Abstract
In this study, we investigated the prompt release of acetaldehyde and other oxygenated volatile organic compounds (VOCs) from leaves of Grey poplar [Populus x canescens (Aiton) Smith] following light-dark transitions. Mass scans utilizing the extremely fast and sensitive proton transfer reaction-mass spectrometry technique revealed the following temporal pattern after light-dark transitions: hexenal was emitted first, followed by acetaldehyde and other C6-VOCs. Under anoxic conditions, acetaldehyde was the only compound released after switching off the light. This clearly indicated that hexenal and other C6-VOCs were released from the lipoxygenase reaction taking place during light-dark transitions under aerobic conditions. Experiments with enzyme inhibitors that artificially increased cytosolic pyruvate demonstrated that the acetaldehyde burst after light-dark transition could not be explained by the recently suggested pyruvate overflow mechanism. The simulation of light fleck situations in the canopy by exposing leaves to alternating light-dark and dark-light transitions or fast changes from high to low photosynthetic photon flux density showed that this process is of minor importance for acetaldehyde emission into the Earth's atmosphere.
Trees emit numerous volatile organic compounds (VOCs), including a large number of oxygenated compounds, into the atmosphere (Fehsenfeld et al., 1992; Kesselmeier and Staudt, 1999). It is estimated that the biogenic emission of VOCs other than methane and isoprenoids, including oxygenated volatile organic compounds such as aldehydes, ketones, alcohols, and carboxylic acids, amounts to around 24% of the total VOC budget in forest ecosystems (Guenther et al., 1994, 1995) and contributes with approximately 260 teragram C year−1 to the global budget (Guenther et al., 1995). Accordingly, cuvette measurements demonstrated that the leaves of trees may act as significant acetaldehyde emitters (Hahn et al., 1991; Kesselmeier et al., 1997; Kreuzwieser et al., 1999, 2000, 2001; Janson et al., 1999; Janson and de Serves, 2001) showing emission rates even comparable to those reported for isoprene emissions under particular conditions (Holzinger et al., 2000; Kreuzwieser et al., 2000).
VOCs, including carbonyls, play a significant role in the atmosphere's chemistry (Thompson, 1992). Since VOCs may alter the concentrations of hydroxyl radicals, they are supposed to increase the lifetime of climate-sensitive trace gases such as methane (Brasseur and Chatfield, 1991). Short-chained carbonyls, like acetaldehyde, formaldehyde, and acetone, are also involved in the production of tropospheric ozone and peroxyacetyl nitrates (PAN-family compounds) that are known for their adverse effects on plant growth and human health (Kotzias et al., 1997). The oxidation of carbonyls, particularly of acetaldehyde and formaldehyde by OH-radicals, also causes the generation of acetic and formic acid, which contribute to the atmosphere's acidity (Bode et al., 1997; Kesselmeier et al., 1997).
The metabolic origin of acetaldehyde emitted by forest trees is still a matter of debate. Laboratory studies showed that acetaldehyde emission correlates with root flooding (Kreuzwieser et al., 1999; Holzinger et al., 2000) and xylem sap ethanol concentrations (Kreuzwieser et al., 2001; Cojocariu et al., 2004). Ethanol formed in anoxic roots during flooding is transported to leaves by the transpiration stream (MacDonald and Kimmerer, 1991) and is oxidized in the leaves to acetaldehyde by alcohol dehydrogenase (ADH). A small portion of this acetaldehyde can be emitted, while the bulk is further metabolized by aldehyde dehydrogenase (ALDH; Kreuzwieser et al., 2000) to acetate and acetyl-CoA.
Recently, strong transient acetaldehyde bursts during light-dark transitions were reported for some tree species (Holzinger et al., 2000; Karl et al., 2002a). These acetaldehyde bursts are thought to be a result of a pyruvate overflow mechanism (Karl et al., 2002a). In the proposed mechanism, pyruvate decarboxylase (PDC) acts as a safety valve to convert excess cytosolic pyruvate into acetaldehyde, which is subsequently oxidized by ALDH to acetate (Tadege et al., 1999). Such an excess of cytosolic pyruvate may be the result of transiently decreased transport rates of pyruvate equivalents (i.e. phosphoenolpyruvate [PEP]) into organelles, or reduced pyruvate utilization in leaf cells immediately after darkening (Karl et al., 2002a).
The objective of this study was to characterize in more detail the acetaldehyde emissions from Grey poplar [Populus x canescens (Aiton) Smith, earlier referred to as P. tremula × P. alba] leaves after darkening. Moreover, it was aimed to test whether accumulation of cytosolic pyruvate causes increased acetaldehyde emissions as expected from the pyruvate overflow mechanism and to study whether acetaldehyde emissions during light-dark transitions can be of ecological significance, as proposed by Karl et al. (2002a).
