Abstract
The tomato (Lycopersicon esculentum) mutant def-1, which is deficient in induced jasmonic acid (JA) accumulation upon wounding or herbivory, was used to study the role of JA in the direct and indirect defense responses to phytophagous mites (Tetranychus urticae). In contrast to earlier reports, spider mites laid as many eggs and caused as much damage on def-1 as on wild-type plants, even though def-1 lacked induction of proteinase inhibitor activity. However, the hatching-rate of eggs on def-1 was significantly higher, suggesting that JA-dependent direct defenses enhanced egg mortality or increased the time needed for embryonic development. As to gene expression, def-1 had lower levels of JA-related transcripts but higher levels of salicylic acid (SA) related transcripts after 1 d of spider mite infestation. Furthermore, the indirect defense response was absent in def-1, since the five typical spider mite-induced tomato-volatiles (methyl salicylate [MeSA], 4,8,12-trimethyltrideca-1,3,7,11-tetraene [TMTT], linalool, trans-nerolidol, and trans-β-ocimene) were not induced and the predatory mite Phytoseiulus persimilis did not discriminate between infested and uninfested def-1 tomatoes as it did with wild-type tomatoes. Similarly, the expression of the MeSA biosynthetic gene salicylic acid methyltransferase (SAMT) was induced by spider mites in wild type but not in def-1. Exogenous application of JA to def-1 induced the accumulation of SAMT and putative geranylgeranyl diphosphate synthase transcripts and restored MeSA- and TMTT-emission upon herbivory. JA is therefore necessary to induce the enzymatic conversion of SA into MeSA. We conclude that JA is essential for establishing the spider mite-induced indirect defense response in tomato.
Plants protect themselves against herbivores through a combination of constitutive and inducible defenses that decrease herbivore performance. Induced defenses are characterized by changes in morphology and increases in secondary metabolites or defense-associated proteins. If such changes lower the food quality for herbivores they are referred to as direct defense. Plants may also acquire protection indirectly, for example via the production of herbivore-induced volatiles that attract the natural enemies of the herbivores, such as predators and parasitoids (for review, see Dicke et al., 1998; Baldwin and Preston, 1999; Sabelis et al., 1999).
In tomato (Lycopersicon esculentum), jasmonic acid (JA) and Systemin (Sys) are the key molecules that mediate local and systemic signaling, leading to direct defense-related gene expression. In the model proposed by Li et al. (2002a), herbivory induces proteolytic release of mobile Sys from its immobile precursor prosystemin. Sys then interacts with a plasma membrane-bound receptor (Scheer and Ryan, 2002) leading to a signaling cascade that results in the lipase-mediated release of linolenic acid from membrane lipids. Linolenic acid is converted into JA via a sequence of enzymes (lipoxygenase, allene oxide synthase, allene oxide cyclase, 12-oxo-phytodienoic acid [OPDA]-reductase, and β-oxidation enzymes), referred to as the octadecanoid cascade. JA but also the JA-precursor OPDA and the JA-derivate methyl jasmonate (MeJA; Farmer et al., 1992) are active as regulators of tomato defense genes.
The role of jasmonate in tomato defense signaling has been studied by making use of the JA-biosynthesis mutants def-1, spr-1 and spr-2 and the JA-perception mutant jai1-1 (Li et al., 2002a, 2002b, 2004). Jai1-1 has been shown to be the tomato homolog of the Arabidopsis COI1 gene and is referred to as LeCoi1. The jai1-1 mutant failed to express defense-related genes in response to wounding, MeJA and spider mite-herbivory, was insensitive to the phytotoxin coronatine and exhibited increased resistance to virulent strains of Pseudomonas syringae (Li et al., 2004). The JA-synthesis mutant def-1 was deficient in induced accumulation of JA and defense proteins (proteinase inhibitors), while the spider mite Tetranychus urticae had a higher reproductive success on def-1 and caused more feeding-damage than on wild-type plants. In undamaged def-1, the JA-content was similar to that in the wild type but after 2 d of infestation by spider mites, JA levels in the wild type increased almost 3-fold, whereas those in def-1 remained unchanged. In contrast to jai1-1, treatment of the def-1 mutant with MeJA restored resistance to spider mite-feeding and reduced spider mite-fecundity. Moreover, plants expressing a 35S::prosystemin transgene, which constitutively activates the octadecanoid pathway in a DEF-1-dependent manner, were highly resistant to the cell content-feeders T. urticae and western flower thrips (Frankliniella occidentalis). These results provided strong evidence that tomato defense to cell content-feeding herbivores is regulated by the octadecanoid signaling pathway (Li et al., 2002b).
It has been established that jasmonate precursors and derivatives or JA itself can induce the emission of volatiles similar to those induced by herbivory such as 4,8-dimethyl-1,3,7-nonatriene (DMNT) and 4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT) as well as the monoterpenes linalool and β-ocimene in detached lima bean leaves (Koch et al., 1999). In addition, JA or MeJA-vapor elicited the emission of volatiles such that predatory mites were attracted (Dicke et al., 1999; Gols et al., 2003). JA probably induced the synthesis of these volatiles rather than their release from storage. For example, in cotton, MeJA induced the emission of β-ocimene, linalool, α- and β-farnesene, and TMTT, but it did not cause release of the stored monoterpenes α-pinene, β-pinene, α-humulene, and β-caryophyllene, which were only released after mechanical damage (Rodriguez-Saona et al., 2001). However, the formation of type VI trichomes and the volatile organics in them (α-pinene, β-pinene, limonene, and cis-β-ocimene) is reduced in tomato jai1-1 and could be therefore dependent on JA (Li et al., 2004).
The beet army worm (Spodoptera exigua) induced lower amounts of α-pinene, β-pinene, 2-carene, β-phellandrene, and β-caryophyllene in def-1 than in the wild type, while the predatory mite Phytoseiulus persimilis was not attracted to Spodoptera-infested def-1 plants, only to wild-type plants. However, when def-1 was treated with exogenous JA the plants also attracted predatory mites (Thaler et al., 2002). These results led to several interesting conclusions. First, they showed that the Spodoptera-induced emission of tomato mono- and sesquiterpenes was DEF-1-dependent. Second, they showed that JA promoted the emission of unidentified volatiles that attracted predatory mites to tomato, therefore it is unknown whether the same volatiles were emitted as by Spodoptera-infested plants. It has been shown that predatory mites respond to several herbivore-induced volatiles when offered in pure form (Dicke et al., 1990, 1998). Thus, the response of predatory mite to JA-treated tomato plants (def-1 or wild type) cannot be taken as proof for the presence of the same attractants. To exclude the possibility that herbivores and exogenous JA induce different attractants of predators in tomato, an approach is required combining analysis of transcript levels, volatiles metabolites and predator behavior.
Here we report that tomato-reared spider mites induced transcription of direct defense genes related to SA primarily, and no indirect defenses in def-1. Nevertheless, the spider mites performed only as well on def-1 as on the wild type. Through combined transcriptomics, volatile-metabolomics and behavioral analyses, we characterized the tomato response to spider mites and we were able to demonstrate that the indirect defense response of tomato to spider mites is orchestrated by JA. Moreover, we provide preliminary evidence for cross talk between JA and SA at the interface of direct and indirect defenses through JA-mediated volatilization of SA.
RESULTS
Spider Mites Perform Equally Well on def-1 and Wild Type
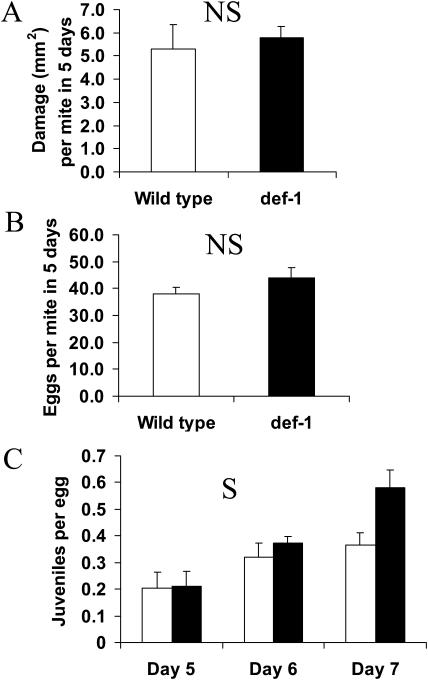
In order to assess whether the def-1 mutation affected the rate in which spider mites damaged leaves and produced offspring, we measured the area of chlorotic lesions caused by spider mite-feeding and counted the number of eggs they produced. Figure 1A shows the damage in mm2 of chlorotic leaf area per mite over a period of 5 d on wild-type and def-1 plants. The damage was 1.06 ± 0.21 mm2 per mite per day on the wild type and 1.16 ± 0.10 mm2 per mite per day on def-1. Each 2-d-old adult female laid on average 38.1 ± 2.3 eggs in 5 d on the wild type and 44 ± 3.7 on def-1 (Fig. 1B).
Figure 1.
