INTRODUCTION
Collagen type VI is a microfibrillar collagen found in many extracellular matrices including those of muscle, skin, tendon, and vessels. It is composed of three α chains encoded by three independent genes on chromosomes 2 and 21 (Table 5.1). Mutations in these three genes have been found to underlie a group of muscle disorders that are now referred to as collagen VI-related myopathies. The collagen VI-related myopathies encompass a spectrum of disorders ranging from the more severe Ullrich congenital muscular dystrophy (UCMD) through phenotypes of transitional severity to the milder Bethlem myopathy (BM). An additional, mostly contractural, phenotype, referred to as myosclerosis, has also been delineated and associated with mutations in COL6A2.
Table 5.1.
The three collagen VI genes and α chains
| α chains | Size (kDa) | Corresponding gene |
Gene position |
|---|---|---|---|
| α1(VI) | 140 | COL6A1 | 21q22.3 |
| α2(VI) | 140 | COL6A2 | 21q22.3 |
| α3(VI) | 260–330* | COL6A3 | 2q37 |
Size variable depending on state of glycosylation.
UCMD was initially described in a series of papers in the 1930s by Otto Ullrich (Ullrich, 1930a, b), who referred to the condition as “atonic-sclerotic muscular dystrophy” because of the characteristic occurrence of weakness and striking joint hypermobility (together imposing an “atony” in the original description) in conjunction with significant and evolving contractures (the “sclerotic” part of the initial description). Ullrich’s disease was maintained as a distinct diagnostic entity mainly in the Japanese and European literature (Furukawa and Toyokura, 1977; Nonaka et al., 1981; Voit, 1998). After collagen VI mutations had been identified in BM it was the realization by Enrico Bertini and Mimma Pepe in 2001 in Italy that some of the clinical features in UCMD were reminiscent of BM that led to the first discovery of collagen VI mutations in this condition as well (Camacho Vanegas et al., 2001).
BM was initially reported in 1976 by Bethlem and van Wijngaarden in the Netherlands as an autosomal dominant early-onset but relatively benign myopathy associated with the development of characteristic contractures. Several others then reported similar cases, including a report of a large French-Canadian kindred, the authors of which then suggested the name Bethlem myopathy (Mohire et al., 1988). Linkage to the collagen VI genes was first established in larger families in Holland and the USA, leading to the identification of collagen VI mutations in these original families in 1996 (Jöbsis et al., 1996) and 1998 (Pan et al., 1998). Genetic analysis in many patients with clinically convincing UCMD and BM has now clarified that the majority of patients with these phenotypes have underlying dominant or recessive mutations in the three known collagen VI genes COL6A1, COL6A2, and COL6A3 (Lampe and Bushby, 2005). This has been true in particular for the clinically more distinct phenotype of UCMD. There is, however, a definite number of patients with convincing clinical features who have no detectable mutations in these three collagen VI genes (Tetreault et al., 2006; Petrini et al., 2007), indicating that there is some degree of genetic heterogeneity underlying an otherwise fairly typical phenotype. This number may indeed be larger in the sometimes less distinct clinical phenotype of Bethlem (K.M.D. Bushby, personal communication).
Prevalence numbers are slowly emerging. In the population followed by the Muscle Centre in Newcastle upon Tyne, UK, the prevalence of UCMD has been calculated as 0.13 per 100000 and that of BM as 0.77 per 100000 (Norwood et al., 2009). In other populations it is now emerging that the collagen VI-related muscle disorders are one of the most common entities subsumed under the category of congenital muscular dystrophy (Okada et al., 2007; Peat et al., 2008).
CLINICAL FEATURES
As introduced above, there are two classical clinical phenotypes associated with mutations in the collagen VI genes: the severe Ullrich congenital muscular dystrophy and the milder Bethlem myopathy (outlined separately below), which were thought to be distinct entities but which are now connected by phenotypes showing clinical features of intermediate severity between the two. Not surprisingly, certain aspects of the clinical phenotype of the collagen VI-related myopathies are shared to a greater or lesser degree by all the disorders in this spectrum, in particular as far as the contractural and joint hypermobility aspects of the phenotypes are concerned. The typical severe congenital presentation in this group of disorders includes congenital weakness and hypotonia, associated with striking joint laxity particularly of the distal joints, whereas more proximal joints such as hips, knees, elbows, and spine may be affected by congenital contractures (Bertini and Pepe, 2002). In the milder presentation of Bethlem myopathy the laxity is less conspicuous but still is often present during the early phases of the disease in childhood. In contrast, later the clinical picture in the typical case of Bethlem myopathy is much more dominated by the prominent contractures that develop over time (Merlini et al., 1994). The relative contribution of laxity and contractures can vary widely in an individual patient, so that patients with a clinical presentation of predominant joint hypermobility as well as patients with a presentation almost entirely governed by contractures (“myosclerosis”) may be seen. Meticulous attention to such connective tissue-related aspects of the phenotype probably provides the best clues for a clinical diagnosis in this group of conditions.
Ullrich congenital muscular dystrophy
Ullrich disease or congenital muscular dystrophy type Ullrich (UCMD; MIM #254090) (Ullrich, 1930a, b) presents with clinical manifestations that are typically readily apparent at birth or during the first year of life. Frequently, however, the diagnosis of a neuromuscular disorder is not readily made at birth but only later when the acquisition of motor milestones is delayed. During pregnancy there may be a history of perceived reduced prenatal movements, but polyhydramnion, indicative of decreased prenatal swallowing as sometimes seen in other congenital disorders of muscle, is rarely reported in Ulllrich CMD (Ullrich, 1930a, b; Nonaka et al., 1981; Voit, 1998; Bertini and Pepe, 2002; Lampe and Bushby, 2005).
Signs and symptoms at birth include hypotonia and weakness associated with extreme distal joint laxity while at the same time contractures and skeletal deformities can be seen in more proximal joints in about 50% of the patients (Figure 5.1). Hands, fingers, and feet are extremely hyperlax, allowing for the fingers to bend back onto the dorsum of the hands with ease, whereas the hand may drop down at the wrist, clinically reminiscent of Ehlers–Danlos syndrome (EDS). Hip dislocation/dysplasia is seen in about 50% of the patients. There may be congenital pes adductus, but, more commonly, the feet may be seen dorsiflexed against the shin because of initial hyperlaxity at the ankle. A prominent calcaneus is often evident at birth and remains a conspicuous feature in a patient with typical UCMD. It is, however, not a specific sign as it can also be seen in other congenital disorders associated with significant hypotonia.
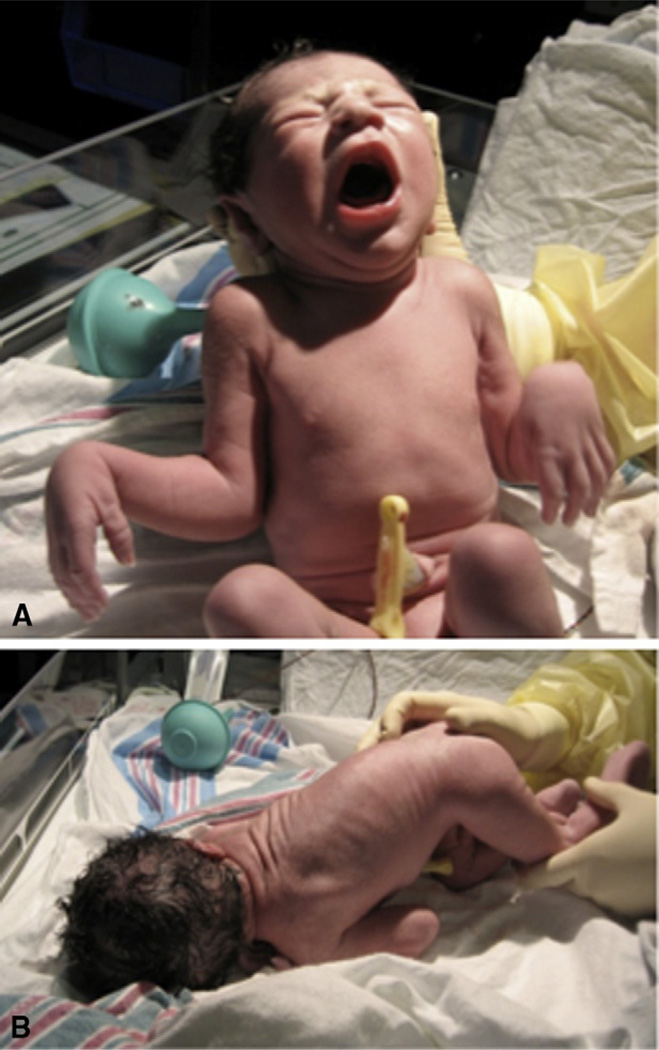
Figure 5.1.
Neonatal presentation of Ullrich congenital muscular dystrophy. Note elbow contracture and flaccid-appearing hands in (A) and kyphosis in (B).
Additional clinical features at birth may include dislocated hips, torticollis, and kyphoscoliosis, as well as contractures of the hips, knees, and elbows. Even though contractures may be present at birth, a clinical picture corresponding to a distally predominant arthrogryposis multiplex congenita (Hall, 1997) is not usually seen. Overall, at birth the presence of hyperlaxity is seen more consistently in UCMD than the presence of contractures. Frequently, the more severe contractures found immediately at birth have a tendency to improve over the first several months of life; however, new and eventually progressive contractures will set in later in most, but not all, patients.
In the most severe cases walking is never achieved, although some patients manage to crawl. More commonly, though, children with UCMD will achieve the ability to walk, often with some delay and sometimes only with the use of assistive devices (Nonaka et al., 1981; Voit, 1998). Walking then is typically lost again during childhood with a median age around 10 years (but as early as 3.5 years of age and as late as early adulthood) (Nadeau et al., 2009), mostly as a result of an increase in weakness and an even more noticeable increase in contractures, in particular in the knees and hips, with the contractures sometime progressing faster than the weakness (Figure 5.2). Thus, some children will have a period in which they prefer to ambulate on their knees as the contractures do not allow for the knees to be straight and yet there remains sufficient strength in the hip muscles to enable this mode of ambulation.
