Abstract
Several plant species defend themselves indirectly from herbivores by producing herbivore-induced volatile compounds that attract the natural enemies of herbivores. Here we tested the effects of elevated atmospheric CO2 (720 μmol mol−1) concentration on this indirect defense, physiological properties, and constitutive and induced emissions of white cabbage (Brassica oleracea ssp. capitata, cvs Lennox and Rinda). We monitored the orientation behavior of the generalist predator Podisus maculiventris (Heteroptera: Pentatomidae) and the specialist parasitoid Cotesia plutellae (Hymenoptera: Braconidae) to plants damaged by Plutella xylostella (Lepidoptera: Plutellidae) in the Y-tube olfactometer. Elevated CO2 levels did not affect stomatal densities but reduced specific leaf area and increased leaf thickness in cv Lennox. In addition to enhanced constitutive monoterpene emission, P. xylostella-damaged cabbages emitted homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene, sesquiterpene (E,E)-α-farnesene, and (Z)-3-hexenyl acetate. Growth at elevated CO2 had no significant effect on the emissions expressed per leaf area, while minor reduction in the emission of homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene and (E,E)-α-farnesene was observed at elevated CO2 in one of two experiments. The generalist predator P. maculiventris discriminated only between the odors of intact and P. xylostella-damaged cv Rinda plants grown at ambient CO2 concentration, preferring the odor of the damaged plants. The specialist parasitoid C. plutellae preferred the odor of damaged plants of both cultivars grown at ambient CO2 but did not detect damaged cv Lennox plants grown at elevated CO2. The results suggest that elevated atmospheric CO2 concentration could weaken the plant response induced by insect herbivore feeding and thereby lead to a disturbance of signaling to the third trophic level.
Several plant species express traits which can indirectly participate in controlling the herbivore population, i.e. the release of volatile compounds after herbivore damage which participate in top-down control of herbivores by attracting predators and parasitoids (e.g. Dicke et al., 1990; Turlings et al., 1991). The emission of these herbivore-induced volatile compounds are strongly affected by abiotic factors such as soil and air humidity, temperature, light, and fertilization rate (Gouinguené and Turlings, 2002) as well as biotic factors such as plant cultivar, growth stage of the leaf, and attacking herbivore species (Takabayashi et al., 1994).
The concentration of atmospheric CO2 is expected to double by the end of this century (Houghton et al., 2001), and a simultaneous increase in the emissions from plants is predicted due to climate warming (Constable et al., 1999a, 1999b). This will affect the hydrocarbon load into the atmosphere and cause changes in ozone and aerosol formation (Andreae and Crutzen, 1997; Peñuelas and Llusià, 2001, 2003). Monoterpenes (MTs) have an antioxidative role protecting plants probably in a wide range of stress situations (Loreto et al., 2004), and these changes in MT formation may well be an important issue to be considered in climate change studies.
Changes in the atmospheric CO2 concentration affect plant physiology: plants are larger, grow faster, have increased carbon:nitrogen ratios and a decreased specific leaf area (SLA) under elevated CO2 (Bazzaz, 1990; Bezemer and Jones, 1998; Poorter and Navas, 2003; Sallas et al., 2003). Presumably higher trophic levels, predators, and parasitoids will also be affected through changes in the host plant quality. An elevated CO2 concentration has evoked variable changes in the MT emission of plants: no effect (Peñuelas and Llusià, 1997; Constable et al., 1999b), MT emission has been higher at elevated CO2 than at ambient air (Staudt et al., 2001), or CO2 exposure has decreased total MT emission (Loreto et al., 2001; Vuorinen et al., 2004). Changes in the emitted MTs and other compounds might be dependent on the availability of photosynthetic carbon which is needed in the production of volatiles (Loreto et al., 2001). Even though an elevated CO2 level stimulates plant growth, the density of stomata might be decreased, as detected with Arabidopsis (Woodward et al., 2002), and this could potentially diminish the emission of volatile compounds.
A variety of induced compounds are released from herbivore-damaged plants (Geervliet et al., 1997; Paré and Tumlinson, 1999; Van Den Boom et al., 2004; Vuorinen et al., 2004). Feeding damage of both specialist Plutella xylostella and generalist Spodoptera littoralis larvae induced the emission of (Z)-3-hexenyl acetate and (E,E)-α-farnesene, but the homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) emission was higher after specialist than generalist damage in cabbages (Brassica oleracea; Vuorinen et al., 2004). Also, larvae of Pieris rapae and Pieris brassicae induced the emission of DMNT and (Z)-3-hexenyl acetate and caused twice as high total emissions as produced by control cabbages (Geervliet et al., 1997). Potting et al. (1999) showed that P. xylostella-damaged Brassica napus plants were attractive to the parasitoid Cotesia plutellae. The induced compounds should contain the chemical information needed by predators and parasitoids to locate their hosts. Although, a number of studies has been conducted on volatiles that attract the natural enemies of herbivores, there has been no report yet on the volatiles emitted from plants grown at elevated CO2 level and the subsequent consequences on the orientation behavior of the natural enemies.
The objective of this study was to evaluate the effects of an elevated CO2 concentration on the emission of cabbage plants damaged by the crucifer specialist, the diamondback moth, P. xylostella, which is one of the most serious pests of cruciferous plants found throughout the world (Talekar and Shelton, 1993). The orientation behaviors of the generalist predator Podisus maculiventris and the specialist parasitoid C. plutellae were determined, since the response of specialists would be expected to differ from that of generalists. These results are important for evaluation and modeling of the effects of climate change on multitrophic signaling by plant volatiles in food chains both in natural and man-made ecosystems.
