, therefore, isoprene emission rate stays constant). When 36.25 mm DOX is fed, the exogenous source exceeds the steady-state consumption, and so even with essentially no endogenous production of DXP, the DMAPP concentration increases, resulting in an increase in the rate of isoprene emission. This could only occur if the steady-state rate of DMAPP was not substantially above the Km of isoprene synthase for DMAPP. From the discussion above, we believe that the very high Kms (in the millimolar range) reported for the isolated enzyme also are true for the enzyme in situ, making it possible for exogenous DOX to increase the rate of isoprene emission by increasing the concentration of DMAPP.
Table I.
Hypothesized effects of DOX-d2 feeding and a heatfleck on isoprene emission and related parameters
| [DXP] | DXS Activity | Xylulose Kinase Activity | Isoprene Synthase Activity | |
|---|---|---|---|---|
| DOX-d2 feeding (2.94 mm) | Remains constant | Reduced to compensate for DXP from DOX | Activity dependent on DOX arrival | Constant, as the DMAPP level is constant |
| DOX-d2 feeding (36.25 mm) | Increases | Nearly zero as DMAPP and DXP levels above set points | Can exceed the regulated rate of DXP synthesis | Increased because [DMAPP] is no longer regulated |
| Heatfleck | Decreases | Increases, reduced feedback | Little affected (substrate limited) | Increased by the heat |
| Post heatfleck | Decreases | Substrate limited | Not affected | Substrate limited |
In earlier work, we found that high emission rates of isoprene were correlated with low chloroplastic pools of DMAPP (Wolfertz et al., 2003). This indicates that the set point for DMAPP concentration may be different in different plants and would require very large variations in the amount of isoprene synthase in leaves. Both the substrate concentration inside chloroplasts and the amount of isoprene synthase (Schnitzler et al., 1997) have effects on the rate of isoprene emission. In future work, we plan to measure DMAPP concentration and isoprene synthase amount and activity in response to changes known to influence isoprene emission rates, including development (Harley et al., 1994; Monson et al., 1994) and weather (Sharkey et al., 1999; Hanson and Sharkey, 2001; Pétron et al., 2001).
MATERIALS AND METHODS
Plant Culture
Leaves were taken from a Eucalyptus globulus tree kept in a greenhouse of the Institute of Plant Pathology at the University of Bonn. During summer, the tree stood outside in the garden of the institute. Leaves were cut under water and held in water for the whole measurements. Measurements were done between July and September 2003.
Gas Exchange, Leaf Sampling, and DMAPP Assay
Gas exchange measurements were carried out as described in Kühnemann et al. (2002) but using a leaf chamber with temperature control plus a fan to reduce the boundary layer resistance to heat transfer. We found that that Eucalyptus emits isoprene in the ppmv level, dominating the spectral absorption of the photoacoustic spectrometer. Given the selectivity and sensitivity of the photoacoustic spectrometer for isoprene and isoprene-d2, other monoterpene emissions of Eucalyptus leaves can be neglected. Isoprene, isoprene-d2, and CO2 levels were continuously determined with a 12CO2-laser-photoacoustic spectrometer. The data were analyzed by a least squares fit described by Gäbler (1998), and the error bars on the photoacoustic data give the uncertainty of the fit. All measurements were made at 30°C (except for heatfleck measurements) and 1,000 μmol m−2 s−1 light. After turning on light, basal isoprene emission rate was determined before adding exogenous DOX-d2. After isoprene-d2 reached its constant level, leaves were removed from the chamber and quickly frozen in liquid nitrogen and stored in liquid nitrogen (covered by aluminum foil) until they were used for DMAPP assays.
For determining the DMAPP amount, we used the method of Fisher et al. (2001); briefly, leaf tissue was ground in liquid nitrogen and afterward distilled water was added. The samples were then centrifuged in a microfuge for 15 min at 4°C. The supernatant was injected into a glass vial already filled with sulfuric acid (8 m), flushed with gaseous nitrogen, and sealed. After incubating for 45 min in a water bath at 50°C, the assay vial was flushed again with nitrogen and the headspace gas passed through a cooling trap at −90°C before entering the photoacoustic spectrometer in order to determine isoprene-d2 concentration. This method converts 5% of the DMAPP to isoprene as determined by Wolfertz et al. (2003), and this correction was used to calculate the DMAPP-d2 from the isorpene-d2 measurement.
