Abstract
Indirect defense of plants against herbivores often involves the induced emission of volatile infochemicals including terpenoids that attract natural enemies of the herbivores. We report the isolation and characterization of a terpene synthase cDNA (LjEβOS) from a model legume, Lotus japonicus. Recombinant LjEβOS enzyme produced (E)-β-ocimene (98%) and its Z-isomer (2%). Transcripts of LjEβOS were induced in L. japonicus plants infested with two-spotted spider mites (Tetranychus urticae), coinciding with increasing emissions of (E)-β-ocimene as well as other volatiles, (Z)-3-hexenyl acetate and (E)-4,8-dimethyl-1,3,7-nonatriene, by the infested plants. We suggest that LjEβOS is involved in the herbivore-induced indirect defense response of spider mite-infested L. japonicus via de novo formation and emission (E)-β-ocimene. Mechanical wounding of the leaves or application of alamethicin (ALA), a potent fungal elicitor of plant volatile emission, also induced transiently increased levels of LjEβOS transcripts in L. japonicus. However, wounding or ALA did not result in elevated release of (E)-β-ocimene. Differences in volatile emissions after herbivory, mechanical wounding, or treatment with ALA suggest that neither a single mechanical wounding event nor ALA simulate the effect of herbivore activity and indicate that herbivore-induced emission of (E)-β-ocimene in L. japonicus involves control mechanisms in addition to up-regulation of LjEβOS transcripts.
The herbivore-induced emission of plant volatiles can attract carnivorous natural enemies of herbivores thus potentially protecting the plant (Paré and Tumlinson, 1999; Kessler and Baldwin, 2001; Pichersky and Gershenzon, 2002). Low-molecular-weight terpenoids such as monoterpenes (C-10), sesquiterpenes (C-15), and homoterpenes (C-11 or C-16) are among the most common plant volatile compounds induced and emitted upon herbivory. These terpenoid volatiles, or in case of the homoterpenes, their precursors, are formed by the activity of families of terpenoid synthases (TPS) from polyprenyl diphosphate precursors (Bohlmann et al., 1998; Bouwmeester et al., 1999; Degenhardt and Gershenzon, 2000; Arimura et al., 2004). The acyclic monoterpene hydrocarbon compound (E)-β-ocimene is one of the most common volatile chemicals released from plants in response to herbivory (Paré and Tumlinson, 1999; Pichersky and Gershenzon, 2002). For example among the legumes, lima bean plants (Phaseolus lunatus) infested with two-spotted spider mites (Tetranychus urticae) release (E)-β-ocimene, which attracts a carnivorous natural enemy of two-spotted spider mites, predatory mites (Phytoseiulus persimilis; Dicke et al., 1990a, 1990b, 1999; Ozawa et al., 2000a). (E)-β-ocimene was also shown to act as a possible plant-to-plant signal which up-regulates the signaling pathway of jasmonic acid and ethylene in uninfested lima bean plants (Arimura et al., 2000, 2002). To our knowledge, TPS genes for the formation of (E)-β-ocimene have been cloned as cDNAs only from Arabidopsis (Fäldt et al., 2003) and from snapdragon (Antirrhinum majus; Dudareva et al., 2003). These ocimene synthases represent two different subfamilies of the TPS gene family (Dudareva et al., 2003).
Lotus japonicus is emerging as a model system for molecular and genetic studies in legumes due to its small genome size, its short generation times, self-compatibility, and due to the large expressed sequence tag (EST) resources and efforts toward sequencing the L. japonicus genome (Handberg and Stougaard, 1992; Endo et al., 2002). L. japonicus also serves as a useful system to study indirect plant-herbivore defense mechanisms in a legume. We have previously demonstrated that shoots of L. japonicus (ecotype Gifu B-129) infested with spider mites released a blend of volatiles, which contributed significantly to the attraction of predator mites (Ozawa et al., 2000b). We report here the cloning and functional characterization of a cDNA encoding (E)-β-ocimene synthase from L. japonicus ecotype Miyakojima MG-20. Effects of herbivory on transcript levels of (E)-β-ocimene synthase and increased emissions of (E)-β-ocimene are described. We discuss results of wound- and elicitor-induced regulation of (E)-β-ocimene biosynthesis at the transcriptional and emission levels.