RESULTS AND DISCUSSION
Light-Dark Transitions Cause Transient Emissions of Different Oxygenated VOC
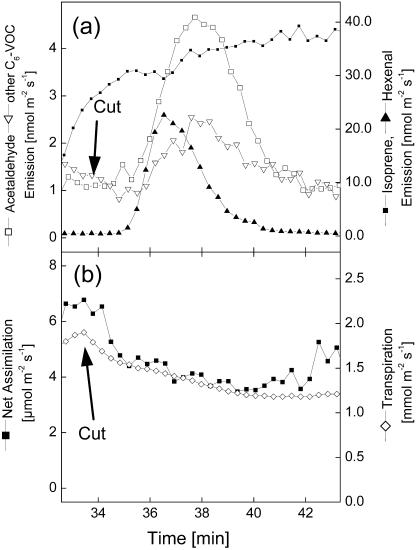
In order to test whether other volatiles, in addition to acetaldehyde, are released from intact poplar leaves during light-dark transitions, mass scans up to 150 atomic mass units were performed before and immediately after darkening. These scans indicated that, in addition to acetaldehyde (mass 45) species, which produce major ion signals in a proton transfer reaction-mass spectrometry (PTR-MS) instrument at mass 81 (hexenal) and mass 83 (hexanal, hexenols, and hexenyl acetates, termed other C6-VOCs), were released in considerable magnitude after darkening. VOC emissions during different light-dark transitions always showed the same temporal pattern, with hexenal being released as the first compound approximately 30 s after the light was switched off (Fig. 1a). Acetaldehyde was released somewhat later (after approximately 50 s), together with other C6-VOCs (Fig. 1a). Whereas hexenal and acetaldehyde emissions occurred as sharp peaks, other C6-VOC emissions lasted over a longer period of time, disappearing after approximately 15 min. In contrast to increased emission of oxygenated VOCs, the emission of isoprene (mass 69) dropped rapidly, in parallel to the decline of net assimilation and transpiration (Fig. 1, a and b).
Figure 1.
VOC emission (a) and gas exchange (b) of intact poplar leaves following rapid light-dark transitions. During the time indicated by the gray background, the light was switched off. One typical sequence out of 12 independent experiments is shown.
During some of the experiments performed, poplar leaves had to be excised from the trees in order to apply enzyme inhibitors via the transpiration stream. Surprisingly, cutting off the leaves caused transient emissions of hexenal, acetaldehyde, and other C6-VOCs in the same temporal order as observed during light-dark transitions (Fig. 2a). At the same time, rates of net assimilation and transpiration dropped due to cutting off the leaf (Fig. 2b). It is unlikely that the transient emission of these compounds was due to a transport of wound-VOCs or their precursors from the cut end of the petiole to the leaf because the emissions appeared much faster (after approximately 30 s) than could be expected from transport processes and because they were also observed in intact leaves after light-dark transitions (Fig. 1a). As seen in the same experiments, feeding [U-13C]Glc via cut ends of the petioles required 6 to 10 min until 13C appeared in isoprene (Schnitzler et al., 2004). Consistently, ethanol fed via cut petioles was detectable in the cuvette atmosphere after approximately 10 min (Kreuzwieser et al., 2001). The observed release of acetaldehyde (Holzinger et al., 2000; Karl et al., 2002a) and wound-VOCs (Charron et al., 1996) after darkening confirms previous findings with different tree species and lettuce, respectively. By contrast, the release of VOCs due to cutting at a distant part of the plant has not yet been described. Since the patterns of VOC emissions were quite similar, the same mechanism can be assumed for both phenomena.
Figure 2.
Effect of cutting a poplar leaf on VOC release (a) and gas exchange (b). At the time indicated, the leaf was excised and the cut end of the petiole placed in a solution. One typical sequence out of five independent experiments is shown.
Effects of Anoxia on Acetaldehyde and C6-Volatiles at Light-Dark Transitions
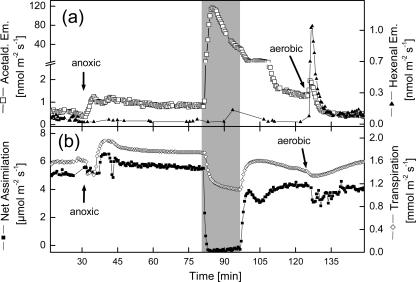
It was surprising that light-dark transitions caused the emission of the typical wound-VOC, hexenal, and other C6-VOCs, together with acetaldehyde in poplar leaves. This is even more surprising since Karl et al. (2002a) reported that the closely related species quaking aspen (Populus deltoides) did not release any of these compounds besides acetaldehyde. This difference may be species specific or depend on different growth conditions of the experimental plants. To clarify whether the formation of hexenal and other C6-VOCs was connected to the lipoxygenase (LOX) pathway, which requires molecular oxygen (Hatanaka, 1993; Bate and Rothstein, 1998), experiments under anoxic conditions were performed (Fig. 3). When the cuvette environment was switched from aerobic to anoxic (N2/360 ppm CO2) conditions, acetaldehyde emission rates in illuminated poplar leaves increased by a factor of 2, from 0.4 to 0.6 nmol m−2 s−1 to 0.9 to 1.3 nmol m−2 s−1 (Fig. 3a). The increased acetaldehyde emissions are a consequence of an actual production due to a switch from mitochondrial respiration to alcoholic fermentation producing acetaldehyde from the decarboxylation of pyruvate (Vartapetian and Jackson, 1997). Steady emission rates after changing to an anoxic atmosphere were observed after approximately 7 min. Light-dark transitions under these conditions dramatically increased acetaldehyde emissions up to 123 nmol m−2 s−1. Transient acetaldehyde emission rates peaked approximately 2 min after darkening. In none of the experiments performed under anoxia did darkening induce either hexenal or other C6-VOC emissions (Fig. 3a). This lack of emissions under anoxic conditions strongly indicates the involvement of the LOX pathway (Hatanaka, 1993) in the formation of hexenal and other C6-VOCs during light-dark transitions, and further suggests that the generation of acetaldehyde after darkening is independent from the formation of volatile LOX products.
Figure 3.