Performance of spider mites on wild-type (white bars) and def-1 (black bars) plants. Leaf area damaged by spider mites (A) was determined from five sets of plants infested with four adult female spider mites. There was no significant (NS) difference between the damage inflicted on wild-type leaves and def-1 leaves (ANOVA with df = 1; F = 0.22; P = 0.65). Spider mite fecundity (B) was determined from 12 sets of plants infested with seven adult female spider mites on one leaf per plant. Eggs were counted daily. The figure shows the average total egg production per mite in 5 d. There was no significant (NS) difference between the number of eggs produced on wild-type and def-1 (ANOVA with df = 1; F = 2.0; P = 0.17). The numbers of juveniles that emerged per egg (C) was calculated for days 5, 6, and 7. Egg hatching was not observed before day 5. The hatching rate on def-1 was significantly (S) higher than on the wild type with P = 0.014 (repeated measures ANOVA with df = 1; F = 7.4).
Spider Mite Eggs Produced on def-1 Are More Viable
To determine whether the def-1 mutation affected spider mite-egg viability, we counted the number of juveniles (protonymphs) that had emerged on day 5 (the 1st d eggs hatched), 6, and 7 after infesting wild-type and def-1 plants. Repeated measures ANOVA (df = 1) revealed that the hatching rate from day 5 to day 7 on def-1 was significantly higher than on the wild type (F = 7.4; P = 0.014). The ratio of juveniles to eggs on day 7 was 0.37 ± 0.05 on the wild type and 0.58 ± 0.07 on def-1 (Fig. 1C).
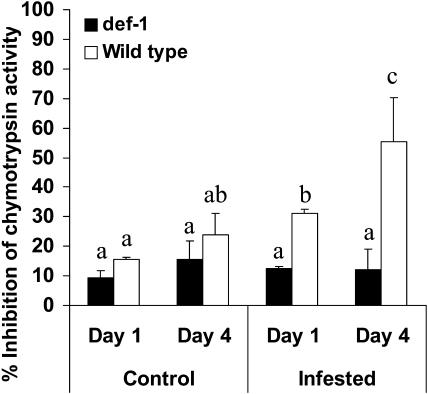
Spider Mites Do Not Induce Proteinase Inhibitor Activity in def-1
As a measure of the direct defense, we determined the proteinase activity in infested and uninfested leaves. We performed a full factorial ANOVA on the data, discriminating between plant-type (wild type and def-1), treatment (control and infested), and time (day 1 and day 4). ANOVA (df = 1) showed that all three factors were significantly different (plant-type, F = 31.76; P = 0.0001; treatment, F = 11.74; P = 0.009; time, F = 8.04; P = 0.022). We subsequently performed a Fisher post hoc test (df = 8) on the separate samples. The infested wild-type samples from day 1 differed significantly from all other samples (P < 0.05), except from the uninfested wild-type sample for day 4 (P = 0.3). The infested wild type day 4 samples differed from all other samples (P < 0.007). No other samples were significantly different from each other (Fig. 2). These data confirm that def-1, unlike wild-type tomato, was unable to produce proteinase inhibitor activity in response to spider mite herbivory, as previously reported (Li et al., 2002b).
Figure 2.
Proteinase inhibitor activity in tomato leaves. The figure shows the percentage of inhibition of chymotrypsin activity in wild-type (white bars) and def-1 (black bars) leaves. Samples of infested and control plants were taken after 1 and 4 d of spider mite infestation. Bars annotated with the same letter were not significantly different (P > 0.05) after ANOVA and the Fisher post hoc test.
SA-Related Transcript Accumulation Is Higher in def-1
DNA microarray analyses were used to determine the effect of spider mite-infestation on the induction of gene-expression, in def-1 and wild-type plants. A dedicated microarray with 428 tomato expressed sequence tags (ESTs) was used, as described by Kant et al. (2004). These ESTs were selected on the basis of their potential relevance in plant-herbivore and plant-pathogen interactions and signaling in general. Data on the ESTs that were used and the details of the microarray experiments can be found in the supplemental data (available at www.plantphysiol.org). The microarray slide also contained 839 PCR-amplified cDNA fragments and 170 ESTs from petunia (Petunia hybrida) flowers for unrelated experiments and 46 controls. Day 1 of infestation was chosen to establish the early response of the tomato plants to spider mite feeding. Transcript levels of 28 genes (17 from tomato and 11 from petunia) were at least 1.6 times higher in spider mite-infested def-1 plants than in uninfested def-1 (three independent experiments; Table I). We have previously determined that a 1.6-fold difference in transcript level of both tomato-clones as well as cross-hybridizing petunia-clones is reliable and relevant (Kant et al., 2004). Subsequently, up- or down-regulated clones had to meet two additional criteria, a P-value (adjusted for multiple testing) smaller than 0.05 and a signal to noise (S:N) ratio larger than 2.0. Compared to our previous comparison of infested and uninfested wild-type plants (Kant et al., 2004) the list of spider mite-responsive genes was shorter for the def-1 mutant (60 for wild type versus 42 for the def-1 mutant). Although def-1 is deficient in induced JA-accumulation, Table I still includes JA- and wound-responsive genes such as wound-induced proteinase inhibitor I and II, Leu aminopeptidase, and allene oxide cyclase. These genes were not induced in the jai1-1 mutant after MeJA treatment (Li et al., 2004). The increase in transcript levels of proteinase inhibitors corresponded with the slight, yet not significant, increase in proteinase inhibitor activity in def-1 on day 1 (Fig. 2). Various PR-proteins and a chitinase, which were previously identified as SA-responsive, were induced as well. Transcript levels of genes related to anthocyanin production such as chalcone isomerase A and chalcone synthase A were also up-regulated. Correspondingly, several tomato genes homologous to petunia cDNAs regulating the transcription of genes involved in the biosynthesis of anthocyanins (an1, an2, an11; Quattrocchio et al., 1993; Spelt et al., 2000, 2002), were up-regulated by spider mites in def-1.
Table I.
Spider mite-regulated genes in def-1
| Plant | TC/GB/AT | Annotation | Category | Ratio | P |
|---|---|---|---|---|---|
| Le | TC115911 | PR-P6 | SA | 17.3 | 0.000 |
| Le | TC121229 | PR-P4 | SA | 8.1 | 0.002 |
| Le | TC117270 | Hsr203 | SA | 4.6 | 0.003 |
| Le | TC124809 | Chitinase | SA | 3.1 | 0.024 |
| Le | TC116545 | PR-P23 | SA | 2.4 | 0.007 |
| Le | TC115998 | Osmotin like | SA | 2.0 | 0.029 |
| Le | TC118684 | MLO-like protein | SA,PATHO | 3.3 | 0.000 |
| Le | TC124387 | Wound-induced proteinase inhibitor I | JA | 6.8 | 0.000 |
| Le | TC124100 | Leu-aminopeptidase | JA | 4.1 | 0.000 |
| Le | TC124388 | Wound-induced proteinase inhibitor I | JA | 3.3 | 0.000 |
| Le | TC124098 | Proteinase inhibitor; auxin-induced | JA | 3.1 | 0.009 |
| Le | TC117620 | Allene oxide cyclase | JA | 2.4 | 0.000 |
| Le | TC123946 | Cathepsin D inhibitor | JA | 1.9 | 0.053 |
| Le | TC116772 | GDSL-motif lipase | LIPASE | −1.6 | 0.003 |
| Le | AA092256 | Amino transferase | MET | 2.2 | 0.000 |
| Le | TC115813 | Hydroxymethylglutaryl CoA-synthase | SEC MET | 1.8 | 0.000 |
| Le | TC116498 | Tuberisation-related protein | MISC | 1.7 | 0.000 |
| Le | TC115865 | Catalase | ST | 1.7 | 0.000 |
| Ph | N/A | Patatin-like, pat1 | JA | 1.7 | 0.005 |
| Ph | TC125626 | Pro rich protein | PATHO | −1.6 | 0.002 |
| Ph | AF146702 | MYB-like transcription factor, an2 | SEC MET | 1.9 | 0.001 |
| Ph | AF260919 | bHLH transcription factor, an1 | SEC MET | 1.8 | 0.009 |
| Ph | S80857 | Chalcone synthase, chsA | SEC MET | 1.8 | 0.020 |
| Ph | Y07721 | Glutatione S-transferase, an9 | SEC MET | 1.7 | 0.002 |
| Ph | N/A | Anthocyanin synthase, as | SEC MET | 1.7 | 0.003 |
| Ph | AF155332 | Flavonoid 3′hydroxylase, ht1 | SEC MET | 1.7 | 0.050 |
| Ph | AF233638 | Chalcone isomerase, chiA | SEC MET | 1.7 | 0.003 |
| Ph | U9478 | MYB-like transcription factor, an11 | SEC MET | 1.7 | 0.022 |
| Ph | CAA79856 | DAPH synthase | SEC MET | 1.6 | 0.029 |
| Ph | N/A | Thyoredoxin | ST | 1.6 | 0.000 |
| Ph | N/A | Prohibitin | ST | −1.6 | 0.001 |
| Ph | N/A | ZF-HD homeobox protein | ST | −2.8 | 0.022 |
The cDNAs from the dedicated microarray that were either 1.6-fold up- or down-regulated on day 1, are shown. The ratios indicate the relative transcript abundance in spider mite-infested plants over uninfested plants. The cDNAs were either from tomato (Le) or Petunia (Ph). Where possible the corresponding TC-numbers, GeneBank-numbers (GB), or AT-numbers with the highest homology are shown (N/A, not applicable). The cDNAs were subdivided into various categories according to their function: JA, jasmonate related; SA, salicylic acid related; PATHO, pathogen related; SEC MET, secondary metabolism; ST, signal transduction; and MISC, miscellaneous. The P-values (adjusted for multiple testing) denote the significant difference of the average ratios (Ratio) of infested over uninfested plants in three independent experiments.