RESULTS
Isolation of L. japonicus Monoterpene Synthase Full-Length cDNA LjEβOS
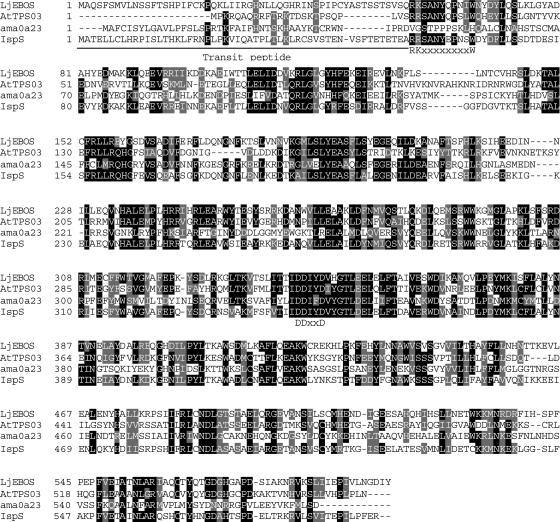
An EST clone for a putative mono-TPS (LjTPS1) was identified in the L. japonicus EST database (http://www.kazusa.or.jp/en/plant/lotus/EST/; accession no. AV409220). The subsequent BLAST search using the sequence of LjTPS1 against the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/BLAST/) resulted in the discovery of a transformation-competent artificial chromosome (TAC) clone (GenBank accession no. AP006119), which contains a gene with high similarity to known monoterpene synthases (LjEβOS). The nucleotide sequences of LjTPS1 and LjEβOS share 81% identity over a length of 1,328 bp. Compared to the nucleotide sequences of the open reading frames (ORFs) of other known mono-TPSs (Bohlmann et al., 1998; Aubourg et al., 2002), the two putative L. japonicus mono-TPS sequences lack each approximately 400 bp from the 5′-ends of their predicted ORFs. The 5′-end of cDNA corresponding to LjEβOS was obtained by a rapid amplification of cDNA ends (RACE) followed by recovery of a full-length cDNA of 1,843 bp (GenBank accession no. AY575970). However, RACE did not lead to obtain the 5′-end of cDNA corresponding to LjTPS1, and this transcript may not be expressed in the L. japonicus shoots used in our research. The ORF of full-length LjEβOS from nucleotide 56 to nucleotide 1,843 encodes for a predicted protein of 595 amino acids, a molecular mass of approximately 68.8 kD, and a calculated pI of 6.26 (Fig. 1). The predicted protein encoded by LjEβOS contains a modification of the conserved motif RRx8W motif of plant monoterpene synthase (Bohlmann et al., 1998; Williams et al., 1998) in the form of an RKx8W motif at amino acid positions 56 to 66 from the N terminus (Fig. 1). The N-terminal region upstream of the RKx8W motif is indicative of the presence of a plastid transit peptide. We also found the DDxxD active-site motif located at amino acids 343 to 347 from the N terminus. Based on sequence relatedness, LjEβOS is a member of the TPS-b group of angiosperm mono- and hemi-TPSs of the plant TPS-gene family (Bohlmann et al., 1998; Aubourg et al., 2002).
Figure 1.
Amino acid sequence alignment of LjEβOS (LjEBOS), Arabidopsis (E)-β-ocimene synthase (AtTPS03, GenBank accession no. AY151086), snapdragon (E)-β-ocimene synthase (ama0a23, AY195607), and Populus alba × Populus tremula isoprene synthase (IspS, AJ294819). Amino acid residues that are identical among at least three sequences are indicated by white letters on black background. Other conserved residues are shaded in gray. Dashes indicate sequence gaps introduced to optimize the alignment. Conserved motifs R(R/K/G)x8W and DDxxD are indicated.
