Abstract
Many plants have an indirect defense against herbivores by emitting volatiles that attract carnivorous enemies of the herbivores. In cucumber (Cucumis sativus) the production of carnivore attractants can be induced by herbivory or jasmonic acid spraying. From the leaves of cucumber plants with and without spider mite infestation, two subtractive cDNA libraries were made that were enriched in cDNA fragments up- or down-regulated by spider mite infestation. A total of 713 randomly selected clones from these libraries were used to make a cDNA microarray. Subsequently, cucumber plants were sprayed with jasmonic acid, mechanically damaged, infested with spider mites, or left untreated (control). Leaf samples were taken at a range of different time points, and induced volatile compounds and mRNA (from the same leaves) were collected. cDNAs prepared from the mRNA were hybridized to the clones on the microarray. The resulting gene expression profiles were analyzed in combination with volatile production data in order to gain insight in the possible involvement of the studied genes in the synthesis of those volatiles. The clones on the microarray and the induced cucumber volatiles could be grouped into a number of clusters in which specific biosynthetic genes clustered with the product of that pathway. For example, lipoxygenase cDNA clones clustered with the volatile (Z)-3-hexenyl acetate and the volatile sesquiterpene (E,E)- α-farnesene clustered with an up-regulated sesquiterpene synthase fragment. This fragment was used to screen a cDNA library which resulted in the cloning of the cucumber (E,E)-α-farnesene and (E)-β-caryophyllene synthases. The use of combined global gene expression analysis and metabolite analysis for the discovery of genes involved in specific biosynthetic processes is discussed.
Plants have evolved the capacity to respond to herbivory with the production and emission of volatile compounds that attract predators of the herbivores (Dicke et al., 1990; Turlings et al., 1990). Thus, plants can defend themselves against herbivory not only by the use of direct defense (such as toxicity), but also by mobilizing carnivorous organisms in the surrounding environment (Turlings et al., 1995; Takabayashi and Dicke, 1996; De Moraes et al., 1998; Kessler and Baldwin, 2001). There is ample evidence that plants respond differently to mechanical damage than to herbivore feeding and that this is caused by elicitors released by the herbivore (e.g. Turlings et al., 1995; Takabayashi and Dicke, 1996; De Moraes et al., 1998; Van Poecke and Dicke, 2004). The many parameters that affect a plant's response to herbivory suggest the involvement of a sophisticated defense system that optimizes response depending on the intruding organism. The impact of individual compounds in a volatile blend emitted by herbivore-infested plants on predator behavior is difficult to investigate. Nevertheless, single compounds have in a number of cases been shown to attract predators (Dicke et al., 1990; Kessler and Baldwin, 2001), and careful exploitation of differential induction of volatiles by herbivory and jasmonic acid have enabled to study the role of individual compounds within the total volatile blend emitted by the plant (De Boer and Dicke, 2004). However, the latter method may be used to investigate the role of some compounds but not the role of many others. The biosynthesis of many of the induced volatiles occurs de novo (Paré and Tumlinson, 1997) and involves the induction of enzyme activity (Bouwmeester et al., 1999; Degenhardt and Gershenzon, 2000). The cloning of genes that encode key enzymes regulating the biosynthesis of specific volatile compounds followed by the design of transgenic plants with changed expression of these genes (overexpression or knock-outs) provides an exciting approach to elucidate the impact of the different volatiles in a blend and improve plant indirect defense (Bouwmeester et al., 2003).
Cucumber (Cucumis sativus) has been demonstrated by several authors to produce a limited number of compounds upon spider mite infestation, and the role of volatile production in predator attraction is well characterized (Takabayashi et al., 1994; Janssen et al., 1998; Agrawal et al., 2002). We have also demonstrated that an enzyme involved in volatile formation, viz. (3S)-(E)-nerolidol synthase, is induced in cucumber upon spider mite feeding (Bouwmeester et al., 1999). To identify cucumber genes of which the expression changes upon herbivory, we decided to use an untargeted approach. Global gene expression techniques like cDNA microarrays have been developed to follow changes in the transcriptome (Leung and Cavalieri, 2003). Thousands of genes can be scanned simultaneously in one experiment using minute amounts of mRNA from the tissue to be analyzed. In plant science, microarrays have been used extensively for the genetic model organism Arabidopsis (e.g. Reymond et al., 2000). Using microarrays with Arabidopsis, essential information about transcriptionally regulated genes e.g. promoter-sequences, gene structure, amino acid motifs, and protein structure etc. can be obtained in silica. Although some of the above possibilities cannot be fully accessed in genetically less well-characterized organisms, the technique is still a powerful approach for the study of gene regulation in these organisms. Techniques like differential display reverse transcription PCR and microarrays have already been used to identify genes with induced or up-regulated transcription upon herbivory (Arimura et al., 2000; Reymond et al., 2000; Hermsmeier et al., 2001), but in the present paper we have used subtractive cDNA libraries (suppression subtractive hybridization [SSH]) as a source for clone selection in the microarray preparation procedure to increase the proportion of regulated target clones. The use of microarrays to track new biosynthetic genes in plants was successfully applied to find genes involved in rose flower secondary metabolite formation (fragrance; Guterman et al., 2002). RNA from specific organs (petals) was prepared for the selection of spotted clones and preparation of the hybridizing cDNA, thereby minimizing the amount of background mRNA used in the procedures of clone selection and hybridization.
A next challenge in further refining the search for biosynthetic genes is to make a parallel analysis of transcript and metabolite profiles. Significant correlations between the metabolic contents and the expression of relevant genes have been demonstrated in a system using potato tubers (Urbanczyk-Wochniak et al., 2003), and future potential of this combination is discussed elsewhere (Roessner et al., 2002). Synergistic effects for combined analysis of gene expression and metabolites can also be found in recent work on the responses of tomato to the initial phases of spider mite infestation (Kant et al., 2004). Here, we apply cDNA microarray analysis, in combination with a metabolic approach to the study of spider mite induced gene expression in C. sativus L. cv Corona with special focus on the induced volatiles that attract predators of the herbivores. Spider mite infestation and treatment with jasmonic acid induce a similar but not identical defense response. Therefore, these treatments were used in order to reveal transcriptional variation of genes involved in plant defense. Transcriptome (microarray analysis) and metabolome (volatile analysis) data were analyzed using correlation coefficients in combination with self organizing maps (SOMs) in order to link genes to specific induced volatiles, emitted by cucumber leaves upon the different treatments.
RESULTS
Microarray and Metabolite Analysis
The procedure for constructing the SSH-library cuts each cDNA several times, and most clones had a size of 50 to 400 bp. Sequencing of 96 randomly selected clones from the SSH+ cDNA library showed that about 20% of the clones represented the cucumber lipoxygenase (T10085) and another 10% had a high homology with a PR-1 gene from Brassica napus (T08154; Table I). The limited size of the SSH cDNAs hindered cDNA identification using GenBank, resulting in a large number of unknowns.
Table I.