To identify genes regulated by DEF-1, microarray slides were hybridized simultaneously with probes from def-1 and Castlemart, the corresponding wild type, after 1 d of spider mite infestation, in three independent experiments. Only nine genes were induced or repressed in the wild type compared to def-1 (Table II). Wound inducible-proteinase inhibitor I and II and Leu aminopeptidase were more highly expressed in the wild type, illustrating their dependence on JA. Remarkably, two SA-inducible PR-genes (PR-P4, Fidantsef et al., 1999; and PR-P6, Tornero et al., 1997) were higher expressed in def-1 after 1 d. For P6 we confirmed this by RNA gel-blot analysis for the three sets of RNA used for the microarrays (ratio def-1:wild type 2.3 ± 0.7). This finding indicates that DEF-1 is important in up-regulating JA-responsive genes and down-regulating SA-regulated genes. Moreover, the def-1 mutation seems to affect only a specific set of genes since the differences between the transcriptome of def-1 and wild type were very limited (Table II).
Table II.
Microarray analysis of genes induced by spider mites in wild-type versus def-1 plants
| Plant | TC | Annotation | Category | Ratio | P |
|---|---|---|---|---|---|
| Le | K03291 | Wound-induced proteinase inhibitor II | JA | 8.6 | 0.001 |
| Le | TC124388 | Wound-induced proteinase inhibitor I | JA | 6.2 | 0.000 |
| Le | TC124387 | Wound-induced proteinase inhibitor I | JA | 4.3 | 0.006 |
| Le | TC124100 | Leu-aminopeptidase | JA | 3.4 | 0.001 |
| Le | TC121229 | PR-P4 | SA | −1.7 | 0.005 |
| Le | TC115911 | PR-P6 | SA | −2.0 | 0.004 |
| Le | TC116018 | Phe ammonia lyase | SEC MET | 1.6 | 0.020 |
| Le | TC125673 | Lipid transfer protein | MISC | −2.0 | 0.020 |
The cDNAs from the dedicated microarray that were either 1.6-fold up- or down-regulated on day 1 are shown. The P-values (adjusted for multiple testing) denote the significant differences in the average ratios (Ratio) of spider mite-infested wild-type plants over spider mite-infested def-1 plants in three independent experiments.
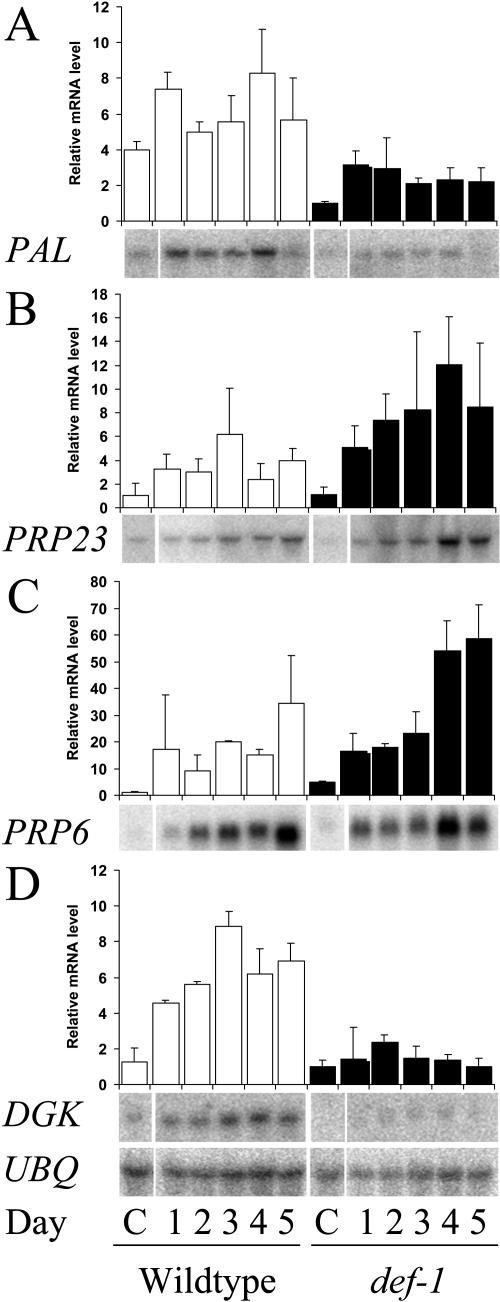
To visualize transcript levels for a selection of genes over 5 d, the whole period of spider mite-infestation, RNA gel-blot analysis was used and the difference in expression between wild type and def-1 was evaluated by means of ANOVA. Expression of the Phe ammonia lysase (PAL) gene, identified by microarray analysis, remained higher in wild type over the period of 5 d (Fig. 3A; P < 0.001), confirming that the expression of this gene is influenced by the def-1 mutation. The transcript levels of the SA-related gene PR-P23 (Rodrigo et al., 1993), were different in def-1 and wild type (Fig. 3B; P = 0.0013), with the largest difference on day 4 (Fisher post hoc P = 0.01). Expression of PR-P6 was also different between wild type and def-1 (Fig. 3C; P = 0.007) with the largest difference also on day 4 (P = 0.04). This indicates that the deficiency of inducible JA--accumulation in def-1 (Li et al., 2002b) leads to higher expression levels of SA-related genes. For spider mite infested lima beans it is known that SA levels increase between day 1 and day 3 while JA levels remain constant during that period after an initial increase during the 1st d of infestation (Arimura et al., 2002). This increase in SA levels during the later periods of spider mite-infestation could correspond with the expression of SA-related genes in tomato (Fig. 3, B and C). We also analyzed the transcript levels of a member of the diacylglycerol kinase (DGK) family (Fig. 3D), since this gene was induced by spider mites in wild-type tomato (Kant et al., 2004). Transcript levels of DGK were higher in the wild type on all days during spider mite infestation (Fisher post hoc test; P < 0.001 for all days), indicating that the expression of this gene this is DEF1-dependent. This illustrates that the rigorous criteria of our microarray analysis can eliminate clones with low and variable expression levels.
Figure 3.
Spider mite-induced gene expression in wild type and def-1. RNA gel-blot analysis of genes related to secondary metabolism (PAL), SA signaling (PR-P6 and PR-P23) and lipid signaling (DGK) in wild type and def-1 during 5 d of spider mite-infestation. For both plants the day 0 control (C) is shown, which is representative for all other days of the control plants. To check for equal loading, blots were hybridized with a probe for polyubiquitin (UBQ; TC115895). The bar graphs represent quantification (means and SEs of duplicated experiments) of the hybridizing bands, which were normalized for ubiquitin. The results were evaluated by means of ANOVA. Expression between wild type and def-1 was significantly different for P6 (P = 0.007), P23 (P = 0.013), DGK (P < 0.0001), and PAL (P < 0.0001).
Spider Mites Do Not Induce Emission of Volatiles in def-1 Plants
Because jai1-1 has low constitutive levels of (mono) terpenes (Li et al., 2004), we analyzed the total content of internal volatile organic compounds through pentane extraction of uninfested def-1 and wild-type leaves (n = 6). We identified 8 monoterpenes, 13 sesquiterpenes, 2 aromatics, and 2 homoterpenes. The amounts of one monoterpene (β-phellandrene) and two sequiterpenes (β-caryophyllene and α-gurjunene) were significantly higher in wild-type leaf tissue than in def-1 (Table III).
Table III.
The total leaf volatile-content of uninfested plants
| Compound | Total Amount in Wild-Type Leaf Tissue | Total Amount in Def-1 Leaf Tissue | P-Value (Wild Type versus def-1) |
|---|---|---|---|
| μg/g FW | μg/g FW | ||
| 2-Carene | 1.7 ± 0.5 | 1.3 ± 0.5 | 0.4 |
| α-Terpinene | 1.0 ± 0.2 | 0.6 ± 0.2 | 0.2 |
| p-Cymene | 0.5 ± 0.2 | 0.1 ± 0.1 | 0.09 |
| α-Phellandrene | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.5 |
| β-Phellandrenea | 8.0 ± 1.0 | 3.2 ± 0.5 | 0.0002* |
| cis-β-Ocimene | 0.04 ± 0.02 | 0.04 ± 0.04 | 0.9 |
| trans-β-Ocimene | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.9 |
| γ-Terpinolene | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.8 |
| Terpinolene | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.9 |
| DMNT | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.5 |
| MeSA | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.6 |
| δ Elemenea | <0.01 | <0.01 | 0.1 |
| α-Cubebene | <0.01 | 0.03 ± 0.02 | 0.06 |
| α-Copaene | <0.01 | <0.01 | 0.4 |
| β-Elemenea | 0.02 ± 0.02 | 0.06 ± 0.02 | 0.2 |
| Longifolenea | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.1 |
| β-Caryophyllene | 0.8 ± 0.2 | 0.2 ± 0.1 | 0.0003* |
| α-Gurjunenea | 0.05 ± 0.01 | <0.01 | 0.003* |
| β-Farnesene | 0.03 ± 0.02 | <0.01 | 0.2 |
| α-Humulene | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.7 |
| γ-Muurolenea | 0.02 ± 0.01 | <0.01 | 0.2 |
| α-Muurolenea | 0.08 ± 0.06 | <0.01 | 0.7 |
| cis-Nerolidol | 0.1 ± 0.04 | 0.2 ± 0.1 | 0.6 |
| trans-Nerolidol | 0.09 ± 0.03 | 0.03 ± 0.02 | 0.2 |
| TMTT | 0.09 ± 0.06 | 0.07 ± 0.03 | 0.7 |
Leaf volatiles were obtained by extraction with pentane and analyzed by means of GC-MS (n = 6). The values indicate the means and ses of each volatile identified. The results were evaluated by means of ANOVA. P-values marked with an asterisk (*) denote compounds that were found in significant different amounts. Compounds marked with footnote a designation were not available as pure standard and their amounts had to be estimated on the basis of the compound closest on the retention time scale of the chromatogram, providing it was of the same chemical class and a pure standard was available.