Expression in Escherichia coli and Functional Characterization of LjEβOS
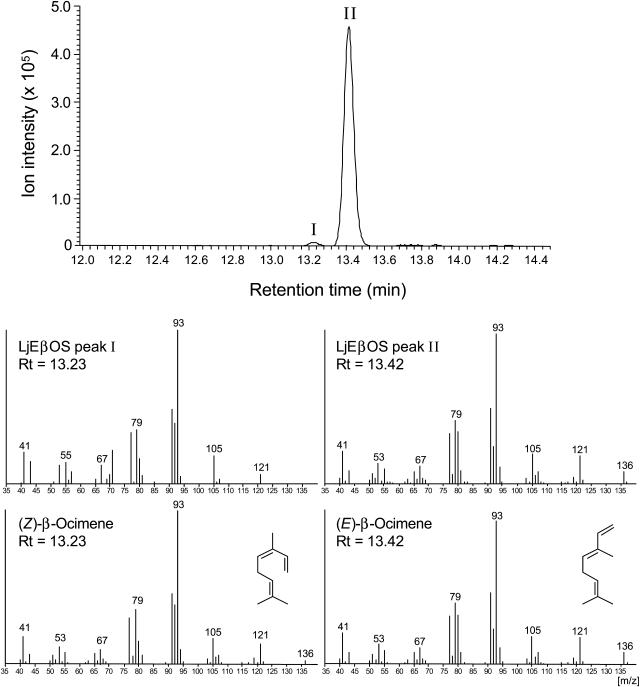
For functional identification of LjEβOS, the cDNA was expressed in E. coli strain BL21-CodonPlus(DE3) transformed with pET101/D-LjEβOS. For expression of LjEβOS without a transit peptide, a starting Met was introduced immediately upstream of the RKx8W motif. Protein extracts of induced E. coli BL21-CodonPlus(DE3)/pET101/D-LjEβOS cells were assayed with three different prenyl diphosphate substrates: geranyl diphosphate (GPP), farnesyl diphosphate, and geranylgeranyl diphosphate. Assays with GPP as the substrate yielded a monoterpene hydrocarbon product profile composed of approximately 98% of (E)-β-ocimene and 2% of (Z)-β-ocimene as identified by gas chromatography-mass spectrometry (GC-MS) using authentic standards for comparison of retention times and mass spectra (Fig. 2). A control extract prepared from E. coli BL21(DE3) transformed with pET101/D-TOPO without the LjEβOS insert did not produce monoterpene hydrocarbon products (data not shown). Recombinant LjEβOS enzyme was not active with farnesyl diphosphate or geranylgeranyl diphosphate. The LjEβOS enzyme was thereby identified as (E)-β-ocimene synthase.
Figure 2.
Expression in E. coli of recombinant LjEβOS protein and GC-MS analysis of monoterpene products formed by recombinant LjEβOS enzyme activity in vitro. Total ion chromatogram and mass spectra are of LjEβOS in vitro enzyme activity products with GPP as substrate. The mass spectrum of the most abundant product (Peak II) and a minor compound (Peak I), identified as (E)- and (Z)-β-ocimene, respectively, are shown together with that of the respective of authentic standards.
Curiously, the deduced protein sequence of LjEβOS has relatively low sequence similarities with two other previously identified (E)-β-ocimene synthases from Arabidopsis (At4g16740; Fäldt et al., 2003; 42% identity [ID] and 61% similarity [SI]) and from snapdragon (AY195607; Dudareva et al., 2003; 33% ID and 51% SI). In contrast, it exhibits much higher similarity with isoprene synthase, a hemiterpene synthase, from Populus alba × Populus tremula (AJ294819; Miller et al., 2001) with 55% ID and 71% SI. LjEβOS also has relatively high similarities with other angiosperm mono-TPSs, e.g. 47% ID and 65% SI with Citrus limon (−)-β-pinene synthase (AF514288; Lücker et al., 2002); and 46% ID and 65% SI with C. limon γ-terpinene synthase (AF514286; Lücker et al., 2002). This finding supports a concept of multiple origins of (E)-β-ocimene synthases within the plant TPS-b group. The formation of (E)-β-ocimene from GPP does not involve a cyclization reaction and conceivably does not require the initial isomerization reaction of monoterpene cyclases (Wise and Croteau, 1999). It is therefore possible that (E)-β-ocimene synthases, like myrcene synthases and linalool synthase, could arise as default functions after mutations occurring in positions that are essential in monoterpene cyclization.
