Abstract
The starch excess phenotype of Arabidopsis mutants defective in the starch phosphorylating enzyme glucan, water dikinase (EC 2.7.9.4) indicates that phosphorylation of starch is required for its degradation. However, the underlying mechanism has not yet been elucidated. In this study, two in vivo systems have been established that allow the analysis of phosphorylation of transitory starch during both biosynthesis in the light and degradation in darkness. First, a photoautotrophic culture of the unicellular green alga Chlamydomonas reinhardtii was used to monitor the incorporation of exogenously supplied 32P orthophosphate into starch. Illuminated cells incorporated 32P into starch with a constant rate during 2 h. By contrast, starch phosphorylation in darkened cells exceeded that in illuminated cells within the first 30 min, but subsequently phosphate incorporation declined. Pulse-chase experiments performed with 32P/31P orthophosphate revealed a high turnover of the starch-bound phosphate esters in darkened cells but no detectable turnover in illuminated cells. Secondly, leaf starch granules were isolated from potato (Solanum tuberosum) plants grown under controlled conditions and glucan chains from the outer granule layer were released by isoamylase. Phosphorylated chains were purified and analyzed using high performance anion-exchange chromatography and matrix-assisted laser desorption/ionization mass spectrometry. Glucans released from the surface of starch granules that had been isolated from darkened leaves possessed a considerably higher degree of phosphorylation than those prepared from leaves harvested during the light period. Thus, in the unicellular alga as well as in potato leaves, net starch degradation is accompanied with an increased phosphorylation of starch.
Starch is the predominant carbohydrate reserve in plants and is deposited as semicrystalline particles. One can distinguish reserve starch in storage organs and transitory starch in photosynthetic organs. Whereas reserve starch can be stored over months or years, transitory starch is normally accumulated during the day and degraded in the following dark period. Starch consists of the two Glc polymers, amylose and amylopectin. Amylose comprises predominantly linear chains of α-1,4-linked Glc residues, whereas amylopectin exists as a branched α-1,4:α-1,6 d-glucan polymer. Amylopectin accounts for about 75% of the starch weight in reserve starch (Ball et al., 1998) and typically more than 90% in transitory starch (Zeeman et al., 2002).
Amylopectin contains small amounts of phosphate monoesters (Hizukuri et al., 1970). We have recently shown that the phosphorylation of starch-like polyglucans is catalyzed by the glucan, water dikinase (GWD, formerly designated as R1, EC 2.7.9.4; Ritte et al., 2002):
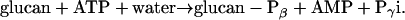 |
Under in vitro conditions this reaction leads to the formation of glucan-bound Glc6P and Glc3P residues in a ratio of approximately 2:1, which is also found in native starch (Tabata and Hizukuri, 1971). The phosphorylation level of starch is quite low. In cereal endosperm starch, the amounts of Glc6P and Glc3P residues are at or below the detection limit, and even in potato (Solanum tuberosum) tuber starch, which is regarded as highly phosphorylated, only 0.2% to 0.5% of the Glc residues are phosphorylated (Tabata and Hizukuri, 1971; Blennow et al., 2002). The phosphorylation level of leaf starch is 0.1% or less (Yu et al., 2001).
The physiological relevance of starch phosphorylation is not yet fully understood. However, the analyses of plants with reduced GWD activity indicate that the phosphorylation of starch affects its in vivo degradability. Starch breakdown is strongly impaired in GWD antisense potato plants and in the GWD-deficient starch-excess 1 (sex1) mutants of Arabidopsis (Lorberth et al., 1998; Yu et al., 2001; Ritte et al., 2003). The link between phosphorylation and degradation of starch is presently poorly understood. It has been suggested that starch phosphorylation leads to increased hydrophilicity of the starch particle, thereby making it more easily accessible for degrading enzymes (Yu et al., 2001). However, experimental evidence is lacking.
Nielsen et al. (1994) have shown that potato tuber starch is phosphorylated during biosynthesis. This result, in combination with the more recent findings (Lorberth et al., 1998; Yu et al., 2001; Ritte et al., 2002), suggests that phosphate incorporation during starch synthesis somehow determines the degradability of the starch particle. However, it is not yet known whether phosphorylation of starch is restricted to the period of biosynthesis. If so, GWD in leaves would be expected to be active only during the light period. However, binding of GWD to transitory starch granules increases during starch degradation (Ritte et al., 2000a), suggesting a more active role of the enzyme during the dark period. Therefore, we examined in vivo starch phosphorylation in photosynthetically competent cells. For these analyses we chose the unicellular green alga Chlamydomonas reinhardtii, which is excellently suited for short-term labeling studies, and potato leaves, in which we have documented before that the amount of granule-bound GWD strongly increases during starch breakdown (Ritte et al., 2000a). Using isolated transitory starch granules, we established a method to liberate near-surface glucan chains and to analyze their phosphate esters using chromatography and mass spectrometry.
RESULTS
In Vivo Starch Phosphorylation in Chlamydomonas
Genomic analysis provides evidence for the existence of GWD-like sequences in Chlamydomonas (Mikkelsen et al., 2004). Supportive evidence for a GWD protein in Chlamydomonas was derived from western-blot analysis. An antibody directed against the potato GWD clearly recognized a Chlamydomonas protein very similar in size to the potato GWD (data not shown). Thus, Chlamydomonas appears to be suited to study starch phosphorylation in vivo. In addition, short-term labeling studies can be easily performed with this unicellular organism. We chose the cell wall defective cw15 mutant for the in vivo labeling studies, as the cells can be lysed more rapidly than the wild type. Cells were grown photoautotrophically under a 12-h light period. Twenty-eight hours before the start of the labeling experiment, cells were transferred to a medium lacking phosphate to induce the high affinity phosphate uptake system (Shimogawara et al., 1999). Under these conditions, the photosynthetic capacity is not significantly affected (Wykoff et al., 1998). At the end of the light period, algae were harvested by centrifugation, resuspended in fresh medium containing 20 mm NaHCO3, placed in microtubes, and agitated on a rotating wheel in light or darkness.