As a marker for the indirect defense, we assessed the temporal emission of volatile organic compounds by infested and uninfested intact plants over a period of 5 d (n = 9). We identified 9 monoterpenes, 13 sesquiterpenes, 2 aromatics, and 2 homoterpenes. Uninfested wild-type plants emitted higher amounts of β-caryophyllene and β-phellandrene while uninfested def-1 plants emitted more of α-gurjunene, although none of these differences were significant. The emitted compounds β-myrcene, linalool, and limonene (Table IV) were not identified in the internal pools (Table III), while α-phellandrene and cis-β-ocimene were not detected in the headspace of tomato. We did not find significant differences between volatiles emitted by spider mite-infested and uninfested def-1. The sesquiterpene α-copaene was emitted in higher amounts by def-1 than by wild type (Fisher post hoc test: P = 0.009) but its emission was independent of spider mite infestation (Fisher post hoc test: P = 0.99). α-Copaene was present at very low amounts in the internal pool of volatiles (Table III). When expressed in microgram emission per gram fresh weight per 5 d, over 75% of the tomato headspace consisted of TMTT and MeSA while the internal pool of wild type and def-1 consisted primarily out of the monoterpenes 2-carene (±11%), α-terpinene (±7%), and β-phellandrene (±54%). However, 2-carene and α-terpinene were only present in low amounts in the headspace of tomato.
Table IV.
Volatiles emitted by tomato plants
| Compound | Total Emission Wild-Type Control | Total Emission def-1 Control | Total Emission Wild-Type Infested | Total Emission def-1 Infested | P-Value# |
|---|---|---|---|---|---|
| μg/g FW/5 d | μg/g FW/5 d | μg/g FW/5 d | μg/g FW/5 d | ||
| β-Myrcene | <0.01 | <0.01 | 0.01 ± 0.01 | <0.01 | 0.12 |
| 2-Carene | 0.03 ± 0.03 | <0.01 | 0.2 ± 0.1 | 0.04 ± 0.04 | 0.32 |
| α-Terpinene | <0.01 | <0.01 | 0.02 ± 0.02 | <0.01 | 0.41 |
| p-Cymene | 0.3 ± 0.2 | 0.02 ± 0.01 | 0.1 ± 0.03 | 0.3 ± 0.02 | 0.29 |
| β-Phellandrenea | 9.8 ± 2.9 | 4.5 ± 1.1 | 9.8 ± 2.8 | 5.7 ± 2.3 | 0.79 |
| Limonene | 2.5 ± 1.5 | 0.8 ± 0.5 | 1.7 ± 0.9 | 1.1 ± 0.6 | 0.14 |
| trans-β-Ocimene | 0.1 ± 0.02 | 0.03 ± 0.02 | 0.6 ± 0.2 | 0.03 ± 0.03 | 0.004* |
| γ-Terpinene | 0.3 ± 0.2 | 0.2 ± 0.06 | 0.2 ± 0.1 | 0.1 ± 0.03 | 0.74 |
| Terpinolene | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.2 ± 0.03 | 0.1 ± 0.01 | 0.11 |
| Linalool | 0.1 ± 0.02 | 0.03 ± 0.02 | 0.3 ± 0.05 | 0.03 ± 0.02 | 0.016* |
| DMNT | 0.3 ± 0.2 | 0.1 ± 0.03 | 0.5 ± 0.2 | 0.1 ± 0.03 | 0.24 |
| MeSA | 18.8 ± 5.6 | 2.0 ± 0.5 | 72.1 ± 28.4 | 3.5 ± 1.2 | 0.049* |
| δ Elemenea | 0.5 ± 0.2 | 0.2 ± 0.06 | 0.7 ± 0.2 | 0.3 ± 0.1 | 0.63 |
| α-Cubebene | <0.01 | <0.01 | 0.02 ± 0.01 | 0.03 ± 0.03 | 0.36 |
| α-Copaene | 1.4 ± 0.7 | 5.2 ± 1.1 | 1.6 ± 0.5 | 5.9 ± 1.5 | 0.74 |
| β-Elemenea | 0.7 ± 0.3 | 1.4 ± 0.5 | 0.9 ± 0.3 | 1.3 ± 0.4 | 0.52 |
| Longifolenea | 0.4 ± 0.1 | 0.3 ± 0.09 | 0.6 ± 0.2 | 0.3 ± 0.09 | 0.29 |
| β-Caryophyllene | 1.3 ± 0.3 | 0.8 ± 0.2 | 1.9 ± 0.4 | 1.1 ± 0.3 | 0.4 |
| α-Gurjunenea | 1.7 ± 0.8 | 2.2 ± 1.1 | 2.3 ± 1.1 | 1.5 ± 0.6 | 0.21 |
| β-Farnesene | 0.1 ± 0.05 | 0.1 ± 0.02 | 0.1 ± 0.03 | 0.1 ± 0.02 | 0.76 |
| α-Humulene | 1.1 ± 0.7 | 0.8 ± 0.6 | 1.1 ± 0.6 | 0.5 ± 0.2 | 0.26 |
| γ-Muurolenea | 0.5 ± 0.2 | 0.9 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.47 |
| α-Muurolenea | 1.5 ± 0.6 | 0.8 ± 0.3 | 1.4 ± 0.8 | 0.4 ± 0.2 | 0.61 |
| cis-Nerolidol | 0.2 ± 0.04 | 0.1 ± 0.02 | 0.2 ± 0.05 | 0.1 ± 0.02 | 0.069 |
| trans-Nerolidol | 0.2 ± 0.03 | 0.2 ± 0.03 | 0.5 ± 0.1 | 0.2 ± 0.03 | 0.012* |
| TMTT | 172 ± 85 | 46 ± 8 | 345 ± 109 | 56 ± 11 | 0.043* |
Emitted volatiles were collected during 5 d at 24-h intervals. The values indicate the means and ses of each treatment group. The results of the 5 sampling days were pooled (n = 9) and were evaluated by means of a factorial ANOVA using the factor plant (wild type and def-1) and the factor treatment (spider mite-infested and uninfested). #, P-values denote the significance of the interaction (plant × treatment) and those marked with an asterisk (*) denote compounds that significantly depend on both factors. Compounds marked with footnote a designation were not available as pure standard and their amounts had to be estimated on the basis of the compound closest on the retention time scale of the chromatogram, providing it was of the same chemical class and a pure standard was available.
We did not find volatiles that were emitted exclusively by wild type or def-1 but for several volatiles we found significant quantitative differences. The emission of 5 volatiles (trans-β-ocimene, linalool, trans-nerolidol, TMTT, and MeSA) were induced by spider mites in wild type plants (Table IV). Moreover, none of these volatiles were induced by spider mites in def-1. Full factorial ANOVA showed that induced-emission of all five compounds was mutually dependent on induction by spider mites and on DEF1 (Table IV), strongly suggesting that their induction by spider mites is JA-dependent.
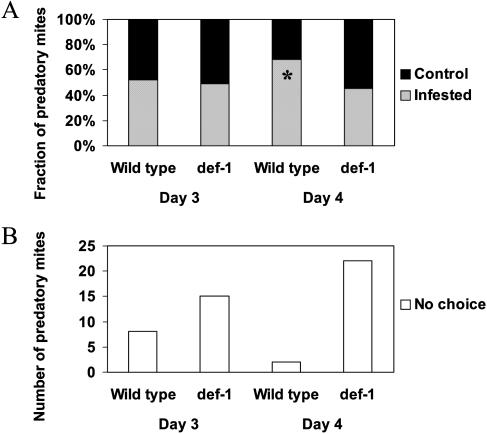
Predatory Mites Are Not Attracted to Spider Mite-Infested def-1
We investigated whether predatory mites (the natural enemies of spider mites) discriminated between odors from infested and uninfested wild-type plants and odors from infested and uninfested def-1 plants. We recorded the behavioral response of predatory mites submitted to the odors of plants infested with spider mites and uninfested plants, by using a Y-tube olfactometer (Bruin et al., 1992). The experiments were performed, three and 4 d after spider mite-infestation and were analyzed by a replicated test for goodness of fit (Sokal and Rohlf, 1995). All replicates tested homogenous (no significance for heterogeneity since 0.11 > P < 0.98 at df = 1) and could therefore be pooled. A significant fraction of predatory mites chose spider mite-infested wild-type odors on day 4 (P = 0.001). No significant differences were found for the responses of predatory mites to wild-type plants on day 3 or for def-1 plants (Fig. 4A) on either of the 2 d (0.84 > P < 0.98). In the experiments with def-1, a relatively high number of predatory mites did not make a choice within 5 min (Fig. 4B). This behavior is typical in the absence of a positive stimulus (Drukker et al., 2000).