This work was supported by the University of Wisconsin-Madison/University of Bonn Exchange program and by the U.S. National Science Foundation (grant no. IBN–0212204).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043737.
References
- Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affek HP, Yakir D (2003) Natural abundance carbon isotope composition of isoprene reflects incomplete coupling between isoprene synthesis and photosynthetic carbon flow. Plant Physiol 131: 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso WR, Croteau R (1991) Purification and characterization of the monoterpene cyclase [gamma]-terpinene synthase from Thymus vulgaris. Arch Biochem Biophys 286: 511–517 [DOI] [PubMed] [Google Scholar]
- Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH (1997) Terpenoid biosynthesis from 1-deoxy-D-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA 94: 10600–10605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CA, Canvin DT (1971) Photosynthesis and CO2 evolution by leaf discs: gas exchange, extraction and ion-exchange fractionation of 14C-labelled photosynthetic products. Can J Bot 49: 1225–1234 [Google Scholar]
- Dahnke H, Kahl J, Schüler G, Boland W, Urban W, Kühnemann F (2000) On-line monitoring of biogenic isoprene emissions using photoacoustic spectroscopy. Appl Phys B 70: 275–280 [Google Scholar]
- Delwiche CF, Sharkey TD (1993) Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ 16: 587–591 [Google Scholar]
- Fehsenfeld FC, Calvert J, Fall R, Goldan P, Guenther A, Hewitt C, Lamb B, Liu S, Trainer M, Westberg H, et al (1992) Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Global Biogeochem Cycles 6: 389–430 [Google Scholar]
- Fischbach RJ, Zimmer I, Steinbrecher R, Pfichner A, Schnitzler JP (2000) Monoterpene synthase activities in leaves of Picea abies (L.) Karst. and Quercus ilex L. Phytochemistry 54: 257–265 [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Rosenstiel TN, Shirk MC, Fall R (2001) Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem 2001 292: 272–279 [DOI] [PubMed] [Google Scholar]
- Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Botteneheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A, et al (2000) Biogenic hydrocarbons in the atmospheric boundary layer: a review. B Am Meteorol Soc 81: 1537–1575 [Google Scholar]
- Gäbler R (1998) Aufbau eines transportablen photoakustischen Spektrometers für den empfindlichen und kontinuierlichen Ethylennachweis in der Pflanzenphysilogie. PhD thesis. University of Bonn, Bonn, Germany
- Guenther A, Geron C, Pierce T, Lamb B, Harley P, Fall R (2000) Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos Environ 34: 2205–2230 [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, et al (1995) A global model of natural volatile organic compound emissions. J Geophys Res 100: 8873–8892 [Google Scholar]
- Hanson DT, Sharkey TD (2001) Rate of acclimation of the capacity for isoprene emission in response to light and temperature. Plant Cell Environ 24: 937–946 [Google Scholar]
- Harley PC, Litvak ME, Sharkey TD, Monson RK (1994) Isoprene emission from velvet bean leaves. Interactions among nitrogen availability, growth photon flux density, and leaf development. Plant Physiol 105: 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Fall R, Rosenstiel TN, Prazeller P, Larsen B, Seufert G, Lindinger W (2002) On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 215: 894–905 [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33: 23–88 [Google Scholar]
- Kreuzwieser J, Graus M, Wisthaler A, Hansel A, Rennenberg H, Schnitzler J-P (2002) Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol 156: 171–178 [DOI] [PubMed] [Google Scholar]
- Kühnemann F, Wolfertz M, Arnold A, Lagemann M, Popp A, Schüler G, Jux A, Boland W (2002) Simultaneous online detection of isoprene and isoprene-d2 using infrared photoacoustic spectroscopy. Appl Phys B 75: 397–403 [Google Scholar]
- Laule O, Furholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehning A, Zimmer I, Steinbrecher R, Brüggemann N, Schnitzler JP (1999) Isoprene synthase activity and its relation to isoprene emission in Quercus robur L-leaves. Plant Cell Environ 22: 495–504 [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126: 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Brancaleoni E, Ciccioli P (2004) 13C labelling reveals chloroplastic and extra-chloroplastic pools of dimethylallyl piroposphate and their contribution to isoprene formation. Plant Physiol 135: 1903–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1993) On the relationship between isoprene emission and photosynthetic metabolites under different environmental conditions. Planta 189: 420–424 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213: 483–487 [DOI] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R (1994) Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia 99: 260–270 [DOI] [PubMed] [Google Scholar]