Spider Mites Induce Increased Transcript Levels of LjEβOS and Emissions of (E)-β-Ocimene in L. japonicus Plants
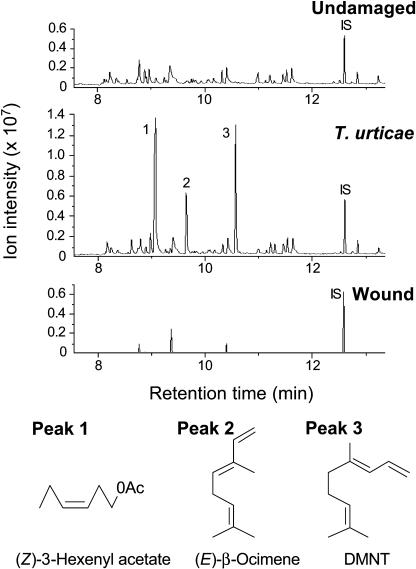
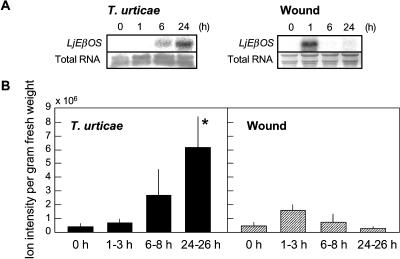
Emission of (E)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), and (Z)-3-hexenyl acetate from L. japonicus (ecotype Miyakojima MG-20) plants was induced 24 h after initiation of a spider mite infestation but not by a single mechanical wounding event (Fig. 3). This finding suggests that one or more herbivore-specific signals are required for the induced emission of these volatiles in addition to a wound stimulus. To test if spider mite-induced emission of (E)-β-ocimene is the result of induced levels of LjEβOS transcripts and to test if differences in wound- and spider mite-induced volatile emissions are reflected in differences at the transcript level, we measured accumulation of LjEβOS transcript abundance as well as (E)-β-ocimene emissions over a time course in L. japonicus shoots exposed to spider mites or after wounding. Transcripts of LjEβOS were induced at 6 and 24 h after first contact with spider mites (Fig. 4A). We also found significantly higher emission of (E)-β-ocimene from spider mite-infested plants than from uninfested plants at 24 to 26 h (P < 0.05, Dunnet's test; Fig. 4B). For comparison with continuous feeding by spider mites, a single mechanical wounding event resulted in a very transient induction of LjEβOS transcripts detected at 1 h after wounding, coinciding with a slightly increased, but not significant (P > 0.05, Dunnet's test), higher level of (E)-β-ocimene emissions at 1 to 3 h after wounding.
Figure 3.
GC profiles of volatiles emitted from L. japonicus plants after 24 h of spider mite infestation or mechanical wounding compared with undamaged plants. Identification of compounds: 1, (Z)-3-hexenyl acetate; 2, (E)-β-ocimene; 3, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT); internal standard (IS), n-tridecane.
Figure 4.
LjEβOS transcripts and (E)-β-ocimene emissions in L. japonicus plants in response to spider mite infestation or mechanical wounding. A, Northern analysis of transcripts in the L. japonicus shoots exposed to spider mites or wounded mechanically for 0, 1, 6, and 24 h. B, Volatiles were collected over 2 h at time points, 1 to 3 h, 6 to 8 h, and 24 to 26 h from the headspace of L. japonicus exposed to spider mites or wounded mechanically and subjected to GC-MS. Data represents the mean ± se (n = 4–9, ion intensity of (E)-β-ocimene per gram fresh weight). Untreated plants served as control. A bar with asterisk is significantly different from the untreated control within each sampling interval (Dunnet's test at the 0.05 level).
Effects of Alamethicin on Transcripts of LjTPS1, LjEβOS, and LjSqS
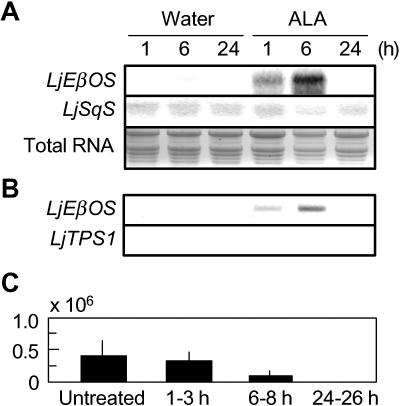
Treatment of L. japonicus plants with alamethicin (ALA), an elicitor of the plant pathogenic fungus Trichoderma viride, induced LjEβOS transcripts at 1 h with a maximum in the shoots at 6 h (Fig. 5A). In contrast to the mono-TPS LjEβOS, transcripts of squalene synthase (LjSqS; AB102688; Akamine et al., 2003), the committed enzyme of the triterpenoid branch of terpenoid biosynthesis, was not induced by ALA, indicating some specificity in the response to ALA. Analyses of transcripts by reverse transcription (RT)-PCR using a pair of LjEβOS-specific primers confirmed the temporal patterns of LjEβOS-transcripts in ALA-treated shoots (Fig. 5B). However, volatile collection analyses indicated that application of ALA to L. japonicus shoots did not induce a significant emission of (E)-β-ocimene from plants for up to 24 h (P > 0.05, Dunnet's test; Fig. 5C). In contrast to the LjEβOS-transcripts, RT-PCR using the LjTPS1-specific primers did not exhibit any detectable signals suggesting absence or very low levels of the LjTPS1 transcripts in constitutive and ALA-treated plants, which is in agreement with the failure of recovering LjTPS1 cDNA by RACE (see above).
Figure 5.