Figure 4.
Olfactory response of Phytoseiulus persimilis to tomato plants infested with Tetranychus urticae and uninfested control plants. A, Percentages of predatory mites that chose for odors of infested plants (black bars) or for odors of uninfested plants (gray bars) after 3 or 4 d (tests with wild type and def-1 were conducted independently). Results were analyzed with a replicated test for goodness of fit. An asterisk (*) denotes P = 0.001. None of the other experiments tested significantly different (0.84 > P < 0.98). B, Total number of predatory mites per experiment that did not make a choice within 5 min (white bars).
Exogenous JA Restores Induction of MeSA and TMTT Emission in def-1
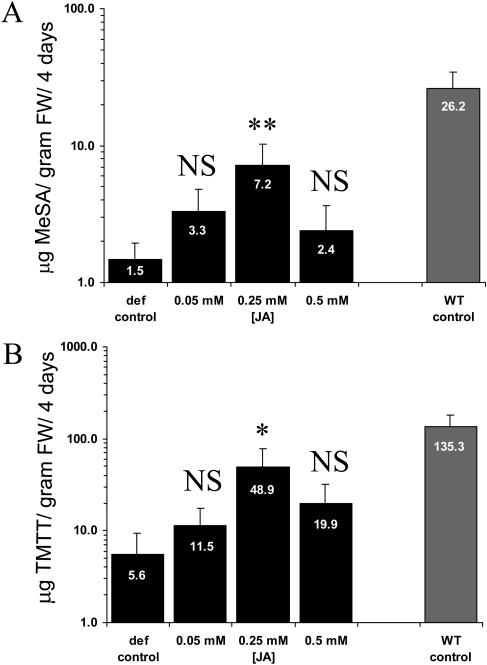
To test whether volatile emission by def-1 could be restored, we pretreated intact def-1 plants with three different JA concentrations and infested them with spider mites. We sprayed intact plants with 3 mL JA (treatment) or with 3 mL water (control) 2 h prior to infesting them with spider mites. The absolute amounts of emitted volatiles were evaluated by means of ANOVA and Dunnett's post hoc test. We found a significant increase in MeSA (Fig. 5A) and TMTT (Fig. 5B) emission by plants treated with 0.25 mm JA. TMTT emission was approximately nine times higher than from the infested def-1 control, but approximately three times lower than from infested wild-type plants. Induced emission of none of the other identified volatiles was significantly affected by the JA-pretreatments.
Figure 5.
MeSA and TMTT emission by JA-treated def-1 plants. The data shown represents the amounts of MeSA (A) and TMTT (B) emitted per gram fresh weight (FW) of spider mite-infested def-1 pretreated with 3 mL water (def control), 3 mL of 0.05, 0.25, or 0.5 mm JA and spider mite-infested wild type (WT control) during 4 d. Data was evaluated using ANOVA followed by Dunnett's post hoc test for comparing a control mean to other group means (separate ANOVAs on MeSA and TMTT, P < 0.04). A single asterisk (*) denotes P < 0.05 and double asterisks (**) denote P < 0.01 after Dunnett's test (df = 8). Vertical bars indicate the means and ses. NS denotes not significant different from def control. The infested wild-type data were obtained from an independent experiment (Kant et al., 2004) and are shown for comparison.
Exogenous JA Restores Induction of SAMT and GGPPS Transcripts in def-1
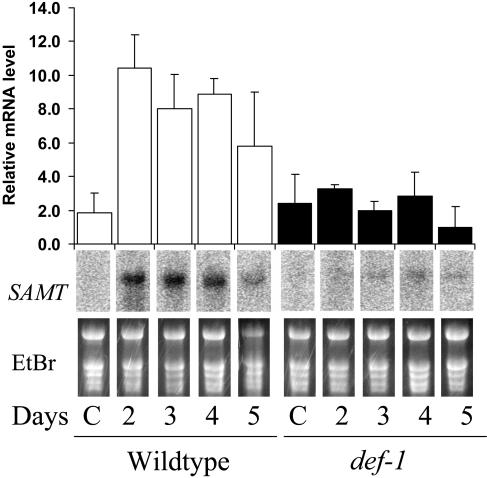
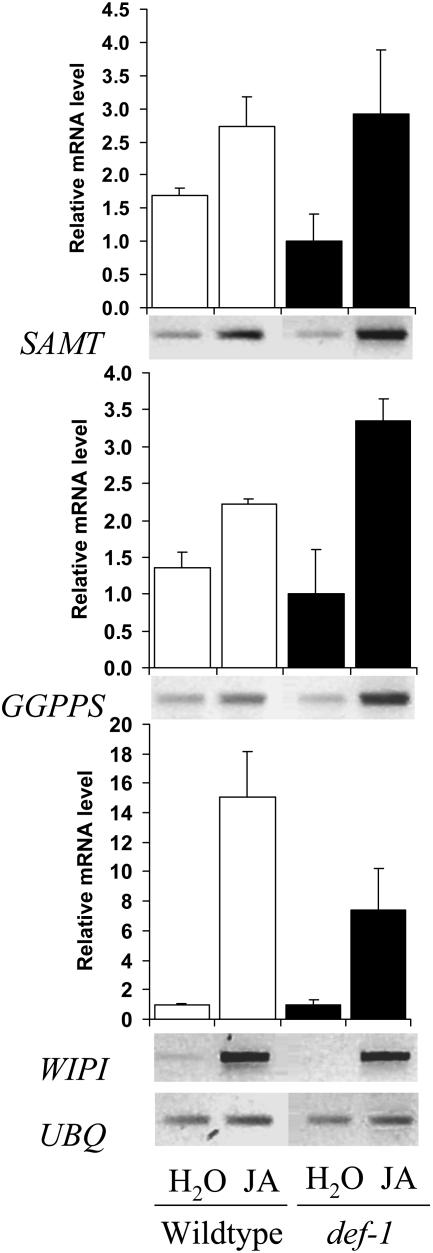
In addition to measuring induction of MeSA emission, we investigated whether spider mites could induce transcription of SAMT in def-1 and wild type. SAMT was up-regulated in wild-type plants (approximately 4-fold after 2 d) but not in def-1 (Fig. 6; Fisher post hoc test; for all days of infestation P < 0.02), indicating a dependence on JA for induction. We then determined the effect of exogenous JA on the transcript levels of SAMT. We sprayed intact plants with 3 mL 0.25 mm JA (treatment) or with 3 mL water (control) 2 h prior to infesting them with spider mites and sampled after 24 h. Quantitative reverse transcription (RT)-PCR analysis showed that SAMT was expressed on average 1.7-times higher in untreated, spider mite-infested wild type than def-1 after 24 h (Fig. 7). In plants treated with JA, levels of SAMT increased in both wild type and def-1 to matching levels (Fig. 7). JA also induced the expression of WIPI but expression levels in def-1 were not as high as in wild-type plants. We also investigated the transcript levels of a putative geranylgeranyldiphosphate synthase (GGPPS). Geranylgeranyldiphosphate is a precursor of diterpenes and TMTT (Boland et al., 1998). Transcript levels of GGPPS also increased by application of exogenous JA, in both wild type and def-1 (Fig. 7). ANOVA and a Fisher post hoc showed that JA induced SAMT, GGPPS, and WIPI significantly in def-1 with P = 0.03, 0.003, and 0.04, respectively.
Figure 6.
Spider mite-induced expression of SAMT in def-1 and wild type. RNA gel-blot analysis of the gene encoding SAMT during spider mite infestation. For all plants the control for day 2 (C) is shown, which is representative for all other days of the control plants. Ethidium bromide-stained gels are shown to indicate equal loading. The bar graph represents quantification (means and SEs of duplicated experiments) of the hybridizing bands.
Figure 7.
Spider mite-induced expression of SAMT, GGPPS, and WIPI genes in JA-treated wild type and def-1. Intact infested wild-type (WT) and def-1 plants had been pretreated with 0.25 mm JA (+) or water (−). RT-PCR analysis is shown for SAMT, GGPPS, WIPI, and UBQ. The bar graphs represent quantification (means and ses of duplicated experiments) of the hybridizing bands that were normalized for ubiquitin.
DISCUSSION
In this study we have shown that the spider mite-induced indirect defense response in tomato is JA-dependent. First, we have shown that tomato-reared spider mites perform equally well on def-1 and wild-type tomato, but that the offspring is more viable on def-1. Second, spider mite-induced gene expression in def-1 was predominantly related to the SA-mediated defense-response. Third, predatory mites were only attracted to spider mite-infested wild-type plants but not to def-1. Fourth, transcription of SAMT and GGPPS was enhanced in spider mite-infested def-1 after pretreatment with JA, concomitant with restoration of spider mite-induced MeSA and TMTT emission.