VOC emission (a) and gas exchange (b) of poplar leaves following light-dark transitions under anoxic conditions. At the time indicated by the arrow, the cuvette atmosphere was switched to anoxic conditions (N2, 360 ppm CO2). Later the light was switched off (gray bar) and, after 20 min, the light was turned on again and the atmosphere switched back to aerobic conditions (arrow). One typical sequence out of three independent experiments is shown.
When the light was turned on again in these experiments, acetaldehyde emission rates decreased to levels comparable to those observed before the light-dark transition (Fig. 3a). Rapid changes to an aerobic atmosphere caused a transient increase in acetaldehyde emission, followed by the return of emission rates to initial values of approximately 0.5 nmol m−2 s−1. The transiently increased acetaldehyde emissions could be a product of oxidation of ethanol that was synthesized during anoxic conditions (data not shown). Conversion of ethanol into acetaldehyde has been observed during postanoxic stress in roots of plants after periods of flooding (Zuckermann et al., 1997; Boamfa et al., 2003). This oxidation reaction is proposed to be catalyzed by catalase or ADH (Monk et al., 1987), both of which are constitutively expressed in poplar leaves (Kreuzwieser et al., 1999).
A strong transient burst of hexenal was observed when oxygen was resupplied (Fig. 3). It can be assumed that the precursors of the wound-VOCs, linolenic acid and linoleic acid (Hatanaka, 1993), were released during the light-dark transition, although a conversion into hexenal and other C6-VOCs by LOX was not possible in the absence of oxygen. When the cuvette atmosphere was switched back to aerobic conditions, LOX activity was enabled to produce hexenal and other C6-VOCs. A quite similar phenomenon was observed when leaves were wounded under a N2 atmosphere (Fall et al., 1999); under such conditions, wound-VOCs were not emitted as long as oxygen was not available for the injured leaf.
Effects of ALDH and Pyruvate Dehydrogenase Inhibitors on VOC Emissions
It was suggested that acetaldehyde may be a product of a pyruvate dehydrogenase (PDH) bypass funneling acetaldehyde into general metabolism (Tadege et al., 1999). Acetaldehyde emissions would result from a leak between its formation by the decarboxylation of pyruvate by PDC and its consumption by cytosolic or mitochondrial ALDH (Tadege et al., 1999; Karl et al., 2002a). Pyruvate accumulation in the cytosol should therefore trigger acetaldehyde emission. It was recently assumed that this process occurs as a consequence of reduced PDH activity during light-dark transitions (Karl et al., 2002a). Inhibiting PDH activity, therefore, should have the same effects: accumulated cytosolic pyruvate concentrations accompanied by increased acetaldehyde emissions rates. This effect was indeed observed in this study (Fig. 4), when poplar leaves were treated with acetylphosphinate, a potent and specific inhibitor of the PDH complex (Laber and Amrhein, 1987). If, in addition to PDH, ALDH was inhibited, 5 times higher acetaldehyde emissions were observed than if PDH was inhibited alone (Fig. 4), indicating an accumulation of acetaldehyde under these conditions.
Figure 4.
Effects of enzyme inhibitors on acetaldehyde emission of poplar leaves. At the times indicated, the leaf was excised and the cut petiole placed in a solution containing either 0.65 mm of the ALDH inhibitor disulfiram, 1 mm of the PDH inhibitor acetylphosphinate, or both inhibitors. Acetaldehyde emissions after 2 h of inhibitor treatment were determined using DNPH-coated silica gel cartridges. Means ± sd of five independent experiments are shown. Statistically significant differences at P < 0.05, as calculated by Tukey's test under ANOVA, are indicated by different letters.
It has been proposed that cytosolic PEP is transported into chloroplasts by a PEP/inorganic phosphate antiporter (Flügge, 1999), where it is used as a precursor of isoprene (Sharkey and Yeh, 2001; Rosenstiel et al., 2003, 2004). Isoprene formation has been assumed to act as a metabolic safety valve, helping to maintain metabolic homeostasis in the competition for PEP by cytosolic processes, such as the production of organic acids like oxalacetate via PEP carboxylase that itself serves as a precursor for amino acid biosynthesis (Rosenstiel et al., 2004). The findings of significantly increased isoprene emission rates under (1) anoxic rather than aerobic conditions (Fig. 5, b and c) and (2) conditions of inhibited ALDH activity (Fig. 5a) clearly support this hypothesis. Net assimilation rates were widely unaffected during these experiments, except when ALDH-inhibited leaves were transferred from aerobic to anoxic conditions (Fig. 5b). These decreased assimilation rates were probably caused by reduced stomatal conductance as often observed if plants are exposed to anoxic conditions (Vartapetian and Jackson, 1997).
Figure 5.
Effects of anoxia and the ALDH inhibitor disulfiram on isoprene emission of poplar leaves. An excised poplar leaf was placed into a cuvette and was then fed with 0.65 mm disulfiram (a); fed via the transpiration stream, with 0.65 mm disulfiram under normoxic conditions and then switched to anoxic conditions (b); and exposed to anoxic conditions (N2, 360 ppm CO2) and then switched to normoxic conditions (c). Changes of conditions are indicated by vertical lines. One typical experiment out of three is shown.