BlastX hits for cDNA clones and contigs, discussed in this paper
| Clone ID | BlastX Annotation (Accession No.) | E Value | No. of Copies |
|---|---|---|---|
| Contig 1 | B. napus, PR-1 (T08154) | 7e–26 | 47 |
| Contig 7 | C. sativus, Lipoxygenase (T10085) | 4e–16 | 37 |
| Contig 8 | C. sativus, Lipoxygenase (T10085) | 7e–93 | 17 |
| Contig 9 | C. sativus, Lipoxygenase, 3′-UTR (U36339) | 2e–15 | 10 |
| Contig 10 | C. sativus, Lipoxygenase 1, (AAC61785.1) | 7e–34 | 6 |
| 5 G 8 | C. sativus, (E,E)-α-Farnesene synthase (AY640154) | –a | |
| 8 D 11 | C. sativus, (E,E)-α-Farnesene synthase (AY640154) | – | |
| 3 B 8 | C. sativus, Peroxidase (T10444) | 6e–;17 | |
| 5 E 2 | C. sativus, Peroxidase (T10444) | 1e–44 | |
| 6 B 1 | C. sativus, Peroxidase (T10444) | 1e–51 | |
| 6 C 12 | C. sativus, Peroxidase (T10444) | 2e–50 | |
| 6 H 4 | Arabidopsis, 1-deoxy-d-xylulose 5-phosphate reductoisomerase (T52570) | 6e–20 |
Dash indicates no value.
When comparing RNA expression levels in leaves after 168 h of spider mite infestation versus noninfested leaves of cucumber plants, more than 40% of SSH+ clones printed on the chip had an induced expression in the spider mite infested leaf material. The majority of the clones that are transcriptionally up-regulated by spider mite infestation were transcribed close to background levels in the control material. Some clones were induced from background levels in the control material to levels of maximum detection in spider mite infested leaves (e.g. the lipoxygenase fragments). The strongest regulated clones on the microarray were identified as being the same as those redundant in the SSH+ cDNA library [lipoxygenase (T10085) and the putative PR-1 gene (T08154)]. Genes that were up-regulated after spider mite infestation were detected in the SSH+ cDNA library, whereas down-regulated clones were found in the SSH− library, which validates the quality of the subtraction process (Fig. 1). Around 30% of the cDNAs represented on the microarray fell below the detection limit for our system under all conditions tested.
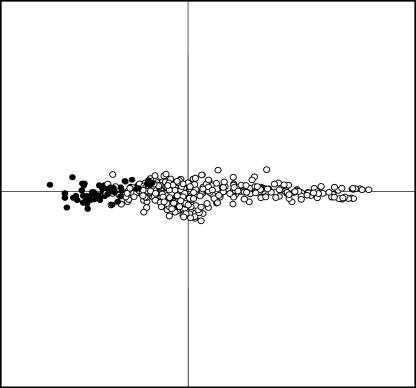
Figure 1.
Score plot (85% of dynamics) of the overall difference in transcriptional behavior of clones printed on the chip after subtraction of average of columns and rows for the PCA. Data points are from SSH−- (black circles) and SSH+- (white circles) cDNA libraries. The more separated two plotted cDNA clones are, the more diverged is their gene expression profile.
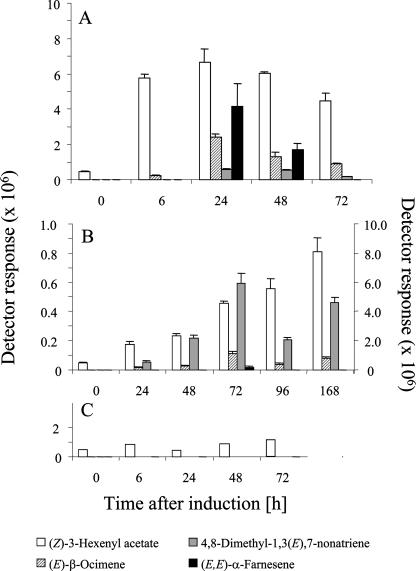
Metabolite analysis showed a quantitatively and qualitatively different emission pattern for the spider mite infested and the jasmonic acid sprayed plants (Fig. 2, A and B). For example, (E)-β-ocimene is the first and (E,E)-α-farnesene the strongest induced terpenoid volatile in the jasmonic acid treated plants, while 4,8-dimethyl-1,3(E),7-nonatriene is most characteristic for the spider mite infested plants. Although (E,E)-α-farnesene is the most dominant terpenoid in the jasmonic acid treated plants, it is only detected in one of the spider mite infested plants in this experimental series (Fig. 2B). From 24 h onwards, the volatile emission from the plants sprayed with jasmonic acid declined, while volatile emission from spider mite infested plants more or less continuously increased with time (Fig. 2B). A short decline in the increasing trend was observed at 96 h in the spider mite infested plants. Plants treated with water never produced any detectable levels of terpenoids, and the levels of (Z)-3-hexenyl acetate were substantially lower than for jasmonic acid and spider mite infested plants (Fig. 2C).
Figure 2.
Volatile profile over time emitted from four leaf discs after spraying with 1 mm jasmonic acid (collected 0, 6, 24, 48, and 72 h after treatment; A), after spider mite infestation (collected 0, 6, 24, 48, 72, 96, and 168 h after treatment; B) or after spraying with 0.01% Tween 20 in water (C). In B, the right y axis represents data for (Z)-3-hexenyl acetate. Error bars indicate se of three replicates (A and B).
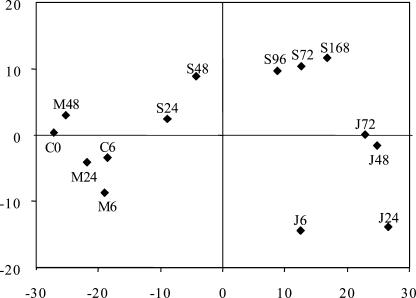
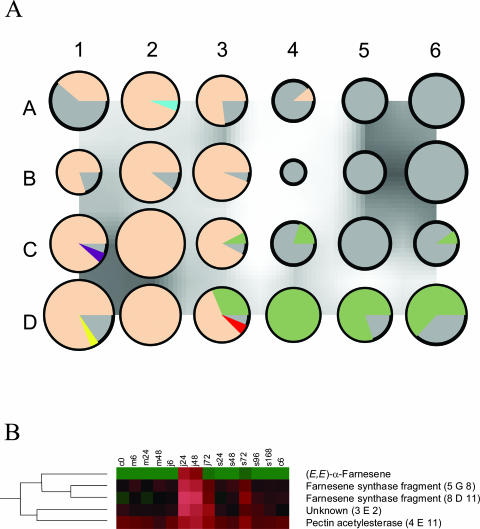
Only clones with an expression difference of more than 2.4-fold (the level of experimental plus biological variation found in our hybridization experiments) between the lowest and the highest level within the 14 treatments were included in principal component analysis (PCA)-analysis. A two-dimensional PCA plot based on the overall gene expression in each of the different treatments explained 77% of the gene expression dynamics in the system (Fig. 3). Jasmonic-acid sprayed plants clearly separated from the control plants along the x-component. The second component (y) showed the strongest difference in the overall expression between the early jasmonic acid sprayed plants (6 and 24 h) and the spider mite infested plants by the end of the experiment. The decreased production of volatiles collected at 96 h in spider mite infested plants (Fig. 2) was also reflected in the data-points representing expression of the spider mite induced transcriptome in Figure 3. These points shift from left to right along the x-component up to S72, then move back to the left for S96 and then resume to move to the right to S168 (Fig. 3). A dendrogram of the gene expression data had a first branch point between spider mite down-regulated and spider mite up-regulated genes (data not shown). Within the spider mite up-regulated cDNAs two major expression profiles can be distinguished. Both groups were up-regulated by spider mite infestation, but one was induced at a relatively later stage by the jasmonic acid treatment (“spider mite induced/late jasmonic acid induced”) than the other (“spider mite induced/early jasmonic acid induced”). The difference in the transcriptional profiles of these two groups segregates them in the SOM (Fig. 4A). In a dendrogram based on the cDNA expression patterns and including the metabolite analysis, (E,E)-α-farnesene was directed to a well-defined cluster of four cDNAs (Fig. 4B). Also, all cDNAs spotted on the array can be ranked on the basis of their Pearson correlation coefficients for example relative to the (E,E)-α-farnesene emission data. Expression data of the two clones correlating best to the (E,E)-α-farnesene data, 5G8 and 8D11, show correlation coefficients of 0.93 and 0.89, (P ≤ 0.001) respectively. Sequence analysis of these clones revealed that they had a high sequence homology to a putative terpene synthase (AAM00426.1) and the (+)-δ-cadinene synthase (Q43714). When the expression data were assigned to organize into 24 (4 × 6) separate groups in a SOM, the farnesene-related data were allocated into the same group, A2, as the two cDNA fragments (Fig. 4A). (E,E)-α-farnesene and the two cDNA-fragments have a characteristic expression pattern that differs from most other cDNAs (Fig. 5).
