Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders in children. However, the pathogenesis of ADHD remains unclear. Iron, an important trace element, is implicated in brain function and dopaminergic activity. Recent studies have investigated the association between iron deficiency and ADHD, but the results are inconsistent.
Methods
A systemic search of MEDLINE, EMBASE, Web of Science and Cochrane Library databases was supplemented by manual searches of references of key retrieved articles. Study quality was evaluated using the Newcastle-Ottawa Scale. The standardised mean difference (SMD) and 95% confidence intervals (CIs) were calculated using a random-effects model. H2 and I2 were used to evaluate the heterogeneity, and sensitivity, subgroup and meta-regression analyses were conducted to explore the reason of heterogeneity.
Results
The search yielded 11 studies published before July 25, 2016. Of these, 10 studies, comprising 2191 participants and 1196 ADHD cases, reported serum ferritin levels, and six studies, comprising 617 participants and 369 ADHD cases, reported serum iron levels. Serum ferritin levels were lower in ADHD cases (SMD = -0.40, 95% CI = -0.66 to -0.14). However, we found no correlation between serum iron levels and ADHD (SMD = -0.026, 95% CI = -0.29 to 0.24). Meta-regression analysis indicated that publication year, age, gender, sample size, and Hb levels did not significantly influence the pooled estimates of serum ferritin.
Conclusion
Lower serum ferritin rather than serum iron is associated with ADHD in children.
Introduction
As one of the most common childhood psychiatric disorders, attention-deficit/hyperactivity disorder (ADHD) is estimated to affect approximately 5.9–7.1% of children and adolescents worldwide [1]. Inattention, impulsivity, and/or hyperactive/impulsive behaviors are key features of ADHD. It has also been associated with other psychiatric comorbidities, such as disruptive behaviors, antisocial activities, and poor verbal working memory disorders, resulting in impaired learning and social ability [2, 3]. ADHD is commonly considered to develop as a result of interactions between genetic and environmental factors [4]. However, the specific underlying etiology of ADHD remains unclear.
Several studies of the neurobiology and treatment of ADHD have suggested that nutritional factors, such as glucose metabolism, fatty acid metabolism, and mineral or vitamin deficiencies, may affect brain function and are implicated in the pathogenesis of the disorder [5–7]. Of these nutritional factors, iron deficiency has attracted more attention as iron plays an important role in the regulation of dopaminergic activity, which is associated with the pathogenesis and symptoms of ADHD [8].
In 1997, Sever et al. [9] found significantly increased serum ferritin levels and decreased ADHD symptom scores in children with ADHD after iron supplementation, suggesting that nonanemic children with ADHD may benefit from iron supplementation. Subsequently, other researchers attempted to explore the relationship between iron deficiency and ADHD. However, their conclusions were often inconsistent. Some authors reported that mean serum ferritin levels were lower in children with ADHD than in healthy controls [8, 10, 11]. Furthermore, Juneja et al. [10] reported that serum ferritin levels were inversely correlated with ADHD symptom severity. Moreover, Cortese et al. [8] compared brain iron levels in children with ADHD and healthy controls using magnetic resonance imaging (MRI), reporting significantly lower estimated brain iron in the bilateral thalami of children with ADHD. Similarly, Adisetiyo et al. [11] found that medication-naïve patients with ADHD had lower estimated brain iron in the striatum and thalamus than did healthy controls. However, other authors have failed to find a relationship between serum ferritin and ADHD [12–14].
In 2012, Cortese et al. [15] published a systematic review of studies investigating the correlation between iron and ADHD. However, they did not perform statistical analyses on indices of iron status. Moreover, several studies with large sample sizes have been published after 2012. We therefore conducted a systematic review and meta-analysis, including more recent data, to estimate the association between iron status and ADHD.
Materials and Methods
Study Retrieval
We searched PubMed, EMBASE, Web of Science and Cochrane Library from inception to July 25, 2016. The search strategy combined Medical Subject Heading (MeSH) terms and free text terms, including ‘‘attention-deficit/hyperactivity disorder” or ‘‘ADHD” or “attention deficit disorders with hyperactivity” or ‘‘hyperkinetic disorder” or “hyperkinetic syndrome” and “iron” or “siderophilin” or “serotransferrin” or “transferrin” or “ferritin” or “iron deficiency anemia” or “ID.” We only included studies on humans published in English, but study location was not restricted. Additionally, the references of included studies and previous reviews were screened manually to identify additional appropriate studies for inclusion.
Study Selection
Two investigators independently selected the studies. Initially, clearly irrelevant studies were excluded by scanning titles and abstracts. The full texts of the remaining articles were then evaluated carefully according to our eligibility criteria. Where required, any disagreement on eligibility for inclusion was resolved by a third author.
We included studies that met all the following criteria: 1) they assessed serum ferritin or serum iron in children or adults with ADHD and non-ADHD controls; 2) patients with ADHD were diagnosed according to formal criteria (e.g. the Diagnostic Statistical Manual of Mental Disorders [DSM]-IV [TR] or previous versions); 3) they provided the mean and standard deviation for raw data. We excluded case reports, conference abstracts, reviews, and animal studies. Studies without raw data or reporting iron status in tissues other than the blood were also excluded.