- Monson RK, Jaeger CH, Adams III WW, Driggers EM, Silver GM, Fall R (1992) Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98: 1175–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétron G, Harley P, Greenberg J, Guenther A (2001) Seasonal temperature variations influence isoprene emission. Atmos Environ 28: 1707–1710 [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W (1998) Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew Chem Int Ed 37: 2478–2481 [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Fisher AJ, Fall R, Monson RK (2002) Differential accumulation of dimethylallyl diphosphate in leaves and needles of isoprene- and methylbutenol-emitting and nonemitting species. Plant Physiol 129: 1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK (2003) Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421: 256–259 [DOI] [PubMed] [Google Scholar]
- Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH (1998) Differential incorporation of 1-deoxy-D-xylulose into monoterpenes and carotenoids in higher plants. Chem Commun 2: 221–222 [Google Scholar]
- Sanchez JC, Gimenez R, Schneider A, Fessner WD, Baldoma L, Aguilar J, Badia J (1994) Activation of a cryptic gene encoding a kinase for L-xylulose opens a new pathway for the utilization of L-lyxose by Escherichia coli. J Biol Chem 269: 29665–29669 [PubMed] [Google Scholar]
- Schnitzler J-P, Arenz R, Steinbrecher R, Lehning A (1996) Characterization of an isoprene synthase from leaves of Quercus petraea (Mattuschka) Liebl. Bot Acta 109: 216–221 [Google Scholar]
- Schnitzler J-P, Lehning A, Steinbrecher R (1997) Seasonal pattern of isoprene synthase activity in Quercus robur leaves and its significance for modeling isoprene emission rates. Bot Acta 110: 240–243 [Google Scholar]
- Schwender J, Zeidler J, Groner R, Muller C, Focke M, Braun S, Lichtenthaler FW, Lichtenthaler HK (1997) Incorporation of 1-deoxy-D-xylulose into isoprene and phytol by higher plants and algae. FEBS Lett 414: 129–134 [DOI] [PubMed] [Google Scholar]
- Sharkey TD (1990) Feedback limitation of photosynthesis and the physiological role of ribulose bisphosphate carboxylase carbamylation. Bot Mag Tokyo 2: 87–105 [Google Scholar]
- Sharkey TD, Chen XY, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125: 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Loreto F, Delwiche CF (1991) The biochemistry of isoprene emission from leaves during photosynthesis. In TD Sharkey, EA Holland, HA Mooney, eds, Trace Gas Emissions from Plants. Academic Press, San Diego, pp 153–184
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374: 769 [Google Scholar]
- Sharkey TD, Singsaas EL, Lerdau MT, Geron C (1999) Weather effects on isoprene emission capacity and applications in emissions algorithms. Ecol Appl 9: 1132–1137 [Google Scholar]
- Sharkey TD, Vanderveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91: 679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52: 407–436 [DOI] [PubMed] [Google Scholar]
- Silver GM, Fall R (1991) Enzymatic synthesis of isoprene from dimethylallyl diphosphate in aspen leaf extracts. Plant Physiol 97: 1588–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver GM, Fall R (1995) Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem 270: 13010–13016 [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD (1998) The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ 21: 1181–1188 [Google Scholar]
- Wildermuth MC, Fall R (1996) Light-dependent isoprene emission – characterization of a thylakoid-bound isoprene synthase in Salix discolor chloroplasts. Plant Physiol 112: 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Fall R (1998) Biochemical characterization of stromal and thylakoid-bound isoforms of isoprene synthase in willow leaves. Plant Physiol 116: 1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191: 180–190 [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F, Yeh S, Weise SE (2003) Biochemical regulation of isoprene emission. Plant Cell Environ 26: 1357–1364 [Google Scholar]
- Zeidler J, Schwender J, Müller C, Wiesner J, Weidemeyer C, Beck E, Jomaa H, Lichtenthaler HK (1998) Inhibition of the non-mevalonate 1-deoxy-D-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch 53: 980–986 [Google Scholar]