Effects of ALA treatment on transcripts accumulation and emission of (E)-β-ocimene in L. japonicus. A, Northern hybridization signals for LjEβOS and LjSqS in the L. japonicus shoots treated with water or ALA solution. B, RT-PCR analysis using the gene-specific primers of LjEβOS or LjTPS1 followed the same time courses shown in A. C, (E)-β-Ocimene emissions from L. japonicus plants treated with ALA solution. Data represent the mean ± se (n = 3–6, ion intensity of (E)-β-ocimene per gram fresh weight).
DISCUSSION
We describe the identification, cloning, and functional characterization of a cDNA for (E)-β-ocimene synthase from L. japonicus. Increased emissions of (E)-β-ocimene from spider mite-infested L. japonicus plants seems to be associated with increased transcript levels of (E)-β-ocimene synthase, suggesting that herbivorous spider mites induce monoterpene volatile emissions by a mechanism that involves induced TPS gene expression. This is similar to findings in other species such as corn (Zea mays; Shen et al., 2000; Schnee et al., 2002) or poplar (Populus trichocarpa × deltoides; Arimura et al., 2004), where herbivore-induced emission of terpenoid volatiles is associated with induced transcript levels of the corresponding TPS genes. Dicke et al. (1990b) reported that a high ratio of the E-isomer in a mixture of (E)- and (Z)-β-ocimene (E/Z ratios of 97:3 and 70:30) results in the attraction of predator mites, which are natural enemies of spider mites, whereas a lower 60:40 E/Z ratio acts as a repellent for predator mites. Interestingly, only the E-isomer of β-ocimene was found in the headspace of spider mite-infested L. japonicus plants (Fig. 3), suggesting that the activity of LjEβOS, which almost exclusively produces (E)-β-ocimene (approximately 98%), leads to the emission of monoterpene volatiles in spider-mite infested L. japonicus that is highly enriched for one isomer of β-ocimene and could play a role in attracting carnivorous mites.
In contrast to L. japonicus plants infested with spider mites, only a brief induction of LjEβOS transcripts and a very weak emission of (E)-β-ocimene was found following a single mechanical wounding event of L. japonicus leaves (Fig. 4). One interpretation of this result is that cutting leaves with scissors may not be a good imitation of spider mite feeding. It is possible that continuous mechanical wounding, the presence of herbivore-derived elicitors, and/or a puncturing or sucking mode of damage may be required to trigger the same response with regard to volatile emission and transcript accumulation as is induced by real feeding of spider mites on L. japonicus.
Since the application of exogenous ALA, an ion channel-forming fungal elicitor, induced (E)-β-ocimene synthase transcripts in L. japonicus plants (Fig. 5), it is possible that not only herbivore feeding but also pathogen infections contribute to the up-regulation of (E)-β-ocimene synthesis in L. japonicus. Curiously, even 24 h after treatment with ALA, the treated L. japonicus leaves did not produce any of the induced volatile compounds (data not shown). This is in disagreement with findings in the lima bean system where ALA elicits the emission of linalool, DMNT, (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, and methyl salicylate (Engelberth et al., 2001; Kunert et al., 2002). Mechanisms such as the regulation of substrate availability, in addition to induced TPS transcripts, also contribute to the control of terpenoid emissions, as was previously concluded from studies of emission of β-ocimene and myrcene in snapdragon flowers (Dudareva et al., 2003), in studies with herbivore-induced terpenoid emission in poplar leaves (Arimura et al., 2004), and in studies of terpenoid emissions in TPS-overexpressing Arabidopsis plants (Aharoni et al., 2003). It cannot be excluded that additional (E)-β-ocimene synthase gene(s) exist in L. japonicus that could contribute to regulation of herbivore-induced volatile emission in this system. For example, two myrcene synthases have been described in snapdragon with different roles in the regulation floral scent formation (Dudareva et al., 2003).
Our finding of spider mite-induced (E)-β-ocimene synthase transcript accumulation and (E)-β-ocimene emission in the L. japonicus MG-20 ecotype provides a foundation for future investigation of molecular genetic control of volatile emission in other ecotypes. In contrast to MG-20 plants, the L. japonicus ecotype Gifu B-129 characterized by Ozawa et al. (2000b) did not show emission of (E)-β-ocimene after 3 d of feeding by spider mites. Both ecotypes also differ in several other traits, such as anthocyanin contents, trichome density, overall growth habit, and organ shape (Kawaguchi et al., 2001), making this a suitable system for future comparative adaptive trait analysis including an analysis of factors that determine ecotype-specific differences of herbivore-induced volatile emissions.