Data Extraction
Two reviewers independently extracted information on the first author, publication year, country, study design, exclusion criteria, age (mean±SD), number of cases and controls, proportion of female and male participants, analytical technology, ferritin (mean±SD), serum iron (mean±SD), and units of measure.
Quality Assessment
Study quality was rated by two authors using the Newcastle-Ottawa Scale, which is recommended for quality assessment of cohort and case-control studies, and has a maximum score of nine. Studies scoring 7 to 9, 4 to 6, and 0 to 3 are regarded as high quality, moderate quality, and low quality, respectively.
Statistical Analysis
The standardised mean difference (SMD) was used to assess the association between blood iron status and ADHD. Due to the high heterogeneity between studies, we chose a random-effects model to calculate the pooled SMD. Heterogeneity across studies was assessed using H2 [16] and I2 [17]. H2>1 or I2 >50% indicated high heterogeneity. We also used Galbraith radial plots to explore which studies may be contributing to heterogeneity. Additionally, we carried out sensitivity analyses excluding studies that might contribute to high heterogeneity indicated by the Galbraith radial plot.
Subgroup analyses stratified studies by treatment, comorbidities, and assay method. Publication bias was assessed by visual inspection of funnel plots and Egger’s tests. For Egger’s tests, P <0.05 was considered statistically significant. We also performed unrestricted maximum likelihood random-effects meta-regressions, including publication year, mean age, gender (% female), and sample size as regressors. All statistical analyses were performed using Stata 14.0 (Stata Corp, College Station, Texas, USA)
Results
Literature Search and Selection
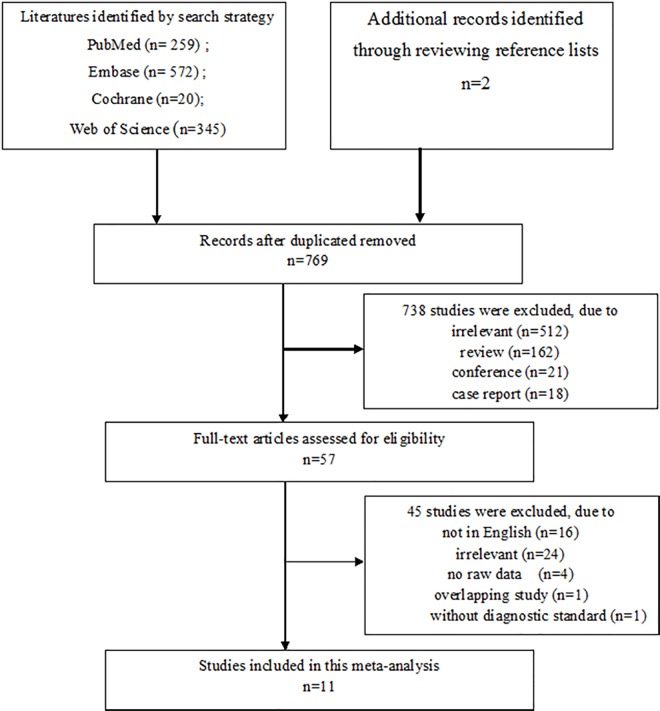
We screened a total of 1198 articles from PubMed, EMBASE, Cochrane Database, and Web of Science, as well as 2 additional articles from manual searches of the references of articles. After removing 427 duplicates, 162 reviews, 512 irrelevant studies, 18 case reports, and 21 conference abstracts, we retained 57 studies for full-text review. After further screening, 45 studies were excluded, including 16 studies not in English, 24 irrelevant studies, 2 studies without raw data, 1 overlapping study and 1 study without a diagnostic standard. Finally, we included 11 studies (Fig 1). Of these, 10 studies, comprising 2191 participants and 1196 ADHD cases, analyzed serum ferritin and 6 studies, comprising 617 participants and 369 ADHD cases, analyzed serum iron.
Fig 1. The flow diagram of the selection process.
Study Characteristics
Table 1 presents the characteristics of the 11 included studies, published from 2004 to 2016. All were case-control studies. The number of participants varied across studies, and ranged from 27 to 1260. Four studies were conducted in Europe [8, 13, 14, 18], one in the USA [11], one in Egypt [19], one in Brazil [20], and four in Asia [10, 21–23]. With the exception of three studies [8, 22, 23], all included studies reported medication use in the patients. As far as we know, psychoeducation as well as drug therapy and/or behavioral intervention are the main strategies for the treatment of ADHD. Drug therapy mainly included stimulants such as such as methylphenidate or atomoxetine, we categorized those patients with ADHD who were medication-naïve or without drug medication for at least one month into the without treatment group.