Apparently, elevated cytosolic pyruvate and PEP concentrations promote both enhanced PEP uptake into the chloroplast accompanied by enhanced isoprene formation, as well as enhanced acetaldehyde production via a pyruvate overflow mechanism (Karl et al., 2002a). Consequently, if the acetaldehyde released during light-dark transitions is a result of accumulated pyruvate in the cytosol (Karl et al., 2002a), inhibition of ALDH by disulfiram should cause considerably increased acetaldehyde emissions during such transitions because acetaldehyde consumption is inhibited. Enhanced acetaldehyde emissions were not observed in this study (Table I). Moreover, inhibition of ALDH led to significantly reduced acetaldehyde amounts and emission rates compared to controls without the enzyme inhibitor (Table I). In these experiments, it was crucial that disulfiram was an effective ALDH inhibitor. In animal cells, disulfiram was shown to be very specific in inducing the formation of an intramolecular disulfide bond (Lipsky et al., 2001) and also to be effective in herbaceous plants (Op den Camp and Kuhlemeier, 1997) and poplar leaves (Kreuzwieser et al., 2001). To test the functioning of disulfiram, inhibitor-treated illuminated leaves were exposed to anoxia. Under these conditions, acetaldehyde emissions were considerably higher (up to 340 nmol m−2 s−1) than in anoxic controls without disulfiram (up to 123 nmol m−2 s−1; Fig. 3a) since the acetaldehyde produced could not be oxidized to acetate by ALDH (Tadege et al., 1999; Kreuzwieser et al., 2001). From these results, together with the finding of dramatically enhanced acetaldehyde emissions by inhibition of both PDH plus ALDH than by inhibition of PDH alone, it seems most likely that disulfiram effectively inhibited ALDH in these experiments (Table I).
Table I.
Effects of anoxia and the ALDH inhibitor disulfiram on acetaldehyde emission during light-dark transitions
| Control | Disulfiram | Anoxia | |
|---|---|---|---|
| Acetaldehyde released during light-dark transition [nmol m−2 leaf area] | 2.48 ± 1.52 | 0.50 ± 0.06* | 33 ± 18* |
| Maximal peak emission rate [nmol m−2 s−1] | 18 ± 12 | 3.7 ± 0.5* | 93 ± 40* |
An excised poplar leaf was either fed via the transpiration stream without (control) or with 0.65 mm disulfiram for 60 min, or exposed to anoxic conditions. The light was then switched off and acetaldehyde emissions were determined. Acetaldehyde peaks were integrated to determine the absolute amounts released. Means ± sd of three to four independent experiments are shown. Statistically significant differences compared to controls at P < 0.05, as calculated by Student's t test, are indicated by asterisks.
In conclusion, these data indicate that the strong transient release of acetaldehyde following light-dark transitions is not directly related to the cytosolic pyruvate pool, as proposed by Karl et al. (2002a). It still remains to be seen what processes cause the appearance of acetaldehyde together with wound-VOCs during light-dark transitions, but also during cutting at a distant part of the plant. The emission of volatiles can be caused either from the release from storage pools (Niinemets et al., 2004) or from an actual production from metabolic precursors. It seems unlikely that light-dark transitions cause such enormous physicochemical changes in cell compartments, thereby triggering transiently increased acetaldehyde emissions. This assumption is supported by the fact that leaf temperatures slightly decreased after switching off the light; according to Henry's law, this increases the water solubility of acetaldehyde and promotes reduced, instead of increased, emissions of acetaldehyde. From the present results, it is therefore assumed that changes in actual production rates trigger changed emissions. The observation that C6-VOC emission is accompanied by the release of acetaldehyde as a consequence of plant wounding (Kimmerer and Kozlowski, 1982; Kirstine et al., 1998; Fall et al., 1999; De Gouw et al., 2000) supports the view of connected metabolic pathways and further suggests that similar processes take place during light-dark transitions and wounding. It may be proposed that the strong artificial changes from saturating photosynthetic photon flux density (PPFD) to darkness led to disturbances in membranes, thereby inducing the typical wounding responses, including the oxidation of the unsaturated fatty acids linolenic acid and linoleic acid (Hatanaka, 1993) in the absence of any visible leaf injury. Acetaldehyde may be released from acetyl-CoA, which reacts in this wounding process with hexenal (the compound appearing as the first after light-dark transitions) to form hexenyl-acetate (Hatanaka, 1993). This hypothesis is supported by considerably increased acetaldehyde emissions under anoxic compared to normoxic conditions (Table I). Since anoxia causes a lack of substrate (hexenal) for this reaction, a release of acetaldehyde from excess acetyl-CoA or acetate could be promoted. Acetyl-CoA can be generated in the cytosol, e.g. by the action of ATP-citrate lyase (Nikolau et al., 2000), or can be exported from plastids and mitochondria as acetate (Groenveld et al., 1991). Generally, the conversion of acetyl-CoA by an ALDH (Sanchez, 1998) and by the esterase activity of ALDH (Kitson and Kitson, 1997) into acetaldehyde has been shown in Archaezoa and animals. Future experiments, however, have to clarify whether this is also possible in higher plants and explain the acetaldehyde emission after light-dark transitions. Fumigation of leaves with 13CO2 (Karl et al., 2002a) caused about 60% 13C-labeling of acetaldehyde being released during light-dark transitions. This finding is consistent with the view of acetaldehyde release from acetyl-CoA, since a fast mixing of cytosolic triose phosphate pools by 13C-labeled triose phosphate from the chloroplasts must be assumed (Hoefnagel et al., 1998; Karl et al., 2002b; Rosenstiel et al., 2003, 2004), subsequently leading to 13C-labeling of acetyl-CoA pools. However, more experimental data have to be collected to further test this hypothesis.