MATERIALS AND METHODS
Plants and Spider Mites
L. japonicus (ecotype Miyakojima MG-20) was grown in plastic pots (diameter = 8 cm, depth = 6.5 cm) each containing four plants in a growth chamber (12 h light:12 h dark, 25°C ± 1°C) for 2 months. Two-spotted spider mites (Tetranychus urticae) were obtained from a laboratory-maintained culture reared on kidney bean plants (Phaseolus vulgaris cv Nagauzuramame) in a greenhouse (25 ± 2°C).
Plant Treatment
Plants without flowers were used for each treatment. For mechanical wounding, leaves were wounded by cutting 20 leaves transversely at the widest point with scissors. ALA (100 ng/mL, Sigma-Aldrich, St. Louis) in 10 mL of water was sprayed onto four plants. Controls were sprayed with 10 mL of water. For spider mite infestation, we placed approximately 400 spider mite females on the L. japonicus shoots in a pot. Treatments were at 11 am. During treatment experiments, temperature was held constant at 25°C ± 1°C and the photoperiod was 16 h light:8 h dark with light turning on at 7 am and turning off at 11 pm.
Sequence Analysis
The putative splice sites and a potential coding region in the sequence of the TAC clone (AP006119) from L. japonicus chromosome 3 were predicted using the software NetGene2 (http://www.cbs.dtu.dk/services/NetGene2/) and GeneMark.hmm (http://opal.biology.gatech.edu/GeneMark/eukhmm.cgi). Alignments of deduced amino acid sequences were created using CLUSTALW, and visualized using BOXSHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html).
5′-RACE and Full-Length cDNA Cloning of LjEβOS
Cloning of the 5′-end of LjEβOS was accomplished by 5′-RACE using the First Choice RLM-RACE kit (Ambion, Austin, TX) following the manufacturer's protocol. Total RNA was isolated from ALA-treated leaves by means of the method described by Wang et al. (2000). cDNA was amplified with Turbo Pfu polymerase (Stratagene, La Jolla, CA), using a LjEβOS-reverse primer (5′-GAAGCTCCAATGCATGATTCAC-3′) and a 5′-RACE outer primer (included with the kit). PCR was 2 min at 94°C followed by 35 cycles of 30 s at 94°C, 30 s at 61°C, and 60 s at 72°C, followed by 5 min at 72°C. A LjEβOS-reverse primer was designed from the sequence of the TAC clone (AP006119).
Total RNA from ALA-treated leaves was reverse transcribed into cDNA using Super-Script II RNase H− reverse transcriptase (Invitrogen, Burlington, Canada) following the manufacturer's protocol. The cDNA was amplified by PCR using high fidelity Turbo Pfu polymerase with forward primer (LjEβOS-ORFS+CACCATG, 5′-CACCATGAGAAAATCAGCCAATTACCAA-3′) and reverse primer (LjEβOS-ORFA, 5′-TTAATATATGTCTCCATTGAGAACAATGG-3′). The temperature program for PCR was as follows: 2 min at 94°C followed by 35 cycles of 30 s at 94°C, 30 s at 55°C, and 90 s at 72°C, followed by 5 min at 72°C. LjEβOS cDNA was subcloned into pET101/D-TOPO expression vector (Invitrogen). Recombinant plasmids were transformed into E. coli TOP10 cells. The plasmid pET101/D-LjEβOS was purified, its insert sequenced, and transformed into E. coli BL21-CodonPlus(DE3) (Stratagene) for expression.
Functional Expression of Mono-TPS in E. coli and Enzyme Assay
Bacterial strain E. coli BL21-CodonPlus(DE3)/pET101/D-LjEβOS was grown to A600 = 0.5 at 37°C in 5 mL of Luria-Bertani medium with ampicillin at 100 μg/mL. Cultures were induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside and held overnight at 20°C with shaking at 200 rpm. Cells were pelleted by centrifugation and were resuspended in 1 mL mono-TPS buffer (25 mm HEPES, pH 7.2, 7.5 mm MgCl2, 20 μm MnCl2, 5% glycerol). Resuspended cells were broken by sonication (Branson Sonifier 250, Branson Ultrasonic Corporation, Danbury, CT) at 5 W for 10 s. Cell extracts were cleared by centrifugation and assayed for mono-TPS activity with 55 μm GPP (Echelon Biosciences, Salt Lake City). The assay mixture (1 mL) was overlaid with 1 mL of pentane to trap volatile products. After incubation with gentle shaking at 30°C for 1 h, the pentane layer was passed through a column of equal amounts of anhydrous MgSO4 and silica gel. The assay mixture was extracted with pentane (1 mL) a second time, and the pentane was passed through the column again. Samples were concentrated to <200 μL under a gentle stream of charcoal-filtered nitrogen. Extracts of E. coli BL21-CodonPlus(DE3) transformed with pET101/D-TOPO plasmid without the LjEβOS insert, treated as described above, were used as controls.