RESULTS
Plant Growth and Properties
In the first experiment, the growth of cv Lennox was more responsive to elevated CO2 than growth of cv Rinda, cv Lennox exhibited enhanced shoot dry weight and decreased fresh/dry weight ratios in both intact and P. xylostella-damaged plants and enhanced shoot fresh weight in intact plants (data not shown). Leaf thickness was increased and SLA was decreased in cv Lennox at elevated CO2, but stomatal density was not affected in either of the cultivars (Table I). In the second experiment, leaf area, SLA, shoot fresh weight, and shoot dry weight in intact Rinda and Lennox cvs did not differ between ambient and elevated CO2 concentrations, although SLA of P. xylostella-damaged plants of cv Lennox was decreased. Even though P. xylostella caused more feeding holes in cv Lennox grown at elevated CO2, the shoot dry weight of those plants remained higher at elevated CO2 than at ambient CO2 (Table I).
Table I.
Physiological properties (mean ± sd) of the intact control cabbages in experiments 1 and 2, and P. xylostella-damaged cabbages in experiment 2
| N | Rinda
|
Lennox
|
|||||
|---|---|---|---|---|---|---|---|
| Ambient CO2 | Elevated CO2 | P | Ambient CO2 | Elevated CO2 | P | ||
| Experiment 1 | |||||||
| Control | |||||||
| SLA (cm2 g dw−1) | 6 | 227.1 ± 40.0 | 190.5 ± 31.7 | 0.110 | 139.5 ± 14.8 | 110.2 ± 9.0 | 0.002 |
| Leaf thickness (μm) | 12 | 293.8 ± 40.9 | 293.1 ± 47.9 | 0.967 | 307 ± 33.8 | 350 ± 41.0 | 0.012 |
| Stomatal density (n mm−2) | |||||||
| 3rd youngest leaf | |||||||
| Adaxial | 6 | 146.0 ± 24.1 | 128.2 ± 16.7 | 0.153 | 207.7 ± 51.2 | 239.1 ± 103.0 | 0.518 |
| Abaxial | 6 | 203.2 ± 26.8 | 202.0 ± 36.9 | 0.950 | 254.4 ± 60.1 | 246.4 ± 21.6 | 0.775 |
| 5th youngest leaf | |||||||
| Adaxia | 6 | 232.0 ± 20.7 | 213.2 ± 42.0 | 0.347 | 288.2 ± 25.4 | 291.4 ± 33.4 | 0.860 |
| Abaxial | 6 | 318.9 ± 32.2 | 286.8 ± 77.1 | 0.370 | 345.8 ± 91.3 | 326.3 ± 57.4 | 0.667 |
| Experiment 2 | |||||||
| Control | |||||||
| Leaf area (cm2) | 5 | 272.7 ± 70.0 | 269.8 ± 27.8 | 0.934 | 218.9 ± 24.3 | 228.8 ± 13.9 | 0.451 |
| SLA (cm2 g dw−1) | 5 | 199.1 ± 49.3 | 143.3 ± 24.6 | 0.053 | 168.5 ± 33.8 | 149.8 ± 30.0 | 0.381 |
| Shoot fresh weight (g) | 5 | 15.8 ± 4.9 | 16.8 ± 2.4 | 0.707 | 12.9 ± 1.4 | 14.2 ± 1.3 | 0.163 |
| Shoot dry weight (g) | 5 | 1.7 ± 0.6 | 2.2 ± 0.4 | 0.136 | 2.0 ± 1.1 | 1.8 ± 0.3 | 0.710 |
| Fresh/dry weight ratio | 5 | 9.6 ± 1.4 | 7.6 ± 1.0 | 0.028 | 7.3 ± 2.7 | 7.8 ± 0.9 | 0.717 |
| P. xylostella | |||||||
| Leaf area (cm2) | 5 | 254.8 ± 27.9 | 227.94 ± 29.2 | 0.174 | 189.0 ± 30.6 | 200.0 ± 5.1 | 0.469 |
| SLA (cm2 g−1) | 5 | 172.5 ± 30.4 | 158.2 ± 28.5 | 0.466 | 158.2 ± 34.6 | 105.0 ± 7.0 | 0.025 |
| Shoot fresh weight (g) | 5 | 15.4 ± 2.4 | 14.23 ± 2.5 | 0.470 | 10.8 ± 2.1 | 12.8 ± 0.6 | 0.100 |
| Shoot dry weight (g) | 5 | 1.8 ± 0.4 | 1.78 ± 0.4 | 0.962 | 1.5 ± 0.5 | 2.2 ± 0.2 | 0.026 |
| Fresh/dry weight ratio | 5 | 8.7 ± 1.1 | 8.17 ± 1.1 | 0.458 | 7.4 ± 1.2 | 5.8 ± 0.3 | 0.037 |
| ∅ < 2 mm holes per leaf | 5 | 2.1 ± 1.6 | 2.6 ± 3.3 | 0.777 | 0.8 ± 0.4 | 1.6 ± 0.5 | 0.020 |
| ∅ > 2 mm holes per leaf | 5 | 7.3 ± 4.2 | 5.7 ± 2.4 | 0.496 | 3.1 ± 1.1 | 5.3 ± 1.0 | 0.009 |
Plants were grown at ambient (360 μmol mol−1) or elevated CO2 (720 μmol mol−1) concentration. The CO2 effect was tested with independent samples t test.