Synthesis and degradation of starch were monitored in labeling experiments using NaH14CO3. In illuminated cells, the rate of starch synthesis was high, ranging from 93 to 133 μmol C (mg chlorophyll [Chl])−1 h−1 in four independent experiments. No incorporation of 14C into starch was detectable in darkened cells. If starch was prelabeled with 14C in the light and the cells were then transferred to darkness, we consistently observed a rapid decrease in the labeled starch fraction (Fig. 1). This indicates that the darkened cells rapidly initiate starch mobilization.
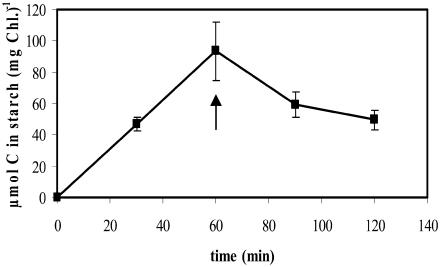
Figure 1.
Starch synthesis in the light and breakdown of the newly synthesized starch in darkness in Chlamydomonas. Algal cells equivalent to 3 μg of Chl were suspended in 0.525 mL of medium supplemented with 20 mm NaHCO3 and 0.25 μCi NaH14CO3. Following agitation for 60 min in the light, cells were transferred to darkness (indicated by an arrow). Cells were lysed at the times indicated, and the radioactivity in starch was determined. Each value is the mean of four replicate samples ± se.
Phosphate incorporation into starch was monitored by adding 32P-orthophosphate to the cell suspension. Following incubation, cells were quickly lysed in 2% (w/v) SDS, and insoluble compounds were pelleted by centrifugation. After several washing steps, starch was solubilized using a mixture of α-amylase and amyloglucosidase. Following this treatment, no particulate material was detectable, and, therefore, the pellets almost exclusively consisted of starch. The algal cells incorporated phosphate into starch during both synthesis and degradation (Fig. 2A). Within the first 30 min of labeling, starch phosphorylation in darkened cells exceeded that in illuminated cells. However, in darkness, net phosphate incorporation markedly slowed down with time. Maximal 32P content was reached after 90 min. By contrast, illuminated cells incorporated phosphate into starch with an essentially constant rate during the 2-h incubation period. Under the experimental conditions used, the phosphate uptake into the cells was slightly higher in the light than in darkness. During 40 min, illuminated and darkened cells took up 1.27 and 1.06 μmol Pi (mg Chl)−1, respectively.
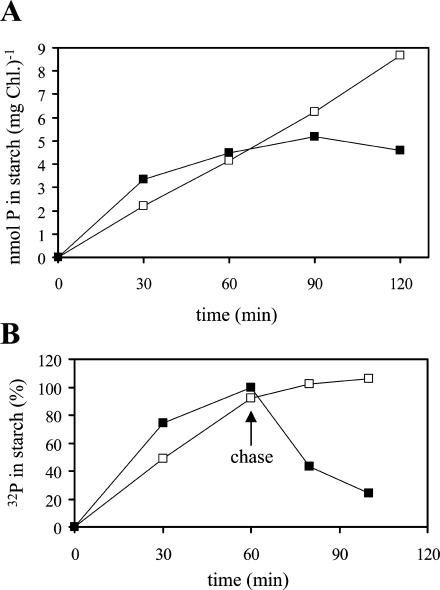
Figure 2.
Starch phosphorylation in light and darkness in Chlamydomonas. Algal cells equivalent to 7.2 μg of Chl were agitated in light (white symbols) or darkness (black symbols) for the times indicated, and the amount of 32P incorporation into starch was determined. The radioactivity in control samples in which the cells were lysed prior to addition of the label was subtracted (<40 cpm). Values are means of duplicate samples. A, Continuous labeling. B, Transient labeling (pulse and chase). Following incubation for 1 h with 40 μm 32P (6 μCi), unlabeled phosphate was added to give a final concentration of 20 mm (chase). The radioactivity incorporated into starch during 1 h in darkness was set to 100%.
The turnover of the starch-bound phosphate was studied by using pulse-chase experiments. After 1 h of incubation with 32P-orthophosphate, a 500-fold excess of nonlabeled phosphate was added. The amount of radioactivity in the starch was analyzed 20 and 40 min after the chase (Fig. 2B). No turnover of the starch-bound phosphate is detectable during starch synthesis in the light. The 32P labeling of the starch even slightly increased during the chase. Presumably, this is due to a delayed decrease in the intracellular specific radioactivity (e.g. in the plastidial ATP pool). By contrast, in darkened algae, more than 50% of the starch-associated radioactivity was lost within 20 min after the chase. This demonstrates that starch phosphorylation during starch breakdown is transient, and the phosphate esters formed underlie a rapid turnover. Thus, starch phosphorylation during degradation cannot be calculated from the net incorporation of radioactivity into starch. Based on the data shown in Figure 2, one can roughly estimate that the rate of phosphorylation during starch breakdown exceeds that during synthesis by (at least) a factor of two. The data obtained by an independent labeling and pulse-chase experiment are included as supplemental material (available at www.plantphysiol.org). From the mean rates of starch synthesis and starch phosphorylation in the light (average of two independent experiments), we calculated an esterification of approximately 0.2 nmol P/μmol Glc. Probably, this value is an underestimation, since the specific radioactivity of the plastidic ATP pool is likely to be (at least temporarily) lower than that of the orthophosphate supplied to the cells. However, this value is similar to starch phosphate contents of leaf starch (Blennow et al., 2000; Yu et al., 2001).