Figure 3.
Loading plot based on the overall gene expression in each of the different treatments. The transcriptional profile for each of the different treatments (columns) as two components explains 77% of the gene expression dynamics (PCA subtracting the average of columns and rows). The larger the distance between two sample-points the more diverged are the overall gene expression profiles for these treatments. Control (C), mechanically wounded (M), jasmonic acid sprayed (J), and spider mite infested (S). The number after each letter indicates hours after start of treatment.
Figure 4.
A, SOM with 24 components reflecting the gene expression and volatile formation patterns; PCA standardized rows, subtraction of average and subtraction of root mean square. Each circle or pie represents a cluster of genes with similar expression pattern. The larger the circle, the more clones in this particular group with a similar expression pattern. Areas where the intercircular space is dark indicate a similar expression profile between the neighboring clusters. The colored area inside the circles can be divided into wedge shaped pieces representing a specific gene or volatile. Intracircular color codes: Spider mite and early jasmonic-acid induced genes (beige), spider mite and late jasmonic-acid induced genes (green), (Z)-3-hexenyl acetate (yellow), (E)-β-ocimene (purple), 4,8-dimethyl-1,3(E),7-nonatriene (red), and (E,E)-α-farnesene (blue). cDNAs down-regulated by both spider mite infestation and jasmonic acid spraying are assigned to groups toward the A6 corner. B, Section of a dendrogram demonstrating (E,E)-α-farnesene production data clustering with four cDNAs, two of which are partial cDNA sequences of the (E,E)-α-farnesene synthase (5G8 and 8D11). Red describes high transcription/(E,E)-α-farnesene production rate and green (via black) color represents low transcription/emission rate. The 14 treatments of the cucumber plants are (left to right): control, 0 h; mechanical wounding, 6, 24, and 48 h; jasmonic acid spraying, 6, 24, 48, and 72 h; and spider mite infested, 24, 48, 72, 96, and 168 h and control 6 h.
Figure 5.
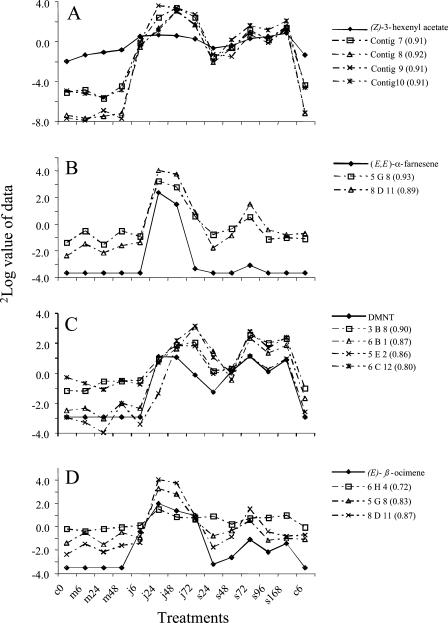
Logarithmic values for collected volatile emission data and cDNA expression data at the different treatments are plotted in the same diagram. (Z)-3-hexenyl acetate and lipoxygenase derived contigs 7, 8, 9, and 10 described in Table I (P ≤ 0.001; A). (E,E)-α-farnesene and cDNA fragments (5G8 and 8D11) from the cloned (E,E)-α-farnesene synthase gene (P ≤ 0.001; B). Dimethyl-1,3(E),7-nonatriene (DMNT) plotted with four peroxidase-like cDNA fragments putatively involved in their biosynthesis (P ≤ 0.001; C). (E)-β-ocimene together with 1-deoxy-d-xylulose 5-phosphate reductase-like cDNA fragment (6H4) (P ≤ 0.005) and the farnesene synthase cDNA fragments (P ≤ 0.001; D). Pearson correlation coefficient for the association of cDNA fragment to the volatile in that diagram is in brackets.
The SOM gives an overview of the distribution of the spotted cDNAs based on their regulation patterns and complements information obtained from a dendrogram (Fig. 4A). The down-regulated genes clustered toward the A6-corner. The four volatiles, i.e. (Z)-3-hexenyl acetate, (E)-β-ocimene, 4,8-dimethyl-1,3(E),7-nonatriene, and (E,E)-α-farnesene, were allocated to different groups in the SOM, reflecting their differential emission profile. (Z)-3-hexenyl acetate was assigned to group D1 and clusters with the four lipoxygenase cDNA contigs 7, 8, 9, and 10 (Table I). (E)-β-ocimene clustering (group C1) did not reveal any obvious gene-candidates that could be involved in its biosynthesis. 4,8-Dimethyl-1,3(E),7-nonatriene was assigned to group D3 between the two major dendrogram groups, “spider mite induced/late jasmonic acid induced” and “spider mite induced/early jasmonic acid induced.” Four cDNA fragments with high sequence homology to cucumber peroxidase (T10444) were also allocated to this group D3 (Table I). When specific volatile data and selected cDNA expression data from the SOM were plotted in the same diagram an associated behavior could be observed (Fig. 5). The Pearson correlation between the volatiles (Z)-3-hexenyl acetate, dimethyl-1,3(E),7-nonatriene, and (E,E)- α-farnesene and the expression patterns of some selected cDNAs is highly significant (P ≤ 0.005; Fig. 5). Although we were not able to identify cDNAs representing genes that are directly involved in the biosynthesis of (E)-β-ocimene, a cDNA fragment 6H4 on the microarray had a high homology (E-value, 6e−20) to 1-deoxy-d-xylulose 5-phosphate reductoisomerase. The expression pattern of this cDNA significantly correlated with the (E)-β-ocimene emission data (Pearson correlation coefficient of 0.72; Fig. 5).