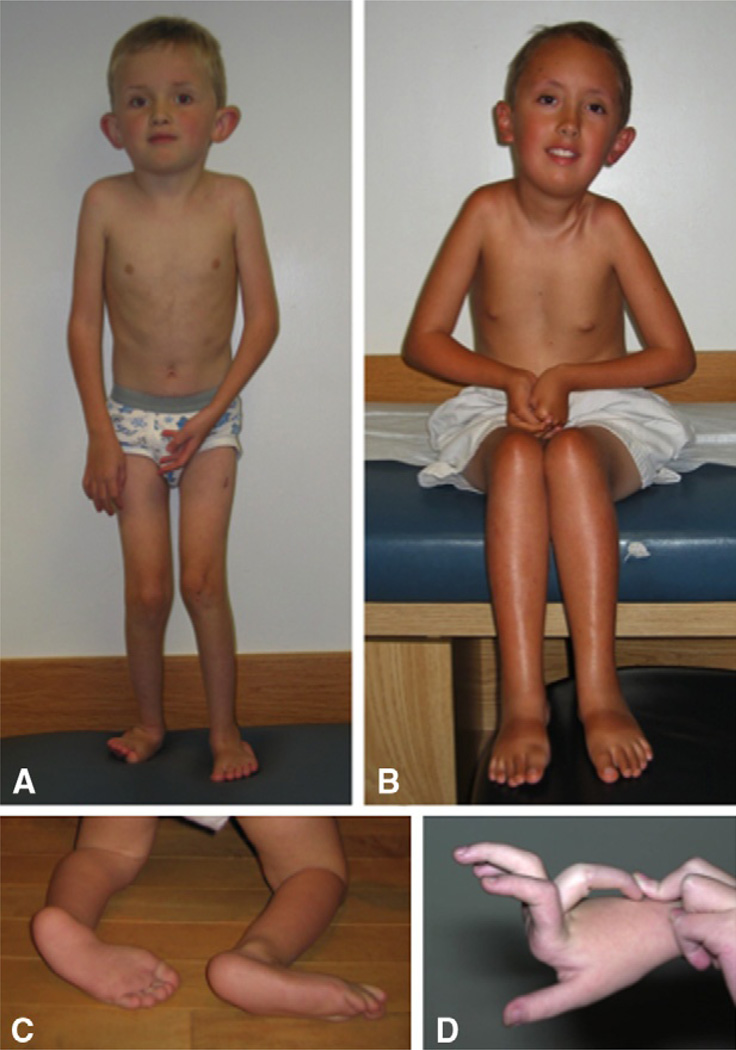
Figure 5.2.
Ullrich congenital muscular dystrophy (UCMD). The patient in (A) is able to walk, but note knee and elbow contractures. This patient lost the ability to walk at 9 years of age. The patient in (B) shows typical contractures in the nonambulatory patient, affecting the pectoralis, elbows, hips, knees, and Achilles tendons. The feet in (C) show the typical prominent calcaneus and the soft plantar skin, whereas (D) demonstrates the striking distal hyperlaxity seen in the fingers in a patient with UCMD. (D: Courtesy of Marjo van der Knaap, Amsterdam, Netherlands)
Even as the contractures progress to involve the spine, pectoralis, elbows, hips, knees, and long finger flexors, the hyperlaxity of the distal joints often persists to very late stages of the disease. This distal hyperlaxity typically involves all interphalangeal joints, including the most distal ones, while at the same time there will be increasing evidence of evolving contractures of the long finger flexors that first become evident upon extension of the fingers in the metacarpophalangeal joint. Scoliosis is often a serious and progressive problem, requiring surgical intervention in many patients. The scoliosis may evolve out of kyphoscoliosis present at birth, or start to develop independently before ambulation is lost (Nadeau et al., 2009), and even more significantly in the nonambulant patient and during the teenage years. Surgical intervention for scoliosis may be necessary before the loss of ambulation, so that changes in functional abilities after surgery will have to be considered as well. We have the impression that prominent rigidity of the spine may sometimes help delay excessive scoliosis. Osteopenia has been observed in a number of patients as early as in Ullrich’s original description (Ullrich, 1930a, b). Weakness tends to be diffuse and can be variable in its relative distribution. Antigravity strength in arms and legs seems initially preserved, even in severely affected infants.
The nature of the contractures, which can progress independently from the weakness, and the simultaneously persistent significant distal hyperlaxity already point to the nature of this condition as a disorder affecting both skeletal muscle and connective tissue. Skin is another significantly affected tissue in collagen VI-related disorders, leading to a number of notable dermatological findings (Kirschner et al., 2005). Excessive scar formation including the formation of large keloids can be a significant problem in some but not all patients, and has to be taken into account when surgical interventions are considered. Keratosis pilaris often is seen prominently on extensor surfaces of the limbs. This dermatological finding is not specific to collagen VI-related disorders, but is quite consistent in the more severely affected patients, so that it is diagnostically useful in the overall context of the clinical presentation. In contrast to the roughness of the keratosis pilaris, the skin in the palms of the hands and feet is notably soft and velvety, akin to the skin texture in EDS. A general tendency for hyperhidrosis that some of the children show was commented upon by Ullrich (1930a, b).
Respiratory involvement manifesting as progressive respiratory insufficiency due to restrictive lung disease occurs in the majority of severely affected patients during the first 15 years of life and is due to a combination of weakness of the diaphragm and accessory muscles as well as stiffness of the chest wall. Early clinical signs are mostly related to night-time hypoventilation, so that sleep studies are the most effective way to monitor for early respiratory insufficiency. Regular pulmonary function testing should also be performed in all patients in order to monitor the decline in predicted forced vital capacity. In most patients there will be a clear decline in forced vital capacity toward the end of the first decade of life and into the beginning of the second decade, when the decline will be less marked on average (Nadeau et al., 2009). In those patients who need it, night-time noninvasive ventilatory support will be necessary during the second decade on average, but may be as early as 3 years of age or delayed into adulthood, reflecting the significant variability in clinical severity seen in these patients. In contrast to Duchenne muscular dystrophy, in some patients with UCMD night-time ventilatory support may become necessary before the ability to walk is lost (Nadeau et al., 2009), although in the majority ambulation is lost before onset of respiratory support.
After the initiation of noninvasive ventilation the respiratory situation tends to be quite stable over many years. Failure of initiation of noninvasive respiratory support in patients with signs of respiratory failure will lead to death in a relatively short amount of time (Nonaka et al., 1981; Nadeau et al., 2009). Cardiac involvement in UCMD usually is not seen, although there has been a rare observation of cardiac arrhythmia in UCMD (F. Muntoni, personal communication) and it is not clear whether this was coincidental or is more common.
Feeding difficulties and, at times, prominent gastroesophageal reflux have been observed in more severely affected infants. Some infants have required transient nasogastric tube feeding, whereas a minority have received G-tube feeding later in life to avert the risk of nutritional deficiency and dehydration with intercurrent illnesses (Nadeau et al., 2009). Swallowing difficulties have been reported and may need to be monitored (Nadeau et al., 2009).
Much of the natural history and the incidence of late complications in patients with severe collagen VI deficiency remain to be fully explored but will probably become clearer as patients now regularly survive well into adulthood with the institution of well-managed ventilatory support.
Intermediate phenotypes
Both UCMD and BM had been described initially as distinct clinical syndromes, but the subsequent discovery of collagen VI mutations in both of these conditions and the availability of better diagnostic tools leads to the realization that there are clinical presentations of transitional severity that are difficult to classify as clearly Ullrich or Bethlem (Demir et al., 2002, 2004; Mercuri et al., 2002). Included in this group are patients with clinical presentations that are more severe than classical Bethlem but milder compared with classical Ullrich, indicating that there is a phenotypic spectrum bridging the two classical presentations. Patients with intermediate phenotypes between Ullrich and Bethlem present with significant weakness in early childhood and often show features typical of both presentations, including the Ullrich-like distal laxity of the most distal interphalangeal joints, while at the same time showing early Bethlem-like contractures of the long finger flexors. Ambulation in these patients is achieved, although weakness can be considerable. Ambulation may be maintained into adulthood – beyond the age at which walking is usually lost in patients with typical UCMD. Walking may become difficult for these patients, however, necessitating aids such as crutches or walkers, and ambulation may be lost as early as late teenage and early adult years. Progressive respiratory impairment is an important feature in the intermediate patients also.
Bethlem myopathy
In the milder Bethlem myopathy (BM, MIM #158810) (Bethlem and van Wijnaarden, 1976) the onset of the disease may also be congenital, but children are affected to a much milder degree (Mohire et al., 1988; Merlini et al., 1994; Jöbsis et al., 1999; Bertini and Pepe, 2002; Lampe and Bushby, 2005). Equinovarus deformity or, more commonly, foot dorsiflexion contractures and torticollis (50%) have been noted at birth in infants born with Bethlem myopathy (Jöbsis et al., 1999). Hypotonia at birth may not have been noted or is remembered only in hindsight. Although surgical release of the torticollis has been performed successfully in a number of cases (Jöbsis et al., 1999), the early contractures, when present, tend to resolve. Children may be found to have only mild weakness during childhood, frequently associated with some degree of notable distal joint laxity.
New contractures then set in, typically towards the end of the first decade of life, affecting Achilles tendons, elbows, pectorales muscles, long finger flexors, and the interphalangeal joints of digits 2 to 5 in particular, as well as in other muscle groups (Jöbsis et al., 1999) (Figure 5.3). Once established, contractures often are stable, or they may progress and become disabling in their own right, including restricted hand function due to the flexion contractures. Compared with the Ullrich phenotype, spinal rigidity is moderate in Bethlem myopathy. The distribution of the muscle weakness often shows a proximal predominance, but there can be distal weakness as well. The weakness is stable or may even improve somewhat in time with the normal increase in strength in puberty. There is, however, a slowly progressive increase in weakness starting in the third to fourth decades of life, so that on average two-thirds of patients over the age of 60 years need assistance with ambulation (Jöbsis et al., 1999). In other patients the onset of symptoms may be delayed into childhood or young adulthood, or the patient may have been noted to be a toe-walker in childhood before the onset of any perceptible weakness. Some family members may not even be aware of the presence of mild contractures or mild weakness (Merlini et al., 1994), so it is important to examine family members closely in order to establish inheritance within a family.
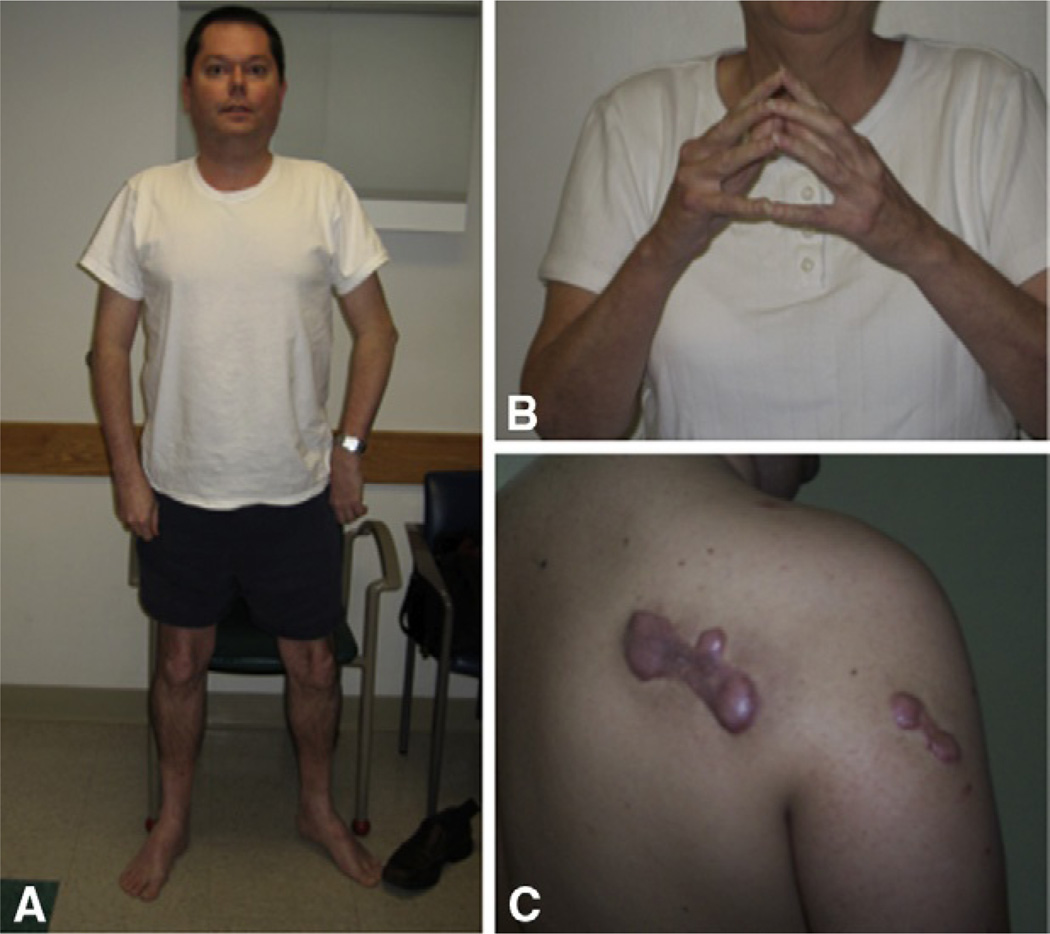
Figure 5.3.