Starch Phosphorylation in Chlamydomonas Occurs at C6 and C3 Position of the Glc Units
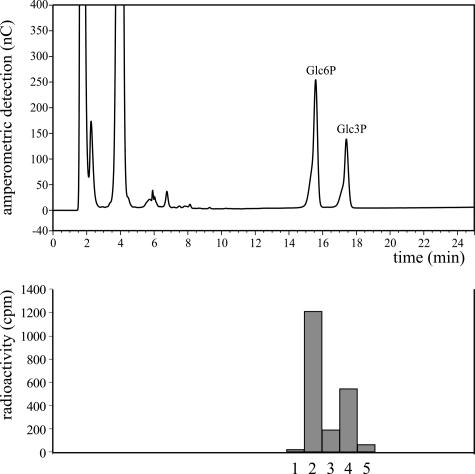
In higher plants, starch phosphorylation leads to the formation of both Glc6P and Glc3P residues (Tabata and Hizukuri, 1971; Yu et al., 2001). To determine whether both esters occur in Chlamydomonas starch, radiolabeled starch extracted from darkened cells was hydrolyzed, and the products were analyzed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD). Since the amounts of the sugar phosphates in the starch hydrolysate were below the limit of accurate amperometric detection, we added authentic Glc6P and Glc3P as internal standards. The peak fractions were collected and the radioactivity was determined. As shown in Figure 3, the 32P label coeluted with the two standards, indicating that in Chlamydomonas starch phosphorylation occurred at C6 and C3 positions of the Glc residues. The ratio of Glc6P to Glc3P is approximately 2:1, which is also typical for higher plants (Tabata and Hizukuri, 1971).
Figure 3.
Phosphorylation of Chlamydomonas starch occurs in C6 and C3 positions of the glucosyl residues. Following incubation of the algae with 32P-orthophosphate in darkness for 2 h, starch was isolated and subjected to acid hydrolysis. An aliquot of the hydrolysate was mixed with authentic Glc6P and Glc3P, and a sample equivalent to 2,100 cpm was analyzed by HPAEC-PAD (top). Fractions were collected, and the radioactivity was determined (bottom). Fractions 2 and 4 comprise material coeluting with Glc6P and Glc3P, respectively. A total of 97% of the radioactivity applied to the column was recovered in fractions 1 to 5. Essentially the same result was obtained in an independent experiment (data not shown).
Starch Phosphorylation in Potato Leaves
Intact higher plants are not suited for short-term labeling studies using phosphate isotopes, thus an alternative experimental system was required to investigate in vivo starch phosphorylation in potato leaves. It is reasonable to assume that most of the starch phosphorylation occurring during net starch degradation (compare with Fig. 2) takes place at the surface of the starch granule. As the phosphate level at the starch granule surface is likely to reflect the actual phosphorylating activity under various physiological conditions, we decided to analyze the glucan chains exposed to the starch granule surface.
Release of Glucan Chains from the Outer Layer of Leaf Starch Granules
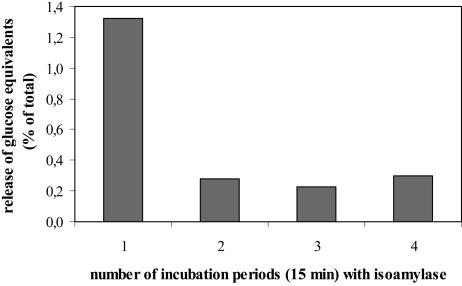
Using appropriate conditions isoamylase (EC 3.2.1.68) from Pseudomonas proved to be a useful tool to release glucan chains exposed at the granule surface (Fig. 4). Because of the instability of the enzyme activity (the enzyme was completely inactivated during 2 h of incubation in the absence of starch granules), the kinetics of the granule degradation was monitored by using a series of short (15-min) incubation periods and repetitive addition of fresh isoamylase solution. Following each incubation, the suspension was centrifuged and the supernatant was collected. The granules were briefly washed in buffer without enzyme and then incubated with fresh enzyme solution for a further 15 min. The glucans released were monitored as Glc following exhaustive hydrolysis. Approximately 1.3% of the total Glc content of the starch granule preparation was released within the first 15-min incubation period. During the three subsequent periods, only 0.2% to 0.3% of the total Glc content was released. Thus, a tiny portion of the α-1,6 linkages in the granule preparation is easily accessible to the enzyme, whereas the majority of the linkages is cleaved with at least 5-fold lower efficiency. This result was confirmed in an independent experiment (data not shown). It is likely that those chains released in the initial phase were exposed to the granule surface at the start of the enzymatic degradation. This assumption is supported by results obtained by an isoamylase treatment of transitory starch granules that had been phosphorylated by purified GWD in vitro. In this case, only surfaces near glucan chains can serve as phosphate acceptors, since exogenously supplied GWD has no access to the interior of the particle. Following phosphorylation of starch granules with 33P-ATP, 50% to 80% of the total incorporated radioactivity were solubilized by isoamylase, whereas no significant degradation of the granules could be detected macroscopically (compare with Fig. 4; 0.25 unit isoamylase/mg starch, t = 30 min, n = 3). Thus, treatment of intact starch granules with isoamylase yields a preparation enriched in chains from the outer layer.
Figure 4.
Quantification of the glucans released from intact starch granules by repetitive isoamylase treatment. Twenty milligrams of potato leaf starch granules were repeatedly incubated with a fresh solution of 5 units isoamylase in 0.5 mL of acetate buffer for 15 min each. At the end of each incubation period, granules and supernatant were separated by centrifugation. The amount of glucans released during each incubation step was determined as Glc following acid hydrolysis of the supernatant. Values are means of duplicate samples.