Cloning and Functional Protein Expression
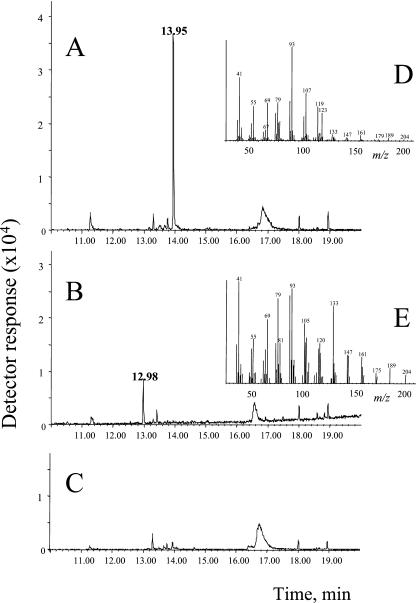
The gene expression profiles of the two cDNA clones, 5G8 and 8D11, correlated very well with the (E,E)-α-farnesene emission data (Fig. 5B). These 2 clones were used to screen a phage cDNA library which resulted in the isolation of 13 clones which proved to be identical upon sequencing. Fragments 5G8 and 8D11 were 100% identical to (different parts of) the cloned (E,E)-α-farnesene synthase showing that both fragments are derived from the same cDNA. Functional Escherichia coli expression of the full-length cDNA isolated in this way followed by an enzymatic assay with farnesyl diphosphate (FDP) as substrate and subsequent product analysis using gas chromatography-mass spectrometry (GC-MS) showed that this sesquiterpene synthase indeed catalyzes the formation of (E,E)-α-farnesene from FDP (Fig. 6A,D). In the process of cloning the (E,E)-α-farnesene synthase, we also picked up another full-length cDNA clone from the cDNA library with a coding sequence similar to a sesquiterpene synthase. Cloning, E. coli expression, and an enzymatic assay with FDP as substrate showed that this is an (E)-β-caryophyllene synthase (Fig. 6, B and E). Alignment of (E)-β-caryophyllene synthase and (E,E)-α-farnesene showed a similarity of only 42% (Fig. 7). The risk of cross-hybridization of this second sesquiterpene synthase to the cDNA fragments on the microarray representing (E,E)-α-farnesene synthase under the stringency conditions used can hence be concluded to be very low. In enzyme assays with GDP as substrate, it turned out that the cucumber (E,E)-α-farnesene synthase can also convert GDP to (E)-β-ocimene with roughly the same efficiency as for the conversion of FDP to (E,E)-α-farnesene over a range of substrate concentration (5–20 μm) chosen to be in the range of the anticipated Km based on published data for other terpene synthases (data not shown). Although the correlation of cDNA fragments 5G8 and 8D11 with (E,E)-α-farnesene is better than with (E)-β-ocimene, the latter is also significant (Fig. 5).
Figure 6.
GC-MS profiles of products formed by the heterologously expressed sesquiterpene synthase CsαFS and CsβCS with FDP as substrate. Chromatogram of product (m/z 93 + 161 + 189 + 204) obtained from assay with lysate of bacteria expressing recombinant CsαFS. The main product peak (Rt 13.95) is (E,E)-α-farnesene (A). Chromatogram of product (m/z 93 + 161 + 189 + 204) obtained from assay with lysate of bacteria expressing recombinant CsβCS. The main product peak (Rt 12.98) is (E)-β-caryophyllene by comparison with the Wiley GC-MS database (B). Chromatogram (m/z 93 + 161 + 189 + 204) of assay with lysate of bacteria expressing pET23c (empty vector; C). Mass spectrum of peak at 13.95 (D). Mass spectrum of peak at 12.98 (E). Compounds were identified by comparison with the Wiley GC-MS database, Adams (1995) and Joulain and König (1998).
Figure 7.
Alignment of the amino acid sequences of CsαFS and CsßCS with four sesquiterpene synthases catalyzing the formation of either (E,E)-α-farnesene (MxdαFS and PtαFS) or (E)-β̃-caryophyllene (AtβCS and AaβCS). MxdαFS and PtαFS are (E,E)-α-farnesene synthases from Malus × domestica (AAO22848) and Pinus taeda (AAO61226), respectively. AaβCS is an (E)-β̃-caryophyllene synthase from Artemisia annua (AAL79181) and AtβCS is an (E)-β-caryophyllene/α-humulene synthase from Arabidopsis (AAO85539). The RR-motif is indicated with **. The position of the cDNA fragments 8D11 and 5G8 (see text) is indicated.
DISCUSSION
Data Analysis
In this work we show that the combination of transcriptome and metabolome analysis can lead to the identification of new genes encoding enzymes involved in specific biosynthetic processes in plants, such as—in this work—genes related to volatile induction and biosynthesis. To our knowledge, the use of statistical software for gene expression analysis to link phenotypic (volatile) data to mRNA expression data in order to facilitate the linking of a selection of genes to specific functions is new. Generally it is assumed that induced volatiles are synthesized de novo (Paré and Tumlinson, 1997), and hence volatile emission reflects the production capacity (enzyme machinery). In a regulated system, such as the induced indirect defense system we have used here, enzyme activities involved can be expected to be proportional to the presence of the corresponding transcripts. The correlation between volatile emission and gene expression patterns can then be used to select interesting genes. In a less dynamic system (for example when endogenous metabolites are analyzed), it may be necessary to calculate metabolite fluxes (increase or decrease between two time points) to get a better correlation with expression data.
When ranking the clones based on how well their expression pattern over all the treatments correlates with the emission rates of specific volatiles, we obtained a list of several cDNA clones with significant correlation values. Based on their expression pattern, volatile data and cDNAs are distributed to 24 groups in a SOM (Fig. 4A). Candidate genes for a role in the biosynthesis of volatiles were selected from this SOM and the Pearson correlation coefficients used to rank genes. For example, there are two different cDNA fragments corresponding to the cDNA of (E,E)-α-farnesene synthase on the chip 5G8 and 8D11. These two cDNA fragments have the highest correlation with the volatile emission data of (E,E)-α-farnesene (Fig. 5B) and also form a separate cluster with (E,E)-α-farnesene (Fig. 4B). The SOM in Fig. 4A also allocates lipoxygenase-gene derived cDNA fragments together with (Z)-3-hexenyl acetate and peroxidase-like fragments to the same group as 4,8-dimethyl-1,3(E),7-nonatriene (Figs. 4 and 5).
Subtractive cDNA Libraries
The two SSH-libraries were clearly enriched in cDNA clones with opposite expression profiles over the experiment (Fig. 1). Some conclusions from the use of randomly selected clones from an SSH cDNA-library on the chip are on the one hand the advantage of giving a higher proportion of clones regulated (40%) compared to when random clones from a conventional cDNA library for a similar system are used. A nonsubtracted cDNA library from spider mite infested/mechanically wounded lima bean leaves contained 5% regulated clones (Arimura et al., 2000). On the other hand, the manufacturers' claim of prevention of clone redundancy was by far not obtained (for example the SSH+ contained around 10% PR-1 and 20% lipoxygenase cDNAs). It is noteworthy that the most redundant cDNA clones were also expressed at remarkably high levels, which might explain the limited success of the normalization procedure when making the up-regulated subtractive cDNA library. The low-expressing mRNAs are enriched by the SSH-technique, which is a positive property, but many of these were below the microarray detection level for our system (in our case up to 30% of spotted clones). Similar observations when using SSH libraries have also been made in mammalian systems (Hida et al., 2000; Boeuf et al., 2001).