Ecological Significance of Light-Dark-Driven Acetaldehyde Emissions
The strong transient acetaldehyde release during light-dark transitions may also contribute to acetaldehyde emission into the atmosphere. It was assumed that, in tree canopies, leaves that are subject to light flecks throughout the day emit small amounts of acetaldehyde with each transition from high light to low light (Karl et al., 2002a). To test this hypothesis, canopy conditions were simulated in this laboratory study. However, repeated light-dark transitions for several times (light off for 23 and 46 s, light on for 23 s) did not cause any enhanced acetaldehyde emission (Fig. 6a). As compared to a complete cutoff of illumination after which acetaldehyde emission appeared approximately 50 s later (Fig. 1a), it seemed that switching on the light before this time completely suppressed acetaldehyde emission (Fig. 6a).
Figure 6.
Influences of alternating light-dark transitions and dark-light transitions on VOC release (a) and gas exchange (b) of poplar leaves. At times indicated by gray bars, the light was switched off (0 μmol m−2 s−1 PPFD) and switched on (1,400 μmol m−2 s−1) again. Duration of dark/light periods: 23 s (dark)/23 s (light) (min 107–114); 46 s (dark)/23 s (light) (min 126–138). One typical sequence out of three independent experiments is shown.
Net assimilation rates quickly reacted to the light-dark cycles applied, whereas transpiration and isoprene emissions adapted to lower levels (Fig. 6). Hexenal emissions seemed to be transiently triggered under prolonged (46 s) periods of darkening. However, whenever the light was switched on, hexenal emissions quickly dropped, suggesting that the production process was immediately stopped by light.
If the light was switched off at the end of such light-dark cycles for a longer period of time (15 min), neither acetaldehyde nor C6-VOC was emitted by the leaves. VOC emission was only detected again when leaves were exposed to light over prolonged periods of time and then darkened (data not shown). Such a pattern may be due to either (1) a pool of precursors (e.g. acetyl-CoA) built up during the light phase, which was then immediately converted into acetaldehyde if the light was switched off, or (2) short-term adaptation mechanisms that prevented acetaldehyde release during repeated light-dark transitions.
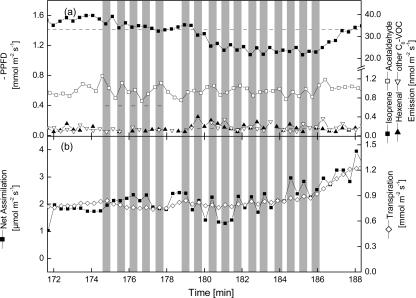
To expose poplar leaves to more realistic scenarios, PPFD was rapidly reduced from light saturating (1,400 μmol m−2 s−1) to lower light levels (395 μmol m−2 s−1) and also below the light compensation point of 115 μmol m−2 s−1 (95 μmol m−2 s−1) determined for the poplar trees used in this study. In none of the cases was increased acetaldehyde or wound-VOC emissions observed (Fig. 7). Whereas reductions to 395 μmol m−2 s−1 only had a small effect on the isoprene emission rate (Fig. 7a) and photosynthetic gas exchange (Fig. 7b), reduction of light intensity to 95 μmol m−2 s−1 PPFD caused fluctuating isoprene emission rates and net assimilation rates. These experiments clearly show that acetaldehyde emissions due to light-dark changes cannot be of significance under field conditions. Similar results have also been obtained from Quercus robur leaves in the laboratory and needles of adult Norway spruce trees in the field (data not shown).
Figure 7.
Influence of changes from high to low light intensities on VOC release (a) and gas exchange (b) of poplar leaves. As indicated by gray bars, PPFD was reduced (395 μmol m−2 s−1) and increased again (1,400 μmol m−2 s−1). In a second series, PPFD was decreased to 95 μmol m−2 s−1 PPFD. Each dark/light phase lasted for 46 s. One typical sequence out of three independent experiments is shown.
CONCLUSIONS
In recent years, different pathways have been elucidated leading to the production and emission of acetaldehyde by the leaves of trees (for overview, see Fig. 8). Besides the oxidation of xylem-derived ethanol, which has been produced in anoxic tissues of stems or roots (Kreuzwieser et al., 1999, 2000, 2001; Holzinger et al., 2000), acetaldehyde may also result from a pyruvate overflow mechanism (Karl et al., 2002a). This process is clearly supported by the results of this study (Fig. 4). However, a third biochemical pathway causing the formation and emission of acetaldehyde must be active during light-dark transitions and involves the release of wound-VOCs by the leaves (Fig. 1). This pathway seems to be independent of cytosolic pyruvate and is suggested to be part of the leaves' responses to wounding. The exact biochemical origin of acetaldehyde emitted during light-dark transitions is not yet clear (Fig. 8). It is still not understood which metabolic pathways are responsible for acetaldehyde emissions from leaves of trees in the field. From this study it is evident that light-dark transitions are unlikely to contribute significantly to these emissions. The good correlations between xylem sap ethanol concentrations and acetaldehyde release in adult Norway spruce, as observed in a field study (Cojocariu et al., 2004), suggest that the oxidation of ethanol may be of significance under field conditions; however, production of acetaldehyde directly from pyruvate via PDC cannot be excluded.
Figure 8.
Possible pathways responsible for the production of acetaldehyde by the leaves of trees. Acetaldehyde can be derived from the oxidation of xylem-transported ethanol (Kreuzwieser et al., 1999), from the decarboxylation from pyruvate (pyruvate overflow mechanism; Karl et al., 2002a), and as hypothesized in this study from conversion of acetyl-CoA during light-dark transitions and wounding. Enzymatic catalyzed reactions inhibited in this study are indicated by crosses (ALDH, PDH complex).