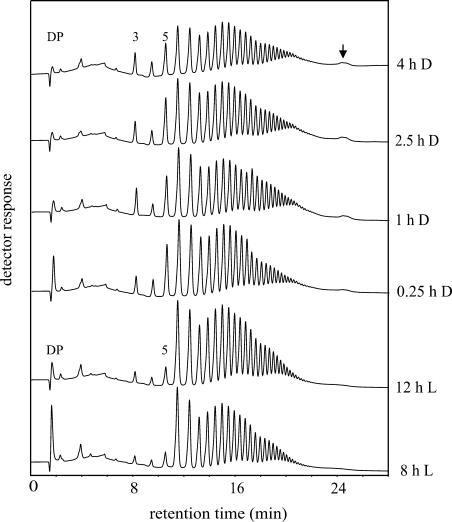
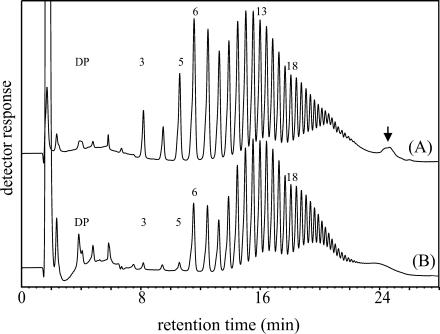
Starch granules were prepared from potato leaves that had been harvested at different times during the day/night cycle. Granules were isolated under denaturating conditions to remove granule surface-associated proteins and to prevent enzymatic modification of the starch during extraction. The granules were then treated with isoamylase for 20 min, and the glucan chains released were analyzed using HPAEC-PAD (Fig. 5). While the total amount of released glucans was very similar in the different samples, two qualitative differences became evident. First, the proportion of the very short chains (DP 3, DP 4, and especially DP 5) strongly increased upon darkening of the leaves. Second, a small late eluting peak (Fig. 5, marked by an arrow) was clearly present in samples from darkened leaves but hardly detectable in those from illuminated leaves. The same characteristic differences were observed in an independent experiment (data not shown). These alterations are restricted to glucan chains at the granule surface. We extracted starch from darkened leaves and compared the patterns of glucan chains released by isoamylase from either intact granules (Fig. 6A) or solubilized starch (Fig. 6B). Solubilized starch is completely debranched by the enzyme. In contrast with the sample derived from intact granules (Fig. 6A), the very short chains and the late eluting peak were hardly detectable in the chain length distribution profile of completely debranched starch (Fig. 6B).
Figure 5.
HPAEC-PAD analysis of glucan chains released by isoamylase from intact potato leaf starch granules. Potato plants were cultivated under a 14-h light period. Leaves were harvested at the times indicated, and starch was isolated. Starch granules (50 mg each) were incubated with 12.5 units isoamylase in a volume of 0.5 mL for 20 min. A total of 90 μL of the material released into the supernatant was analyzed using HPAEC-PAD. DP, Degree of polymerization.
Figure 6.
Side chain patterns from surface near glucans or from total starch. Intact (A) or solubilized (B) starch granules from darkened potato leaves were treated with isoamylase as described in “Materials and Methods.” Glucan chains equivalent to 100 nmol glucosyl residues each were analyzed.
The Phosphorylation Level of the Granule Surface Is Increased during Starch Breakdown
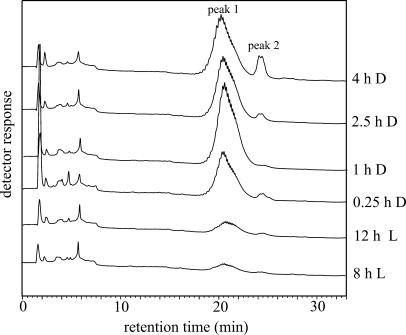
In order to purify phosphorylated side chains, the compounds released by isoamylase were subjected to anion-exchange chromatography using Q Sepharose. Uncharged chains were removed from the column by extensive washing with water. Subsequently, charged chains were eluted using a mixture of NaCl and HCl and were then analyzed by HPAEC-PAD (Fig. 7). In samples derived from darkened leaves, two peaks were eluted between 20 and 25 min. These peaks were hardly detectable in samples prepared from illuminated leaves (Fig. 7). This result was confirmed in an independent experiment (data not shown). When the charged chains purified from darkened leaves (4 h D, compare with Fig. 7) were cochromatographed with the total glucan pool released from granules that had been prepared from illuminated leaves (8 h L, compare with Fig. 5), it became evident that the phosphorylated glucans comprise the shallow late peak (compare with Figs. 5 and 6, marked by arrows) present in the nonfractioned chains in samples from darkened leaves.
Figure 7.
HPAEC-PAD analysis of granule surface exposed phosphorylated chains. Following isoamylase treatment of intact starch granules (compare with Fig. 5), the phosphorylated chains were isolated by anion-exchange. Phospho-oligosaccharides were eluted in one step using 100 mm NaCl, 10 mm HCl.
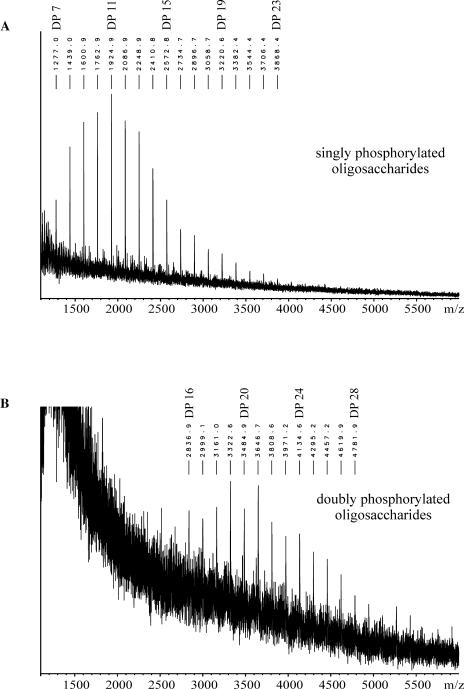
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) analysis confirmed that the glucans purified by anion-exchange chromatography (compare with Fig. 7) are phosphorylated oligosaccharides (Fig. 8). Singly phosphorylated glucan chains ranging from DP 7 to DP 23 (Fig. 8A) were detected in the sample (one-step elution) derived from intact starch granules from darkened leaves (4 h D). A further resolution of the phosphorylated glucans into two fractions (peaks 1 and 2; compare with Fig. 7) by stepwise elution from the anion-exchanger revealed that side chains carrying two phosphate groups were also present. The compounds eluting in peak 2 (compare with Fig. 7) consist of doubly phosphorylated oligosaccharides ranging from DP 14 to DP 32 (Fig. 8B), whereas the material eluting in peak 1 comprises the monophosphorylated glucans. The mass spectra obtained closely resembled that depicted in Figure 8A (data not shown). Thus, in darkened leaves, the phosphate content of the starch granule surface is strongly increased. It should be noted that this increase was poorly reflected on the total starch level. This conclusion was reached when aliquots from the starch granule preparations analyzed in Figures 5, 7, and 8 were subjected to acid hydrolysis and the total Glc6P to Glc ratio was determined (Table I). The total starch phosphate content was only moderately elevated in the starch samples prepared from darkened leaves.