Identification of Products of Mono-TPS Assays by GC-MS
Products of mono-TPS assays were identified and their relative ratios estimated following GC-MS analysis on an Agilent 6890 Series GC System connected to an Agilent 5973 Network Mass Selective detector (70 eV; Palo Alto, CA) using a DB-1MS capillary column (0.25 mm i.d. × 30 m with 0.25-mm film; J&W Scientific, Palo Alto, CA). Injections were splitless and the injector temperature was 200°C. The temperature program for GC was as follows: initial temperature was 40°C and immediately rose at 10°C/min to 200°C then rose at 20°C/min to a final temperature of 300°C, which was held for 1 min. The carrier gas was helium. (E)- and (Z)-β-ocimene were identified by comparisons with mass spectral libraries (ChemStation software, Hewlett-Packard, Wiley library, Palo Alto, CA) and by matching retention time with an authentic standard generously provided by Dr. John H. Borden (Simon Fraser University, Burnaby, Canada).
Northern Analysis
RNA samples (10 μg) were isolated from L. japonicus shoots and separated by electrophoresis through formaldehyde-agarose gels and blotted to nylon membranes (Hybond-N+, Amersham Biosciences, Piscataway, NJ). The cDNA clones for LjEβOS and LjSqS were 32P-labeled by random priming (Strip-EZ DNA, Ambion). Hybridization and washes were carried out according to Arimura et al. (2004). The hybridized, labeled cDNA signals were detected using a Storm 860 phosphorimager (Amersham Biosciences). Ribosomal RNA stained with ethidium bromide was visualized (ChemiImager 5500 with AlphaEaseFC software, Alpha Innotech, San Leandro, CA).
RT-PCR Expression Analysis
Total RNA was reverse transcribed from 1 μg of total RNA, using Super-Script II RNase H− reverse transcriptase following the manufacturer's protocol. The cDNAs were amplified by PCR using Taq polymerase (New England Biolabs, Mississauga, Canada) with 2 μL of RT-products and a pair of LjEβOS-specific primers (LjEβOS-S1, 5′-GGTAATTTCAAGACAAGTCTTGTC-3′ and LjEβOS-A1, 5′-GACCCAAGCATTATTGAGATAATGC-3′) or LjTPS1-specific primers (LjTPS1-S1, 5′-GGTAATTTTGAGGCGAACCTAATTGG-3′ and LjTPS1-A1, 5′-CACCCATGCATTGTTGAGGTAGTCA-3′). The temperature program for PCR was as follows: 5 min at 94°C followed by 35 cycles of 30 s at 94°C, 30 s at 55°C, and 60 s at 72°C, followed by 5 min at 72°C. PCR products were separated by electrophoresis in 1.5% agarose gel including ethidium bromide and visualized by ChemiImager 5500.
Analysis of Plant Volatiles
For headspace analyses, L. japonicus plants in a pot were enclosed together with a piece of filter paper that contained 250 ng of tridecane in n-hexane, as an internal standard, in a glass container (2 L) at 25°C. The volatile compounds were drawn from the headspace of the container into a glass tube packed with Tenax TA adsorbents (100 mg, mesh 20/35) for 2 h at a flow rate of 100 mL/min. The collected volatile compounds were analyzed by GC-MS (GC: Hewlett-Packard 6890 with HP-5MS capillary column, 30 m long, 0.25 mm i.d., and 0.25-μm film thickness; MS: Hewlett-Packard 5973 mass selective detector, 70 eV) equipped with a thermal desorption cold trap injector (TCT; CP4010, Chrompack, Middelburg, The Netherlands). Headspace volatiles collected on Tenax-TA were released in the TCT themo-desorption unit at 220°C for 8 min, within a He flow. The desorbed compounds were collected in the TCT cold trap unit (SIL5CB-coated fused silica capillary) at −130°C. Flash heating of the cold trap unit provided sharp injection of the compounds into the capillary column of the gas chromatograph to which the cold trap unit was connected. The oven temperature of the GC was programmed to rise from 40°C (5-min hold) to 280°C at 15°C/min. The headspace volatiles were identified by comparing their mass spectrums and retention times with those of authentic compounds.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AV409220, AP006119, AY575970, AY151086, AY195607, AJ294819, and AB102688.