(A) Adult patient with Bethlem myopathy (BM). Note typical elbow and Achilles tendon contractures. (B) demonstrates the typical finger flexor contractures in BM that make it impossible to put the fingers close together with the elbows extended. (C) demonstrates excessive keloid formation in a patient with BM. (C: Courtesy of Claudia Castiglione, Santiago, Chile.)
A potential complication associated with progression of Bethlem myopathy is the development of respiratory insufficiency resulting from restrictive pulmonary disease on the basis of a combination of stiffness of the rib cage together with respiratory/diaphragmatic muscle weakness (Haq et al., 1999). Most of the more typically affected patients have milder respiratory symptoms, but should be monitored by regular sleep studies. Cardiac involvement has not been reported so far in patients with Bethlem myopathy, except for one patient in whom asymmetrical septal hypertrophy was seen, probably a coincidental finding (de Visser et al., 1992).
Involvement of the skin is similar to that seen in UCMD, although soft and velvety skin is less typical whereas the development of keloidal scars can be substantial (Nadeau and Muntoni, 2008).
There is wide clinical variability associated with the Bethlem phenotype. Some patients may present with predominantly proximal weakness and very few contractures, thus presenting more akin to a limb-girdle muscular dystrophy (LGMD) (Scacheri et al., 2002), whereas others may show a more contractural picture with relatively mild weakness, a phenotype historically referred to as myosclerosis (Bradley et al., 1973); see below. Later onset with rather minimal clinical manifestation has also been seen. Even within the same family there may be striking degrees of variability in the degree of the contractures and of the weakness.
LGMD presentation of Bethlem myopathy
Some patients have been reported as presenting with mostly proximal weakness, without any significant contractures over the course of the disease or showing development of contractures much later, thus carrying a diagnosis of LGMD on clinical grounds (Scacheri et al., 2002). Dominantly acting collagen VI missense mutations found in this context were located in the COL6A1 and COL6A2 genes (COL6A1: K121R; COL6A2: D630N) in two such families (Scacheri et al., 2002), indicating that collagen VI-related myopathies have to be considered within the differential diagnosis of a patient with proximal muscle weakness and a nonspecifically myopathic muscle biopsy. However, the same mutation seen in one of the families with the LGMD presentation (COL6A2: D630N) was also seen by us in a family with typical Bethlem myopathy in some family members. The LGMD-like presentations as well as contractural presentations may even coexist in the same family. Thus, limb-girdle weakness without significant contractures is best viewed as one possible clinical presentation along the phenotypic spectrum of collagen VI-associated myopathies and not as a separate clinical entity. Nonetheless, it is important to be aware of this presentation as there may be few typical clinical clues pointing towards a collagen VI-associated myopathy.
Myosclerosis
A phenotype that is related to Bethlem myopathy but that also shows some significant differences is known as myosclerosis. The term “myosclerosis” was used by Guillaume Duchenne when he observed that muscle fibers in the biopsy from patients were completely encased by connective tissue (sclerotic) (Duchenne, 1868). A phenotype of myosclerosis was then more described clearly by Lowenthal in 1954, when the term was used to refer to the development of contractures without significant weakness, as well as a certain “woody” feeling upon palpation of the muscles (Bradley et al., 1973). It has been pointed out in the latter report that there was evidence for etiological heterogeneity in patients considered to have this diagnosis on clinical grounds (Bradley et al., 1973). In 2008, however, Merlini described a pair of siblings fulfilling the clinical diagnosis of myosclerosis, who were homozygous for a truncating mutation at the end of the C1 domain of COL6A2 (Merlini et al., 2008b), situated just before the beginning of the alternate splice events that lead to the three C2 domain isoforms recognized in the COL6A2 gene. This mutation has not been seen outside of this phenotype. The patients presented with moderate weakness and significant and progressive contractures of multiple joints, including masseter muscles, neck, shoulders, elbows, fingers, knees, and Achilles tendons, without significant joint hyperlaxity at any point. Muscles had a firm “woody” feel. Biopsies were severely fibrotic with partial collagen VI deficiency in the basement membrane and complete collagen VI deficiency around intramuscular capillaries, which also had a thickened basement membrane. Myosclerosis, with its predominance of contractures and comparatively mild weakness, could be understood as lying at the opposite end of a weakness/contracture spectrum to the LGMD-like presentation, with almost no contractures but significant weakness. Whether myosclerosis will maintain a separate phenotypic and genetic position within the collagen VI-related myopathies remains to be seen as more cases are identified and correlated with collagen VI mutations.
COLLAGEN VI AND MOLECULAR PATHOGENESIS
Collagen VI synthesis and interactions
Collagen VI belongs to the nonfibrillar collagens forming a network of beaded microfibrils in the extracellular matrix (Chu et al., 1990a; Timpl and Chu, 1994). The major three α chains, α1(VI), α2(VI), and α3(VI), are encoded by three genes: COL6A1 and COL6A2 on chromosome 21q22 and COL6A3 on chromosome 2q37 (Weil et al., 1988; Heiskanen et al., 1995) (Figure 5.4). These three chains form the heterotrimeric monomer that is the basic building block of most of collagen VI. All three chains have relatively short triple helical collagenous domains of 335–336 amino acids containing single cysteine residues in the N-terminal part of the triple helical domains that are important for later intracellular assembly of the dimer and tetramer before secretion into the extracellular matrix (Chu et al., 1988).
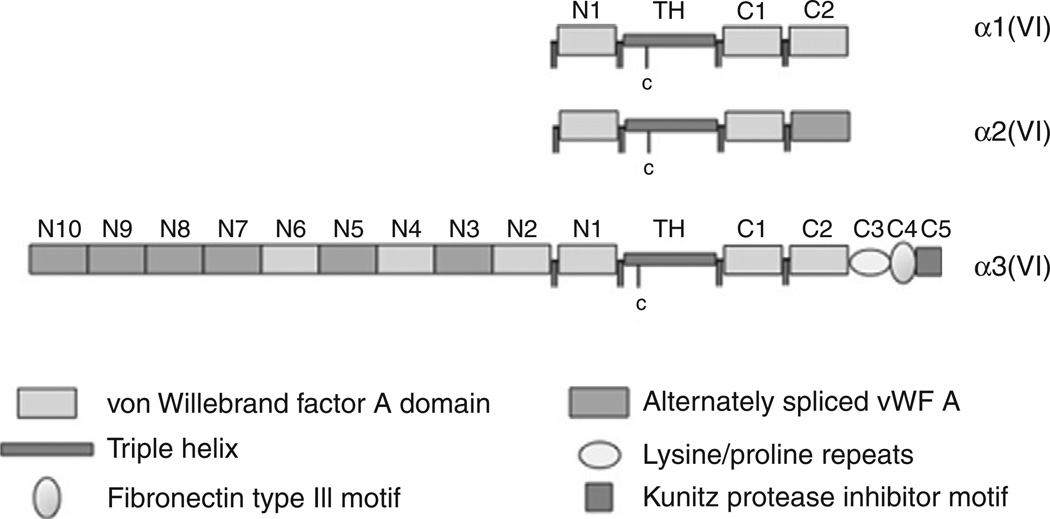
Figure 5.4.
Schematic depiction of the three α chains, α1(VI), α2(VI), and α3(VI), highlighting the domain structure and the triple helical domains with the cysteine residues that are involved in higher-order assembly.
The α1(VI) and α2(VI) chains are related and likely arose by gene duplication on chromosome 21q22 where they are oriented head to tail (Heiskanen et al., 1995). They both have two C-terminal and one N-terminal globular von Willebrand factor A domain (Chu et al., 1990b). The α3(VI) chain on chromosome 2q37 has a larger and extensively spliced N-terminal domain that is again rich in von Willebrand factor A domains (Chu et al., 1990a). The regulation and physiological function of these splice isoforms is not known, but when a stop mutation occurs in one of the alternately spliced exons its effect seems to be lessened compared with that of the nonspliced exons (Demir et al., 2002). The C-terminal domains of the α2(VI) and α3(VI) domain undergo further splicing and post-translational processing respectively.
The COL6A2 gene generates two splice versions of the C2 domain in addition to the full-length version, which is predominant. The C1 domain alone is sufficient for assembly of the monomer, yet lack of the C2 domain or mutations in the C2 domain cause significant disease (Baker et al., 2005; Petrini et al., 2007; Merlini et al., 2008b). The most C-terminal domain of α3(VI) protein undergoes proteolytic processing in the extracellular matrix after secretion. More recently two additional COL6A3-related collagen VI chain genes have been identified: COL6A5 and COL6A6 in human and col6a4, 5, 6 in the mouse (COL6A4 in the human is interrupted no longer functional) (Fitzgerald et al., 2008; Gara et al., 2008).
The expression pattern of these novel chains differs from that of the traditional chains, but there is evidence now that they are able to combine into a hetero-trimer with the α1(VI) and α2(VI) chains (Fitzgerald et al., 2008; Gara et al., 2008). Yet, it seems that there is no clear evidence that this heterotrimer has the ability to ameliorate the phenotype, as the disease in patients with COL6A3 mutations is not obviously milder than that in patients with mutations in the COL6A1 and COL6A2 genes, as would be expected if the COL6A5 or COL6A6 chains were able to compensate for mutations in the COL6A3 gene. It remains to be seen whether mutations in these additional genes will be found to cause disease or whether variation in expression of these genes is capable of modifying the clinical phenotype of patients with mutations in the classical three genes.