The Emission of Volatile Compounds
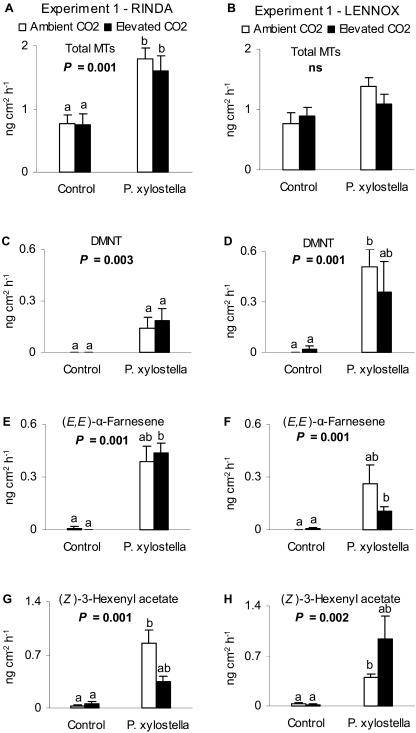
The composition of the cabbage emission profile in both experiments was identical. The emission of intact control cabbages consisted mainly of constitutive MTs (sabinene, limonene, β-pinene + myrcene [these two compounds were not separated on our HP-5 column], 1,8-cineol, α-thujene, and α-pinene), while P. xylostella-damaged cabbages emitted in addition to MTs also induced compounds, DMNT, sesquiterpene (E,E)-α-farnesene, and (Z)-3-hexenyl acetate. The emission data expressed on a leaf area basis and collated from both experiments revealed that growing the plants at elevated CO2 had no effect on the emission of total (Figs. 1 and 2, A and B) or individual (data not shown) MTs of intact control and P. xylostella-damaged cabbage cvs Rinda and Lennox. Emissions of individual MTs of cv Rinda in experiment 1 were significantly higher from herbivore-damaged than intact control plants (main effect one-way ANOVA P < 0.005), while in experiment 2 only the emission of α-thujene was enhanced by P. xylostella-damage (main effect K-W P = 0.037). On the other hand, α-pinene and β-pinene emissions in experiment 1 and the emissions of all individual MTs of cv Lennox in experiment 2 were significantly higher from herbivore-damaged plants than from intact plants.
Figure 1.
Emission of intact control and P. xylostella-damaged cabbages (cvs Rinda and Lennox) grown at ambient or elevated CO2 concentration. A and B, Emissions of total MTs (sabinene, limonene, β-pinene + myrcene, 1,8-cineol, α-thujene, and α-pinene; mean + se). C and D, DMNT. E and F, (E,E)-α-farnesene. G and H, (Z)-3-hexenyl acetate (ng cm−2 h−1) from intact control and P. xylostella-damaged cabbage plants (n = 5) grown at ambient (360 μmol mol−1) or elevated (720 μmol mol−1) CO2 concentration in experiment 1. P-values indicate the main effect tested by Kruskal-Wallis-test. Different letters above the bars indicate significant differences between the treatments by Tukey or Dunnett T3 post hoc tests.
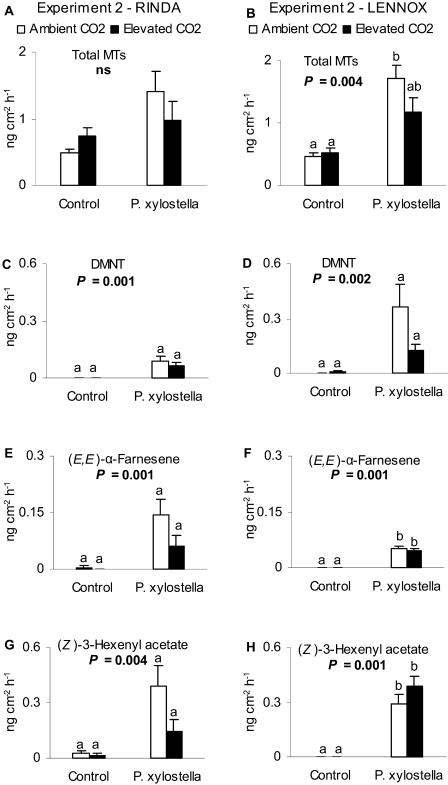
Figure 2.
Emission of intact control and P. xylostella-damaged cabbages (cvs Rinda and Lennox) grown at ambient or elevated CO2 concentration. A and B, Emissions of total MTs (sabinene, limonene, β-pinene + myrcene, 1,8-cineol, α-thujene, and α-pinene; mean + se). C and D, DMNT. E and F, (E,E)-α-farnesene. G and H, (Z)-3-hexenyl acetate (ng cm−2 h−1) from intact control and P. xylostella-damaged cabbage plants (n = 5) grown at ambient (360 μmol mol−1) or elevated (720 μmol mol−1) CO2 concentration in experiment 2. P-values indicate the main effect tested by Kruskal-Wallis-test. Different letters above the bars indicate significant differences between the treatments by Dunnett T3 post hoc test.
In both cultivars and both experiments, the emissions of induced compounds, DMNT, (E,E)-α-farnesene, and (Z)-3-hexenyl acetate, were not particularly responsive to the CO2 concentration (Figs. 1 and 2) even though elevated CO2 seemed to diminish the emission of DMNT from cv Lennox and (E,E)-α-farnesene from cv Rinda in experiment 2. The emissions of induced compounds were naturally higher from herbivore-damaged plants than from intact plants (Figs. 1 and 2, C–H). P. xylostella-damage tended to increase more clearly the emission of total MTs and induced compounds from plants grown at ambient CO2 than at elevated CO2.