Spider Mites Perform Equally Well on def-1 and Wild Type
In our experiments, spider mites performed equally well on def-1 and wild-type plants (±40 eggs per mite after 5 d; see Fig. 1B). This result contrasts with that of Li et al. (2002b) who reported a much higher performance of spider mites on def-1 (±30 eggs per mite after 6 d) than on wild type (±5 eggs per mite after 6 d). This may be because they used spider mites cultivated on bean plants instead of tomato plants as we did. It has been established that spider mites, after transfer to tomato from another host plant species, can adapt to tomato as a consequence of selection (Gotoh et al., 1993; Egas and Sabelis, 2001). Several generations of selection on tomato result in a population that is more resistant to methylketones in tomato's glandular hairs (Chatzivasileiadis et al., 2001) and that exhibits lower mortality and higher fecundity (Magowski et al., 2003). Our results and those of Li et al. (2002b) therefore make a strong case for the JA-mediated defense response being a driving force for spider mite selection on host plants. Since our spider mites had been on tomato for over fifty generations, it is likely that their performance on the wild type was improved by selection and, as a consequence, the positive effect of the JA-deficiency on their performance was much less pronounced. However, we found a higher egg-hatching rate on def-1. Possibly JA-mediated defenses have an effect on egg development, since negative effects on the growth and development of Manduca sexta larvae have been found on tobacco after pretreatment with MeJA (Van Dam et al., 2000). Alternatively, JA-mediated defenses may affect egg quality, since it was found that hatching of Perillus biocalatus (two-spotted stinkbug) eggs decreased as the level of Cys proteinase inhibitors increased (Ashouri et al., 1998). These results suggest that plant defenses can have an impact not only on juvenile development but also on egg viability.
DEF-1 Influences Both JA- and SA-Inducible Genes
The function of DEF-1 is not yet clear, but Howe et al. (1996) suggested that the def-1 mutation disrupts the flux of linolenic acid through the octadecanoid pathway or affects the subcellular compartmentalization of an octadecanoid intermediate. Howe et al. (1996) showed that OPDA and JA induced proteinase inhibitor (PI)-accumulation in def-1 while the upstream precursors linolenic acid and 13(S)-hydroperoxylenolenic acid did not. From our microarray analysis it is clear that def-1 severely compromises the induction of JA-responsive genes by spider mites, but this effect is moderate in comparison with MeJA-treated jai1-1 (Li et al., 2004). This suggests that def-1 still has some residual octadecanoid-signaling capacity. Our study also showed that the def-1 mutation resulted in higher levels of SA-related transcripts upon herbivory by spider mites (Table II), indicating higher SA-responsiveness in infested def-1. Similarly, high levels of induced SA-related PR-gene expression have been found to occur in other JA-mutants as well, such as Pseudomonas syringae-infected jai1-1 (Zhao et al., 2003) and P. syringae-infected Arabidopsis coi1, in which it co-occurred with SA accumulation (Kloek et al., 2001). Also the reduction of downstream JA-responses could allow for higher SA-responsiveness since it was shown that transcription of SA-induced PR-genes could be inhibited by methyl jasmonate (Niki et al., 1998).
JA Is Involved in MeSA and TMTT Emission
This study shows that the def-1 mutation prevented tomato from mounting its indirect defense. The mutation did not result in an overall terpene deficiency in leaves, as in jai1 tomato fruits and sepals (Table III; Li et al., 2004) since def-1 harbors the 5 spider mite-induced volatiles at similar levels as the wild type. Therefore, we conclude that JA-accumulation is essential for spider mite-induced emission of volatiles in tomato. Interestingly, the emission of all volatiles induced in the wild type shared the same JA-dependency, although the metabolic origin of these volatiles is diverse. The monoterpenes linalool, trans-β-ocimene and the diterpene-derivate TMTT are products from the 2-methylerythritol 4-phosphate (MEP) pathway in the plastids whereas trans-nerolidol is a sesquiterpene from the mevalonate (MVE) pathway in the cytosol (Koch et al., 1999). It is not clear to what extent these pathways interact, since the terpene precursor isopentenyl diphosphate can transfer from the plastids to the cytosol (Lichtenthaler, 1999). In contrast, MeSA, the conversion product of SA, is derived from Phe (Shulaev et al., 1997; Seskar et al., 1998) and/or isochorismate (Wildermuth et al., 2001). Although we cannot exclude that the induced emission of (some) terpenoids results from releasing these compounds from their stored form, there is evidence for de novo MeSA and TMTT production upon herbivory (Farag and Pare, 2002; Chen et al., 2003).
Although the def-1 mutation allowed for higher expression of SA-related genes, emission of MeSA in def-1 only increased after treatment with JA. This is striking since emission of MeSA has been shown to be positively dependent on levels of SA in tobacco (Shulaev et al., 1997; Seskar et al., 1998). In addition, caterpillar feeding on NahG Arabidopsis did not induce emission of MeSA (Van Poecke et al., 2001), showing that SA is essential for MeSA production as well. The low inducibility of SAMT in def-1 by spider mites (Fig. 6) suggests that SAMT-expression is dependent on JA, similar to MeJA-inducible AtBSMT1 expression in Arabidopsis (Chen et al., 2003). However, in Atropa belladonna root cultures, SAMT appeared SA-inducible (Fukami et al., 2002). We were able to confirm the JA-dependency, since treatment of def-1 with JA resulted in enhanced spider mite-induced SAMT transcription (Fig. 7) as well as enhanced emission of volatile MeSA (Fig. 5).
The emission of TMTT coincided with that of MeSA and was not induced in def-1 (Table IV). In addition, the emission of TMTT by spider mite-infested def-1 increased significantly after the plants had been treated with exogenous JA (Fig. 5). TMTT is a volatile homoterpene-derivate of geranylgeranyl diphosphate (Boland et al., 1998). A putative GGPPS is induced by spider mites in wild-type plants (Kant et al., 2004). Our microarray analysis showed that transcript levels of this GGPPS were significantly higher in the wild type than in def-1, but only before P-value adjustment (see supplemental data). Treatment of def-1 with JA enhanced spider mite-induced GGPPS-transcript levels and spider mite-induced TMTT emission (Figs. 5 and 7). Taken together, these results suggest that induced emission of both MeSA and TMTT depend on JA at the transcriptional level. In lima bean, however, OPDA rather than JA seems to induce emission of TMTT and MeSA. Koch et al. (1999) showed that OPDA, but not exogenous JA, could induce TMTT in detached lima bean leaves. In addition, Engelberth et al. (2001) showed that JA could induce emission of linalool and β-ocimene, whereas emission of MeSA and TMTT correlated with the accumulation of OPDA and SA. Our results indicate that in def-1 significant induction of MeSA and TMTT requires an optimal concentration of JA (Fig. 5). It is possible that the optimal dose of exogenous JA depends on the amounts of other in planta defense intermediates (such as SA or ethylene) present at the moment of the application since SA may antagonize downstream JA-responses, either through interference with the translocation of octadecanoid intermediates to the peroxisome (Laudert and Weiler, 1998) or through activation of NPR1 (Spoel et al., 2003). It is well known that in lima beans SA levels increase up to 4-fold upon spider mite-infestation (Arimura et al., 2002). To establish whether the increased MeSA emission results in a decrease of the internal SA concentration, a precise quantitative analysis would be necessary. This analysis would also have to include other derivatives of SA, such as its SA-β-glucoside (SAG; Seo et al., 1995) and to include all tissues involved, local and systemic. Preliminary experiments indicate that SA and SAG levels in spider mite-infested leaves are not different in def-1 and wild type, since we measured equal quantities of both after 4 d (data not shown) when MeSA emission is much higher in the wild type.
JA Is Essential for the Indirect Defense
Predatory mites are the natural enemies of spider mites and since they are blind they use induced plant-odors to locate plants containing prey. Thaler et al. (2002) found that predatory mites also chose wild-type plants infested with Spodoptera exigua larvae (which cannot serve as prey for predatory mites) in olfactory choice-assays with Spodoptera-infested def-1 or uninfested wild type as the alternative. This is surprising (but see: Shimoda and Dicke, 2000) since Spodoptera cannot serve as prey for predatory mites. Since predatory mites can only be cultivated on spider mites, this may be taken to suggest that Spodoptera induces tomato-odors similar to spider mites. However, Thaler et al. (2002) reported that Spodoptera induced four monoterpenes and one sesquiterpene in wild-type tomato but not in def-1. None of these terpenes are induced by spider mites and vice versa. In addition, Thaler et al. (2002) found that predatory mites discriminated between tomatoes, pretreated with JA and untreated plants in independent experiments on the wild type and on def-1. However, the volatiles emitted by JA-treated def-1 were not measured. Hence, it is unlikely that the responses of predatory mites to Spodoptera-induced odors and JA-induced odors were caused by the same volatile components. Predatory mites have been found to respond to several different pure volatile compounds (Dicke et al., 1990) and can learn to associate the odor with the absence and presence of food (Drukker et al., 2000). In our experiments, predatory mites that had experience with spider mite-infested wild-type plants did not show a preference for spider mite-infested def-1 (Fig. 4). However, spider mite-infested def-1 pretreated with JA, emitted volatiles that were also induced by spider mites on the wild type (Fig. 5). Therefore, our results together with those of Thaler et al. (2002) provide unambiguous evidence for JA as an essential plant defense-component for the induction of host-plant odors that are associated with spider mite herbivory and thus with potential prey for a predator.
MATERIALS AND METHODS
Plant Material and Arthropod Rearing
Tomato (Lycopersicon esculentum Mill cv Castlemart, cv Moneymaker, and def-1) seedlings were grown in a greenhouse with day/night temperatures of 23°C to 18°C and a 16/8-h light/dark regime. Def-1 was kindly provided by Greg Howe (Department of Energy-Plant Research Laboratory, Michigan, USA) and was previously described in Howe and Ryan (1999) and Li et al. (2002b). Three days prior to each experiment plants were transferred to a climate room at 23°C to 18°C, a 16/8-h light regime with 300 μE m−2 s−1 and 60% relative humidity.