Table 1. Characteristics of included studies.
| Author, Year | Area | Total Sample | Total with ADHD | Age Mean±SDADHD Controls | Sex (%M) ADHD Controls | Serum Ferritin and/ or Serum Iron ADHD/Controls | Exclusion Criteria | Psychiatric Comorbid Disorders | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Chen, 2004 | Taiwan | 110 | 58 | 8.5±2.2; 7.9±2.0 | 91.4; 76.9 | 19.7±6.4/16.1±6.8 (iron umol/L) | N/A | N/A | Drug free for 2 months |
| Konofal, 2004 | France | 80 | 53 | N/A | 84.9; 74.1 | 23±13/44±22 (ferritin ng/ml) | Additional psychiatric disorders, physical diseases, and malnutrition | No | Drug free for 2 months |
| Menegassi2010 | Brazil | 62 | 41 | 9.0±2.6 (group1); 8.8±2.4; (group2); 8.9±2.7 | 80.5; 71.4 | 56.6±19.2/58.8±28.9(ferritin ng/ml) 79.5±27.1/92±31.4 (iron ug/dl) | IQ<70, psychiatric disorders other than ODD and CD, any medical conditions affecting iron | ODD, CD | Group1 without medication use; Group 2 treated for 3 months |
| Juneja, 2010 | India | 50 | 25 | 8.44±1.687.96±1.46 | 84.0; NA | 6.04±3.85/48.96±41.64 (ferritin ng/ml) | IQ<85, any chronic illness, or any acute severe illness in last two weeks | ODD | Newly diagnosed |
| Kwon, 2011 | Korea | 96 | 48 | 6.98±0.397.53±0.63 | 54.2; 58.3 | 35.8±16.6/37.1±18.3(ferritin ng/ml) 80.9±33.3/82.0±28.1(iron ug/dl) | IQ<70, psychiatric comorbidities, and neurological disorders | No | Naive |
| Mahmoud,2011 | Egypt | 73 | 58 | 8.6 ± 1.8; 8.3 ± 1.8 | 44.8; 48.0 | 24.8±14.1/32.6±18.7(ferritin ug/dl) | Co-morbid neurological disorders, severely anemia | No | Without medication for 1 month |
| Cortese, 2011 | France | 27 | 18 | 9.9±1.5; 10.1±2.2 | 88.9; 55.6 | 32.4±13.4/51.6±16.4 (ferritin ng/ml) | IQ<70, neurological diseases, comorbid psychiatric disorders expect ODD | ODD | N/A |
| Donfrance-sco, 2013 | Italy | 194 | 101 | 8.9±2.5; 9.2±3.1 | 91.1; 88.2 | 33.0±17.8/33.1±18.7 (ferritin ng/ml) | IQ<70, neurobiological disease, any medical conditions affecting iron | ODD, anxiety, depression, dysthymic disorder | Naive |
| Adisetiyo,2014 | USA | 49 | 22 | 12.6 ±2.8; 13.3±2.6 | 68.2; N/A | 50.8±25.2/38.2±22.8 (ferritin ng/ml) 76.9±26.0/65.7±28.2 (iron mcg/dl) | Diagnosis of psychotic, major depressive, conduct, tic, or pervasive developmental disorders | Yes | 10 medication naïve patients; 12 patients with medication treatment |
| Bener, 2014 | Qatar | 1260 | 630 | 11.5±3.8; 11.5±3.6 | 50.0; 49.7 | 36.3±5.9/38.2±5.6 (ferritin ng/ml) 82.1±13.6/85.6±12.4(iron ng/ml) | Hb <10 g/dL, calcium supplements or vitamin D intake during the last 6 month; epilepsy or antiepileptic drugs | N/A | N/A |
| Percinel, 2016 | Finland | 300 | 200 | 11.0±2.4; 11.0±3.0 | 63.5; 60.0 | 27.9±15.3/30.8±17.5(ferritin ng/ml) 71.9±31/77.9±30.6 (iron ug/dl) | IQ<80, comorbid psychiatric disorder, any medical conditions affecting iron, Hb<12g/L | No | No |
N/A not available; IQ intelligence quotient; TIBC total iron binding capacity; ODD oppositional defiant disorder; CD conduct disorder.
According to the Newcastle-Ottawa Scale, the overall methodological quality was good, with 10 studies that were high quality and only one study [18] that was of moderate quality (Table 2).
Table 2. The Newcastle-Ottawa scale score of included studies.
| Study | Selection | Comparability | Outcome | Total scores |
|---|---|---|---|---|
| Chen, 2004 | 3 | 2 | 2 | 7 |
| Konofal, 2004 | 3 | 1 | 2 | 6 |
| Menegassi, 2010 | 3 | 2 | 2 | 7 |
| Juneja, 2010 | 3 | 2 | 2 | 7 |
| Kwon, 2011 | 4 | 2 | 2 | 8 |
| Mahmoud, 2011 | 3 | 2 | 2 | 7 |
| Cortese, 2011 | 4 | 2 | 2 | 8 |
| Donfrancesco, 2013 | 4 | 2 | 3 | 9 |
| Adisetiyo, 2014 | 4 | 2 | 2 | 8 |
| Bener, 2014 | 4 | 2 | 2 | 8 |
| Percinel, 2015 | 3 | 2 | 2 | 7 |
Serum Ferritin and ADHD
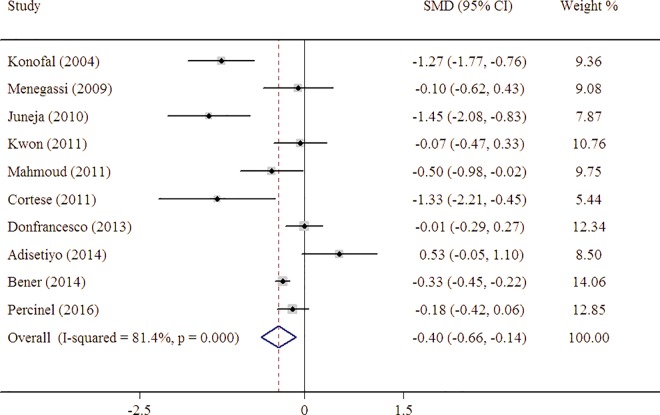
Serum ferritin levels were significantly lower in patients with ADHD compared with healthy controls, with a pooled SMD estimate from 10 studies of -0.40 (95% CI = -0.66 to -0.14) indicating a negative association between serum ferritin and ADHD (Fig 2). However, there was significant statistical heterogeneity across studies (I2 = 8.4%, 95% CI 67–90%; H2 = 4.37).