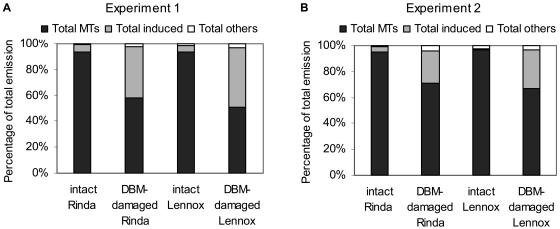
The relative proportions of total MTs, total induced compounds, and total other compounds [hexanal, (Z)-3-hexenol, heptanal, nonanal and decanal] in relation to total volatiles were not affected by the elevated CO2 concentration in either of the experiments. Again, P. xylostella-damage increased significantly (P < 0.001) the proportion of total induced compounds and thereby decreased significantly the proportion of total MTs (P < 0.001) in both cultivars and in both experiments (Fig. 3). In experiment 1, the percentage of induced compounds tended to be larger than in experiment 2 (Fig. 3).
Figure 3.
Percentage of MTs and induced and other compounds in the total emission. A, Emission of total MTs (sabinene, limonene, β-pinene + myrcene, 1,8-cineol, α-thujene, and α-pinene), total induced compounds [DMNT, (E,E)-α-farnesene, (Z)-3-hexenyl acetate, and (Z)-3-hexenol], and total other compounds [hexanal, (Z)-3-hexenol, nonanal, and decanal] in experiment 1 and B, emissions of total MTs, total induced compounds, and total other compounds [hexanal, (Z)-3-hexenol, heptanal and nonanal] in experiment 2 from intact control and P. xylostella-damaged cabbage cvs Rinda and Lennox (n = 10).
Behavioral Response of P. maculiventris and C. plutellae
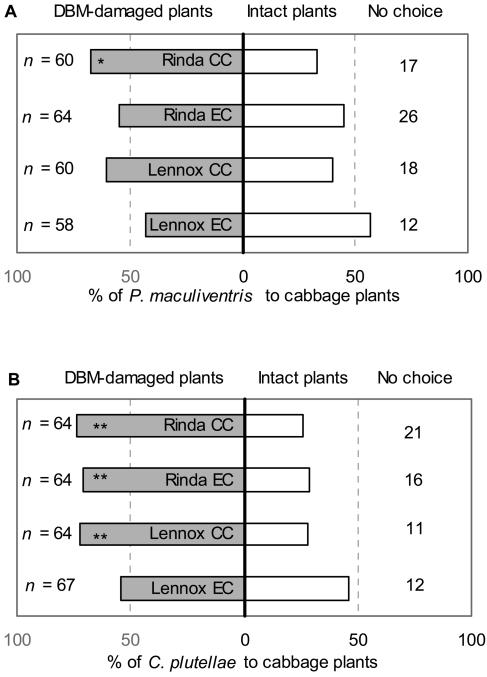
In experiment 1, the generalist predator P. maculiventris discriminated only between the odors of intact and P. xylostella-damaged cv Rinda plants, which were grown at ambient CO2 concentration, preferring the odor of the damaged plants (Fig. 4A). In experiment 2, the specialist parasitoid C. plutellae preferred the odor of damaged plants (both cultivars) when the plants had been grown at ambient CO2 but did not detect damaged cv Lennox plants when they were grown at elevated CO2 (Fig. 4B).
Figure 4.
The orientation behavior of P. maculiventris and C. plutellae. The percentage of A, P. maculiventis in experiment 1 and B, C. plutellae in experiment 2 choosing for P. xylostella-damaged or intact control cabbage (cvs Rinda and Lennox) plants grown at ambient (CC) or elevated CO2 (EC) in the Y-tube olfactometer. Asterisks indicate significant (*P < 0.05, **P < 0.01, in binomial test) preference toward either odor source. n indicates the total number of predators or parasitoids used in the assay including the individuals that did not show any preference for either odor source. DBM, diamondback moth, P. xylostella.
DISCUSSION
Elevated CO2 and Plant Properties
As indicated by the decreased SLA and the increased leaf thickness in experiment 1, cv Lennox was more responsive to elevated CO2 than cv Rinda. The SLA of Lennox was reduced at elevated CO2 in both experiments although in experiment 2 only in the plants fed by P. xylostella. This intraspecific variation in the SLA response to elevated CO2 is well known in the family Brassicaceae (Van Der Kooij et al., 2000). The analysis of more than 100 plant species has revealed that there is an average 11% reduction of stomatal density attributable to doubled atmospheric CO2 (Hetherington and Woodward, 2003). We did not find significantly diminished stomatal density in cabbages grown at elevated CO2, suggesting that the small reduction in the emission of DMNT and (E,E)-α-farnesene from P. xylostella-damaged cabbages is not a consequence of a lower emission rate through stomata but probably is a reflection of restricted synthesis of volatile compounds. However, at the moment the importance of the density and behavior of stomata for controlling total emission is still unclear (Niinemets and Reichstein, 2003).