MATERIALS AND METHODS
Plant Materials
The present experiments were performed with 6-month-old Grey poplar plants [Populus x canescens (Aiton) Smith INRA clone 717 1-B4; earlier referred to as P. tremula × P. alba]. The plants were micropropagated under sterile conditions as described by Leplé et al. (1992). Cultivation proceeded in plastic pots (12 × 10 × 10 cm) filled with a mixture of perlite, sand, and commercial potting soil (2:1:1, v/v/v; Floradur Type 1; Floragard, Oldenburg, Germany) as a substrate. Every 2 weeks plants were fertilized with 100 mL of a nutrient solution containing 6 g L−1 of a complete fertilizer (Hakaphos Blau; Bayer, Leverkusen, Germany) and were watered daily with tap water. Plants were kept under long-day conditions (light intensities between 250 and 800 μmol m−2 s−1, depending on the distance of the leaves to the illumination source) at day and night temperatures of 20°C ± 3°C and relative humidity of 70% ± 10%.
Cuvette Measurements of Gas Exchange
Photosynthetic gas exchange of individual leaves was measured using the dynamic cuvette system described by Schnitzler et al. (2004). The cuvettes of a volume of 1.27 L were made of an aluminum frame with Plexiglas top and bottom covers. The top lid contained a water-cooled Plexiglas bilayer to adjust constant leaf temperature. Two fans ensured homogeneous mixing of the air inside the cuvette. Light was provided by an artificial cool-light source (KL2500 LCD; Schott, Cologne, Germany) of mean intensities of approximately 1,400 μmol m−2 s−1 PPFD at leaf level. Leaf temperatures were held constant at approximately 32°C. The cuvette contained sensors for cuvette air temperature and relative humidity (1400-104; Heinz Walz GmbH, Effeltrich, Germany), leaf temperature (NiCr-Ni temperature transmitters GNTP; Greisinger Electronic GmbH, Regenstauf, Germany), and PPFD (LI-190SA, LI-COR, Lincoln, NB). It was flushed continuously with synthetic air containing 358 ± 7 ppmv CO2 (Messer Austria GmbH, Vienna). For anoxic treatment, the cuvette was flushed with N2 containing 360 ppmv CO2. In aerobic and anoxic treatments, flow rates through the cuvette were kept constant at 3 L min−1. Relative air humidity was adjusted to approximately 50% (Schnitzler et al., 2004). The photosynthetic gas-exchange and transpiration rates were monitored using a differential infrared absorption analyzer (Li-6262).
For gas-exchange measurements, one fully expanded leaf was placed into the cuvette. In some experiments, the leaf was excised and fed via the cut petiole with solutions containing the ALDH inhibitor disulfiram (0.65 mm) or the PDH inhibitor acetylphosphinate (1 mm).
Measurement of Acetaldehyde, C6 Wound Compounds, and Isoprene with PTR-MS
The PTR-MS technique has been described in great detail elsewhere (Hansel et al., 1995; Lindinger et al., 1998). Volume mixing ratios (VMR) of VOCs present in the air stream from the cuvette system were measured with the Innsbruck PTR-MS in a similar setup as described by Kreuzwieser et al. (2002). The total residence time in the inlet line was less than 2 s, which is far less than the typical gas-exchange time (25 s) of the cuvette.
In this study, the PTR-MS technique was used for on-line monitoring of acetaldehyde, C6 wound compounds, and isoprene. Acetaldehyde was detected at protonated molecular mass 45+, and isoprene corresponds to the ion signal at mass 69+. C6 wound compounds are hexyl and hexenyl compounds, which are emitted after leaf wounding (Fall et al., 1999). For many compounds, proton transfer from H3O+ was nondissociative; however, in the case of hexyl and hexenyl compounds, dissociation occurred and several fragment ions were formed. As discussed by Fall et al. (1999), major product ions of hexenals are masses 81 and 99. Therefore, we used the sum of these ions and termed the result hexenal. Other C6-aldehydes, alcohols, and hexenyl acetate formed ions at masses 83, 101, and 143. The sum of mass ions 101+ plus 83+ plus 143+ was used to quantify the sum of hexenols, hexanal, and hexenyl acetates (termed other C6-VOCs).
The entire (background corrected) ion signal at these masses was converted into VMR of the respective VOCs. The PTR-MS instrument was calibrated for acetaldehyde using a calibration standard of 1,183 ppbv (± 5%) acetaldehyde in N2, for isoprene using a calibration standard of 7.9 ppmv (± 10%) isoprene in N2 (Messer, Griesheim, Germany). Both standards were diluted with humidified synthetic air to provide VOC VMR in the ranges that were expected in the experiments. The linearity of the PTR-MS instrument was better than 2%, which was basically equal to the accuracy of the flow dilution system. The accuracy of the isoprene measurements corresponds to the error in the gas standards, which is ± 5% for acetaldehyde and ± 10% for isoprene, respectively. The sensitivities for hexyl and hexenyl compounds were estimated from the sensitivity of acetone, which has a comparable dipole moment. The estimated sensitivities of C6 leaf wound-VOCs are in good agreement with recent calibration measurements of pure compounds performed at the Forschungszentrum Jülich, Germany, in 2003 (J. Beauchamp, personal communication). The accuracy of the hexenal measurements is better than 30%; for the sum of hexenols plus hexanal plus hexenyl acetates, the accuracy is better than 50%.