Fig 2. Forest plot for the random-effects meta-analysis of serum ferritin levels in persons with ADHD and controls.
Serum Iron and ADHD
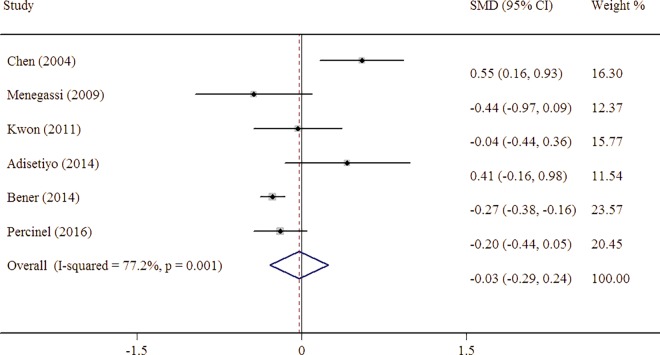
The pooled estimate from six studies showed a serum iron level of -0.026 (95% CI = -0.29 to 0.24), which did not indicate a negative correlation between serum iron and ADHD (Fig 3). There was statistical heterogeneity across studies with I2 77% (95% CI 49–90%).
Fig 3. Forest plot for the random-effects meta-analysis of serum iron levels in persons with ADHD and controls.
Stratified and Sensitivity Analyses of Serum Ferritin
Of the 10 studies included in the meta-analysis, eight included patients without treatment, with a pooled SMD for serum ferritin of -0.29 (95% CI = -1.26 to 0.68), while patients under treatment had a pooled SMD of -0.36 (95% CI = -0.72 to 0.00). The pooled SMD in five studies of patients with ADHD without other psychiatric disorders was -0.48 (95% CI = -0.94 to -0.01), while the pooled SMD in four studies of patients with ADHD and psychiatric comorbidities was -0.42, (95% CI = -1.06 to 0.22) (Table 3).
Table 3. Pooled estimate and heterogeneity in subgroups analyses.
| Subgroup analysis | No. of trails | No. of subjects | Pooled estimate (SMD, 95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ADHD | Controls | I2 | P-value | |||
| Treatment | ||||||
| Without treatment | 8 | 516 | 366 | -0.36 (-0.72 to 0.00) | 82.6 | <0.001 |
| Under treatment | 3 | 50 | 57 | -0.29 (-1.26 to 0.68) | 80.9 | 0.005 |
| Comorbid | ||||||
| Yes | 5 | 207 | 175 | -0.42 (-1.06 to 0.22) | 82.2 | 0.001 |
| No | 4 | 359 | 200 | -0.48 (-0.94 to -0.01) | 86.6 | <0.001 |
| Assay method | ||||||
| ELISA | 4 | 237 | 170 | -0.78 (-1.48 to -0.07) | 89.8 | <0.001 |
| Others | 5 | 911 | 787 | -0.21 (-0.51 to 0.09) | 73.4 | 0.005 |
We also conducted a meta-analysis of the assay methods used, separated into ELISA or other methods. The pooled SMD for serum ferritin levels assessed using ELISA was -0.78 (95% CI = -1.48 to -0.07), while for other methods it was -0.21 (95% CI = -0.51 to 0.09).
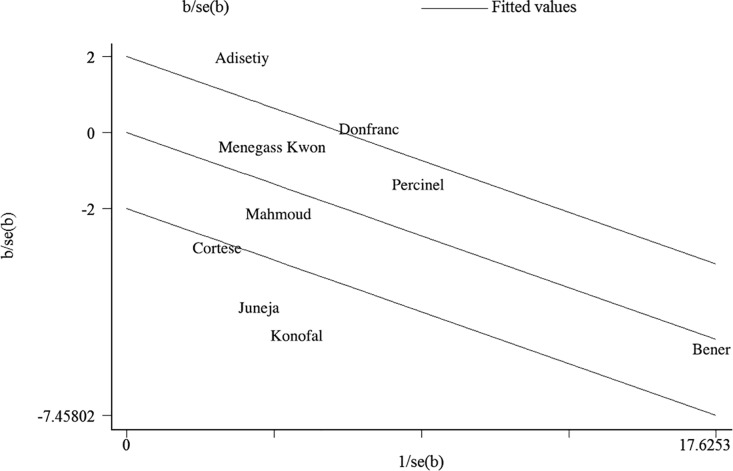
The Galbraith radial plot graph (Fig 4) showed four studies [8, 10, 11, 18] that might contribute to the high heterogeneity across studies. Further sensitivity analysis indicated that the overall pooled results did not vary substantially after omitting each of the four studies one at a time (Table 4).