Induced and Repressed Responses
A number of microarray cDNA fragments with a sequence most similar to a gene encoding a PR-1 protein (T08154) is strongly up-regulated by spider mite infestation and redundant in our SSH+ library. Comparing spider mite infested plants with noninfested plants, we found these cDNAs to be the most up-regulated genes along with the lipoxygenase-gene derived fragments (T10085). Intercomparison of the transcriptome from several treatments makes it possible to segregate the lipoxygenase cDNA and the PR-1-like cDNAs. PR-1 cDNAs are among those later induced by jasmonic acid treatment compared to the lipoxygenase cDNAs. PR-1 proteins are generally associated with salicylic acid induced pathways. However, methyl jasmonate has been shown to induce PR-1 in tobacco (Xu et al., 1994), and also other SA-independent PR-1-inducing factors have been reported (Pieterse and van Loon, 1999). The most strongly down-regulated clones were mainly of housekeeping origin, especially cDNAs involved in photosynthesis. Photosynthesis related genes are also down-regulated in Nicotiana attenuata exposed to Manduca sexta caterpillars (Hermsmeier et al., 2001).
It is known from earlier reports that jasmonic acid and spider mites induce different blends of predator-attracting volatiles in lima bean plants (Dicke et al., 1999), and this was now also shown to be the case for cucumber (Fig. 2). Jasmonic-acid sprayed plants produced larger quantities of volatiles but also a different blend. Schmelz et al. (2001) demonstrated a difference in volatile quantity and composition when comparing volatile release from damaged and intact maize plants. From our experiments, we find that the ratio of terpenoids to (Z)-3-hexenyl acetate was significantly higher in the jasmonic acid-sprayed plants than in the spider mite infested plants at 7 d after infestation. It should be noted that the jasmonic acid-sprayed plants are not mechanically damaged, whereas the spider mite infested plants are being damaged continuously by the herbivores. In Figure 3, we note that the second component (y) mainly reflects the difference in gene expression between jasmonic acid and spider mite treatments, illustrating a difference also in gene expression profile for the treatments. The differences between the jasmonic acid and spider mite treatments in volatile formation are likely caused by the stimulation of a broader spectrum of signal transduction pathways by the herbivore (Ozawa et al., 2000; Horiuchi et al., 2001; Dicke et al., 2003). The transient decline in volatile emission at 96 h is interesting (Fig. 2B) although the reason for this effect is not clear. Nevertheless, the decline occurred at around this time point also when the treatment was repeated. Moreover, the transient decrease in volatile production is also reflected in the transcriptional data, where the trend is interrupted at 96 h and then resumed at 168 h (Fig. 3).
cDNAs Involved in Volatile Biosynthesis
We cloned the (E,E)-α-farnesene synthase (accession no. AY640154) supported by the observation that the product (E,E)-α-farnesene correlated well with two cDNA fragments over the treatments (Figs. 4 and 5). The result demonstrates the applicability of combining metabolic profiling and global gene-expression techniques. (E,E)-α-farnesene has been reported before to be a component in the herbivory-induced volatiles from C. sativus (Takabayashi et al., 1994; Bouwmeester et al., 2003) and is known to attract carnivorous arthropods (Scutarenu et al., 1997). (E,E)-α-farnesene and its synthase had a different induction pattern compared to the other induced volatiles and enzymes (Fig. 5). It is tempting to speculate that the (E,E)-α-farnesene synthase needs a higher threshold of intracellular jasmonic acid to be activated than the other terpene synthases, resulting for example from direct jasmonic acid spraying or very intense spider mite feeding. Maize seedlings show a correlation between the levels of herbivory by beet armyworm caterpillars and jasmonic acid content and sesquiterpene volatile formation (Schmelz et al., 2003), but the authors do not discuss the relationship between jasmonic acid and volatile composition.
We have also cloned, expressed, and identified another sesquiterpene synthase, i.e. (E)-β-caryophyllene synthase (accession no. AY640155). (E)-β-caryophyllene was never detected as an induced volatile in cucumber and, to our knowledge, has not been reported from cucumber in the literature. In many other plant species (E)-β-caryophyllene is an important constituent of the induced volatile blend (Bouwmeester et al., 2003; Van den Boom et al., 2004). An (E)-β-caryophyllene synthase from Artemisia annua has been shown to be up-regulated by mechanical wounding and a fungal elicitor and was suggested to be involved in plant defense although its specific function remains unknown (Cai et al., 2002). The absence of (E)-β-caryophyllene in the volatile blend of induced cucumber could perhaps be due to a low or localized expression, and we are currently investigating if the cloned (E)-β-caryophyllene synthase has a role in the herbivore-induced defense system in cucumber. An amino acid alignment of these two new terpene synthases from cucumber and four other sesquiterpene synthases that are able to convert FDP to either (E,E)-α-farnesene or (E)-β-caryophyllene demonstrate their similarity to other sesquiterpene synthases (Fig. 7). The RR motif close to the N terminal of the three farnesene synthases is hypothesized to be involved in the formation and stabilization of the intermediate nerolidyl-cation (Williams et al., 1998) formed en route to (E,E)-α-farnesene. The motif is changed to RP in the three caryophyllene synthases where a nerolidyl-cation is not involved in the cyclization mechanism. The alignment confirms previous observations that sequence analysis of terpene synthases cannot reveal the identity of the catalyzed end product. The cucumber sesquiterpene synthases have a higher similarity to each other than to synthases from other species with similar catalytic reactions (Fig. 7).
(Z)-3-hexenyl acetate was assigned to cluster D1 (Fig. 4A). This compound is well known to be induced by herbivory in a range of plant species (Turlings et al., 1990; Van den Boom et al., 2004), by spider mite feeding in cucumber in particular (Takabayashi et al., 1994) and by jasmonic acid in e.g. lima bean plants (Dicke et al., 1999). The compound is formed by the action of an acyl-transferase acting on (Z)-3-hexen-1-ol. It is postulated that (Z)-3-hexen-1-ol is derived from the chloroplastic lipids, mainly by the activity of a galactolipid-specific lipase, while a lipoxygenase is catalyzing the subsequent step (Matsui et al., 2000). Lipoxygenases convert linolenic acid to 9- or 13-hydroperoxylinolenic acid which can be converted by other enzymes to several products, such as nonenal, jasmonic acid, and (Z)-3-hexenal (Somerville et al., 2000). Although lipoxygenase products have diverse functions and the wounding (punching) of the leaf discs before volatile capture likely gives high background levels, there is a good correlation between the lipoxygenase cDNA fragments and the production of (Z)-3-hexenyl acetate (Figs. 4 and 5).