Elevated CO2 and Plant Emissions
The elevated CO2 concentration per se had no significant effect on constitutive MT emission from intact control plants, while it seemed to have overall reducing, although nonsignificant, impact on MT emission from herbivore-damaged plants. In an earlier study, we detected decreased MT emissions per shoot dry weight from intact and herbivore-damaged cv Lennox cabbages grown at elevated CO2 (Vuorinen et al., 2004). At least in the case of intact cabbages, the observed decrease in the MT emission might have been a consequence of expressing emission results per shoot dry weight since the intact control plants grown at elevated CO2 concentration had higher shoot dry weights than plants grown at ambient CO2 (Vuorinen et al., 2004). In general, emissions from plants have been expressed on a dry weight basis (e.g. Peñuelas and Llusià, 1997; Constable et al., 1999b; Staudt et al., 2001) or on a leaf area basis (e.g. Loreto et al., 2001, 2004). If the MT emission in this study was expressed on a shoot dry weight basis (data not shown), then it was significantly decreased from herbivore-damaged cabbages grown at elevated CO2.
In earlier studies, the reduction of MT emission from species which do not store terpenes, such as Quercus ilex (Loreto et al., 2001) and cabbage (Vuorinen et al., 2004), has been detected at elevated CO2 concentration. In contrast, Staudt et al. (2001) found that Q. ilex leaves grown at elevated CO2 exhibited higher MT emission per projected leaf area and per leaf dry mass than leaves grown at ambient CO2 regardless of the CO2 concentration during sampling. We collected volatile compounds at ambient CO2. In a trial test, P. xylostella-damaged plants grown and sampled at elevated CO2 had significantly lower MT emission than those plants grown at elevated CO2 and sampled at ambient CO2, while CO2 concentration during sampling of volatile compounds did not affect MT emissions from intact cabbages (data not shown). Assumingly the decrease in emission of MTs at elevated CO2 would have been stronger if the sampling would have been conducted at elevated CO2. When one considers terpene storing plants such as conifers (Constable et al., 1999b) or Rosmarinus officinalis (Peñuelas and Llusià, 1997), elevated CO2 concentration appeared to have no impact on MT emission. Litvak et al. (2002) detected a lower total MT pool size in wounded Douglas fir needles than in intact needles at ambient and elevated CO2 concentrations, suggesting that the rate of MT accumulation decreases in relation to other compounds such as starch at elevated CO2. They also concluded that Douglas fir depends more on constitutive MTs for defense than on induced compounds, which is not the case with cabbage. The Brassica species do not have MT pools in their leaves but are able to produce herbivore-induced volatile compounds.
In our earlier study we found no impact of CO2 levels on the emission of induced compounds (Vuorinen et al., 2004), while in this study CO2 concentration seemed to decrease the emissions of P. xylostella-induced compounds. The variation in the emission of induced compounds between individual plants was notable, and therefore no clear statistically significant CO2 effect was observed. Also, feeding habits and the type of damage caused by P. xylostella larvae is not necessarily equal among the plants that might have great impact on the emission rates of induced compounds. Herbivore-induced compounds are derived mainly from the jasmonic acid signaling pathway, which modulates the lipoxygenase pathway producing green leaf volatiles, and two terpenoid pathways (Dicke et al., 2003), mevalonic acid pathway in the cytosol and the deoxyxylulose phosphate/methylerythritol pathway in plastids (Sponsel, 2002). In parallel to our earlier results (Vuorinen et al., 2004), at least volatile compounds from the jasmonic acid signaling pathway were detectable from cabbage after P. xylostella-damage.
Trophic Interactions
P. maculiventris nymphs preferred the odor of damaged plants grown at ambient CO2, but showed little preference between damaged and intact plants when the cabbages were grown at elevated CO2. According to the opinions of Vet and Dicke (1992) herbivore-derived volatiles are the most reliable cues for generalist predators for prey locating. Therefore predators should not innately react to herbivore-induced plant-derived semiochemicals, which are more abundant but less reliable and which can be used for prey locating after associative learning process. However, Reddy et al. (2002) observed that the generalist predator Chrysoperla carnea is attracted to the odor of (Z)-3-hexenyl acetate, which is released in large amounts from herbivore-damaged cabbages. Furthermore, Dickens (1999) detected that P. maculiventris has olfactory receptors for (Z)-3-hexenyl butyrate which is induced by the Colorado potato beetle, and the predator was also attracted to a blend comprising (E)-2-hexenol, (Z)-3-hexenol, (±)-linalool, nonanal, and MeSA. Sant'Ana et al. (1999) found that third- and fifth-instar nymphs reacted to common plant green leaf volatiles (E)-2-hexenol and (E)-2-hexenal, which is also the dominant odor of the male-produced aggregation pheromone. The fifth-instars responded also to nonanal and (±)-linalool (Sant'Ana et al., 1999). Neither (E)-2-hexenol nor (E)-2-hexenal was emitted from the damaged cabbages in this study. The greatly reduced emission of (Z)-3-hexenyl acetate from P. xylostella-damaged cv Rinda plants grown at elevated CO2 may be an explanation for the reduced host-searching efficiency of P. maculiventris in this study. On the other hand, there seemed to be elevated emission of (Z)-3-hexenyl acetate at elevated CO2 from P. xylostella-damaged cv Lennox plants, but nonetheless the predator still did not show a preference for the odor of damaged plants.