Fig 4. Galbraith radial plot graph of included studies.
Table 4. The sensibility analysis of included studies for serum ferritin.
| Study omitted | Estimate | 95% Confidence Interval | |
|---|---|---|---|
| Konofal (2004) | -0.30 | -0.54 | -0.05 |
| Menegassi (2009) | -0.43 | -0.71 | -0.15 |
| Juneja (2010) | -0.30 | -0.54 | -0.06 |
| Kwon (2011) | -0.44 | -0.73 | -0.16 |
| Mahmoud (2011) | -0.39 | -0.67 | -0.11 |
| Cortese (2012) | -0.34 | -0.60 | -0.08 |
| Donfrancesco (2013) | -0.46 | -0.75 | -0.17 |
| Adisetiyo (2014) | -0.47 | -0.73 | -0.22 |
| Bener (2014) | -0.43 | -0.79 | -0.076 |
| Percinel (2016) | -0.44 | -0.75 | -0.13 |
Meta-regression Analysis
To explore sources of heterogeneity among studies, we performed a meta-regression analysis. This indicated that year of study, sample size, gender, mean age and mean Hb did not significantly influence the pooled estimates of serum ferritin (Table 5).
Table 5. Meta-regression of serum ferritin levels ADHD and controls.
| Moderator | No. of comparisons | P value | 95% Confidence Interval | |
|---|---|---|---|---|
| Publication Year | 10 | 0.07 | -0.01 | 0.23 |
| Age (mean, years) | 10 | 0.38 | -0.17 | 0.41 |
| Gender (% female) | 10 | 0.33 | 0.22 | 59.2 |
| Hb (g/dl) | 8 | 0.10 | -0.15 | 1.28 |
| Sample size | 9 | 0.73 | 0.99 | 1.00 |
Publication Bias
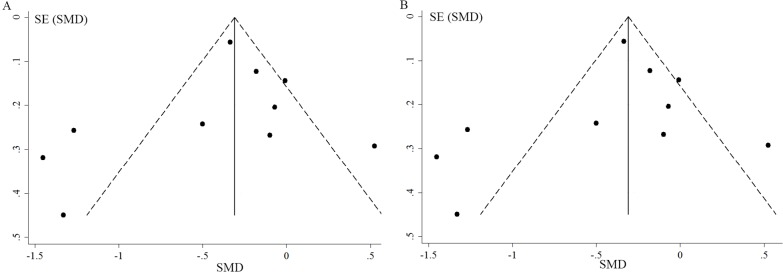
Visual inspection of the funnel plots for included studies of serum iron and serum ferritin revealed some asymmetry (Fig 5A and 5B). However, Egger’s tests did not give significant evidence of publication bias among the included studies (P = 0.42 for serum ferritin, P = 0.35 for serum iron).
Fig 5.
Funnel plot for publication bias test between-group meta-analysis (A) on serum ferritin levels (B) on serum iron levels.
Discussion
Our meta-analysis yielded 10 case-control studies investigating serum ferritin levels in a total of 2191 participants and 1196 ADHD cases. The pooled SMD from the 10 studies indicated that serum ferritin levels were lower in ADHD cases than those in healthy controls. Furthermore, this association remains statistically significant following sensitivity analyses. There was also no publication bias indicated by the funnel plots or Egger’s tests.
Iron, an important trace element, is implicated in many biological processes and plays a crucial role in the development of the brain [24]. Iron deficiency influences the cognitive, motor, social and emotional functions in children. [25] It has been reported that decreased iron concentration in the brain is associated with alterations in the conduction of cortical fibers, changes in serotonergic and dopaminergic systems, and in the formation of myelin [26, 27]. Serum ferritin, an intracellular protein that stores iron, is usually regarded as a reliable indicator of iron stores in body tissues, however, whether the serum ferritin is a good indicator of iron stores in the brain is debatable [28]. The level of serum ferritin is affected by inflammation and food intake [29]. Iron deficiency with or without anemia during childhood, especially in infancy, has a negative impact on cognition, behavior, and motor skills of children [30]. Compared with serum iron, serum ferritin is a more sensitive marker which can be detected at the early stage of iron deficiency even without anemia.