4,8-Dimethyl-1,3(E),7-nonatriene, a terpenoid that attracts the predator Phytoseiulus persimilis of the spider mite Tetranychus urticae (Dicke et al., 1990) clustered into group D3 (Fig. 4). This C11-hydrocarbon is biosynthesized from the terpenoid precursor 3S-(E)-nerolidol by a sequence of oxidative degrading steps (Donath and Boland, 1994, 1995; Bouwmeester et al., 1999; Degenhardt and Gershenzon, 2000). In this, or neighboring groups, we did not find any cDNAs likely to be involved in the biosynthesis of 3S-(E)-nerolidol (i.e. a sesquiterpene synthase). However, group D3 contains four cDNA fragments (3B8, 6B1, 5E2, and 6C12) of putatively peroxidase-type (similar to cucumber acidic peroxidase T10444). Peroxidases have shown to be involved in a wide range of oxidizing reactions and are consequently interesting candidates for a role in the oxidation of 3S-(E)-nerolidol to 4,8-dimethyl-1,3(E),7-nonatriene. For example, decarboxylating peroxidases have been characterized in the catabolism of IAA (Crozier et al., 2000). A possible role for cytochrome P450s in the conversion of nerolidol to 4,8-dimethyl-1,3(E),7-nonatriene has also been suggested (Donath and Boland, 1994, 1995), but we did not detect any cytochrome P450s in group D3.
Based on sequence homology to accession number T52570, a putative 1-deoxy-d-xylulose 5-phosphate reductase was identified. The reducto-isomerase catalyses one of the early steps in the formation of the plastidic terpenoid precursor IDP, which in principle would be a precursor for the monoterpene (E)-β-ocimene. The cDNA is not assigned into the same SOM-cluster as (E)-β-ocimene, but it is induced by spider mite feeding and jasmonic acid. Although the correlation between the expression of this cDNA and (E)-β-ocimene production is somewhat lower than for the other described volatile-associated cDNA fragments, it is still highly significant (P < 0.005; Fig. 5D). The lower correlation is not unexpected considering the position of this enzyme early in the terpenoid pathway in combination with the diverse fates of IDP in the plastids. Otherwise, we have not identified any cDNAs on the microarray that are correlating with the (E)-β-ocimene volatile data nor have we found a terpene synthase-like sequence among the printed cDNA fragments besides the (E,E)-α-farnesene synthase sequence. Interestingly, the additional N terminal coding sequence (44 aa) of (E,E)-α-farnesene synthase has a pI of 8.7, relative to the overall pI of 5.2, which is a common feature of plastid targeting sequences (Keegstra et al., 1989), and according to the iPSORT program (Bannai et al., 2002) the molecule will indeed be targeted to the chloroplast. Moreover, the (E,E)-α-farnesene synthase can catalyze the formation of (E)-β-ocimene from GDP at about equal efficiency as the formation of (E,E)-α-farnesene from FDP (data not shown), and the correlation of the expression pattern of the farnesene cDNA fragments to the β-ocimene is high and significant (Fig. 5D). Thus, this could theoretically imply a dual role for this enzyme, with the targeting signal enabling the pre-enzyme to enter the plastids that in Arabidopsis have been shown to contain GDP as well as some FDP (Aharoni et al., 2003) and subsequently form both (E)-β-ocimene and (E,E)-α-farnesene. Also important to consider is that the (E,E)-β-farnesene synthase from Mentha x piperita (Crock et al., 1997) has a targeting-signal to the chloroplast as well, based on analysis with the iPSORT program, while an (E,E)-α-farnesene synthase from apple fruit (AY182241) is supposed to be cytosolic. The (E,E)-β-farnesene synthase from M. x piperita is also able to convert GDP to monoterpenes, but forms several different mainly cyclic monoterpene products. The reported presence of FDP in Arabidopsis plastids (Aharoni et al., 2003), the plastid targeting signal, the dual product formation in vitro, and the correlations between expression and metabolite formation makes the production of (E)-β-ocimene and/or (E,E)-α-farnesene by one enzyme a serious option. Nevertheless, we cannot exclude the presence of additional terpene synthases that may be involved in spider mite induced (E)-β-ocimene and/or (E,E)-α-farnesene production in cucumber. We are currently further investigating the full role of the (E,E)-α-farnesene synthase in induced volatile formation in cucumber.
In conclusion, the approach to combine global gene expression analysis with metabolite analysis has resulted in the discovery of cucumber genes involved in induced volatile emission and indirect defense. This approach has good potential for identifying more genes involved in induced plant defenses in the future.
MATERIALS AND METHODS
Plant Material and Spider Mites
Cucumber seeds (Cucumis sativus L. cv Corona) were germinated and grown in 1-L pots under greenhouse conditions at a 20°C/18°C, 12/12-h supplemental light/dark cycle (October). Two-spotted spider mites (Tetranychus urticae Koch) were reared on lima bean plants (Phaseolus lunatus; for details, see Dicke et al., 1999). Three separate neighboring greenhouse compartments, with the same light and temperature conditions, were used for the different treatments such that jasmonic acid-treated and spider mite-infested plants were grown in separate compartments. Mechanically wounded and control plants were grown in the same compartment. For spider mite infestation, a lima bean leaf, heavily infested with spider mites, was placed on each of two leaves of approximately 4-week-old cucumber plants for 24 h and then removed. For jasmonic acid treatment, 1 mm jasmonic acid in water with 0.01% Tween 20 was sprayed evenly over the cucumber plants (2 mL per plant). For mechanical wounding, carborundum powder was gently rubbed over the leaf surface, using rubber-gloved hands. Control plants were sprayed with water plus 0.01% Tween 20. Volatile measurements and tissue samples for mRNA preparation were taken at 6, 24, 48, and 72 h after the start of the experiment for the material exposed to mechanical wounding and 0, 6, 24, 48, and 72 h after treatment with jasmonic acid. Spider mite treated material was harvested at 24, 48, 72, 96, and 168 h after the experiment started. Control samples were taken at 0, 6, 24, 48, 72, 96, and 168 h.
Headspace Analysis
A comparison of volatile emission between intact plants and leaf discs was carried out using 3-week-old cucumber plants infected with spider mites for 0, 2, 4, and 6 d. Because the volatile emission patterns were similar for intact plants and leaf discs (particularly for terpenoids; (Z)-3-hexenylacetate production was 2- to 15-fold higher from leaf discs) and because the use of leaf discs allowed the simultaneous collection of samples for volatile analysis and RNA extraction from the same plant, in further experiments leaf discs were used. Four leaf discs (ø 6 cm) were taken from each plant (three replicate plants per treatment) and enclosed in a 1-L glass jar with a Teflon-lined cap with a stainless steel in- and outlet. Jars were placed in a climate room at 23°C and a light intensity of 210 μmol m−2 s−1 and the headspace was sampled during 3 h and analyzed using GC-MS as described previously (Bouwmeester et al., 1999). Remaining parts of the leaf were immediately frozen in liquid nitrogen and used for RNA extraction.