Orientation of the parasitoid C. plutellae toward damaged plant was stronger than that of the generalist predator. The response of parasitoids to plant-herbivore complexes differs among the plant species and herbivores involved. For instance, C. plutellae shows a specific response toward the host-plant complex, unlike C. glomerata, and the presence of the nonhost affects the specificity of the response of the wasps (Shiojiri et al., 2000). Liu and Jiang (2003) showed that volatile compounds from Chinese cabbage were more attractive to female C. plutellae than those from white cabbage when both plant species were either intact or infested with P. xylostella. DMNT and other terpenes appeared to be important cues for orientation of C. plutellae to P. xylostella-damaged plants (Shiojiri et al., 2001). (E,E)-α-Farnesene is not one of the P. xylostella-induced compounds in all cabbage varieties and in this respect differs from DMNT and (Z)-3-hexenyl acetate (Shiojiri et al., 2001). The decreasing impact of elevated CO2 on DMNT and MT emissions from cv Lennox probably explain the reduction in host-searching efficiency of C. plutellae. As our results point to a reduced orientation efficiency of the specialist parasitoid toward herbivore-damaged plants grown at the forecasted elevated atmospheric CO2 concentration, this finding deserves further and more extensive research on other plant species. C. plutellae and other Braconids at the top of food chains maintain important position in terms of global biodiversity (Dolphin and Quicke, 2001). In the future, these parasitoid species may become threatened also by elevated atmospheric CO2 concentration if their host-searching efficiency is impaired.
MATERIALS AND METHODS
The Experiments, Plant Material, and CO2 Exposure
We performed two separate experiments under similar growth conditions for the plants, produced similar Plutella xylostella-damage, sampled volatiles, and used the Y-tube olfactometer in a similar manner in both experiments. In the first experiment, we studied the orientation behavior of the generalist Podisus maculiventris, and in addition to plant growth measurements we also measured leaf thickness and stomatal density from intact control plants. In the second experiment, we tested the orientation behavior of the specialist Cotesia plutellae. Emission data from both experiments are presented.
White cabbage (Brassica oleracea ssp. capitata cvs Lennox and Rinda) seedlings were sown in 1-l plastic pots filled with Sphagnum peat and sand (3:1, v/v) and grown for 24 to 26 d at ambient (360 μmol mol−1) or elevated (720 μmol mol−1) CO2 concentration in growth chambers (Bioklim 2600T, Kryo-Service Oy, Helsinki) at 23°C:18°C, 70%:80% relative humidity, and 22:2-h photoperiod (250–300 μmol m−2 s−1 photosynthetically active radiation during the light period). CO2 enhancement was maintained for 24 h d−1. The CO2 treatments and seedlings were rotated among the two chambers weekly to randomize any systematic chamber effect across the seedlings. The seedlings were watered daily and fertilized weekly with 0.1% of 9-Superex (19:5:20 N:P:K; Kekkilä, Finland) at a rate of 0.05 to 0.1 L plant−1, starting 2 weeks after sowing.
Leaf Properties
The SLA was determined by scanning the leaf with a Logitech-scanner and analyzing the leaf area using a Logitech Photo Touch Color-program (Logitech, Morges, Switzerland) and dividing the leaf area by the leaf dry weight. Leaf thickness was measured from the 3rd and 5th youngest leaves between the veins in the left top one-quarter of the leaf with a micrometer (Mitutoyo Mod. 1D-C112CB, Mitutoyo Kanagawa, Japan). Stomatal density was determined after collection of volatiles by pressing a small proportion of the adaxial and abaxial right top quarter of the leaf surface against a glass microscope slide painted with instant glue (Loctite Super Glue +, Henkel Loctite, Cleveland). After drying for 1 to 2 min, the leaf was peeled off and replicas were examined at 200× magnification with a light microscope (Oksanen, 2003).
Insects and P. xylostella-Damage
Larvae of the crucifer specialist P. xylostella (Lepidoptera: Plutellidae) were reared at 25°C, 50% relative humidity, and 16:8-h photoperiod on broccoli (B. oleracea ssp. italica) seedlings. Feeding damage was caused by transferring eight third-instar larvae onto five randomly selected plants grown at ambient or elevated CO2 for 48 h. Five intact plants from both CO2 concentrations were used as controls. Plants with the feeding larvae were kept in a separate growth chamber in similar environmental conditions as described earlier.
Spined soldier bug P. maculiventris (Hemiptera: Pentatomidae) nymphs were obtained from Koppert Biological Systems, Berkel en Rodenrijs, The Netherlands. The nymphs were fed mainly with larvae of yellow mealworm, Tenebrio molitor (Coleoptera: Tenebrionidae) at 23°C ± 1°C, 75% ± 5% relative humidity, and a 16:8-h photoperiod. Fifth instars were used in the behavioral assay.
C. plutellae Kurdjumov (Hymenoptera: Braconidae) pupae were obtained from the Centre de Coopération Internationale en Recherche Agronomique pour le Développement (Montpellier, France). Second-instar larvae of P. xylostella feeding on broccoli were offered to C. plutellae females for egg laying at 25°C ± 1°C, 50% relative humidity, and 16:8-h photoperiod. Pupae were placed in a clean cage until emergence. Adults were provided with 20% honey-water solution for feeding. From 3- to 5-d-old C. plutellae females were used in the behavioral assay. Predators and parasitoids had not experienced the odor of P. xylostella-damaged white cabbages before the behavioral assay.
Collection of Volatile Compounds
Intact control plants and P. xylostella-damaged plants from both CO2 concentrations from both cultivars (n = 5) were used for the collection of the volatile compounds. Cabbages with rinsed and a slightly pruned root system in a 15-mL vial filled with tap water were individually enclosed inside 1.5-L glass vessels, which were closed with a glass lid sealed with Teflon tape and parafilm. After 5 min adjustment, a sample was collected for 30 min on approximately 150 mg Tenax-TA adsorbent (Supelco, mesh 60/80) by pulling the sample through 6-mm diameter Teflon tubing with a vacuum pump (KNF Neuberger, Freiburg, Germany, Model N022AN.18). An inlet for purified air and an outlet for sampling were on the top of the vessel. The airflow was calibrated with the mini-Buck calibrator (Model M-5, A.P. Buck, Orlando, FL), and flow rate was set to 0.215 L min−1 for filtered and pressurized air and 0.200 L min−1 for sampling. The collection was performed at 22°C and at 300 μmol m−2 s−1 photosynthetically active radiation at ambient CO2 concentration.