In this meta-analysis, we found lower serum ferritin levels in patients with ADHD than in healthy controls. Although the explicit mechanism of lower iron in ADHD is unclear, several lines of evidence may help to explain the process. First, it has been indicated that iron, a major cofactor of the tyrosine hydroxylase enzyme and a rate-limiting step in dopamine synthesis [31], is implicated in the pathophysiology of ADHD. Second, iron deficiency is associated with decreased dopamine transporter density and activity, resulting in increased extracellular dopamine, as well as reduced dopamine receptors in the striatum [32, 33]. Third, iron deficiency may result in dysfunction in the basal ganglia [34]. Forth, an imbalance between inhibitory/excitatory neurotransmitters is thought to play an important role in the pathophysiology of ADHD [35]. The γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter of the mammalian central nervous system and influence the level of iron in brain [36]. GABA levels were lower in the ADHD patients, which may lead to the reduced iron concentration in basal ganglia [37]. Fifth, lower thalamic iron levels were found in children with ADHD compared to controls detected by MRI, which indicated that decreased iron levels in thalamic may implicated the etiology of ADHD [8].
Furthermore, Konofal et al. [38] and Sever et al. [9] reported that after iron supplementation, the serum ferritin of ADHD patients increased significantly and ADHD symptoms improved. Based on these data it appears that iron supplementation may benefit ADHD; however, more studies with high quality should be conducted to confirm this effect.
Serum iron, which indicates the amount of circulating iron that is bound to transferrin, is markedly reduced in iron deficiency anemia. In this study, we failed to find any relationship between serum iron and ADHD. This may because all included studies excluded subjects with severe anemia, and so levels of Hb in ADHD cases and controls were comparable. Researchers have speculated that psychostimulants used in the treatment of ADHD may lead to appetite loss and reduced nutritional iron uptake, which may result in a reduction in serum ferritin in treated patients with ADHD [39]. However, the studies of Adisetiyo et al. [11], Menegassi et al. [20], and Calarge et al. [40] revealed comparable dietary calorie and iron intake in medicated patients with ADHD and non-medicated patients with ADHD. Our subgroup meta-analysis also showed that serum ferritin levels were not lower in the treated group of patients. However, it should be considered that the treatment subgroup had a much smaller sample size than the group without treatment, which may lead to type II error.
Comorbidities are common in patients with ADHD and Cortese et al. [8] found higher serum ferritin levels in psychiatric disorders other than ADHD. In our study, we also found that patients with ADHD alongside other psychiatric comorbidities had higher serum ferritin levels than those without psychiatric comorbidities. This difference may be explained by different dietary intake patterns and pathophysiologic changes associated with other psychiatric disorders. The choice of analytical technology also affects the tested concentration of serum ferritin. In our subgroup analysis based on assay method, lower serum ferritin was found in patients tested via ELISA.
There are several merits of our study. First, to our knowledge, this is the first comprehensive meta-analysis performed to assess the correlation between serum ferritin levels and ADHD. Second, since the level of unobserved heterogeneity appear to be high by assuming homogeneity method [41], we chose random-effects model and performed sensitivity analysis. Although there is high heterogeneity in our study, the results did not change in the sensitivity analysis, indicating the robustness of our findings.
However, several limitations of our study should be taken into consideration. First, the sample size of subjects in the selected studies was small, which may reduce the power of our analyses. More studies with larger numbers of participants should be conducted to clarify the relationship between serum ferritin and ADHD. Second, we performed publication bias tests, however, the studies included in this meta-analysis for serum ferritin or serum iron was no more than ten, which may decreased the power and tend to draw the conclusion that show larger treatment effects [42]. Third, serum ferritin may not completely reflect actual iron levels in the brain. Using MRI, Adisetiyo et al. [11] found lower levels of iron in the striatum and thalamus of mediation-naïve patients with ADHD. However, serum ferritin levels were not significantly different between mediation-naïve patients with ADHD and healthy controls. To assess brain iron levels correctly, further studies should consider combining more indices reflecting blood iron status (transferrin, total iron binding capacity) or non-invasive methods with high sensitivity and specificity, such as MRI to assess brain iron directly. Fourth, different assay techniques were used in the included studies, such as ELISA, electrochemiluminescence, and competitive binding radioimmunoassay technique. To our knowledge, there has been no comparison of different techniques. Therefore, it is not possible to exclude the possibility that differences in techniques used to measure serum ferritin values may result in variable findings [15]. Fifth, the serum iron level is affected by various factors, such as dietary intake, the disease condition, the time of draw, that were not completely considered in the included studies. Sixth, in this meta-analysis, the small number of included studies declined the power of meta-regression analyses. Although except for meta-regression analyses, we also used a random-effects model, subgroup analyses, however, we failed to identify the factors leading to heterogeneity. Therefore, the results should be interpreted cautiously.
Conclusion
Our meta-analysis shows that serum ferritin levels are lower in patients with ADHD than in healthy controls, which suggests that serum ferritin is correlated with ADHD. In this study, we failed to find a correlation between serum iron and ADHD. This is likely due to the fact that serum iron is affected by various factors that were not completely considered in the included studies. There is a need for more high-quality studies with larger sample sizes, assessed using the same assay techniques, and multiple indices of iron status to provide more conclusive results. The mechanisms leading to iron deficiency in ADHD, and the correlation between brain iron and peripheral iron levels also needs further research.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by the National Science Foundation of China (No. 81330016, 81630038 and 81270724), the Major State Basic Research Development Program (2013CB967404 and 2012BAl04B00), grants from the Ministry of Education of China (IRT0935), a grant from the Science and Technology Bureau of Sichuan province (2014SZ0149 and 2016TD0002), and a grant from the clinical discipline program (neonatology) from the Ministry of Health of China (1311200003303).