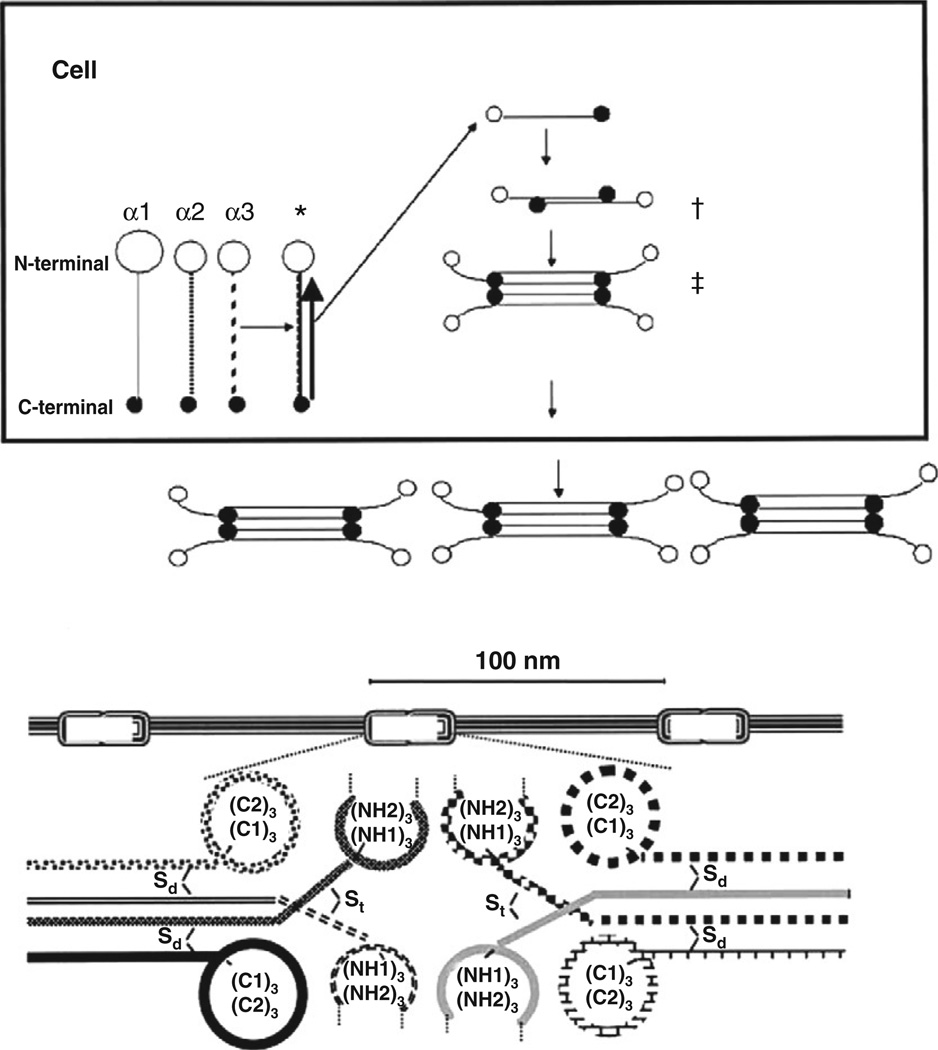
Collagen VI undergoes a complex assembly process inside and outside of the cell (Engvall et al., 1986; Timpl and Chu, 1994) (Figure 5.5). All three primary α chains have to combine to form a heterotrimeric monomer, the basic building block of collagen VI (Engvall et al., 1986). The monomer will thus contain the α1(VI), α2(VI), and α3(VI) chains in equal stoichiometry. Assembly proceeds from the C1 domain along the triple helical domains to the N-terminal domain. Similar to other collagens, the collagenous Gly-X-Y motifs at the C-terminal ends of the individual triple helical domains are crucial for the initiation of the triple helix formation, which successively proceeds from the C-terminal end towards the N-terminal end of the triple helical domains. Two of these triple helical monomers then associate in an antiparallel arrangement mediated by a single cysteine residue located in the N-terminal part of the α1(VI) or α2(VI) collagen chain triple helical domain, interacting with a cysteine residue in one of the C-globular domains (Furthmayr et al., 1983; Chu et al., 1988; Colombatti et al., 1995). Two dimers then associate in a parallel orientation (Engvall et al., 1986), mediated by a similar triple helical cysteine in the α3(VI) chain to form a tetramer (Furthmayr et al., 1983; Chu et al., 1988; Bonaldo et al., 1990). The tetramers are then secreted into the extracellular space, where they associate end-to-end to form the beaded microfibrillar network that is characteristic of collagen VI (Furthmayr et al., 1983; Engvall et al., 1986; Lamande et al., 1998).
Figure 5.5.
Schematic of the collagen VI assembly process. Upper panel depicts intracellular assembly from the heterotrimeric monomer composed of all three α chains (*), via antiparallel dimer (†) to tetramer (‡) formation. The lower panel depicts the formation of the beaded microfilaments with 100-nm periodicity, formed by close interaction of the C- and N-terminal globular domains. Also note the interchain disulfite bridges. (Reproduced with permission from Bertini and Pepe (2002), incorporating a modified schematic from Timpl and Chu (1994).)
The assembled extracellular collagen VI microfibrils have a diameter of 4.5 nm and a periodicity of 100–105 nm (Furthmayr et al., 1983; Chu et al., 1989; Baldock et al., 2003). Collagen VI has a widespread distribution, and is found in most matrices and tissues, including muscle, vessels, skin, intervertebral discs, cartilage, eye, and others. It shows a distinctly pericellular distribution in particular around tendon cells (Senga et al., 1995; Ritty et al., 2003) and also displays a particular affinity for basement membranes (Keene et al., 1988; Kuo et al., 1997). Thus, on immunohistochemical examination, collagen VI immunofluorescence is found to overlap with markers of basement membranes such as perlecan, laminin-γ1 and collagen type IV around endothelium, nerve, and muscle (Pan et al., 2003). Collagen VI interactions that have been suggested include collagen types II (Bidanset et al., 1992), IV (Kuo et al., 1997), and possibly type I, fibulin-2 (Sasaki et al., 1995), fibronectin and perlecan (Tillet et al., 1994), microfibril-associated glycoprotein 1 (Finnis and Gibson, 1997), heparin and hyaluran (Specks et al., 1992), decorin and biglycan (Bidanset et al., 1992; Wiberg et al., 2001), collagen XIV (Brown et al., 1993) and, amongst cell surface receptors, NG2 (Burg et al., 1996), integrin (Aumailley et al., 1991; Pfaff et al., 1993), and CD44. Through decorin and biglycan, collagen VI also interacts indirectly with chondroadherin and matrilin (Wiberg et al., 2003) and possibly also with the sarcoglycan and dystroglycan complexes (Bowe et al., 2000; Rafii et al., 2006). Collagen VI is able to bind to the collagen-binding integrins α1β1 and α2β1 via its native triple helical domain, whereas unfolded collagen VI is able to bind to α5β1 and αβ3 via a cryptic Arg-Gly-Asp (RGD) motif (Aumailley et al., 1991; Pfaff et al., 1993). The membrane-associated chondroitin sulfate proteoglycan NG2 is the other known cell surface receptor for collagen VI (Stallcup et al., 1990; Nishiyama and Stallcup, 1993; Burg et al., 1996). The precise receptor or receptors for collagen VI in skeletal muscle are currently unknown.
Collagen VI functions, molecular pathogenesis
Functions suggested for collagen VI have included roles in cell adhesion (Aumailley et al., 1991; Klein et al., 1995), proliferation, stimulation of DNA synthesis of mesenchymal cells (Atkinson et al., 1996), and neural crest cell migration (Perris et al., 1993). Work in patient biopsies and cell culture has suggested that there is a loss of connection between the basement membrane and the interstitial matrix (Ishikawa et al., 2002, 2004; Pan et al., 2003), resulting in reduced anchorage to the matrix (Kawahara et al., 2007). Abnormal regulation of the putative collagen VI receptor NG2 has been seen (Petrini et al., 2005), although it is currently not clear whether there is a direct link from the muscle via NG2 and collagen VI to the rest of the matrix. In several cellular systems collagen VI seemed to infer resistance to apoptosis through as yet poorly understood pathways (Ruhl et al., 1999).
Further analysis of a mouse model of collagen VI deficiency generated by homologous inactivation of the col6a1 locus (Bonaldo et al., 1998) revealed apoptosis in the muscle mediated by mitochondrial changes (Irwin et al., 2003). In this mouse model there was evidence for easy breakdown of mitochondrial potential because of dysfunction of the mitochondrial permeability transition pore (PTP) in mitochondria prechallenged with oligomycin. There also were morphological differences in the mitochondria, which looked abnormally ballooned, as well as abnormalities of the sarcoplasmic reticulum. This inappropriate opening of the PTP is mediated by cyclophilin D and was correctable by the addition of wild-type collagen VI and of the cyclophilin D inhibitor ciclosporin A (Irwin et al., 2003) and the ciclosporin analog Debio25 (Tiepolo et al., 2009). Crossing the collagen VI null mouse with a cyclophilin D knockout mouse to create a collagen VI cyclophilin D double-knockout situation also prevented the inappropriate opening of the PTP and significantly decreased apoptosis in the muscle, reaffirming the central role of cyclophilin D in this part of the disease process (Palma et al., 2009). The same phenomenon of increased apoptosis because of inappropriate opening of the PTP has also been described in human cell culture and in biopsies obtained from human patients with collagen VI mutations (Angelin et al., 2007; Merlini et al., 2008a). Thus, an apoptotic mechanism, rather than dystrophy due to an unstable plasma membrane, appears to be a major contributor to myofiber loss in this condition, although this mechanism involving cyclophilin D is not specific to collagen VI deficiency and was also observed in a merosin-deficient CMD mouse model (Millay et al., 2008). The pathways mediating between the presence of collagen VI in the matrix and the mechanisms controlling apoptosis are less clear and remain to be worked out in detail.
MUTATIONAL SPECTRUM/GENOTYPE–PHENOTYPE CORRELATIONS
Bethlem myopathy
Many mutations have now been described in the three collagen VI genes both in patients with UCMD and in those with BM, so that a number of observations about genotype–phenotype correlations are beginning to emerge. Many of the mutations have been summarized by Lampe and colleagues (Lampe and Bushby, 2005) in great detail. Thus far, mutations in BM have mostly been dominant, i.e., acting from one allele either as an inherited change in families with Bethlem myopathy or as de novo mutations. Typical mutation types seen in BM are missense mutations of glycine residues of the collagenous Gly-X-Y motif at the N-terminal end of the triple helical domain (Jöbsis et al., 1996; Pepe et al., 1999a; Scacheri et al., 2002; Lampe et al., 2005; Lucioli et al., 2005). This mutation introduces a kink into the triple helical domain of the assembled tetramer, thus exerting a dominant negative effect on the structure of the tetramer (Lamande et al., 2001). The effect of these N-terminal triple helical glycine mutations is variable, correlating with the variable clinical phenotype associated with this type of mutation (Pace et al., 2008). If the effect on assembly is more profound, the phenotype will be more severe and fall into the intermediate or even Ullrich range of severity (Okada et al., 2007; Pace et al., 2008). The other recurrent type of mutation in BM is an inframe deletion of exon 14 in the α1(VI) chain (Pepe et al., 1999b, 2006; Vanegas et al., 2002; Pan et al., 2003; Lampe et al., 2005; Lucioli et al., 2005). This mutation removes one of the cysteines necessary for dimer assembly, blunting its dominant negative effect on the assembly (Pan et al., 2003; Baker et al., 2007). As outlined below, cysteine-sparing mutations in this location are otherwise associated with the more severe phenotype of UCMD because of the more pronounced dominant negative effect. It should be noted, however, that similar to the effect of dominant glycine missense mutations at the N-terminal end of the triple helical domain skipping of exon 14 in the α1(VI) chain can also result in phenotypic severities in the interim range between Ullrich and Bethlem. A range of other mutations has been described in BM, including missense mutations that do not affect the triple helical glycine residues (Scacheri et al., 2002; Lucioli et al., 2005), including in the families with a LGMD-like presentation.