The samples were analyzed by gas chromatography-mass spectrometry (Hewlett-Packard GC type 6890, MSD 5973). Trapped compounds were desorbed (Perkin Elmer ATD400 Automatic Thermal Desorption system; Norwalk, CT) at 250°C for 10 min, cryofocused at −30°C, and injected onto a HP-5 capillary column (50 m × 0.2 mm i.d. × 0.5-μm film thickness; Hewlett-Packard, Palo Alto, CA). The carrier gas was helium. The temperature program began at 40°C for 1 min, followed by increases of 5°C min−1 to 210°C and 20°C min−1 to 250°C. Compounds were identified by comparison of the mass spectra with those in the Wiley library and pure standards. For quantification, commercially available reference substances were used. The reference substances for α-thujene, DMNT, and (E,E)-α-farnesene were not available; therefore, the concentration of these compounds were calculated by assuming that the responses would be the same as the responses of α-pinene, (Z)-ocimene, and (E)-β-farnesene, respectively. After sampling, the leaf area of the plants was determined and emissions were calculated as ng cm−2 h−1.
Behavioral Assay
The Y-tube olfactometer (main arm 10.5 cm, other arms 10 cm, i.d. 1.6 cm, and angle between two arms approximately 90°) was used to test the behavior of P. maculiventris and C. plutellae. The insects were given a choice between P. xylostella-damaged and intact cabbages grown at ambient or elevated CO2 concentration. The plants were carefully placed in the 1-l vessels which were closed with Teflon sealed lids with two inlets. The pressurized air was purified with activated carbon and divided via Teflon tubing into two separate flows (0.500 L min−1) which both passed through a 1-l glass vessel containing one cabbage as an odor source and leading to either arm of the Y-tube. The airflow was adjusted with pressure and needle valves and calibrated daily with M-5 mini-Buck calibrator. The insects were introduced to the downwind end of the Y-tube and observed for 5 min or until they made their final choice. The choice was recorded when the insect passed two-thirds or the far end of the Y-tube arm for P. maculiventris and C. plutellae, respectively. Odor sources, vessels, Y-tube, and lids were replaced after testing six P. maculiventris or eight C. plutellae to avoid any errors caused by the olfactometer system itself. All the glassware was heat-treated at 120°C before use, and the Y-tube was turned horizontally around after each tested insect and rinsed with 96% ethanol at least after every third tested insect. In the experiments, approximately 60 P. maculiventris nymphs and approx. 64 C. plutellae females were tested on 10 and 8 different odor source pairs, respectively, from both CO2 concentrations and cultivars.
Statistical Analyses
Statistical analyses were performed using SPSS 11.5 for Windows statistical package. The main effect of treatments was tested by nonparametric Kruskal-Wallis-test and one-way ANOVA and multiple comparisons by Dunnett T3 and Tukey post hoc tests. ARCSIN-transformed relative proportions of compound groups were tested by independent samples t test. The data from the behavioral assay were analyzed with the nonparametric binomial test to test whether there was a significant difference in attraction between the two odor sources from plants receiving different treatments (test proportion was set to 0.50).
This work was supported by the Research Council for Biosciences and Environment, by the Academy of Finland (decision no. 202300 to T.V., A.-M.N., M.A.I., and J.K.H. and decision no. 75323 to G.V.P.R.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.047084.
References
- Andreae MO, Crutzen PJ (1997) Atmospheric aerosols: biogeochemical sources and role in atmospheric chemistry. Science 276: 1052–1058 [Google Scholar]
- Bazzaz FA (1990) The response of natural ecosystems to the rising global CO2 levels. Annu Rev Ecol Syst 21: 167–196 [Google Scholar]
- Bezemer TM, Jones TH (1998) Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos 82: 212–222 [Google Scholar]
- Constable JVH, Guenther AB, Schimel DS, Monson RK (1999. a) Modelling changes in VOC emission in response to climate change in the continental United States. Global Change Biol 5: 791–806 [Google Scholar]
- Constable JVH, Litvak ME, Greenberg JP, Monson RK (1999. b) Monoterpene emission from coniferous trees in response to elevated CO2 concentration and climate warming. Global Change Biol 5: 255–267 [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA (1990) Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol 16: 3091–3118 [DOI] [PubMed] [Google Scholar]
- Dicke M, Van Poecke RMP, de Boer JG (2003) Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl Ecol 4: 27–42 [Google Scholar]
- Dickens JC (1999) Predator-prey interactions: olfactory adaptations of generalist and specialist predators. Agric For Entomol 1: 47–54 [Google Scholar]
- Dolphin K, Quicke DLJ (2001) Estimating the global species richness of an incompletely described taxon: an example using parasitoid wasps (Hymenoptera: Braconidae). Biol J Linn Soc Lond 73: 279–286 [Google Scholar]
- Geervliet JBF, Posthumus MA, Vet LEM, Dicke M (1997) Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J Chem Ecol 23: 2935–2954 [Google Scholar]