References
- 1.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9. PubMed Central PMCID: PMCPMC3441936. 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biederman J, Petty CR, Monuteaux MC, Fried R, Byrne D, Mirto T, et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. The American journal of psychiatry. 2010;167(4):409–17. Epub 2010/01/19. 10.1176/appi.ajp.2009.09050736 [DOI] [PubMed] [Google Scholar]
- 3.McGough JJ, Smalley SL, McCracken JT, Yang M, Del'Homme M, Lynn DE, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. The American journal of psychiatry. 2005;162(9):1621–7. Epub 2005/09/02. 10.1176/appi.ajp.162.9.1621 [DOI] [PubMed] [Google Scholar]
- 4.Banaschewski T, Becker K, Scherag S, Franke B, Coghill D. Molecular genetics of attention-deficit/hyperactivity disorder: an overview. European child & adolescent psychiatry. 2010;19(3):237–57. PubMed Central PMCID: PMCPMC2839490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rucklidge JJ, Johnstone J, Kaplan BJ. Nutrient supplementation approaches in the treatment of ADHD. Expert review of neurotherapeutics. 2009;9(4):461–76. 10.1586/ern.09.7 [DOI] [PubMed] [Google Scholar]
- 6.Pelsser LM, Frankena K, Toorman J, Savelkoul HF, Pereira RR, Buitelaar JK. A randomised controlled trial into the effects of food on ADHD. European child & adolescent psychiatry. 2009;18(1):12–9. Epub 2008/04/24. [DOI] [PubMed] [Google Scholar]
- 7.Sinn N. Nutritional and dietary influences on attention deficit hyperactivity disorder. Nutrition reviews. 2008;66(10):558–68. Epub 2008/10/02. 10.1111/j.1753-4887.2008.00107.x [DOI] [PubMed] [Google Scholar]
- 8.Cortese S, Azoulay R, Castellanos FX, Chalard F, Lecendreux M, Chechin D, et al. Brain iron levels in attention-deficit/hyperactivity disorder: a pilot MRI study. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2012;13(3):223–31. Epub 2011/05/19. [DOI] [PubMed] [Google Scholar]
- 9.Sever Y, Ashkenazi A, Tyano S, Weizman A. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35(4):178–80. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 10.Juneja M, Jain R, Singh V, Mallika V. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian pediatrics. 2010;47(11):955–8. Epub 2010/05/11. [DOI] [PubMed] [Google Scholar]
- 11.Adisetiyo V, Jensen JH, Tabesh A, Deardorff RL, Fieremans E, Di Martino A, et al. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: a noninvasive biomarker that responds to psychostimulant treatment? Radiology. 2014;272(2):524–32. Epub 2014/06/18. PubMed Central PMCID: PMCPMC4263268. 10.1148/radiol.14140047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konofal E, Lecendreux M, Arnulf I, Mauren MC. Iron deficiency in children with attention-deficit/hyperactivity disorder. Archives of Pediatrics and Adolescent Medicine. 2004;158(12):1113–5. 10.1001/archpedi.158.12.1113 [DOI] [PubMed] [Google Scholar]
- 13.Donfrancesco R, Parisi P, Vanacore N, Martines F, Sargentini V, Cortese S. Iron and ADHD: time to move beyond serum ferritin levels. Journal of attention disorders. 2013;17(4):347–57. Epub 2012/02/01. 10.1177/1087054711430712 [DOI] [PubMed] [Google Scholar]
- 14.Percinel I, Yazici KU, Ustundag B. Iron Deficiency Parameters in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Child psychiatry and human development. 2016;47(2):259–69. Epub 2015/06/21. 10.1007/s10578-015-0562-y [DOI] [PubMed] [Google Scholar]
- 15.Cortese S, Angriman M, Lecendreux M, Konofal E. Iron and attention deficit/hyperactivity disorder: What is the empirical evidence so far? A systematic review of the literature. Expert review of neurotherapeutics. 2012;12(10):1227–40. Epub 2012/10/23. 10.1586/ern.12.116 [DOI] [PubMed] [Google Scholar]
- 16.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ (Clinical research ed). 2007;335(7626):914–6. Epub 2007/11/03. PubMed Central PMCID: PMCPMC2048840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittlbock M, Heinzl H. A simulation study comparing properties of heterogeneity measures in meta-analyses. Statistics in medicine. 2006;25(24):4321–33. Epub 2006/09/23. 10.1002/sim.2692 [DOI] [PubMed] [Google Scholar]
- 18.Konofal E, Lecendreux M, Arnulf I, Mouren MC. Iron deficiency in children with attention-deficit/hyperactivity disorder. Archives of pediatrics & adolescent medicine. 2004;158(12):1113–5. Epub 2004/12/08. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud MM, El-Mazary AA, Maher RM, Saber MM. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Italian journal of pediatrics. 2011;37:60 Epub 2011/12/31. PubMed Central PMCID: PMCPMC3268715. 10.1186/1824-7288-37-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menegassi M, Mello ED, Guimaraes LR, Matte BC, Driemeier F, Pedroso GL, et al. Food intake and serum levels of iron in children and adolescents with attention-deficit/hyperactivity disorder. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999). 2010;32(2):132–8. Epub 2009/10/20. [DOI] [PubMed] [Google Scholar]