Extraction of mRNA and Preparation of cDNA Libraries
Two grams of ground leaf material were homogenized in 8 mL extraction/binding (Dynal) buffer and then mixed with 0.2 g PVPP and centrifuged for 10 min at 18 000g. mRNA was extracted from the supernatant using polyT-magnetic beads according to Genoprep protocol. mRNA was quality assured on gel and the concentration was determined at three different dilutions for each sample using Ribogreen (Molecular Probes, Eugene, OR). cDNA synthesis was performed with Reverse Transcriptase (gibco-BRL) using AA-dNTP-mix (including aminoallyl-dUTP). To enrich for cDNAs involved in indirect plant defense reactions two SSH cDNA libraries were made, according to manufacturer's instructions (CLONTECH, Palo Alto, CA) and cloned into pGEMT easy (Promega, Madison, WI). One library was enriched with cDNAs up-regulated (SSH+) and one with cDNAs down-regulated (SSH−) by spider mite infestation. For the subtraction procedure, cDNA was prepared from cucumber leaf material (168 h after infestation) from infested and noninfested plants. The spider mites and their eggs on infested leaf material were removed with a brush before mRNA preparation. From the same spider mite infested leaf material, a lambda phage cDNA library was made according to manufacturer's instructions (CLONTECH).
cDNA Microarrays
A total of 713 randomly selected clones from the SSH+ and the SSH− library were printed on amino-coated glass slides in double copies, using a 2-pin print head and a custom-built arraying robot (Van Hal et al., 2000). Inserts from the subtractive libraries, cloned in pGEMT easy, were amplified using vector primers in a colony PCR reaction. PCR reaction conditions: Colony PCR reaction corresponding to approximately 10 ng plasmid, 20 nmol dNTP, 100 pmol forward/reverse primer, 2.5 units Taq (Gibco BRL, Cleveland), reaction buffer with MgCl2 according to manufacturer's recommendations, and H2O in a final volume of 100 μL. PCR program 94°C 30 s, (94°C 30 s; 55°C 30 s, and 72°C 2.5 min) × 30. Qiaquick PCR BioRoBot kit (Qiagen, Venlo, The Netherlands) was used for DNA purification followed by complete liquid evaporation. DNA was dissolved in 10 μL 5× SSC before being arrayed in duplicates onto amino silane coated glass slides (PixSys 7500 BioDot; Genomic Solutions, Ann Arbor, MI). On the array, 140 clones from the SSH− library, 573 clones from the SSH+ library, and 44 background (yeast and human origin) and reference (luciferase) clones were spotted. Full-length luciferase cDNA clones spotted on the array were used to normalize expression values derived from the Cy3- or the Cy5 dyes respectively. The background threshold level was determined by the use of a set of nonhybridizing, human (five) and yeast (three) clones. Arrays were dried overnight and then rehydrated with steam and snap-dried (95°C–100°C) and UV cross-linked (150 mJ). The transcriptome from each sample was compared to a common reference made of a mixture of all mRNA samples. Care was taken to use the same amount of RNA for each treatment. Cy3 and Cy5, dissolved in dimethyl sulfoxide, were covalently bound to the incorporated amino-groups in a 0.1 m Na2CO3 buffer pH 9.3. Unincorporated dye was removed by 2× ethanol precipitation and finally dissolved in ddH2O to a concentration of 0.5 μg/μL. A solution of 50% formamide, 5× Denhardt's reagent, 5× SSC, 0.2% SDS, 0.1 mg/mL denatured fish DNA, and the Cy3- and Cy5-labeled cDNA was used for the hybridization to the microarrays during 24 h at 42°C. Washing was performed in the dark once in 1× SSC and 0.1% SDS (5 min), once in 0.1× SSC and 0.1% SDS (5 min), and a brief rinse in 1× SSC.
Expression Data Analysis
Microarrays were scanned (ScanArray 3000, General Scanning, Watertown, MA) for fluorescence emission. The integrated optical density for each dye was measured within a defined circle of each spot, using AIS software (Imaging Research, St. Catherines, Canada). Microsoft Excel was used for organizing data and for statistical analyses to ensure the quality of the expression data. Sequence analysis was performed using DNA-Star. By comparing the transcriptome from each of the 14 treatments to a common reference mix the levels of gene expression can be compared between the different treatments. Only clones with an expression difference of at least 2.4-fold between the lowest and the highest level within the 14 treatments were included in PCA analysis. cDNA fragments present in three or more copies were removed and exchanged with a contig (average values from clones in the contig were applied). Standard Pearson correlation coefficients were determined for data obtained from 14 different measurements. A two-sided t test was used to determine the significance of associations. Data from all 14 treatments were used to determine correlation values, as also treatments that do not induce volatiles are important for the expressional characterization of cDNA clones. Cluster and correlation analysis were performed using Genemaths software (Applied Maths, Sint-Martens-Latem, Belgium) and Microsoft Excel. The correlation analysis using Genemaths software was used to make dendrograms and SOMs. PCA analysis gives complementary information to ranking lists based on correlation coefficients to specific volatiles, especially for cDNAs with high associations to more than one volatile. Significance level for correlations was set at P ≤ 0.005. Log-values for the amounts of volatiles (quantified in area units under the curve) were calculated after being divided with the average volatile expression calculated from all experiments, and were subsequently included in data sheets listing the expression data. When compounds could not be detected on GC-MS, a value of 10,000 area units (estimated minimum detection quantity) was applied for calculations.
Cloning and Characterization of Terpene Synthases
The two clones having a sesquiterpene homolog found in the cluster including the volatile (E,E)-α-farnesene were used as a probe to screen a phage cDNA library. Full-length cDNAs of terpene synthases were cloned into expression vector pET-23c in such a way that no additional amino acids were fused to the expected open reading frame. The Escherichia coli strain BL21(DE3)plysE was used for expression (Stratagene, La Jolla, CA). Functional protein expression was essentially carried out as has been described before (Mercke et al., 2000). A culture of 50 mL was lyzed in 2.5-mL buffer (25 mm HEPES [pH 7.5], 10% v/v glycerol, 10 mm β-mercaptoethanol, 2 mm MgCl2) and finally run on a PD-10 column (Pharmacia, Piscataway, NJ) and eluted in 3.5 mL. For enzyme product identification and analysis: 1 mL of protein extract and 1 mL of buffer together with 15 nmol FDP with an overlay of approximately 1 mL redistilled pentane and incubated for 3 h in 30°C in a 10 mL teflon-lined screw cap glass tube. The assay was extracted twice more with 1 mL pentane. The pentane phase was run over an MgSO4-column and concentrated to approximately 100 μL before GC-MS analysis as described before (Bouwmeester et al., 1999). Compounds were identified using mass spectra and retention indices (Adams, 1995; Joulain and König, 1998). In an assay for comparing the identity and amount of products formed after incubation with the substrates GDP and FDP, samples were incubated for 1 h at 30°C.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AY640154 and AY640155.
This work was supported by a Marie Curie Individual Fellowship (MCFI–2000–01234 to P.M.), by the Dutch Ministry of Agriculture, Nature Management and Fisheries (DWK 333 to F.W.A.V. and H.J.B.), and by the Dutch Technology Foundation (STW project WPB.5479 to I.F.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.048116.