- Gouinguené SP, Turlings TCJ (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129: 1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, Noguer M, Van Der Linden PJ, Xiaosu D, Maskell K, Johnson CA (2001) Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, UK
- Litvak ME, Constable JVH, Monson RK (2002) Supply and demand processes as controls over needle monoterpene synthesis and concentration in Douglas fir (Pseudotsuga menziesii (mirb.) Franco). Oecologia 132: 382–391 [DOI] [PubMed] [Google Scholar]
- Liu SS, Jiang LH (2003) Differential parasitism of Plutella xylostella (Lepidoptera: Plutellidae) larvae by the parasitoid Cotesia plutellae (Hymenoptera: Braconidae) on two host plant species. Bull Entomol Res 93: 65–72 [DOI] [PubMed] [Google Scholar]
- Loreto F, Fischbach RJ, Schnitzler JP, Ciccioli P, Brancaleoni E, Calfapietra C, Seufert G (2001) Monoterpene emission and monoterpene synthase activities in the Mediterranean evergreen oak Quercus ilex L. grown at elevated CO2 concentrations. Global Change Biol 7: 709–717 [Google Scholar]
- Loreto F, Pinelli P, Manes F, Kollist H (2004) Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol 24: 361–367 [DOI] [PubMed] [Google Scholar]
- Niinemets U, Reichstein M (2003) Controls on the emission of plant volatiles through stomata: a sensitivity analysis. J Geophys Res 108: doi 10.1029/2002JD00262014686320
- Oksanen E (2003) Physiological responses of birch (Betula pendula) to ozone: a comparison between open-soil-grown trees exposed for six growing seasons and potted seedlings exposed for one season. Tree Physiol 23: 603–614 [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–331 [PMC free article] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J (1997) Effects of carbon dioxide, water supply, and seasonality on terpene content and emission by Rosmarinus officinalis. J Chem Ecol 23: 979–993 [Google Scholar]
- Peñuelas J, Llusià J (2001) The complexity of factors driving volatile organic compound emissions by plants. Biol Plant 44: 481–487 [Google Scholar]
- Peñuelas J, Llusià J (2003) BVOCs: plant defense against climate warming? Trends Plant Sci 8: 105–109 [DOI] [PubMed] [Google Scholar]
- Poorter H, Navas M-L (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157: 175–198 [DOI] [PubMed] [Google Scholar]
- Potting RPJ, Poppy GM, Schuler TH (1999) The role of volatiles from cruciferous plants and pre-flight experience in the foraging behaviour of the specialist parasitoid Cotesia plutellae. Entomol Exp Appl 93: 87–95 [Google Scholar]
- Reddy GVP, Holopainen JK, Guerrero A (2002) Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J Chem Ecol 28: 131–143 [DOI] [PubMed] [Google Scholar]
- Sallas L, Luomala E-M, Utriainen J, Kainulainen P, Holopainen JK (2003) Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol 23: 97–108 [DOI] [PubMed] [Google Scholar]
- Sant'Ana J, da Silva RFP, Dickens JC (1999) Olfactory reception of conspecific aggregation pheromone and plant odors by nymphs of the predator, Podisus maculiventris. J Chem Ecol 25: 1813–1826 [Google Scholar]
- Shiojiri K, Takabayashi J, Yano S, Takafuji A (2000) Flight response of parasitoids toward plant-herbivore complexes: a comparative study of two parasitoid-herbivore systems on cabbage plants. Appl Entomol Zool (Jpn) 35: 87–92 [Google Scholar]
- Shiojiri K, Takabayashi J, Yano S, Takafuji A (2001) Infochemically mediated tritrophic interaction webs on cabbage plants. Popul Ecol 43: 23–29 [Google Scholar]
- Sponsel VM (2002) The deoxyxylulose phosphate pathway for the biosynthesis of plastidic isoprenoids: early days in our understanding of the early stages of gibberellin biosynthesis. J Plant Growth Regul 20: 332–345 [DOI] [PubMed] [Google Scholar]
- Staudt M, Joffre R, Rambal S, Kesselmeier J (2001) Effect of elevated CO2 on monoterpene emission of young Quercus ilex trees and its relation to structural and ecophysiological parameters. Tree Physiol 21: 437–445 [DOI] [PubMed] [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA (1994) Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol 20: 1329–1354 [DOI] [PubMed] [Google Scholar]
- Talekar NS, Shelton AM (1993) Biology, ecology and management of diamondback moth. Annu Rev Entomol 38: 275–301 [Google Scholar]
- Turlings TCJ, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE (1991) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol 17: 2235–2251 [DOI] [PubMed] [Google Scholar]
- Van Den Boom CEM, Van Beek TA, Posthumus MA, De Groot A, Dicke M (2004) Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. J Chem Ecol 30: 69–89 [DOI] [PubMed] [Google Scholar]
- Van Der Kooij TAW, De Kok LJ, Stulen I (2000) Intraspecific variation in the response of Arabidopsis thaliana lines to elevated atmospheric CO2. Phyton -Ann Rei Bot 40: 125–132 [Google Scholar]
- Vet LEM, Dicke M (1992) Ecology of infochemicals use by natural enemies in tritropic context. Annu Rev Entomol 37: 141–172 [Google Scholar]
- Vuorinen T, Reddy GVP, Nerg A-M, Holopainen JK (2004) Monoterpene and herbivore-induced emissions from cabbage plants grown at elevated atmospheric CO2 concentration. Atmos Environ 38: 675–682 [Google Scholar]
- Woodward FI, Lake JA, Quick WP (2002) Stomatal development and CO2: ecological consequences. New Phytol 153: 477–484 [DOI] [PubMed] [Google Scholar]