Figure 8.
MALDI-MS analysis of phosphorylated chains purified from starch of darkened potato leaves (4 h D). A, Phospho-oligosaccharides released by the one-step elution. B, Phosphoglucans were fractionated using the three-step elution (see “Materials and Methods”), and phospho-oligosaccharides comprising peak 2 (compare with Fig. 7) were analyzed. Masses are calculated as follows: singly phosphorylated chains, [DP × (Glc − H2O) + H2O + 2 Na+ + H2PO4−]+; doubly phosphorylated chains, [DP × (Glc − H2O) + H2O + 3 Na+ + 2 H2PO4−]+.
Table I.
Glc6P contents of starch from potato leaves
| Time of Leaf Harvest | Phosphate Content (nmol Glc6P/μmol Glc) |
|---|---|
| 8 h L | 0.36 |
| 12 h L | 0.39 |
| 0.25 h D | 0.44 |
| 1 h D | 0.44 |
| 2.5 h D | 0.50 |
| 4 h D | 0.46 |
For each time point, two aliquots (15 mg each) of the granule samples used in the previous experiments (compare with Figs. 5 and 7) were hydrolyzed. The contents of Glc6P and Glc were determined in duplicate. Data are means of the total of four measurements per time point. The variation in the Glc6P to Glc ratios between the two aliquots is ≤11%.
Starch Granules from Darkened Leaves Are Better Substrates for GWD in Vitro Compared with Granules from Illuminated Leaves
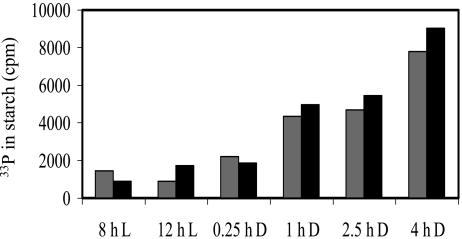
The data shown in Figure 7 strongly indicate that, similar to the results obtained in Chlamydomonas, the level of starch phosphorylation is higher during starch breakdown than during starch biosynthesis. However, in principle an alternative explanation is possible: If neutral glucan chains are degraded more rapidly than phosphorylated ones, the latter would be expected to increase relative to the uncharged chains. Two lines of evidence indicate that this is very unlikely to be relevant. First, in vitro phosphorylation of the granule samples with recombinant GWD revealed that starch granules extracted from darkened leaves were phosphorylated with an up to 7-fold higher rate compared with granules from illuminated leaves (Fig. 9). This increase was not caused by altered surface-to-mass ratios because the mean granule diameters were comparable in the different starch samples (revealed by size distribution analysis using a Coulter Counter; data not shown). Second, in vivo the amount of granule surface-associated GWD strongly increased under conditions of starch degradation in potato leaves, whereas the total amount of GWD, which is predominantly soluble, is unchanged (Ritte et al., 2000a). This result was confirmed using starch and soluble protein prepared under nondenaturating conditions from aliquots of the leaf samples that had been used for the analysis of the surface-bound glucan chains (data not shown).
Figure 9.
In vitro phosphorylation of starch granules by recombinant GWD. Starch was extracted from leaves harvested at the times indicated. Granules (2 × 5 mg each) were incubated with 25 μm ATP containing 1.2 106 cpm [βγ-33P]ATP and 1 μg of GWD for 50 min. Gray and black bars represent the individual measurements of duplicate determinations. The radioactivity present in control samples lacking GWD was subtracted.
DISCUSSION
In photosynthesizing Chlamydomonas cells, starch is phosphorylated concurrently with starch biosynthesis without detectable turnover. Likewise, in potato leaves, starch phosphorylation occurs also during biosynthesis, as indicated by the similar levels of starch-bound Glc6P in light and darkness (Table I). Thus, transitory starch is phosphorylated during synthesis, as it has previously been documented for reserve starch in potato amyloplasts (Nielsen et al., 1994; Wischmann et al., 1999). However, here we show that during breakdown of transitory starch, phosphorylation occurs at an even higher rate. Phosphorylation of Chlamydomonas starch under conditions of net starch breakdown is transient as it is subjected to a substantial turnover. Probably, this is due to the fact that both phosphorylation and degradation take place at the granule surface.
Elevated starch phosphorylation during breakdown of the granule implies that GWD plays an active role during starch degradation. In leaves of Arabidopsis, potato, and pea, the total amounts of the GWD protein are apparently unchanged during light and darkness (Ritte et al., 2000a; Yu et al., 2001). However, for the latter two species, we have shown that the proportion of granule-associated GWD drastically increases during starch breakdown. Modifications of the granule surface are involved in the increased binding of GWD to starch, as demonstrated by in vitro binding assays using purified recombinant GWD (Ritte et al., 2000a). The results presented here show that there is a coincidence of (1) increased binding of GWD with a higher phosphorylation level of the granule surface in vivo and of (2) increased in vitro binding of the recombinant GWD with enhanced phosphorylating activity. The modifications of the granule surface occurring during starch breakdown and leading to elevated binding and activity of GWD are not yet known. Those granules that under in vitro conditions are more effective GWD substrates already display an elevated phosphorylation level at their surface. Thus, starch phosphorylation might be a self-enhancing process. However, the phosphorylation levels of the granule samples D 0.25 h, D 1 h, D 2.5 h, and D 4 h vary about 2-fold (Fig. 7), whereas the rates of in vitro phosphorylation in these granule samples vary approximately 4-fold (Fig. 9). Furthermore, phosphate free granules of the Arabidopsis sex1-3 mutant (Yu et al., 2001) are a suitable substrate for purified GWD in vitro (data not shown). The increase in surface-bound phosphate is, therefore, not (solely) responsible for an enhanced in vitro phosphorylation. Possibly, phosphorylation and degradation of starch are interdependent processes. Phosphorylation of the granule surface by GWD could facilitate the attack of degrading enzymes. By the activity of the latter, new phosphorylation targets could become accessible. These phosphorylation targets are not yet clearly defined. Mikkelsen et al. (2004) found that the amylopectin chain length distribution strongly affected the in vitro activity of GWD. The authors concluded that GWD phosphorylates long chains (DP 30–100). However, the chain length distribution profiles of the outer granule layer did not show pronounced variations in the longer chains during the day/night cycle (Fig. 5). Furthermore, in vitro phosphorylation studies performed with Arabidopsis sex1-3 starch and recombinant GWD did not indicate a strong preference for longer chains (A. Scharf, S. Habel, M. Steup, and G. Ritte, unpublished data).