Acknowledgments
We thank Dr. Sébastien Aubourg for prediction of potential coding regions in the genomic sequence of LjEβOS, Kimberley-Ann Godard for helpful comments, the National Agricultural Research Center for Hokkaido Region for L. japonicus seeds, and the Kazusa DNA Research Institute for Department of Plant Gene Research for a L. japonicus EST clone of LjTPS1.
This work was supported by the program for Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation (grant to J.T.), by the Japan Society for the Promotion of Science (fellowship to G.A.), and by the Natural Sciences and Engineering Research Council (NSERC) of Canada (grant to J.B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.042929.
References
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FW, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15: 2866–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamine S, Nakamori K, Chechetka SA, Banba M, Umehara Y, Kouchi H, Izui K, Hata S (2003) cDNA cloning, mRNA expression, and mutational analysis of the squalene synthase gene of Lotus japonicus. Biochim Biophys Acta 1626: 97–101 [DOI] [PubMed] [Google Scholar]
- Arimura G, Huber DPW, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. Plant J 37: 603–616 [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kühnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J 29: 87–98 [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defense genes in lima bean leaves. Nature 406: 512–515 [DOI] [PubMed] [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267: 730–745 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95: 4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Verstappen FW, Posthumus MA, Dicke M (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol 121: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt J, Gershenzon J (2000) Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210: 815–822 [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25: 1907–1922 [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990. a) Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol 16: 3091–3118 [DOI] [PubMed] [Google Scholar]
- Dicke M, van Beek TA, Posthumus MA, Ben Dom N, van Bokhoven H, de Groot AE (1990. b) Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. Involvement of host plant in its production. J Chem Ecol 16: 381–396 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Fäldt J, Miller B, Bohlmann J (2003) (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15: 1227–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Matsubara H, Kokubun T, Masuko H, Takahata Y, Tsuchiya T, Fukuda H, Demura T, Watanabe M (2002) The advantages of cDNA microarray as an effective tool for identification of reproductive organ-specific genes in a model legume, Lotus japonicus. FEBS Lett 514: 229–237 [DOI] [PubMed] [Google Scholar]
- Engelberth J, Koch T, Schüler G, Bachmann N, Rechtenbach J, Boland W (2001) Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol 125: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt J, Arimura G, Gershenzon J, Takabayashi J, Bohlmann J (2003) Functional identification of AtTPS03 as (E)-β-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 216: 745–751 [DOI] [PubMed] [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487–496 [Google Scholar]
- Kawaguchi M, Motomura T, Imaizumi-Anraku H, Akao S, Kawasaki S (2001) Providing the basis for genomics in Lotus japonicus: the accessions Miyakojima and Gifu are appropriate crossing partners for genetic analyses. Mol Genet Genomics 266: 157–166 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kunert M, Biedermann A, Koch T, Boland W (2002) Ultrafast sampling and analysis of plant volatiles by a hand-held miniaturised GC with pre-concentration unit: kinetic and quantitative aspects of plant volatile production. J Sep Sci 25: 677–684 [Google Scholar]
- Lücker J, El Tamer MK, Schwab W, Verstappen FW, van der Plas LH, Bouwmeester HJ, Verhoeven HA (2002) Monoterpene biosynthesis in lemon (Citrus limon). cDNA isolation and functional analysis of four monoterpene synthases. Eur J Biochem 269: 3160–3171 [DOI] [PubMed] [Google Scholar]
- Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213: 483–487 [DOI] [PubMed] [Google Scholar]
- Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000. a) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41: 391–398 [DOI] [PubMed] [Google Scholar]
- Ozawa R, Shimoda T, Kawaguchi M, Arimura G, Horiuchi J, Nishioka T, Takabayashi J (2000. b) Lotus japonicus infested with herbivorous mites emits volatile compounds that attract predatory mites. J Plant Res 113: 427–433 [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–331 [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol 130: 2049–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Zheng Z, Dooner HK (2000) A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: characterization of wild-type and mutant alleles. Proc Natl Acad Sci USA 97: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Hunter W, Plant A (2000) Isolation and purification of functional total RNA from woody branches and needles of Sitka and white spruce. Biotechniques 28: 292–296 [DOI] [PubMed] [Google Scholar]
- Williams DC, McGarvey DJ, Katahira EJ, Croteau R (1998) Truncation of limonene synthase preprotein provides a fully active ‘pseudomature’ form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry 37: 12213–12220 [DOI] [PubMed] [Google Scholar]
- Wise ML, Croteau R (1999) Monoterpene biosynthesis. In DE Cane, ed, Comprehensive Natural Product Chemistry, Vol 2, Isoprenoids Including Carotenoids and Steroids. Pergamon Press, Oxford, pp 97–153