Not all of the hitherto reported mutations associated with BM have been fully analyzed as to their pathogenic effect on collagen VI. Particular caution is necessary as there are many polymorphisms in the collagen VI genes that have not yet been fully catalogued, so that it may be hard to determine whether a newly encountered sequence change in a patient is in fact pathogenic or a polymorphism. Most recently, recessive mutations in the COL6A2 gene have been seen in patients with typical Bethlem myopathy (Foley et al., 2009; Gualandi et al., 2009). These patients (from three independent families to date) were carrying a null mutation on one allele and a missense mutation on the other, and all these mutations have so far occurred in the COL6A2 gene. The mutations were asymptomatic in the carrier state. Homozygosity for the null allele would, of course, give rise to the more severe phenotype of UCMD, so that the missense mutation on the other allele appears to ameliorate the phenotype into the Bethlem range. Thus, both clinical and genetic data clearly support the notion of a clinical and molecular spectrum of the collagen VI-associated myopathies.
Ullrich congenital muscular dystrophy
In UCMD the first mutations that were detected were recessive null mutations, leading to an absence of collagen VI in muscle biopsy sections and in culture of dermal fibroblasts (Camacho Vanegas et al., 2001; Higuchi et al., 2001). A larger variety of recessively acting mutations, mostly leading to premature termination codons, has subsequently been described, including some with milder manifestations because of their localization in alternatively spliced exons (Demir et al., 2002; Giusti et al., 2005; Lampe et al., 2005; Okada et al., 2007). Splice-site mutations may lead to out-of-frame exon skipping, thus acting as recessive null mutations (Camacho Vanegas et al., 2001; Ishikawa et al., 2002; Lucarini et al., 2005). Haploinsufficiency for one of the collagen VI chains in general does not lead to a clinical phenotype, so that carriers of these null mutations are not significantly affected clinically (Camacho Vanegas et al., 2001; Higuchi et al., 2001; Peat et al., 2007; Foley et al., 2009). Subsequently, it has become evident that de novo dominant mutations in all three collagen VI genes are responsible for a substantial proportion of patients presenting with sporadic UCMD (Pan et al., 2003; Baker et al., 2005; Lampe et al., 2005, 2008; Okada et al., 2007). These mutations are typically inframe exon-skipping mutations (on the basis of splice-site mutations or genomic deletions; Pepe et al., 2006) of exons coding for the N-terminal part of the triple helical domain, but sparing the cysteine residues responsible for higher-order assembly of the basic heterotrimer into the dimer and tetramer state (Pan et al., 2003; Lampe et al., 2008). As the skipped exon is located at the N-terminal end of the triple helical domain, the deleted chain is effectively incorporated into the heterotrimeric monomer. Owing to the fact that the deleted chain preserves the cysteine, the following higher-order assembly will include the mutant containing monomers, so that 15 of 16 secreted tetramers will then include at least one mutant chain as the basis of the strong dominant negative effect that these mutations exert (Pan et al., 2003; Baker et al., 2005; Lampe et al., 2008). In contrast, inframe exon skipping mutations that occur more towards the C-terminal end of the triple helical domain will be excluded from assembly into even the basic heterotrimeric monomer and, therefore, act in a recessive way (Demir et al., 2002; Ishikawa et al., 2004; Baker et al., 2005; Lampe et al., 2005, 2008). These dominant negative mutations in collagen VI are associated with a phenotypic range approaching the severity of recessive null mutations; however, patients with dominant negative mutations may be more likely to at least achieve some limited time of ambulation, compared with those with a complete null situation. In the dominant negative mutations, immunohistochemical analysis of the muscle biopsy will show presence of collagen VI in the extracellular matrix; however, careful labeling with markers of the basement membrane shows that the normally tight connection between collagen VI and basement membrane has been lost (Pan et al., 2003; Ishikawa et al., 2004). This double-labeling technique has emerged as the most sensitive immunohistochemical technique to suggest the presence of a collagen VI disorder (see below).
DIAGNOSIS, DIFFERENTIAL DIAGNOSIS, GENETIC COUNSELING, AND TREATMENT
Clinical diagnosis and differential diagnosis
The first step in the diagnosis of a collagen VI-related condition is the recognition of the salient clinical features that raise the index of suspicion for the presence of UCMD, BM, or a related disorder of intermediate severity. In a juvenile or adult patient with the findings of a prominent contractural phenotype associated with weakness, a collagen VI-related myopathy of intermediate severity, Bethlem myopathy, and myosclerosis are definite diagnostic considerations.
The most important differential diagnosis to consider in this scenario are the Emery–Dreifuss muscular dystrophies (EDMDs), caused by mutations in emerin for the X-linked form and lamin A/C for the autosomal form. There can be considerable overlap in the pattern of contractures, making a reliable distinction based on that feature alone difficult (Bonne et al., 2000), although, in our experience, contractures of the long finger flexors, in particular, are seen more commonly in the collagen VI-related disorders compared with the Emery–Dreifuss group. There is no Emery–Dreifuss typical cardiac involvement in the collagen VI disorders, whereas clinically relevant involvement of the skin seen in the collagen VI-related disorders does not occur in the Emery–Dreifuss group of disorders. For the UCMD group, in the younger child with EDS-like hyperlaxity together with contractures and weakness, this differential diagnosis of EDMD disorders is less relevant, although a CMD presentation of lamin A/C mutations is now being recognized and can be the basis of some confusion (Quijano-Roy et al., 2008). Usually the hyperlaxity of the distal joints is sufficiently striking to provide a high enough index of suspicion for the presence of a collagen VI disorder; however, hyperlaxity can also be a feature of other neuromuscular disorders (Voermans et al., 2009). For example, core disorders such as multi-minicore disease or more severe neonatal central core disease can also lead to a high degree of joint laxity in some patients and may have to be considered in the differential diagnosis (Ferreiro et al., 2002). The presence of additional and purely dermatological findings, such as striking keloid formation and extensive keratosis pilaris, is a strong indicator of the presence of a collagen VI-related disorder (Kirschner et al., 2005).
In the child, adolescent, or young adult with a predominantly rigid spine presentation, the differential diagnosis to consider in addition to the collagen VI-related disorders includes again the EDMDs caused by emerin and lamin A/C mutations, FHL1-related myopathies (reducing body myopathy and an EDMD-like presentation of FHL1 mutations), partial merosin deficiency, the contractural phenotype of LGMD2A (calpain mutations), and early-onset myofibrillar myopathies.
Muscle imaging
Muscle imaging can be helpful in the clinical differentiation of these disorders as there are suggestive patterns of muscle involvement associated with each of the conditions (Deconinck et al., 2010; Mercuri et al., 2010). Imaging in the collagen VI disorders will reveal a picture with characteristic fatty and connective tissue replacement of muscle starting around the fascias surrounding or traversing the muscle, as seen on muscle magnetic resonance imaging (Mercuri et al., 2005). The rectus femoris and vastus lateralis muscles show this pattern most consistently. A similar appearance can be appreciated on muscle ultrasonography, where the degeneration around the central fascia in the rectus femoris generates the appearance of a “central cloud” (Bönnemann et al., 2003). This peculiar “outside-in” picture of degeneration seen on muscle imaging is helpful when present, although it is not seen in all patients or may no longer be discernible in advanced cases, such as in late BM, or in more severely involved UCMD (Mercuri et al., 2005).
Muscle biopsy and dermal fibroblast analysis
Muscle biopsy findings in the collagen VI-related disorders can be quite variable and range from close to normal or mildly myopathic-appearing muscle with atrophic fibers and some degree of fiber-type disproportion (Schessl et al., 2008), to more dramatically myopathic pictures with variability of fiber diameter including the appearance of sometimes extremely atrophic fibers and build-up of extracellular connective and fat tissue. These histological abnormalities usually become more evident with increasing age of the patient. Evidence for myofiber degeneration also becomes more evident later in the disease, although it is never a strikingly prominent aspect of the histological picture. Core-like abnormalities in the myofibers may also be seen on occasion and can be source of confusion with the true core myopathies (personal observations).
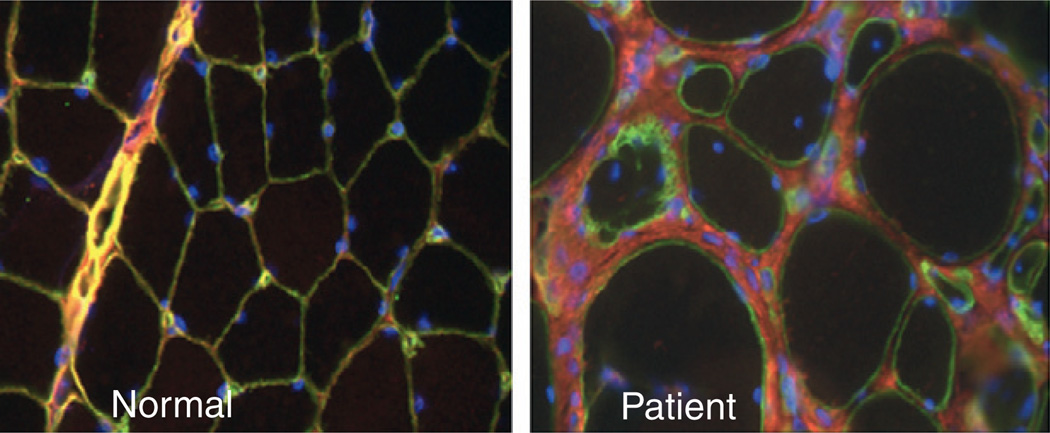
Collagen VI immunhistochemistry on muscle biopsy sections can be performed and may be helpful, particularly in recessive cases of UCMD where staining is absent or severely reduced. In the case of dominant mutations in UCMD there will be strong labeling for collagen VI immunoreactivity in the matrix, but the proper overlap of collagen VI with the basement membrane will be lost, indicating that the mutant collagen VI protein is being secreted but is not capable of proper function (Pan et al., 2003; Ishikawa et al., 2004) (Figure 5.6). Careful double labeling of the basement membrane with antibodies to collagen IV, laminin γ1 or perlecan is thus important in order to assess collagen VI localization in the tissue properly. In the milder BM, this lack of connection between collagen VI and the basement membrane may only be partial and sometimes may not be apparent at all (Pan et al., 2003), thus the muscle immunohistochemical analysis can appear to be normal. Analysis of collagen VI production in dermal fibroblast cultures can also be helpful in implicating collagen VI as being involved, ranging from completely absent or severely reduced with intracellular retention in UCMD to more subtle abnormalities in BM (Jimenez-Mallebrera et al., 2006; Hicks et al., 2008).