References
- Adams RP (1995) Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publishing, Carol Stream, IL
- Agrawal AA, Janssen A, Bruin J, Posthumus MA, Sabelies MW (2002) An ecological cost of plant defence: attractiveness of bitter cucumber plants to natural enemies of herbivores. Ecol Lett 5: 377–385 [Google Scholar]
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FWA, Verhoeven HA, Jongsmaa MA, Schwab W, Bouwmeester HJ (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15: 2866–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Tashiro K, Kuhara S, Nishioka T, Ozawa R, Takabayashi J (2000) Gene responses in bean leaves induced by herbivory and by herbivore-induced volatiles. Biochem Biophys Res Commun 277: 305–310 [DOI] [PubMed] [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S (2002) Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18: 298–305 [DOI] [PubMed] [Google Scholar]
- Boeuf S, Klingenspor M, Van Hal NL, Schneider T, Keijer J, Klaus S (2001) Differential gene expression in white and brown preadipocytes. Physiol Genomics 7: 15–25 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Kappers IF, Verstappen FW, Aharoni A, Luckerhoff LLP, Lücker J, Jongsma M, Dicke M (2003) Exploring multi-trophic plant-herbivore interactions for new crop protection methods. In J Pickett, ed, Proceedings of the International Congress Crop Science and Technology, November 10–12, 2003, Glasgow, Scotland, Vol 2. British Crop Protection Council, Farnham, UK, pp 1123–1134
- Bouwmeester HJ, Verstappen FW, Posthumus MA, Dicke M (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol 121: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Jia JW, Crock J, Linn ZX, Chen XY, Croteau R (2002) A cDNA clone for beta-caryophyllene synthase from Artemisia annua. Phytochemistry 61: 523–529 [DOI] [PubMed] [Google Scholar]
- Crock J, Wildung M, Croteau R (1997) Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha × piperita, L.) that produces the aphid alarm pheromone (E)-β-farnesene. Proc Natl Acad Sci USA 94: 12833–12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier A, Kamiya Y, Bishop G, Yokota T (2000) Biosynthesis of hormones and elicitor molecules. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry & Molecular Biology of Plants. Courier Companies, Rockville, MD, pp 850–929
- De Boer JG, Dicke M (2004) Prey searching behavior of the predatory mite Phytoseiulus persimilis: the role of methyl salicylate. J Chem Ecol 30: 255–271 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570–573 [Google Scholar]
- Degenhardt J, Gershenzon J (2000) Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210: 815–822 [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25: 1907–1922 [Google Scholar]
- Dicke M, van Beek TA, Posthumus MA, Ben Dom N, van Bokhoven H, de Groot AE (1990) Isolation and identification of volatile kairomone that affects acarine predator prey interactions. Involvement of host plant in its production. J Chem Ecol 16: 381–396 [DOI] [PubMed] [Google Scholar]
- Dicke M, van Poecke RMP, de Boer JG (2003) Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl Ecol 4: 27–42 [Google Scholar]
- Donath J, Boland W (1994) Biosynthesis of acyclic homoterpenes in higher-plants parallels steroid-hormone metabolism. J Plant Physiol 143: 473–478 [Google Scholar]
- Donath J, Boland W (1995) Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry 39: 785–790 [Google Scholar]
- Guterman I, Shalit M, Menda N, Piestun D, Dafny-Yelin M, Shalev G, Bar E, Davydov O, Ovadis M, Emanuel M, et al (2002) Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell 14: 2325–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsmeier D, Schittko U, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol 125: 683–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida K, Wada J, Zhang H, Hiragushi K, Tsuchiyama Y, Shikata K, Makino H (2000) Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res 41: 1615–1622 [PubMed] [Google Scholar]
- Horiuchi J, Arimura G, Ozawa R, Shimoda T, Takabayashi J, Nishioka T (2001) Exogenous ACC enhances volatiles production mediated by jasmonic acid in lima bean leaves. FEBS Lett 509: 332–336 [DOI] [PubMed] [Google Scholar]
- Janssen A, Pallini A, Venzon M, Sabelisi MW (1998) Behaviour and indirect interactions in food webs of plant-inhabiting arthropods. Exp Appl Acarol 22: 497–521 [Google Scholar]
- Joulain D, König WA (1998) The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. E.B.-Verlag, Hamburg, Germany
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K, Olsen LJ, Theg SM (1989) Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol Plant Mol Biol 40: 471–501 [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Leung YF, Cavalieri D (2003) Fundamentals of cDNA microarray data analysis. Trends Genet 19: 649–658 [DOI] [PubMed] [Google Scholar]
- Matsui K, Kurishita S, Hisamitsu A, Kajiwara T (2000) A lipid-hydrolysing activity involved in hexenal formation. Biochem Soc Trans 28: 857–860 [PubMed] [Google Scholar]
- Mercke P, Bengtsson M, Bouwmeester HJ, Brodelius PE (2000) Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381: 173–180 [DOI] [PubMed] [Google Scholar]
- Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41: 391–398 [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114: 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4: 52–58 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Willmitzer L, Fernie AR (2002) Metabolic profiling and biochemical phenotyping of plant systems. Plant Cell Rep 21: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Tumlinson JH (2001) The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214: 171–179 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Alborn HT, Banchio E, Tumlinson JH (2003) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216: 665–673 [DOI] [PubMed] [Google Scholar]
- Scutarenu P, Drukker B, Bruin J, Posthumus MA, Sabelis MW (1997) Volatiles from Psylla-infested pear trees and their possible involvement in attraction of anthocorid predators. J Chem Ecol 23: 2241–2260 [Google Scholar]
- Somerville C, Browse J, Jaworski JG, Ohlrogge JB (2000) Lipids. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry & Molecular Biology of Plants. Courier Companies, Rockville, MD, pp 456–527
- Takabayashi J, Dicke M (1996) Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci 1: 109–113 [Google Scholar]
- Takabayashi J, Dicke M, Takahashi S, Posthumus MA, van Beek TA (1994) Leaf age affects composition of herbivore-induced synomones and attraction of predatory mites. J Chem Ecol 20: 373–386 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Loughrin JH, McCall PJ, Rose USR, Lewis WJ, Tumlinson JH (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92: 4169–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253 [DOI] [PubMed] [Google Scholar]
- Urbanczyk-Wochniak E, Luedemann A, Kopka J, Selbig J, Roessner-Tunali U, Willmitzer L, Fernie AR (2003) Parallel analysis of transcript and metabolic profiles: a new approach in systems biology. EMBO J 4: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Boom CEM, Van Beek TA, Posthumus MA, De Groot A, Dicke M (2004) Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. J Chem Ecol 30: 69–89 [DOI] [PubMed] [Google Scholar]
- Van Hal NLW, Vorst O, van Houwelingen AMML, Kok EJ, Peijnenburg A, Aharoni A, van Tunen AJ, Keijer J (2000) The application of DNA microarrays in gene expression analysis. J Biotechnol 78: 271–280 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Dicke M (2004) Indirect defence of plants against herbivores: using Arabidopsis thaliana as a model plant. Plant Biol 6: 387–401 [DOI] [PubMed] [Google Scholar]
- Williams DC, Mcgarvey DJ, Katahira EJ, Croteau R (1998) Truncation of limonene synthase preprotein provides a fully active ‘pseudomature’ form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry 37: 12213–12220 [DOI] [PubMed] [Google Scholar]
- Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6: 1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]