In addition to modifications of the granule surface, posttranslational modification of GWD might also be involved in the increased glucan phosphorylation during starch breakdown. In leaves, the regulation of starch synthesis involves redox modulation of ADP-Glc pyrophosphorylase activity in response to light and sugars (Hendriks et al., 2003). Whether changes in sugar levels or in the plastidial redox state during the light-to-dark transition also affect the activity of GWD remains to be identified.
Our analyses of intact potato leaf starch granules using isoamylase demonstrate that the outer layer of the granules differs from the remainder. This is indicated by the kinetics of isoamylase activity using intact starch granules (Fig. 4). Possibly, the outermost layer displays a lower level of crystallinity and is thus more easily attacked by the enzyme. Furthermore, the distribution profile of glucan chains released by isoamylase from intact granules or solubilized granules, respectively, differs (Fig. 6). Nielsen et al. (2002) demonstrated that side chains with six Glc residues are the shortest abundant chains formed during starch synthesis in Arabidopsis. Obviously, this also holds true for potato. Chains of DP 6 were the shortest abundant chains present at the outermost layer of potato starch granules in the light. By contrast, as a consequence of starch breakdown the amount of chains shorter than six Glc residues strongly increased in darkness (Fig. 5).
When the surface phosphate levels are compared with the total starch phosphate contents, as determined following hydrolysis of complete granules, it is obvious that increased surface phosphorylation does not necessarily lead to a significant increase in total starch phosphate. Thus, the overall starch phosphate content mainly reflects starch phosphorylation during synthesis, and changes occurring at the surface during degradation are probably too small to significantly alter the total phosphate content of starch. GWD seems to be ubiquitous in higher plants and green algae (Ritte et al., 2000b; Yu et al., 2001; Reimann et al., 2002; Li et al., 2003). However, the total starch phosphate content does not correlate with the amount of GWD in various plant species. Despite the presence of GWD in considerable amounts in seeds of pea and maize and in banana fruits, the respective starches contained only minute amounts of covalently bound phosphate (Ritte et al., 2000b). Obviously, the extent at which starch is phosphorylated during synthesis varies in different plant species and different plant organs. Especially high levels of starch phosphorylation have been documented for some tuberous starches, e.g. curcuma and potato (Blennow et al., 1998). The starch-bound phosphate makes up a large proportion of the total phosphate content in potato tubers, and its level is affected by the phosphate supply during plant growth (Kossmann and Lloyd, 2000). One might speculate that the biosynthesis-associated starch phosphorylation in tubers of potato and curcuma serves a function in long-term phosphate storage. In other plant organs and other plant species, however, starch phosphorylation might be more restricted to the period of degradation. The analysis of the phosphorylation level of the starch granule surface as described here could be a tool to investigate in vivo starch phosphorylation in tubers, seeds, and fruits.
MATERIALS AND METHODS
Chemicals and Enzymes
[γ-33P]ATP (10 mCi/mL; 3,000 Ci/mmol), [32P]phosphoric acid (54 mCi/mL, carrier free), and [14C]NaHCO3 (0.25 mCi/mL; 57 mCi/mmol) were all purchased from Hartmann Analytic (Braunschweig, Germany). Amyloglucosidase from Aspergillus niger and α-amylase from Bacillus amyloliquefaciens were obtained by Roche (Mannheim, Germany); isoamylase from Pseudomonas was from Megazyme (Wicklow, Ireland). Glc3P was synthesized as described elsewhere (Ritte et al., 2002).
Plant Material and Growing Conditions
The cell wall deficient mutant cw15 (Hyams and Davies, 1972) of Chlamydomonas reinhardtii Dangeard was grown under a continuous light-dark cycle (12 h light, 150 μmol quanta m−2 s−1, 12 h dark; 25°C) and aerated with air containing 2% (v/v) CO2. Cells were cultivated for 3 d in high salt minimal medium (Sueoka, 1960) supplemented with trace elements as described in Kuhl (1962). To induce phosphate starvation, the cells were pelleted by centrifugation (800g, 15 min, 4°C), washed once in P-starvation medium (same as above, but phosphate was replaced by 25 mm HEPES-NaOH, pH 7.2), and subsequently grown for a further 28 h in the P-starvation medium under otherwise unchanged conditions.
Potato plants (Solanum tuberosum L. cv Desiree) were cultivated in a growth cabinet under controlled conditions (14 h light, approximately 250 μmol quanta m−2 s−1 [20°C]; 10 h dark [16°C]). Leaves from plants in the early flowering stage were analyzed.
Labeling Experiments using Chlamydomonas
Following P-starvation, cells were harvested by centrifugation (10 min at 1,000g, 4°C) and suspended in fresh P-starvation medium to give an OD800 of 2. The cell suspension (0.5 mL each) was transferred into 1.5-mL microtubes. Labeling experiments were started by adding 25 μL of a mixture of NaHCO3 (final concentration 20 mm) and sodium phosphate (final concentration 40 μm). To monitor synthesis or phosphorylation of starch, 0.25 μCi NaH14CO3 or 3 to 6 μCi 32P-orthophosphate were included in this mixture. The tubes were then agitated on a rotating wheel in the light (170 μmol m−2 s−1) or in darkness (<2 μmol m−2 s−1) at 22°C as stated. In the pulse-chase experiments, 50 μL of 230 mm sodium phosphate, pH 7.2, or 50 μL of a mixture of 230 mm sodium phosphate, pH 7.2, and 57.5 mm NaHCO3 were added to cell suspensions (volume = 525 μL) that had been incubated with 32P (see above) for 1 h in darkness or light, respectively. Subsequently, cells were further incubated as stated. Following incubation, cells were lysed by adding one-fifth volume of a 10% (w/v) SDS solution. In control samples, SDS was added prior to the addition of the radioactivity. Following centrifugation for 5 min at 15,000g, the supernatant was discarded and the pellet was washed three times in 0.9 mL of 2% (w/v) SDS, once in 10 mm sodium phosphate, pH 7.2, and two times in 50 mm sodium acetate, pH 5, 5 mm CaCl2. Each washing step was performed for at least 20 min under agitation. The starch was recovered by centrifugation as above. To solubilize the starch, the pellets were resuspended in 0.2 mL of 50 mm sodium acetate, pH 5, 5 mm CaCl2 containing 10 units amyloglucosidase and 140 units α-amylase and were incubated for 2 to 3 h at 37°C under agitation. The samples, which now had a clear appearance, were centrifuged, and the entire supernatant or aliquots of it were mixed with 3 mL of scintillation fluid (Ready Save; Beckman Coulter, Fullerton, CA). Radioactivity was counted using a Beckman Coulter LS6500 scintillation counter.