In experiments with enzyme inhibitors (Fig. 4), acetaldehyde was quantified using dinitrophenyl hydrazine (DNPH)-coated silica gel cartridges. Acetaldehyde present in the air reacted with acidified DNPH, forming the corresponding hydrazone, which was eluted from the cartridges with 3 mL of 66% acetonitrile, and then quantified by HPLC as described by Kreuzwieser et al. (1999).
Acknowledgments
Armin Wisthaler is grateful to the Verein zur Förderung der wiss. Ausbildung und Tätigkeit von Südtirolern an der Landesuniversität Innsbruck for postdoctoral support. We greatly appreciate the donation of acetylphosphinate by Prof. Dr. Nikolaus Amrhein, ETH Zurich.
This work was supported by the German Federal Ministry of Education and Research (BMBF; in the frame of BEWA2000 [Biogenic emissions of volatile organic compounds from forest ecosystems], a subproject of the national joint research project AFO2000 [Atmosphären-Forschungsprogramm 2000]).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043240.
References
- Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16: 561–569 [DOI] [PubMed] [Google Scholar]
- Boamfa EI, Ram PC, Jackson MB, Reuss J, Harren FJM (2003) Dynamic aspects of alcoholic fermentation of rice seedlings in response to anaerobiosis and to complete submergence: relationship to submergence tolerance. Ann Bot (Lond) 91: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode K, Helas G, Kesselmeier J (1997) Biogenic contribution to atmospheric organic acids. In G Helas, J Slanina, R Steinbrecher, eds, Biogenic Volatile Organic Compounds in the Atmosphere. SPB Academic Publishing, Amsterdam, pp 157–170
- Brasseur GP, Chatfield RB (1991) The fate of biogenic trace gases in the atmosphere. In TD Sharkey, B Holland, HA Mooney, eds, Trace Gas Emissions by Plants. Academic Press, New York, pp 1–28
- Charron CS, Cantliffe DJ, Wheeler RM, Manukian A, Heath RR (1996) A system and methodology for measuring volatile organic compounds produced by hydroponic lettuce in a controlled environment. J Am Soc Hortic Sci 121: 483–487 [PubMed] [Google Scholar]
- Cojocariu C, Kreuzwieser J, Rennenberg H (2004) Correlation of the short chained carbonyls emitted from Picea abies (L.) Karst with physiological and environmental factors. New Phytol 162: 717–727 [DOI] [PubMed] [Google Scholar]
- De Gouw JA, Howard CJ, Custer TG, Baker BM, Fall R (2000) Proton-transfer chemical ionization mass spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environ Sci Technol 34: 2640–2648 [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W (1999) Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. J Geophys Res 104: 15963–15974 [Google Scholar]
- Fehsenfeld F, Calvert J, Fall R, Goldan P, Guenther AB, Hewitt CN, Lamb B, Liu S, Trainer M, Westberg H, et al (1992) Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Global Biogeochem Cycles 6: 389–430 [Google Scholar]
- Flügge U-I (1999) Phosphate translocators in plastids. Annu Rev Plant Physiol Plant Mol Biol 50: 27–45 [DOI] [PubMed] [Google Scholar]
- Groenveld HW, Binnekamp A, Sekens D (1991) Cardenolide biosynthesis from acetate in Asclepias uassavica. Phytochemistry 30: 2577–2585 [Google Scholar]
- Guenther AB, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley PC, Klinger L, Lerdau M, McKay WA, et al (1995) A global model of natural volatile organic compound emissions. J Geophys Res 100: 8873–8892 [Google Scholar]
- Guenther AB, Zimmerman PR, Wildermuth M (1994) Natural volatile organic compound emission rates for U.S. woodland landscapes. Atmos Environ 28: 1197–1210 [Google Scholar]
- Hahn J, Steinbrecher R, Slemr J (1991) Study of the emission of low molecular-weight organic compounds by various plants. EUROTRAC Annual Report, Part 4. BIATEX, Garmisch-Partenkirchen, Germany, pp 230–235
- Hansel A, Jordan A, Holzinger R, Prazeller P, Vogel W, Lindinger W (1995) Proton transfer reaction mass spectrometry: on-line trace gas analysis at ppb level. Int J Mass Spec Ion Proc 149: 609–619 [Google Scholar]
- Hatanaka A (1993) The biogeneration of green odour by green leaves. Phytochemistry 34: 1201–1218 [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT (1998) Interdependence between chloroplasts and mitochondria in the light and dark. Biochim Biophys Acta 1366: 235–255 [Google Scholar]
- Holzinger R, Sandoval-Soto L, Rottenberger S, Crutzen PJ, Kesselmeier J (2000) Emissions of volatile organic compounds from Quercus ilex L. measured by proton transfer reaction mass spectrometry (PTR-MS) under different environmental conditions. J Geophys Res 105: 20573–20579 [Google Scholar]
- Janson R, de Serves C (2001) Acetone and monoterpene emissions from the boreal forest in northern Europe. Atmos Environ 35: 4629–4637 [Google Scholar]
- Janson R, de Serves C, Romero R (1999) Emission of isoprene and carbonyl compounds from a boreal forest and wetland in Sweden. Agric For Meteorol 98–99: 671–681 [Google Scholar]
- Karl T, Curtis AJ, Rosenstiel TN, Monson RK, Fall R (2002. a) Transient releases of acetaldehyde from tree leaves – products of a pyruvate overflow mechanism? Plant Cell Environ 25: 1121–1131 [Google Scholar]