The two-spotted spider mite Tetranychus urticae Koch was originally obtained in 1993 from tomato plants in a greenhouse (Houten, The Netherlands; Gotoh et al., 1993) and was maintained on the cultivar Moneymaker ever since (the base-colony). In 2001, 1 year prior to our experiments, we replaced the Moneymaker plants by the cultivar Castlemart. For the experiments involving RNA gel blots, microarray analysis, volatile-sampling, and olfactory choice-assays, adult females were randomly collected from this culture.
The colony of the predatory mite Phytoseiulus persimilis was originally maintained in the laboratory on detached lima bean-leaves infested with spider mites for more than 3 years. Prior to all experiments predatory mites were transferred to intact tomato plants (cultivar Castlemart) infested with spider mites and maintained for approximately 1 month in a climate room at 23°C, a 16/8-h light regime with 100 μE m−2 s−1 and 70% relative humidity. Adult female predatory mites were collected from this culture for the olfactory-choice assays.
Plant Treatments
For the microarray, RNA gel-blot and volatile-sampling experiments, adult female spider mites were collected from the base culture. The mites were placed gently on the adaxial surface of the fully expanded terminal leaflets using a soft-bristle paintbrush. In each experiment 15 mites were introduced per leaflet, on each plant 3 leaflets in total, except for the leaf damage and fecundity assay where respectively 4 and 7 mites were introduced per leaflet. Plants were always 3 weeks old and contained 4 fully expanded leaves, which were chosen for infestation, and 2 emerging leaves. The infestation procedure was performed without wounding or damaging the plant. We never observed spider mites dispersing to adjacent leaflets during the period of the experiment.
Leaf Damage, Spider Mite Fecundity, and Egg Hatching Assays
The total area of chlorotic lesions on spider mite-infested leaves was measured as described in Kant et al. (2004). To determine the number of eggs and juveniles emerging on infested leaves, we inspected sets of 12 plants (containing seven 2-d-old female spider mites on one leaf per plant) daily by means of a stereomicroscope. Eggs and juveniles on both sides of infested leaves of intact plants were counted. We calculated the hatching rate on day 5 by dividing the number of juveniles counted on day 5 by the number of eggs produced on day 1. For day 6 we took the total number of eggs produced on day 1 and 2, subtracted the number of juveniles that had emerged on day 5 and divided the remaining number of eggs by the number of juveniles that had emerged on day 6. A similar calculation was performed for day 7. The experiments were performed in a climate room at 23°C, a 16/8-h light regime with 100 μE m−2 s−1 and 60% relative humidity.
PI Activity
PI activity assays were performed in duplo on wild-type plants and def-1 as described in Kant et al. (2004) using the protocol of Stout et al. (1998).
Olfactory Choice Assays
Olfactory-choice assays for predatory mites were conducted using the Y-tube olfactometer, as previously described by Bruin et al. (1992) with some minor modifications. The Y-tube olfactometer was connected directly to the outlet of the volatile sampling set-up. The olfactory choice-assays were performed on day 3 and day 4 of the volatile-sampling experiments. Two hours prior to each experiment, predatory mites were placed on a petri dish in the absence of prey. Predatory mites were allowed to make a choice between the odors of three infested plants and three uninfested plants within 5 min and were otherwise scored as ‘no choice’. A minimum of 24 mites was tested per replicate. Experiments were performed in twice (for the wild type day 3 experiments n = 24 and 34, the wild type day 4 replicates n = 50 and 60, for the def-1 day three replicates n = 28 and 28 and for the def-1 day 4 replicates n = 30 and 38). The replicates of the experiments presented here were not heterogeneous (statistics not shown) and therefore pooled. Results were analyzed using a replicated test for goodness-of-fit as described in Sokal and Rohlf (1995) with df = 1.
Transcriptome: Microarray and RNA Gel-Blot Analysis
Microarray analysis was performed as described in Kant et al. (2004) with some minor modifications. For each replicate experiment three leaves of three plants (nine in total) were pooled and total RNA was isolated and used for microarray hybridization. The data in this article represent three hybridizations of three independent replicates for the mite infested wild type and mite infested def-1 comparison as well as three hybridizations of three independent replicates for the uninfested def-1 and mite infested def-1 comparison.
The spot signal intensities of the arrays were corrected by subtracting the local background as assessed by ArrayVision software. In addition, we calculated for each clone the average signal-to-noise ratio (S:N). The background-subtracted spot signal intensities were normalized using a Lowess-normalization procedure. From this data, the average signal intensity ratio (infested wild type versus infested def-1 and infested def-1 versus uninfested def-1) and se of these ratios was calculated. For calculation of the significance of up- or down-regulation we used a nested-design ANOVA. The obtained P-values for all clones were adjusted for multiple testing, using Benjamini and Hochberg's (1995) step-up procedure for controlling the false discovery rate (fdr). Clones were selected on the basis of three criteria: (1) the significance of the adjusted P-values (α ≤ 0.05); (2) an average S/N ≥ 2; and (3) the minimal treatment-1/treatment-2 ratio of >1.6 or <-1.6 on the basis of our RNA gel-blot controls. All cDNAs in Table I and II were sequenced.
Northern analysis on wild-type cv Castlemart and def-1 were performed as described in Verdonk et al. (2003). Hybridizing bands were quantified by phosphoimaging (Molecular Dynamics, Sunnyvale, CA), and normalized for ubiquitin, after background subtraction using Image Quant Software 1.11 (Molecular Dynamics). Measurements of two independent experiments were evaluated by means of ANOVA and a Fisher post hoc test.
For RT-PCR determination of transcript levels, 10 μg of total RNA was used to synthesize first-strand cDNA using an 18-mer dT-primer and SuperScript II RNase H-reverse transcriptase (Invitrogen, Carlsbad, CA) in the supplied buffer at 42°C. For each gene we determined the range of PCR amplification that was still linear in order to quantify the amounts of PCR product. Therefore various number of PCR-cycles were run for each gene after which the PCR products were separated on agarose gels, blotted onto nylon membranes and hybridized with the corresponding radioactively labeled probe. Subsequently the PCR products were quantified by phosphoimaging (see above) to determine the number of cycles at which linear amplification occurred. PCR amplification of UBQ (TC116081) fragments (0.723 kb) comprised 15, 18, and 20 cycles (each cycle: 94°C for 60 s, 55°C for 45 s, and 72°C for 75 s) with forward primer, 5′-GATTCTCTCTCATCAATCAATTCG-3′ and reverse primer, 5′-GCATCCAAACTTTACAGACTCTC-3′; for WIPI-2 (0.601 kb) 10, 14, and 17 cycles with forward primer, 5′-GACAAGGTACTAGTAATCAATTAT-3′ and reverse primer, 5′-CACATAACACACAACTTTGATGCC-3′; for SAMT (0.440 kb) 18, 20, and 25 cycles with forward primer, 5′-CAATAAGAGATCAAGCCATAAG-3′ and reverse primer, 5′-CTTGGTGGACTTGTACTTGCC-3′ and 25, 30, and 35 cycles for GGPPS (0.323 kb) with forward primer, 5′-GCAATCAATGTAAACAAAGCAC-3′ and reverse primer, 5′-CAAAGATATAAGTGCATCCCATC-3′. The cDNA for SAMT (TC 125218) and for GGPPS (TC 130320) were obtained from The Institute for Genomic Research (TIGR).
Analysis of Tomato Volatiles
Volatiles of def-1 and Castlemart wild-type plants were collected, identified, and quantified on the basis of an internal standard and synthetic external standards of known concentration as described in Kant et al. (2004). In short, 3 plants were enclosed for 5 d in airtight glass containers while a stable air stream was led through. At the outlet an internal standard (benzyl acetate) was added to the air stream and volatiles were trapped in sampling tubes on 300 mg Tenax TA during 24-h intervals. Volatiles were eluded from sampling tubes using 2 mL pentane-diethyl ether (4:1) and 1 μL was analyzed using gaschromatograph mass-spectrometry. At the end of the experiment the fresh weights of the plants and their detached leaves were determined.
For determination of total terpene content, 250 mg leaf material was harvested, frozen in liquid nitrogen, and ground to a fine powder. Care was taken to select leaves from the same age. After addition of 0.1 μg benzyl acetate as an internal standard, samples were extracted in 2 mL pentane while vigorous shaking for 1 h. Pentane extracts were dehydrated with 250 mg Na2SO4 and 10 times concentrated by a stream of N2 at 4°C. One μL was injected into an Optic (ATAS, GL International, Zoetermeer, The Netherlands) injection port at 50°C, which was heated to 275°C at 4°C s−1. The split flow was 0 mL min−1 for 2 min and then 25 mL min−1 until the end of the run. Compounds were separated on a capillary DB-5 column (10 m × 180 μm, film thickness 0.18 μm; Hewlett-Packard, Palo Alto, CA) at 40°C for 3 min and then at 30°C min−1 to 250°C with He (37 kPa) as the carrier gas. The column flow was 3 mL min−1 for 2 min and 1.5 mL min−1 thereafter. Mass spectra of eluting compounds were collected on a Time-of-Flight-MS (Leco, Pegasus III) with a 60 s solvent delay at −1597 eV (ion source at 200°C), at an acquisition rate of 20 spectra s−1. Compounds were identified and quantified on the basis of the internal standard and synthetic external standards of known concentration as described in Kant et al. (2004).