Isolation of Potato Leaf Starch Granules
For the analysis of starch granule-associated proteins, starch was extracted from potato leaves according to Ritte et al. (2000a). For structural analyses and in vitro phosphorylation of starch, this method was modified: The extraction buffer was replaced by 2% (w/v) SDS, and the extraction was performed at room temperature. The crude starch pellet was washed two times in 2% SDS for 15 min and then filtered through nylon nets of 60, 30, and 20 μm mesh width. Subsequently, the filtered starch was washed two times in water and then passed through a Percoll cushion (Ritte et al., 2000a). Following four washing steps with water, the starch was dried under vacuum and stored at −20°C until use.
Isoamylase Treatment of Leaf Starch
For intact starch granules, granules were suspended in 5 mm sodium acetate, pH 4.0, as indicated, and isoamylase (0.25 unit/mg starch) was added. Following incubation at 40°C as stated, the starch granules were pelleted by centrifugation (5 min, 20,000g). The supernatant was collected and heated (5 min, 95°C).
For solubilized granules, 2 mg starch were solubilized by adding 375 μL of 75 mm NaOH and incubation for 2 h at 95°C. Following neutralization with 2 n HCl, 100 mm sodium acetate, pH 4.0, was added to give a final concentration of 5 mm. The solubilized starch was debranched with 5 units isoamylase for 30 min at 40°C.
To measure the amount of glucosyl residues present in starch or released from starch granules by isoamylase, starch granules or aliquots of the solubilized glucan chains were hydrolyzed in 1 n HCl for 2 h at 100°C. Following neutralization with KOH, the amount of Glc was assayed according to Waffenschmidt and Jaenicke (1987).
Separation of Phosphorylated Oligosaccharides
Minicolumns containing 200 μL of Q-Sepharose-FF (Amersham Biosciences, Freiburg, Germany) were prepared using 1-mL plastic pipette tips. A filter paper and a thin layer of glass beads (212–300 microns; Sigma, St. Louis) served as frit to prevent leakage of the Sepharose. The isolation of phosphorylated glucans is based on the method described by Viksø-Nielsen et al. (1998). The column was equilibrated with 5 mm MES-NaOH, pH 8.0. Four hundred microliters of sample were applied that had been adjusted to pH 8.0 using 0.5 n NaOH. Neutral chains were eluted from the column with 10 volumes of water. Phosphorylated chains were then recovered using two volumes of a mixture containing 100 mm NaCl and 10 mm HCl (one-step elution). Alternatively, phosphoglucans were resolved by successive elution with 3 column volumes each of 50 mm NaCl, 100 mm NaCl, and, finally, 150 mm NaCl (three-step elution).
HPAEC-PAD Analyses
HPAEC-PAD analysis was performed as described (Ritte et al., 2000b). However, a Dionex (Sunnyvale, CA) DX 600 equipped with a CarboPac PA 100 column was used. Prior to analysis, samples were centrifuged through 10-kD membranes (Ultrafree MC; Millipore, Bedford, MA) that had been washed once with water. The DP of glucan chains was determined by comparison with standards of known DP (dextrin from potato starch, type IV; Sigma).
MALDI-MS Analyses
Purified phospho-oligosaccharides were dialyzed against water using Spectra/Por Float-A-Lyzer membranes (Spectrum, Breda, The Netherlands) with a cutoff pore size of 500 D. One hundred microliters of sample were then concentrated under vacuum to approximately 10 μL. An aliquot of the concentrated sample (0.5 μL) was mixed on the target with 0.7 μL of a matrix solution containing 2,5-dihydroxybenzoic acid (15 mg mL−1) dissolved in 30% (v/v) aqueous ethanol and was dried under a gentle stream of air. Mass spectra were recorded on a Bruker Reflex II (Bruker Daltonik, Bremen, Germany) in the positive ion mode. For ionization, a nitrogen laser (337 nm, 3 ns pulse width, 3 Hz) was used. For optimization of the mass spectra, the laser was aimed either at the central area of the sample or at the outmost edge of the crystal rim. All spectra were measured in the reflector mode using external calibration.
In Vitro Phosphorylation of Starch Granules Using Recombinant GWD
GWD from potato was heterologously expressed in Escherichia coli and was subsequently purified as described (Ritte et al., 2002). [γ-33P]ATP was enzymatically converted to a mixture of [γ-33P]ATP and [β-33P]ATP (designated as [βγ-33P]ATP) according to Ritte et al. (2002), however, 10 μL [γ-33P]ATP (instead of 1 μL) and 90 μL of buffer (instead of 350 μL) were used to increase the specific radioactivity. Five milligrams of starch granules that had been extracted under denaturing conditions were mixed with 0.5 mL of 50 mm HEPES-KOH (pH 7.5), 1 mm EDTA, 6 mm MgCl2, 0.025 mm ATP, and 1.2 106 cpm [βγ-33P]ATP. The reaction was started by adding 1 μg of GWD. The samples were agitated using a rotating wheel. To terminate the reaction, 0.12 mL of 10% (w/v) SDS were added, the samples were centrifuged (5 min, 10,000g), and the supernatant was removed. The starch was washed two times with 0.8 mL each of 10% SDS and four times with 2 mm ATP. The granules were resuspended in 0.1 mL of water, mixed with 3 mL scintillation fluid, and the radioactivity was counted.
