Abstract
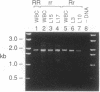
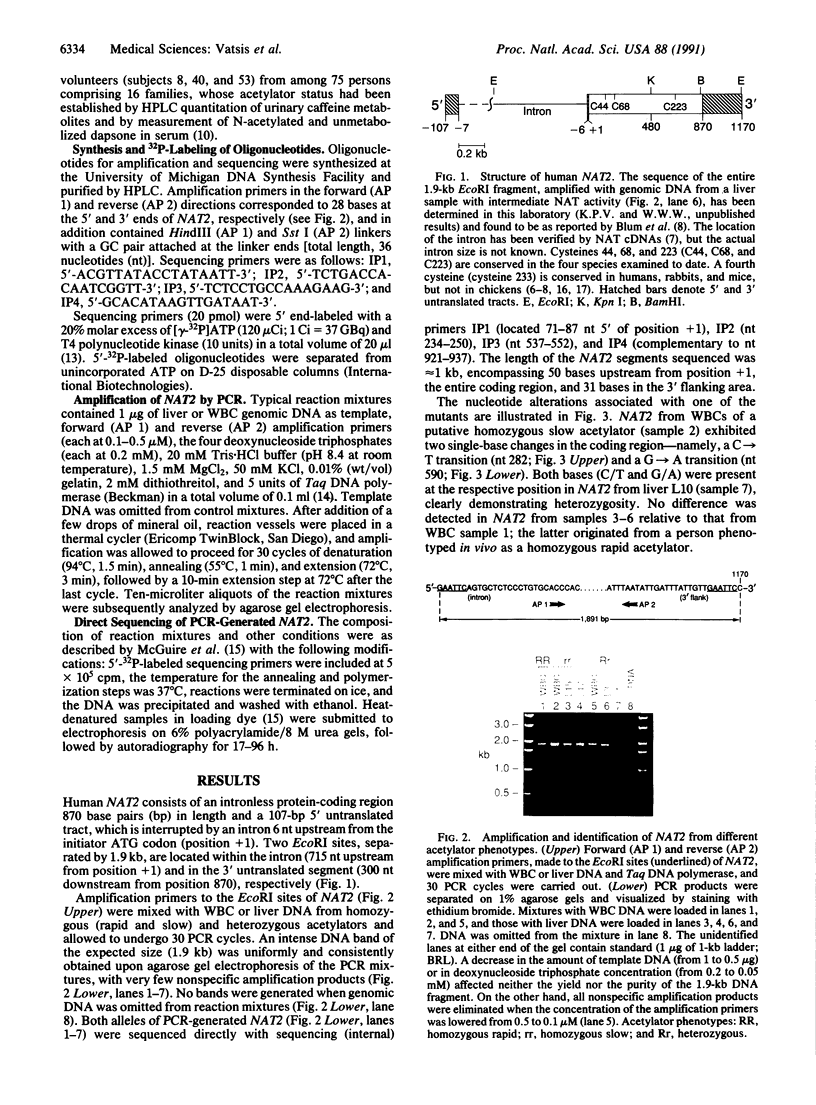
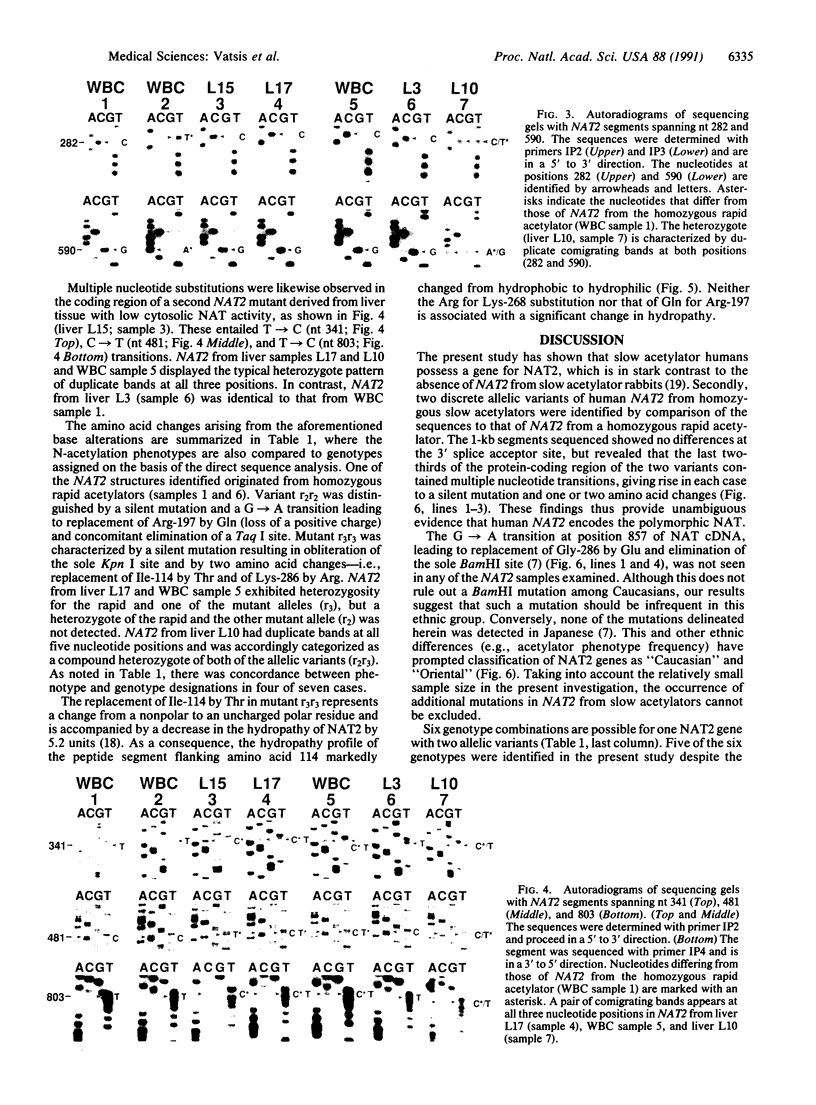
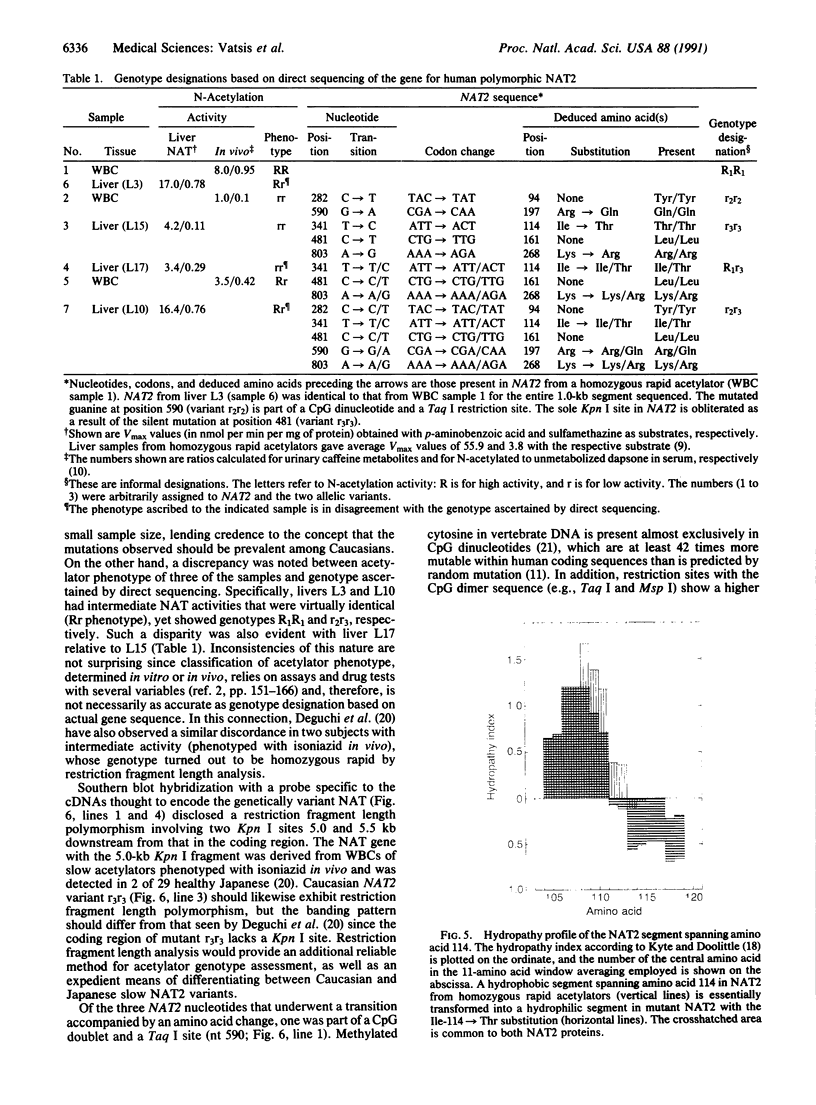
Classification of humans as rapid or slow acetylators is based on hereditary differences in rates of N-acetylation of therapeutic and carcinogenic agents, but N-acetylation of certain arylamine drugs displays no genetic variations. Two highly homologous human genes for N-acetyltransferase (NAT; arylamine acetyltransferase, acetyl CoA:arylamine N-acetyltransferase, EC 2.3.1.5), NAT1 and NAT2, presumably code for the genetically invariant and variant NAT proteins, respectively. In the present investigation, 1.9-kilobase human genomic EcoRI fragments encoding NAT2 were generated by the polymerase chain reaction with liver and leukocyte DNA from seven subjects phenotyped as homozygous and heterozygous acetylators. Direct sequencing revealed multiple point mutations in the coding region of two distinct NAT2 variants. One of these was derived from leukocytes of a slow acetylator and was distinguished by a silent mutation (codon 94) and a separate G----A transition (position 590) leading to replacement of Arg-197 by Gln; the mutated guanine was part of a CpG dinucleotide and a Taq I site. The second NAT2 variant originated from liver with low N-acetylation activity. It was characterized by three nucleotide transitions giving rise to a silent mutation (codon 161), accompanied by obliteration of the sole Kpn I site, and two amino acid substitutions: Thr for Ile (codon 114) and Arg for Lys (codon 268). Heterozygosity was detected in three NAT2 samples: two were heterozygous for the rapid and one of the allelic variants, and the third was a compound heterozygote of both mutant alleles. The results show conclusively that the genetically variant NAT is encoded by NAT2.