- 21.Kwon HJ, Lim MH, Ha M, Kim EJ, Yoo SJ, Kim JW, et al. Transferrin in korean children with attention deficit hyperactivity disorder. Psychiatry investigation. 2011;8(4):366–71. Epub 2012/01/05. PubMed Central PMCID: PMCPMC3246146. 10.4306/pi.2011.8.4.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bener A, Kamal M, Bener H, Bhugra D. Higher prevalence of iron deficiency as strong predictor of attention deficit hyperactivity disorder in children. Annals of medical and health sciences research. 2014;4(Suppl 3):S291–7. Epub 2014/11/05. PubMed Central PMCID: PMCPMC4212392. 10.4103/2141-9248.141974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. The Journal of nutritional biochemistry. 2004;15(8):467–72. Epub 2004/08/11. 10.1016/j.jnutbio.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 24.Beard JL. Why iron deficiency is important in infant development. The Journal of nutrition. 2008;138(12):2534–6. Epub 2008/11/22. PubMed Central PMCID: PMCPMC3415871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.<Long-lasting neural and behavioral effects of iron deficiency.pdf>. [DOI] [PMC free article] [PubMed]
- 26.Ben-Shachar D, Livne E, Spanier I, Zuk R, Youdim MB. Iron modulates neuroleptic-induced effects related to the dopaminergic system. Isr J Med Sci. 1993;29(9):587–92. [PubMed] [Google Scholar]
- 27.Beard J. Iron deficiency alters brain development and functioning. The Journal of nutrition. 2003;133(5 Suppl 1):1468s–72s. Epub 2003/05/06. [DOI] [PubMed] [Google Scholar]
- 28.Oner O, Oner P, Bozkurt OH, Odabas E, Keser N, Karadag H, et al. Effects of zinc and ferritin levels on parent and teacher reported symptom scores in attention deficit hyperactivity disorder. Child psychiatry and human development. 2010;41(4):441–7. Epub 2010/03/20. PubMed Central PMCID: PMCPMC3399584. 10.1007/s10578-010-0178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurnham DI, Northrop-Clewes CA. Inflammation and biomarkers of micronutrient status. Current opinion in clinical nutrition and metabolic care. 2016;19(6):458–63. 10.1097/MCO.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 30.Jáuregui-Lobera I. Iron deficiency and cognitive functions. Neuropsychiatric Disease and Treatment. 2014:2087. [DOI] [PMC free article] [PubMed]
- 31.Sachdev P. The neuropsychiatry of brain iron. J Neuropsychiatry Clin Neurosci. 1993;5(1):18–29. 10.1176/jnp.5.1.18 [DOI] [PubMed] [Google Scholar]
- 32.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. The Journal of nutrition. 2000;130(11):2831–7. [DOI] [PubMed] [Google Scholar]
- 33.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. The Journal of nutrition. 2011;141(4):740s–6s. Epub 2011/02/25. PubMed Central PMCID: PMCPMC3056585. 10.3945/jn.110.131169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youdim MB, Ben-Shachar D, Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. The American journal of clinical nutrition. 1989;50(3 Suppl):607–15; discussion 15–7. [DOI] [PubMed] [Google Scholar]
- 35.Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. The lancet Psychiatry. 2016;3(6):555–67. Epub 2016/05/18. PubMed Central PMCID: PMCPMC4893880. 10.1016/S2215-0366(16)00096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2012;69(7):750–3. Epub 2012/07/04. PubMed Central PMCID: PMCPMC3970207. 10.1001/archgenpsychiatry.2011.2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill JM. Iron concentration reduced in ventral pallidum, globus pallidus, and substantia nigra by GABA-transaminase inhibitor, gamma-vinyl GABA. Brain research. 1985;342(1):18–25. Epub 1985/09/02. [DOI] [PubMed] [Google Scholar]
- 38.Konofal E, Lecendreux M, Deron J, Marchand M, Cortese S, Zaim M, et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatric neurology. 2008;38(1):20–6. Epub 2007/12/07. 10.1016/j.pediatrneurol.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 39.D'Amato TJ. Is iron deficiency causative of attention-deficit/hyperactivity disorder? Archives of pediatrics & adolescent medicine. 2005;159(8):788; author reply Epub 2005/08/03. [DOI] [PubMed] [Google Scholar]
- 40.Calarge C, Farmer C, DiSilvestro R, Arnold LE. Serum ferritin and amphetamine response in youth with attention-deficit/hyperactivity disorder. Journal of child and adolescent psychopharmacology. 2010;20(6):495–502. Epub 2010/12/29. PubMed Central PMCID: PMCPMC3003494. 10.1089/cap.2010.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PloS one. 2013;8(7):e69930 Epub 2013/08/08. PubMed Central PMCID: PMCPMC3724681. 10.1371/journal.pone.0069930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology. 2000;53(11):1119–29. Epub 2000/12/07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.