- Karl T, Fall R, Rosenstiel TN, Prazeller P, Larsen B, Seufert G, Lindinger W (2002. b) On-line analysis of the 13CO2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 215: 894–905 [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Bode K, Hofmann U, Müller H, Schäfer L, Wolf A, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M, et al (1997) Emission of short chained organic acids, aldehydes and monoterpenes from Quercus ilex L. and Pinus pinea L. in relation to physiological activities, carbon budget and emission algorithms. Atmos Environ 31: 119–133 [Google Scholar]
- Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33: 23–88 [Google Scholar]
- Kimmerer TW, Kozlowski TT (1982) Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiol 69: 840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstine W, Galbally I, Ye Y, Hooper M (1998) Emissions of volatile organic compounds (primarily oxygenated species) from pasture. J Geophys Res 103: 10605–10619 [Google Scholar]
- Kitson TM, Kitson KE (1997) Studies of the esterase activity of cytosolic aldehyde dehydrogenase with resorufin acetate as substrate. Biochem J 322: 701–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzias D, Konidari C, Sparta C (1997) Volatile carbonyl compounds of biogenic origin – emission and concentration in the atmosphere. In G Helas, J Slanina, R Steinbrecher, eds, Biogenic Volatile Organic Carbon Compounds in the Atmosphere. SPB Academic Publishing, Amsterdam, pp 67–78
- Kreuzwieser J, Graus M, Wisthaler A, Hansel A, Rennenberg H, Schnitzler J-P (2002) Xylem-transported glucose as additional carbon source for leaf isoprene formation in Quercus robur. New Phytol 156: 171–178 [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Harren FJM, Laarhoven L-J, Boamfa I, te Lintel-Hekkert S, Scheerer U, Hüglin C, Rennenberg H (2001) Acetaldehyde emission by the leaves of trees – correlation with physiological and environmental parameters. Physiol Plant 113: 41–49 [Google Scholar]
- Kreuzwieser J, Kühnemann F, Martis A, Rennenberg H, Urban W (2000) Diurnal pattern of acetaldehyde emission by flooded poplar trees. Physiol Plant 108: 79–86 [Google Scholar]
- Kreuzwieser J, Scheerer U, Rennenberg H (1999) Metabolic origin of acetaldehyde emitted by poplar (Populus tremula x P. alba) trees. J Exp Bot 50: 757–765 [Google Scholar]
- Laber B, Amrhein N (1987) Metabolism of 1-aminoethylphosphinate generates acetylphosphinate, a potent inhibitor of pyruvate dehydrogenase. Biochem J 248: 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L (1992) Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11: 137–141 [DOI] [PubMed] [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998) Proton-transfer-reaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chem Soc Rev 27: 347–354 [Google Scholar]
- Lipsky JJ, Shen ML, Naylor S (2001) Overview – in vitro inhibition of aldehyde dehydrogenase by disulfiram and metabolites. Chem Biol Interact 130–132: 81–91 [DOI] [PubMed] [Google Scholar]
- MacDonald RC, Kimmerer TW (1991) Ethanol in the stems of trees. Physiol Plant 82: 582–588 [Google Scholar]
- Monk LS, Braendle R, Crawford RMM (1987) Catalase activity and post-anoxic injury in monocotyledonous species. J Exp Bot 38: 233–246 [Google Scholar]
- Niinemets U, Loreto F, Reichstein M (2004) Physiological and physico-chemical controls on foliar volatile organic compound emissions. Trends Plant Sci 9: 180–186 [DOI] [PubMed] [Google Scholar]
- Nikolau BJ, Oliver DJ, Schnable PS, Wurtele ES (2000) Molecular biology of acetyl-CoA metabolism. Biochem Soc Trans 28: 591–593 [PubMed] [Google Scholar]
- Op den Camp R, Kuhlemeier C (1997) Aldehyde dehydrogenase from tobacco pollen. Plant Mol Biol 35: 355–364 [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK (2004) Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biol 6: 12–21 [DOI] [PubMed] [Google Scholar]
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK (2003) Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature 421: 256–259 [DOI] [PubMed] [Google Scholar]
- Sanchez LB (1998) Aldehyde dehydrogenase (CoA-acetylating) and the mechanism of ethanol formation in the amitochondriate protest, Giardia lamblia. Arch Biochem Biophys 354: 57–64 [DOI] [PubMed] [Google Scholar]
- Schnitzler J-P, Graus M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A (2004) Contribution of different carbon sources to isoprene biosynthesis in poplar (Populus × canescens) leaves. Plant Physiol 135: 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Yeh SS (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52: 407–436 [DOI] [PubMed] [Google Scholar]
- Tadege M, Dupuis I, Kuhlemeier C (1999) Ethanolic fermentation: new functions for an old pathway. Trends Plant Sci 4: 320–325 [DOI] [PubMed] [Google Scholar]
- Thompson AM (1992) The oxidizing capacity of the Earth`s atmosphere: probable past and future changes. Science 256: 1157–1165 [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB (1997) Plant adaptations to anaerobic stress. Ann Bot (Lond) 79: 3–20 [Google Scholar]
- Zuckermann H, Harren FJM, Reuss J, Parker DH (1997) Dynamics of acetaldehyde production during anoxia and post-anoxia in red bell pepper studied by photoacoustic techniques. Plant Physiol 113: 925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]