Treatment of def-1 with JA
A stock solution of 0.1 m JA was prepared by dissolving (±)-jasmonic acid (Duchefa, Haarlem, The Netherlands) in ethanol and diluting the solution 10 times with water. A solution of 10% ethanol was used as a stock for the control. The 0-, 0.05-, 0.25-, and 0.5-mm working solutions were prepared by diluting the stock solutions with water. For measuring the volatiles emitted by JA-treated plants, sets of three plants were misted with 3 mL of solution in such a way that all leaflets carried clearly visible droplets. The plants were allowed to dry in a climate room for 2 h before the introduction of spider mites. The volatiles emitted during the first 24 h after infestation were collected as described in the volatile analysis section.
To obtain leaves from intact plants treated with JA for RNA extraction, the same procedure was followed as described above, except that only the 0.25 mm JA solution and the water control were used for spraying. Two plants were used per treatment. After 24 h plants were removed from the volatile-sampling set up and leaves were harvested, frozen in liquid nitrogen and stored at −80°C.
Policy Statement
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requestor.
Supplementary Material
Acknowledgments
We thank Greg Howe (Department of Energy-Plant Research Laboratory, East Lansing, Michigan) for providing us with def-1. We also thank Rene Braakman (ATAS Benelux, Zoetermeer, The Netherlands) and Alan Musgrave for their valuable comments. Natali Rianika Mustafa (RUL, Leiden, The Netherlands) is kindly thanked for the SA and SAG assays.
This work was supported by NWO, The Netherlands Organization for Scientific Research (ALW 812.04.004 to M.R.K.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.048694.
References
- Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kuhnemann F, Takabayashi J (2002) Herbivore-induced volatiles induce emission of ethylene in neighboring lima bean plants. Plant J 29: 87–98 [DOI] [PubMed] [Google Scholar]
- Ashouri A, Overney S, Michaud D, Cloutier C (1998) Fitness and feeding are affected in the two spotted stinkbug, Perillus bioculatus, by the cysteine proteinase inhibitor, oryzacystatin I. Arch Insect Biochem Physiol 38: 74–83 [Google Scholar]
- Baldwin IT, Preston CA (1999) The eco-physiological complexity of plant responses to insect herbivores. Planta 208: 137–145 [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300 [Google Scholar]
- Boland W, Gabler A, Gilbert M, Feng Z (1998) Biosynthesis of C11 and C16 homoterpenes in higher plants; stereochemistry of the C-C-bond cleavage reaction. Tetrahedron 54: 14725–14736 [Google Scholar]
- Bruin J, Dicke M, Sabelis MW (1992) Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia 48: 525–529 [Google Scholar]
- Chatzivasileiadis EA, Egas M, Sabelis MW (2001) Resistance to 2-tridecanone in Tetranychus urticae: effects of induced resistance, cross-resistance and heritability. Exp Appl Acarol 25: 717–730 [DOI] [PubMed] [Google Scholar]
- Chen F, D'Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E (2003) An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J 36: 577–588 [DOI] [PubMed] [Google Scholar]
- Dicke M, Vanbeek TA, Posthumus MA, Bendom N, Vanbokhoven H, De Groot AE (1990) Isolation and identification of volatile kairomone that affects acarine predator-prey interactions: involvement of host plant in its production. J Chem Ecol 16: 381–396 [DOI] [PubMed] [Google Scholar]
- Dicke M, Takabayashi J, Posthumus MA, Schutte C, Krips OE (1998) Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: variation in production of cues and in responses of predatory mites. Exp Appl Acarol 22: 311–333 [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25: 1907–1922 [Google Scholar]
- Drukker B, Bruin J, Jacobs G, Kroon A, Sabelis MW (2000) How predatory mites learn to cope with variability in volatile plant signals in the environment of their herbivorous prey. Exp Appl Acarol 24: 881–895 [DOI] [PubMed] [Google Scholar]
- Egas M, Sabelis MW (2001) Adaptive learning of host preference in a herbivorous arthropod. Ecol Lett 4: 190–195 [Google Scholar]
- Engelberth J, Koch T, Schuler G, Bachmann N, Rechtenbach J, Boland W (2001) Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol 125: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Pare PW (2002) Green leaf C6-volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61: 545–554 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Johnson RR, Ryan CA (1992) Regulation of expression of proteinase-inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol 98: 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidantsef AL, Stout MJ, Thaler JS, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogensis-related protein P4 in the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol 54: 97–114 [Google Scholar]
- Fukami H, Asakura T, Hirano H, Abe K, Shimomura K, Yamakawa T (2002) Salicylic acid carboxyl methyltransferase induced in hairy root cultures of Atropa belladonna after treatment with exogeneously added salicylic acid. Plant Cell Physiol 43: 1054–1058 [DOI] [PubMed] [Google Scholar]
- Gols R, Roosjen M, Dijkman H, Dicke M (2003) Induction of direct and indirect plant responses by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J Chem Ecol 29: 2651–2666 [DOI] [PubMed] [Google Scholar]
- Gotoh T, Bruin J, Sabelis MW, Menken SBJ (1993) Host race formation in Tetranychus urticae: genetic differentiation, host plant preference and mate choice in a tomato and a cucumber strain. Entomol Exp Appl 68: 171–178 [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is comprised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Ryan CA (1999) Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics 153: 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN (2001) Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J 26: 509–522 [DOI] [PubMed] [Google Scholar]
- Koch T, Krumm T, Jung V, Engelberth J, Boland W (1999) Differential induction of plant biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol 121: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudert D, Weiler EW (1998) Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signaling. Plant J 15: 675–684 [DOI] [PubMed] [Google Scholar]
- Li L., Li C, Lee GI, Howe GA (2002. a) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA 99: 6416–6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Williams MM, Loh Y-T, Lee GI, Howe GA (2002. b) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signalling pathway. Plant Physiol 130: 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses and glandular trichome development. Plant Cell 16: 126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Magowski W, Egas M, Bruin J, Sabelis MW (2003) Intraspecific variation in induction of feeding preference and performance in a herbivorous mite. Exp Appl Acarol 29: 13–25 [DOI] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y (1998) Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis related (PR) proteins in wounded mature tobacco leaves. Plant Cell Physiol 39: 500–507 [Google Scholar]
- Quattrocchio F, Wing JF, Leppen H, Mol J, Koes RE (1993) Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes. Plant Cell 5: 1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo I, Vera P, Tornero P, Hernandez-Yago J, Conejero V (1993) cDNA cloning of viroid-induced tomato pathogenesis-related protein P23. Plant Physiol 102: 939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Crafts-Brander SJ, Pare PW, Henneberry TJ (2001) Exogenous methyl jasmonate induces volatile emissions in cotton plants. J Chem Ecol 22: 679–695 [DOI] [PubMed] [Google Scholar]
- Sabelis MW, Janssen A, Pallini A, Venzon M, Bruin J, Drukker B, Scutareanu P (1999) Behavioral responses of predatory and herbivorous arthropods to induced plant volatiles: from evolutionary ecology to agricultural implications. In AA Agrawal, S Tuzun, E Bent, eds, Induced Plant Defenses against Pathogens and Herbivores. The American Phytopathological Society Press, St. Paul, pp 269–296
- Scheer JM, Ryan CA (2002) The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR repector kinase family. Proc Natl Acad Sci USA 99: 9585–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seskar M, Shulaev Y, Raskin I (1998) Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol 116: 387–392 [Google Scholar]
- Seo S, Ishizuka K, Ohashi Y (1995) Induction of salicylic acid β-glucosidase in tobacco leaves by exogenous salicylic acid. Plant Cell Physiol 36: 447–453 [Google Scholar]
- Shimoda T, Dicke M (2000) Attraction of a predator to chemical information related to nonprey: when can it be adaptive? Behav Ecol 11: 606–613 [Google Scholar]
- Shulaev V, Silverman P, Raskin I (1997) Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 385: 718–721 [Google Scholar]
- Sokal RR, Rohlf FJ (1995) Replicated test of goodness of fit. In Biometry: The Principles and Practice of Statistics in Biological Research. W.H. Freeman and Company, New York, pp 715–743
- Spelt C, Quattrocchio F, Mol JN, Koes R (2000) Anthocyanin1 of petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 12: 1619–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelt C, Quattrocchio F, Mol J, Koes R (2002) ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell 14: 2121–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux J-P, Brown R, Kazan K, et al (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MJ, Brovont RA, Duffey SS (1998) Effect of nitrogen availability on expression of constitutive and inducible defenses in tomato, Lycopersicon esculentum. J Chem Ecol 24: 945–963 [Google Scholar]
- Thaler JS, Farag MA, Pare PW, Dicke M (2002) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5: 764–774 [Google Scholar]
- Tornero P, Gadea J, Conejero V, Vera P (1997) Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol Plant Microbe Interact 10: 624–634 [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Hadwich K, Baldwin IT (2000) Induced responses in Nicotiana attenuata affect behavior and growth of the specialist herbivore Manduca sexta. Oecologia 122: 371–379 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Posthumus MA, Dicke M (2001) Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J Chem Ecol 27: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Verdonk JC, de Vos CHR, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC (2003) Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA (2003) Virulence system of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J 36: 485–499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.