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres H. H., Klem A. J., Schopfer L. M., Harrison J. K., Weber W. W. On the active site of liver acetyl-CoA. Arylamine N-acetyltransferase from rapid acetylator rabbits (III/J). J Biol Chem. 1988 Jun 5;263(16):7521–7527. [PubMed] [Google Scholar]
- Andres H. H., Vogel R. S., Tarr G. E., Johnson L., Weber W. W. Purification, physicochemical, and kinetic properties of liver acetyl-CoA:arylamine N-acetyltransferase from rapid acetylator rabbits. Mol Pharmacol. 1987 Apr;31(4):446–456. [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., Demierre A., Meyer U. A. N-acetylation pharmacogenetics: a gene deletion causes absence of arylamine N-acetyltransferase in liver of slow acetylator rabbits. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9554–9557. doi: 10.1073/pnas.86.23.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., Demierre A., Meyer U. A. Nucleotide sequence of a full-length cDNA for arylamine N-acetyltransferase from rabbit liver. Nucleic Acids Res. 1989 May 11;17(9):3589–3589. doi: 10.1093/nar/17.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., McBride W., Heim M., Meyer U. A. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. DNA Cell Biol. 1990 Apr;9(3):193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Mashimo M., Suzuki T. Correlation between acetylator phenotypes and genotypes of polymorphic arylamine N-acetyltransferase in human liver. J Biol Chem. 1990 Aug 5;265(22):12757–12760. [PubMed] [Google Scholar]