Analysis of the Granule Size
The size distribution of potato leaf starch granules was analyzed using a Multisizer TM3 Coulter Counter (Beckman Coulter) equipped with a 30-μm aperture. Calibration was performed using latex particles of defined size.
Analytical Techniques
Chlorophyll was determined according to Arnon (1949). Starch bound Glc6P was assayed enzymatically (Nielsen et al., 1994) following acid hydrolysis of starch (Ritte et al., 2000b).
Supplementary Material
Acknowledgments
We thank Kerstin Pusch for skillful technical assistance and Silke Gopp for plant cultivation.
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. SFB–429–TP–B2 to M.S. and TP–B7 to G.R.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041301.
References
- Arnon DJ (1949) Copper enzymes in isolated chloroplasts. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SG, van de Wal MHBJ, Visser RGF (1998) Progress in understanding the biosynthesis of amylose. Trends Plant Sci 3: 462–467 [Google Scholar]
- Blennow A, Bay-Schmidt MA, Wischmann B, Olsen CE, Møller BL (1998) The degree of starch phosphorylation is related to chain length distribution of neutral and phosphorylated chains of amylopectin. Carbohydr Res 307: 45–54 [Google Scholar]
- Blennow A, Engelsen SB, Munck L, Møller BL (2000) Starch molecular structure and phosphorylation investigated by a combined chromatographic and chemometric approach. Carbohydr Polym 41: 163–174 [Google Scholar]
- Blennow A, Engelsen SB, Nielsen TH, Baunsgaard L, Mikkelsen R (2002) Starch phosphorylation: a new front line in starch research. Trends Plant Sci 7: 445–450 [DOI] [PubMed] [Google Scholar]
- Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by post-translational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizukuri S, Tabata S, Nikuni Z (1970) Studies on starch phosphate. Part 1. Estimation of glucose 6-phosphate residues in starch and the presence of other bound phosphate(s). Starch/Staerke 10: 338–343 [Google Scholar]
- Hyams J, Davies DR (1972) Induction and characterization of cell-wall mutants of Chlamydomonas reinhardi. Mutat Res 14: 381–389 [Google Scholar]
- Kossmann J, Lloyd J (2000) Understanding and influencing starch biochemistry. Crit Rev Plant Sci 19: 171–226 [PubMed] [Google Scholar]
- Kuhl A (1962) Zur Physiologie der Speicherung kondensierter anorganischer Phosphate in Chlorella. In Deutsche Botanische Gesellschaft, ed, Beiträge zur Physiologie und Morphologie der Algen. Fischer Verlag, Stuttgart, Germany, pp 157–166
- Li CY, Weiss D, Goldschmidt EE (2003) Effects of carbohydrate starvation on gene expression in citrus root. Planta 217: 11–20 [DOI] [PubMed] [Google Scholar]
- Lorberth R, Ritte G, Willmitzer L, Kossmann J (1998) Inhibition of a starch-granule-bound protein leads to modified starch and repression of cold sweetening. Nat Biotechnol 16: 473–477 [DOI] [PubMed] [Google Scholar]
- Mikkelsen R, Baunsgaard L, Blennow A (2004) Functional characterization of α-glucan, water dikinase, the starch phosphorylating enzyme. Biochem J 377: 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TH, Baunsgaard L, Blennow A (2002) Intermediary glucan structures formed during starch granule biosynthesis are enriched in short side chains, a dynamic pulse labeling approach. J Biol Chem 277: 20249–20255 [DOI] [PubMed] [Google Scholar]
- Nielsen TH, Wischmann B, Enevoldsen K, Møller BL (1994) Starch phosphorylation in potato tubers proceeds concurrently with de novo biosynthesis of starch. Plant Physiol 105: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann R, Ritte G, Steup M, Appenroth KJ (2002) Association of α-amylase and the R1-protein with starch granules precedes the initiation of net starch degradation in turions of Spirodela polyrhiza. Physiol Plant 114: 2–12 [DOI] [PubMed] [Google Scholar]
- Ritte G, Eckermann N, Haebel S, Lorberth R, Steup M (2000. b) Compartmentation of the starch-related R1 protein in higher plants. Starch/Staerke 52: 179–185 [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch related R1 protein is an α-glucan, water dikinase. Proc Natl Acad Sci USA 99: 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Lorberth R, Steup M (2000. a) Reversible binding of the starch-related R1 protein to the surface of transitory starch granules. Plant J 21: 387–391 [DOI] [PubMed] [Google Scholar]
- Ritte G, Steup M, Kossmann J, Lloyd JR (2003) Determination of the starch-phosphorylating enzyme activity in plant extracts. Planta 216: 798–801 [DOI] [PubMed] [Google Scholar]
- Shimogawara K, Wykoff DD, Usuda H, Grossman AR (1999) Chlamydomonas reinhardtii mutants abnormal in their responses to phosphorous deprivation. Plant Physiol 120: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proc Natl Acad Sci USA 46: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata S, Hizukuri S (1971) Studies on starch phosphate. Part 2. Isolation of glucose-3-phosphate and maltose phosphate by acid hydrolysis of potato starch. Starch/Staerke 23: 267–272 [Google Scholar]
- Viksø-Nielsen A, Blennow A, Nielsen TH, Møller BL (1998) Phosphorylated α(1,4) glucans as substrate for potato starch branching enzyme I. Plant Physiol 117: 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waffenschmidt S, Jaenicke L (1987) Assay of reducing sugars in the nanomole range with 2,2′-bicinchoninate. Anal Biochem 165: 337–340 [DOI] [PubMed] [Google Scholar]
- Wischmann B, Nielsen TH, Møller BL (1999) In vitro biosynthesis of phosphorylated starch in intact potato amyloplasts. Plant Physiol 119: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff DD, Davies JP, Melis A, Grossman AR (1998) The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117: 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T-S, Kofler H, Häusler RE, Hille D, Flügge U-I, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13: 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Tiessen A, Pilling E, Kato KL, Donald AM, Smith AM (2002) Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol 129: 516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.