Figure 5.6.
Immunolocalization of collagen VI in the muscle of a patient with a dominant negative mutation in collagen VI. In the normal biopsy, collagen VI (red) overlaps with the basement membrane (green), resulting in a yellow color. In the patient’s biopsy there is a considerable amount of collagen VI immunoreactivity in the matrix; however, the colocalization with the basement membrane is lost, resulting in the green color of the basement membrane. Nuclear counterstain in blue.
Molecular genetic analysis
Mutational analysis can now be achieved on genomic DNA by sequencing all exons for all three collagen VI chains (Lampe et al., 2005). As alluded to before, it is important to point out that this analysis will not infrequently generate sequence changes that may be unknown in their significance, i.e., a given change may not yet have been seen as a disease-associated mutation or as a polymorphism. In this situation, it is important to trace the mutation in the family to see whether it follows the expected pattern of inheritance for a disease causing change in a given family.
In patients in whom the clinical suspicion for the presence of a collagen VI-related myopathy is strong, but, in using genomic sequencing, no mutations in the collagen VI genes are found or a change of uncertain significance is uncovered, it may also be useful to analyze a dermal fibroblast culture for collagen VI production and deposition as well as for possible effects on splicing, by performing reverse transcription–polymerase chain reaction analysis on RNA isolated from the dermal fibroblasts. This latter analysis may be important, as a change of unknown significance may influence splicing or there may be a deep intronic mutation interfering with splicing that is not detected on exon-based genomic sequencing (Lucarini et al., 2005; Martoni et al., 2009).
Lastly, it has become apparent recently that larger genomic deletions can occur, particularly in the COL6A1 and COL6A2 loci, that also will not be detected on exon-based genomic sequencing (which is dosage insensitive); therefore, these deletions require dosage-sensitive techniques such as multiplex ligation-dependent probe amplification or genomic single nucleotide polymorphism arrays for detection (Foley et al. in press 2010). Latest-generation molecular tools including chip-based sequencing arrays will be able to detect large genomic deletions as well as deep intronic sequence changes.
Genetic counseling
Genetic counseling in BM usually assumes the presence of an autosomal dominant condition with a risk of 50% for an affected individual of passing on the mutant allele. Although this assumption is holding up for the majority of patients, a potential caveat is the possibility of recessively acting mutations underlying Bethlem myopathy (Foley et al., 2009; Gualandi et al., 2009), which obviously carry a very different recurrence risk for the offspring of an affected patient. Thus, sporadic patients with BM likely carry a de novo dominant mutation, but they may rarely carry recessive mutations. The identification of the actual collagen VI mutation will help greatly in understanding the inheritance pattern so mutation analysis should always be attempted. In such a patient, in whim one missense mutation is detected that is inherited from an asymptomatic parent, the presence of a larger genomic deletion of the other allele should be suspected and pursued appropriately (see above). In the sporadic patient with UCMD both recessive mutations as well as a de novo dominant negative mutations can be expected with about equal likelihood, depending on the patient population of origin. Patients from populations with a degree of consanguinity or common ancestry are more likely to present with recessive UCMD, whereas patients from populations with mixed heritage are more likely to present with de novo dominant mutations, although either mutation type is possible in both settings.
Recessive versus de novo dominant mutations are obviously associated with greatly different recurrence risk estimations for future pregnancies of a couple with a single (sporadic) affected child and a negative family history. The risk of recurrence would be 25% for the recessive scenario, whereas for the a de novo dominant mutation only the theoretical risk of germ-line mosaicism has to be assumed. Only by the definitive identification of the causative mutations in the collagen VI genes can this situation be clarified.
In general, counseling for the possibility of a certain degree of individual clinical variability is important in families with collagen VI-related disorders, even if the mutation found is a known disease-associated mutation with a given phenotype. Prenatal diagnosis is possible by haplotype analysis and collagen VI staining of a chorionic villus biopsy (Brockington et al., 2004), but is much more straightforward and reliable once the disease-causing mutation(s) are known in the family, allowing for direct testing of the pregnancy.
Management and therapeutic interventions
Therapeutic intervention in the collagen VI disorders currently consists mainly of careful clinical and preventive management of the various clinical aspects of these conditions. Contractures are usually addressed initially by an aggressive stretching program in combination with dynamic splinting. Such a program is helpful in delaying worsening of the contractures; however, rarely can the progression of the contractures be entirely stopped. Surgical release of the contractures can be helpful, in particular in the Achilles tendons to preserve normal walking in patients with Bethlem myopathy, although the contractures have a tendency to recur after surgery. Very importantly, surgical release of other joint contractures, such as flexion contractures in the knees, has less clear efficacy and there is much less underlying clinical experience.
Management of early and progressive scoliosis can be challenging. Bracing may have a temporizing effect but never stops the eventual progression of the scoliosis. Experience with newer scoliosis surgical techniques such as the VEPTER (vertical expandable prosthetic titanium rib) is limited; however, it will likely be explored more in the future and will likely have a place in the surgical management of young children with UCMD and early progressive scoliosis. Careful respiratory monitoring by pulmonary function testing (upright and supine to assess for diaphragmatic involvement) as well as by sleep studies with timely institution of respiratory support is of prime importance and usually consists of noninvasive ventilatory support such as bilevel positive airway pressure. Respiratory insufficiency is clearly progressive in the collagen VI-related myopathies (Wallgren-Pettersson et al., 2004, Nadeau 2009); however, once ventilatory support is instituted, there will be a long period of stability in respiratory status. Adequate nutritional support is of great importance as many of the more severely affected children will have inadequate oral intake of food and fluids, even though there is no frank dysphagia. It is important to monitor bone density and consider calcium and vitamin D supplementation when appropriate. In selected children with collagen VI-related myopathies, a temporary percutaneous gastrostomy tube has become necessary.
Myofiber apoptosis has already been identified as a potential therapeutic target in the animal model of collagen VI deficiency. Pharmacological agents that may counteract the apoptosis that is part of the downstream effect of the collagen VI dysfunction are thus under investigation. A recent uncontrolled study of five patients with collagen VI mutations treated for 1 month with ciclosporin A (acting as a inhibitor of the mitochondrial permeability transition pore) demonstrated decreased apoptosis and increased stability of the mitochondrial permeability transition pore, although strength improvement was not recorded (Merlini et al., 2008a). Antiapoptotic agents with less long-term toxicity are now under active clinical investigation and may enter clinical trials in these patients.
A particular challenge lies in the combined predominance of dominant mutations in all of the collagen VI-related disease. In this situation, gene replacement approaches obviously will not work, and other strategies such as inactivation of the dominant negative allele will have to be devised. Stem cell therapy will have to take into account that the collagen VI cell of origin in muscle is predominantly the muscle interstitial fibroblast (Zou et al. 2008). As mentioned above, there are a minority of patients with UCMD for whom mutations in the three collagen VI genes have been ruled out, indicating that there should be additional genes causing their phenotype that still await discovery. Very recently reduced autophagocytic flow has also been implicated in the pathogenesis of collagen VI deficiency (Grumati P, Coletto L, Sabatelli P et al. (2010). Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med 16: 1313–1320.)
References
- Angelin A, Tiepolo T, Sabatelli P, et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc Natl Acad Sci U S A. 2007;104:991–996. doi: 10.1073/pnas.0610270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JC, Ruhl M, Becker J, et al. Collagen VI regulates normal and transformed mesenchymal cell proliferation in vitro. Exp Cell Res. 1996;228:283–291. doi: 10.1006/excr.1996.0328. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Specks U, Timpl R. Cell adhesion to type VI collagen. Biochem Soc Trans. 1991;19:843–847. doi: 10.1042/bst0190843. [DOI] [PubMed] [Google Scholar]
- Baker NL, Morgelin M, Peat R, et al. Dominant collagen VI mutations are a common cause of Ullrich congenital muscular dystrophy. Hum Mol Genet. 2005;14:279–293. doi: 10.1093/hmg/ddi025. [DOI] [PubMed] [Google Scholar]
- Baker NL, Morgelin M, Pace RA, et al. Molecular consequences of dominant Bethlem myopathy collagen VI mutations. Ann Neurol. 2007;62:390–405. doi: 10.1002/ana.21213. [DOI] [PubMed] [Google Scholar]
- Baldock C, Sherratt MJ, Shuttleworth CA, et al. The supramolecular organization of collagen VI microfibrils. J Mol Biol. 2003;330:297–307. doi: 10.1016/s0022-2836(03)00585-0. [DOI] [PubMed] [Google Scholar]
- Bertini E, Pepe G. Collagen type VI and related disorders: Bethlem myopathy and Ullrich scleroatonic muscular dystrophy. Eur J Paediatr Neurol. 2002;6:193–198. doi: 10.1053/ejpn.2002.0593. [DOI] [PubMed] [Google Scholar]
- Bethlem J, van Wijnaarden GK. Benign myopathy, with autosomal dominant inheritance: a report on three pedigrees. Brain. 1976;99:91–100. doi: 10.1093/brain/99.1.91. [DOI] [PubMed] [Google Scholar]
- Bidanset DJ, Guidry C, Rosenberg LC, et al. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250–5256. [PubMed] [Google Scholar]
- Bonaldo P, Russo V, Bucciotti F, et al. Structural and functional features of the alpha 3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry. 1990;29:1245–1254. doi: 10.1021/bi00457a021. [DOI] [PubMed] [Google Scholar]