- Evans D. A. N-acetyltransferase. Pharmacol Ther. 1989;42(2):157–234. doi: 10.1016/0163-7258(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Kilbane A. J., Petroff T., Weber W. W. Kinetics of acetyl CoA: arylamine N-acetyltransferase from rapid and slow acetylator human liver. Drug Metab Dispos. 1991 Mar-Apr;19(2):503–507. [PubMed] [Google Scholar]
- Kilbane A. J., Silbart L. K., Manis M., Beitins I. Z., Weber W. W. Human N-acetylation genotype determination with urinary caffeine metabolites. Clin Pharmacol Ther. 1990 Apr;47(4):470–477. doi: 10.1038/clpt.1990.59. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Levy G. N., Weber W. W. High-performance liquid chromatographic analysis of 32P-postlabeled DNA-aromatic carcinogen adducts. Anal Biochem. 1988 Nov 1;174(2):381–392. doi: 10.1016/0003-2697(88)90037-1. [DOI] [PubMed] [Google Scholar]
- McGuire M. C., Nogueira C. P., Bartels C. F., Lightstone H., Hajra A., Van der Spek A. F., Lockridge O., La Du B. N. Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase. Proc Natl Acad Sci U S A. 1989 Feb;86(3):953–957. doi: 10.1073/pnas.86.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Cloning and expression of cDNAs for polymorphic and monomorphic arylamine N-acetyltransferases from human liver. J Biol Chem. 1990 Mar 15;265(8):4630–4634. [PubMed] [Google Scholar]
- Ohsako S., Ohtomi M., Sakamoto Y., Uyemura K., Deguchi T. Arylamine N-acetyltransferase from chicken liver II. Cloning of cDNA and expression in Chinese hamster ovary cells. J Biol Chem. 1988 Jun 5;263(16):7534–7538. [PubMed] [Google Scholar]
- Weber W. W., Hein D. W. N-acetylation pharmacogenetics. Pharmacol Rev. 1985 Mar;37(1):25–79. [PubMed] [Google Scholar]