- Bonaldo P, Braghetta P, Zanetti M, et al. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet. 1998;7:2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- Bonne G, Mercuri E, Muchir A, et al. Clinical and molecular genetic spectrum of autosomal dominant Emery–Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol. 2000;48:170–180. [PubMed] [Google Scholar]
- Bönnemann CG, Brockmann K, Hanefeld F. Muscle ultrasound in Bethlem myopathy. Neuropediatrics. 2003;34:335–336. doi: 10.1055/s-2003-44665. [DOI] [PubMed] [Google Scholar]
- Bowe MA, Mendis DB, Fallon JR. The small leucine-rich repeat proteoglycan biglycan binds to alpha-dystroglycan and is upregulated in dystrophic muscle. J Cell Biol. 2000;148:801–810. doi: 10.1083/jcb.148.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley WG, Hudgson P, Gardner-Medwin D, et al. The syndrome of myosclerosis. J Neurol Neurosurg Psychiatry. 1973;36:651–660. doi: 10.1136/jnnp.36.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockington M, Brown SC, Lampe A, et al. Prenatal diagnosis of Ullrich congenital muscular dystrophy using haplotype analysis and collagen VI immunocytochemistry. Prenat Diagn. 2004;24:440–444. doi: 10.1002/pd.902. [DOI] [PubMed] [Google Scholar]
- Brown JC, Mann K, Wiedemann H, et al. Structure and binding properties of collagen type XIV isolated from human placenta. J Cell Biol. 1993;120:557–567. doi: 10.1083/jcb.120.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MA, Tillet E, Timpl R, et al. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- Camacho Vanegas O, Bertini E, Zhang RZ, et al. Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc Natl Acad Sci U S A. 2001;98:7516–7521. doi: 10.1073/pnas.121027598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, Conway D, Pan TC, et al. Amino acid sequence of the triple-helical domain of human collagen type VI. J Biol Chem. 1988;263:18601–18606. [PubMed] [Google Scholar]
- Chu ML, Pan TC, Conway D, et al. Sequence analysis of alpha 1(VI) and alpha 2(VI) chains of human type VI collagen reveals internal triplication of globular domains similar to the A domains of von Willebrand factor and two alpha 2(VI) chain variants that differ in the carboxy terminus. EMBO J. 1989;8:1939–1946. doi: 10.1002/j.1460-2075.1989.tb03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, Pan TC, Conway D, et al. The structure of type VI collagen. Ann N Y Acad Sci. 1990a;580:55–63. doi: 10.1111/j.1749-6632.1990.tb17917.x. [DOI] [PubMed] [Google Scholar]
- Chu ML, Zhang RZ, Pan TC, et al. Mosaic structure of globular domains in the human type VI collagen alpha 3 chain: similarity to von Willebrand factor, fibronectin, actin, salivary proteins and aprotinin type protease inhibitors. EMBO J. 1990b;9:385–393. doi: 10.1002/j.1460-2075.1990.tb08122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombatti A, Mucignat MT, Bonaldo P, et al. Secretion and matrix assembly of recombinant type VI collagen. J Biol Chem. 1995;270:13105–13111. doi: 10.1074/jbc.270.22.13105. [DOI] [PubMed] [Google Scholar]
- Deconinck N, Dion E, Yaou RB, et al. Differentiating Emery–Dreifuss muscular dystrophy and collagen VI-related myopathies using a specific CT scanner pattern. Neuromuscul Disord. 2010;20:517–523. doi: 10.1016/j.nmd.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Demir E, Sabatelli P, Allamand V, et al. Mutations in COL6A3 cause severe and mild phenotypes of Ullrich congenital muscular dystrophy. Am J Hum Genet. 2002;70:1446–1458. doi: 10.1086/340608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Ferreiro A, Sabatelli P, et al. Collagen VI status and clinical severity in Ullrich congenital muscular dystrophy: phenotype analysis of 11 families linked to the COL6 loci. Neuropediatrics. 2004;35:103–112. doi: 10.1055/s-2004-815832. [DOI] [PubMed] [Google Scholar]
- de Visser M, de Voogt WG, la Riviere GV. The heart in Becker muscular dystrophy, facioscapulohumeral dystrophy, and Bethlem myopathy. Muscle Nerve. 1992;15:591–596. doi: 10.1002/mus.880150510. [DOI] [PubMed] [Google Scholar]
- Duchenne GBA. Recherches sur la paralysie musculaire pseudohypertrophique au paralysie myosclerotique. Arch Gen Med. 1868;11:552–588. [Google Scholar]
- Engvall E, Hessle H, Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol. 1986;102:703–710. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro A, Monnier N, Romero NB, et al. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann Neurol. 2002;51:750–759. doi: 10.1002/ana.10231. [DOI] [PubMed] [Google Scholar]
- Finnis ML, Gibson MA. Microfibril-associated glycoprotein-1 (MAGP-1) binds to the pepsin-resistant domain of the alpha3(VI) chain of type VI collagen. J Biol Chem. 1997;272:22817–22823. doi: 10.1074/jbc.272.36.22817. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Rich C, Zhou FH, et al. Three novel collagen VI chains, alpha4(VI), alpha5(VI), and alpha6(VI) J Biol Chem. 2008;283:20170–20180. doi: 10.1074/jbc.M710139200. [DOI] [PubMed] [Google Scholar]
- Foley AR, Hu Y, Zou Y, et al. Autosomal recessive inheritance of classic Bethlem myopathy. Neuromuscul Disord. 2009;19:813–817. doi: 10.1016/j.nmd.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H, Wiedemann H, Timpl R, et al. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983;211:303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Toyokura Y. Congenital, hypotonic–sclerotic muscular dystrophy. J Med Genet. 1977;14:426–429. doi: 10.1136/jmg.14.6.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gara SK, Grumati P, Urciuolo A, et al. Three novel collagen VI chains with high homology to the alpha3 chain. J Biol Chem. 2008;283:10658–10670. doi: 10.1074/jbc.M709540200. [DOI] [PubMed] [Google Scholar]
- Giusti B, Lucarini L, Pietroni V, et al. Dominant and recessive COL6A1 mutations in Ullrich scleroatonic muscular dystrophy. Ann Neurol. 2005;58:400–410. doi: 10.1002/ana.20586. [DOI] [PubMed] [Google Scholar]
- Gualandi F, Urciuolo A, Martoni E, et al. Autosomal recessive Bethlem myopathy. Neurology. 2009;73:1883–1891. doi: 10.1212/WNL.0b013e3181c3fd2a. [DOI] [PubMed] [Google Scholar]
- Hall JG. Arthrogryposis multiplex congenita: etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997;6:159–166. [PubMed] [Google Scholar]
- Haq RU, Speer MC, Chu ML, et al. Respiratory muscle involvement in Bethlem myopathy. Neurology. 1999;52:174–176. doi: 10.1212/wnl.52.1.174. [DOI] [PubMed] [Google Scholar]
- Heiskanen M, Saitta B, Palotie A, et al. Head to tail organization of the human COL6A1 and COL6A2 genes by fiber-FISH. Genomics. 1995;29:801–803. doi: 10.1006/geno.1995.9008. [DOI] [PubMed] [Google Scholar]
- Hicks D, Lampe AK, Barresi R, et al. A refined diagnostic algorithm for Bethlem myopathy. Neurology. 2008;70:1192–1199. doi: 10.1212/01.wnl.0000307749.66438.6d. [DOI] [PubMed] [Google Scholar]
- Higuchi I, Shiraishi T, Hashiguchi T, et al. Frameshift mutation in the collagen VI gene causes Ullrich’s disease. Ann Neurol. 2001;50:261–265. doi: 10.1002/ana.1120. [DOI] [PubMed] [Google Scholar]
- Irwin WA, Bergamin N, Sabatelli P, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Sugie K, Murayama K, et al. Ullrich disease: collagen VI deficiency: EM suggests a new basis for muscular weakness. Neurology. 2002;59:920–923. doi: 10.1212/wnl.59.6.920. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Sugie K, Murayama K, et al. Ullrich disease due to deficiency of collagen VI in the sarcolemma. Neurology. 2004;62:620–623. doi: 10.1212/01.wnl.0000113023.84421.00. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Maioli MA, Kim J, et al. A comparative analysis of collagen VI production in muscle, skin and fibroblasts from 14 Ullrich congenital muscular dystrophy patients with dominant and recessive COL6A mutations. Neuromuscul Disord. 2006;16:571–582. doi: 10.1016/j.nmd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Jöbsis GJ, Keizers H, Vreijling JP, et al. Type VI collagen mutations in Bethlem myopathy, an autosomal dominant myopathy with contractures. Nat Genet. 1996;14:113–115. doi: 10.1038/ng0996-113. [DOI] [PubMed] [Google Scholar]
- Jöbsis GJ, Boers JM, Barth PG, et al. Bethlem myopathy: a slowly progressive congenital muscular dystrophy with contractures. Brain. 1999;122(Part 4):649–655. doi: 10.1093/brain/122.4.649. [DOI] [PubMed] [Google Scholar]
- Kawahara G, Okada M, Morone N, et al. Reduced cell anchorage may cause sarcolemma-specific collagen VI deficiency in Ullrich disease. Neurology. 2007;69:1043–1049. doi: 10.1212/01.wnl.0000271386.89878.22. [DOI] [PubMed] [Google Scholar]
- Keene DR, Engvall E, Glanville RW. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988;107:1995–2006. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner J, Hausser I, Zou Y, et al. Ullrich congenital muscular dystrophy: connective tissue abnormalities in the skin support overlap with Ehlers-Danlos syndromes. Am J Med Genet A. 2005;132:296–301. doi: 10.1002/ajmg.a.30443. [DOI] [PubMed] [Google Scholar]
- Klein G, Muller CA, Tillet E, et al. Collagen type VI in the human bone marrow microenvironment: a strong cytoadhesive component. Blood. 1995;86:1740–1748. [PubMed] [Google Scholar]
- Kuo HJ, Maslen CL, Keene DR, et al. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272:26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- Lamande SR, Sigalas E, Pan TC, et al. The role of the alpha3(VI) chain in collagen VI assembly. Expression of an alpha3(VI) chain lacking N-terminal modules N10–N7 restores collagen VI assembly, secretion, and matrix deposition in an alpha3(VI)-deficient cell line. J Biol Chem. 1998;273:7423–7430. doi: 10.1074/jbc.273.13.7423. [DOI] [PubMed] [Google Scholar]
- Lamande SR, Morgelin M, Selan C, et al. Kinked collagen VI tetramers and reduced microfibril formation as a result of Bethlem myopathy and introduced triple helical glycine mutations. J Biol Chem. 2001;277:1949–1956. doi: 10.1074/jbc.M109932200. [DOI] [PubMed] [Google Scholar]
- Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673–685. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe AK, Dunn DM, von Niederhausern AC, et al. Automated genomic sequence analysis of the three collagen VI genes: applications to Ullrich congenital muscular dystrophy and Bethlem myopathy. J Med Genet. 2005;42:108–120. doi: 10.1136/jmg.2004.023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe AK, Zou Y, Sudano D, et al. Exon skipping mutations in collagen VI are common and are predictive for severity and inheritance. Hum Mutat. 2008;29:809–822. doi: 10.1002/humu.20704. [DOI] [PubMed] [Google Scholar]
- Lowenthal A. A new heredodegenerative group: heredofamilial myosclerosis [in French] Acta Neurol Psychiatr Belg. 1954;52:155–165. [PubMed] [Google Scholar]
- Lucarini L, Giusti B, Zhang RZ, et al. A homozygous COL6A2 intron mutation causes in-frame triple-helical deletion and nonsense-mediated mRNA decay in a patient with Ullrich congenital muscular dystrophy. Hum Genet. 2005;117:460–466. doi: 10.1007/s00439-005-1318-8. [DOI] [PubMed] [Google Scholar]
- Lucioli S, Giusti B, Mercuri E, et al. Detection of common and private mutations in the COL6A1 gene of patients with Bethlem myopathy. Neurology. 2005;64:1931–1937. doi: 10.1212/01.WNL.0000163990.00057.66. [DOI] [PubMed] [Google Scholar]
- Martoni E, Urciuolo A, Sabatelli P, et al. Identification and characterization of novel collagen VI non-canonical splicing mutations causing Ullrich congenital muscular dystrophy. Hum Mutat. 2009;30:E662–E672. doi: 10.1002/humu.21022. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Yuva Y, Brown SC, et al. Collagen VI involvement in Ullrich syndrome: a clinical, genetic, and immunohistochemical study. Neurology. 2002;58:1354–1359. doi: 10.1212/wnl.58.9.1354. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Lampe A, Allsop J, et al. Muscle MRI in Ullrich congenital muscular dystrophy and Bethlem myopathy. Neuromuscul Disord. 2005;15:303–310. doi: 10.1016/j.nmd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Clements E, Offiah A, et al. Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann Neurol. 2010;67:201–208. doi: 10.1002/ana.21846. [DOI] [PubMed] [Google Scholar]
- Merlini L, Morandi L, Granata C, et al. Bethlem myopathy: early-onset benign autosomal dominant myopathy with contractures. Description of two new families. Neuromuscul Disord. 1994;4:503–511. doi: 10.1016/0960-8966(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Merlini L, Angelin A, Tiepolo T, et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci U S A. 2008a;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L, Martoni E, Grumati P, et al. Autosomal recessive myosclerosis myopathy is a collagen VI disorder. Neurology. 2008b;71:1245–1253. doi: 10.1212/01.wnl.0000327611.01687.5e. [DOI] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohire MD, Tandan R, Fries TJ, et al. Early-onset benign autosomal dominant limb-girdle myopathy with contractures (Bethlem myopathy) Neurology. 1988;38:573–580. doi: 10.1212/wnl.38.4.573. [DOI] [PubMed] [Google Scholar]
- Nadeau A, Muntoni F. Skin changes in Ullrich congenital muscular dystrophy. Neuromuscul Disord. 2008;18:982. doi: 10.1016/j.nmd.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nadeau A, Kinali M, Main M, et al. Natural history of Ullrich congenital muscular dystrophy. Neurology. 2009;73:25–31. doi: 10.1212/WNL.0b013e3181aae851. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Stallcup WB. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface. Mol Biol Cell. 1993;4:1097–1108. doi: 10.1091/mbc.4.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka I, Une Y, Ishihara T, et al. A clinical and histological study of Ullrich’s disease (congenital atonic-sclerotic muscular dystrophy) Neuropediatrics. 1981;12:197–208. doi: 10.1055/s-2008-1059651. [DOI] [PubMed] [Google Scholar]
- Norwood EL, Harling C, Chinnery PF, et al. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132(Part 11):3175–3186. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Kawahara G, Noguchi S, et al. Primary collagen VI deficiency is the second most common congenital muscular dystrophy in Japan. Neurology. 2007;69:1035–1042. doi: 10.1212/01.wnl.0000271387.10404.4e. [DOI] [PubMed] [Google Scholar]
- Pace RA, Peat RA, Baker NL, et al. Collagen VI glycine mutations: perturbed assembly and a spectrum of clinical severity. Ann Neurol. 2008;64:294–303. doi: 10.1002/ana.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Tiepolo T, Angelin A, et al. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum Mol Genet. 2009;18:2024–2031. doi: 10.1093/hmg/ddp126. [DOI] [PubMed] [Google Scholar]
- Pan TC, Zhang RZ, Pericak-Vance MA, et al. Missense mutation in a von Willebrand factor type A domain of the alpha 3(VI) collagen gene (COL6A3) in a family with Bethlem myopathy. Hum Mol Genet. 1998;7:807–812. doi: 10.1093/hmg/7.5.807. [DOI] [PubMed] [Google Scholar]
- Pan TC, Zhang RZ, Sudano DG, et al. New molecular mechanism for Ullrich congenital muscular dystrophy: a heterozygous in-frame deletion in the COL6A1 gene causes a severe phenotype. Am J Hum Genet. 2003;73:355–369. doi: 10.1086/377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat RA, Baker NL, Jones KJ, et al. Variable penetrance of C0L6A1 null mutations: implications for prenatal diagnosis and genetic counselling in Ullrich congenital muscular dystrophy families. Neuromuscul Disord. 2007;17:547–557. doi: 10.1016/j.nmd.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Peat RA, Smith JM, Compton AG, et al. Diagnosis and etiology of congenital muscular dystrophy. Neurology. 2008;71:312–321. doi: 10.1212/01.wnl.0000284605.27654.5a. [DOI] [PubMed] [Google Scholar]
- Pepe G, Bertini E, Giusti B, et al. A novel de novo mutation in the triple helix of the COL6A3 gene in a two-generation Italian family affected by Bethlem myopathy. A diagnostic approach in the mutations’ screening of type VI collagen. Neuromuscul Disord. 1999a;9:264–271. doi: 10.1016/s0960-8966(99)00014-0. [DOI] [PubMed] [Google Scholar]
- Pepe G, Giusti B, Bertini E, et al. A heterozygous splice site mutation in C0L6A1 leading to an in-frame deletion of the alpha1(VI) collagen chain in an Italian family affected by Bethlem myopathy. Biochem Biophys Res Commun. 1999b;258:802–807. doi: 10.1006/bbrc.1999.0680. [DOI] [PubMed] [Google Scholar]
- Pepe G, Lucarini L, Zhang RZ, et al. COL6A1 genomic deletions in Bethlem myopathy and Ullrich muscular dystrophy. Ann Neurol. 2006;59:190–195. doi: 10.1002/ana.20705. [DOI] [PubMed] [Google Scholar]
- Perris R, Kuo HJ, Glanville RW, et al. Collagen type VI in neural crest development: distribution in situ and interaction with cells in vitro . Dev Dyn. 1993;198:135–149. doi: 10.1002/aja.1001980207. [DOI] [PubMed] [Google Scholar]
- Petrini S, Tessa A, Stallcup WB, et al. Altered expression of the MCSP/NG2 chondroitin sulfate proteoglycan in collagen VI deficiency. Mol Cell Neurosci. 2005;30:408–417. doi: 10.1016/j.mcn.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Petrini S, D’Amico A, Sale P, et al. Ullrich myopathy phenotype with secondary Co1VI defect identified by confocal imaging and electron microscopy analysis. Neuromuscul Disord. 2007;17:587–596. doi: 10.1016/j.nmd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Pfaff M, Aumailley M, Specks U, et al. Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type VI. Exp Cell Res. 1993;206:167–176. doi: 10.1006/excr.1993.1134. [DOI] [PubMed] [Google Scholar]
- Quijano-Roy S, Mbieleu B, Bönnemann CG, et al. De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann Neurol. 2008;64:177–186. doi: 10.1002/ana.21417. [DOI] [PubMed] [Google Scholar]
- Rafii MS, Hagiwara H, Mercado ML, et al. Biglycan binds to alpha- and gamma-sarcoglycan and regulates their expression during development. J Cell Physiol. 2006;209:439–147. doi: 10.1002/jcp.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritty TM, Roth R, Heuser JE. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure (Camb) 2003;11:1179–1188. doi: 10.1016/s0969-2126(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Ruhl M, Sahin E, Johannsen M, et al. Soluble collagen VI drives serum-starved fibroblasts through S phase and prevents apoptosis via down-regulation of Bax. J Biol Chem. 1999;274:34361–34368. doi: 10.1074/jbc.274.48.34361. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Pan TC, et al. Binding of mouse and human fibulin-2 to extracellular matrix ligands. J Mol Biol. 1995;254:892–899. doi: 10.1006/jmbi.1995.0664. [DOI] [PubMed] [Google Scholar]
- Scacheri PC, Gillanders EM, Subramony SH, et al. Novel mutations in collagen VI genes: expansion of the Bethlem myopathy phenotype. Neurology. 2002;58:593–602. doi: 10.1212/wnl.58.4.593. [DOI] [PubMed] [Google Scholar]
- Schessl J, Goemans NM, Magold Al, et al. Predominant fiber atrophy and fiber type disproportion in early Ullrich disease. Muscle Nerve. 2008;38:1184–1191. doi: 10.1002/mus.21088. [DOI] [PubMed] [Google Scholar]
- Senga K, Kobayashi M, Hattori H, et al. Type VI collagen in mouse masseter tendon, from osseous attachment to myotendinous junction. Anat Rec. 1995;243:294–302. doi: 10.1002/ar.1092430303. [DOI] [PubMed] [Google Scholar]
- Specks U, Mayer U, Nischt R, et al. Structure of recombinant N-terminal globule of type VI collagen alpha 3 chain and its binding to heparin and hyaluronan. EMBO J. 1992;11:4281–4290. doi: 10.1002/j.1460-2075.1992.tb05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin K, Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990;111(Part 2):3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault M, Duquette A, Thiffault I, et al. A new form of congenital muscular dystrophy with joint hyperlaxity maps to 3p23-21. Brain. 2006;129(Part 8):2077–2084. doi: 10.1093/brain/awl146. [DOI] [PubMed] [Google Scholar]
- Tiepolo T, Angelin A, Palma E, et al. The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1 (−/−) myopathic mice. Br J Pharmacol. 2009;157:1045–1052. doi: 10.1111/j.1476-5381.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillet E, Wiedemann H, Golbik R, et al. Recombinant expression and structural and binding properties of alpha 1(VI) and alpha 2(VI) chains of human collagen type VI [published erratum appears in Eur J Biochem 1994; 222: 1064] Eur J Biochem. 1994;221:177–185. doi: 10.1111/j.1432-1033.1994.tb18727.x. [DOI] [PubMed] [Google Scholar]
- Timpl R, Chu ML. Microfibrillar collagen type VI. In: Mecha RP, editor. Extracellular Matrix Assembly and Structure. Orlando: Academic Press; 1994. pp. 207–242. [Google Scholar]
- Ullrich O. Kongenitale atonisch-sklerotische Muskeldystrophie. Monatsschr Kinderheilkd. 1930a;47:502–510. [Google Scholar]
- Ullrich O. Kongenitale atonisch-sklerotische Muskeldystrophie, ein weiterer Typus der heredodegeneration Erkrankungen des neuromuskularen Systems. Z Ges Neurol Psychiatry. 1930b;126:171–201. [Google Scholar]
- Vanegas OC, Zhang RZ, Sabatelli P, et al. Novel COL6A1 splicing mutation in a family affected by mild Bethlem myopathy. Muscle Nerve. 2002;25:513–519. doi: 10.1002/mus.10100. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Bönnemann CG, Hamel BC, et al. Joint hypermobility as a distinctive feature in the differential diagnosis of myopathies. J Neurol. 2009;256:13–27. doi: 10.1007/s00415-009-0105-1. [DOI] [PubMed] [Google Scholar]
- Voit T. Congenital muscular dystrophies: 1997 update. Brain Dev. 1998;20:65–74. doi: 10.1016/s0387-7604(97)00094-6. [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Bushby K, Mellies U, et al. 117th ENMC workshop: ventilatory support in congenital neuromuscular disorders–congenital myopathies, congenital muscular dystrophies, congenital myotonic dystrophy and SMA (II), 4–6 April 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14:56–69. doi: 10.1016/j.nmd.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Weil D, Mattei MG, Passage E, et al. Cloning and chromosomal localization of human genes encoding the three chains of type VI collagen. Am J Hum Genet. 1988;42:435–445. [PMC free article] [PubMed] [Google Scholar]
- Wiberg C, Hedbom E, Khairullina A, et al. Biglycan and decorin bind close to the N-terminal region of the collagen VI triple helix. J Biol Chem. 2001;276:18947–18952. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- Wiberg C, Klatt AR, Wagener R, et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- Zou Y, Zhang RZ, Sabatelli P, et al. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: Implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol. 2008;67:144–154. doi: 10.1097/nen.0b013e3181634ef7. [DOI] [PubMed] [Google Scholar]