Abstract
Human Papillomavirus (HPV) infections are known to cause cervical cancer worldwide, however, limited information is currently available on prevalence, types distribution and risk factors for HPV infection in the Arab countries. We conducted a cross-sectional observational study exclusively of women of Arabic origin residing in Qatar (n = 406) who were selected from the Women’s Hospital at Hamad Medical Corporation (HMC) and Health Centers of the Primary Health Care Corporation in Doha, Qatar over the period March 2013 to August 2014. Socio-demographic, behavioral and clinical data were collected. Four hundred and six cervical smears and 292 blood samples were included in the study. HPV typing was done using HPV type-specific primers-based real-time PCR, and Sanger sequencing. HPV-IgG and IgM were quantified using ELISA assays. The prevalence of HPV infection amongst Qatari and non-Qatari Arab women were 9.8% and 6.1%, respectively and 7.6% and 16.7% in women with normal and abnormal cytology, respectively. HPV 81 was the most commonly found genotype in women with normal cytology (34.5%), whereas HPV 81, 16 and 59 in women with abnormal cytology (25.0% each). All the HPV DNA positive women were seronegative and HPV-IgG prevalence was higher in Qatari women than in non-Qatari Arab women. None of the studied factors had any significant association with HPV-DNA positivity or HPV-IgG seropositivity. The overall identified HPV DNA prevalence and HPV seroprevalence among Arab women in Qatar were on the low side compared to global levels.
Introduction
Human Papillomavirus (HPV) is one of the most prevalent sexually transmitted infections worldwide, and molecular studies have implicated infection with specific HPV high-risk (HR) genotypes as etiological agents of cervical cancer [1–3]. Several factors influence and contribute to the development of cervical cancer. There are over 200 unique HPV genotypes that have been confirmed, of which 30 to 40 are categorized on the basis of their oncogenic potential as HR and low-risk (LR) leading to cervical neoplasia and mild dysplasia, respectively [4]. According to the World Health Organization (WHO), cervical cancer, although can be preventable, is responsible for over 270,000 death annually and is the second commonest type of cancer among women worldwide [5]. It is the fifth most diagnosed cancer amongst women in the State of Qatar [6]. Knowledge of HPV status improves cervical cancer screening and triage for women with mild or borderline cervical smears and HPV vaccination program. However, the guidelines for cervical cancer screening in Qatar are currently under review. As of today, most of the screenig for cervical cancer is opportunistic, in which either the physician is offering or the patient is requesting the test and the frequency is offered every 3 years for low risk patients. HPV vaccines are licensed in Qatar, but currently there is no vaccination program in the country. The vaccine is available however for those who are interested in the vaccine. HPV testing is also not routinely practiced, but it is often performed when a Pap smear result is abnormal or the patient request the test.
Though the incidence of cervical cancer in the Extended Middle East and North Africa (EMENA) shows lower rates compared to the rest of the world [2, 7], the burden of HPV infection still warrants public health interventions. In addition, the age specific HPV prevalence has varied widely across different population and showed two peaks of HPV positivity in younger and older women[8,9]. Among the general population of Arab women with normal or abnormal cytology residing in Qatar, we recently estimated an HPV prevalence rate of 6.1% [10]. We also identified the presence of a varied genotypic profile of HPV with a high prevalence of low-risk HPV genotype 81 [10]. However, HPV DNA testing cannot differentiate between current and previous infection and does not reflect the lifetime risk of HPV infection. Moreover, despite much progress, risk factors influencing the epidemiology of HPV infection are not yet fully understood [11].
During HPV infection, both humoral and cellular immune responses are induced, and antibody production against HPV is important for preventing the spread of infection and re-infection [12,13]. Additionally, it has been shown that the cell-mediated immune response cleared the majority of HPV infections within 1–2 year’s exposure [14]. Despite the fact that a number of seroprevalence studies have been conducted in resource-rich countries and some in resource-poor countries [15–18], no such data appears to be available from the Arab world.
Qatar, a country located in the Arabian Peninsula has in recent years experienced rapid economic growth and globalization resulting in a large influx of foreign expatriates from Western, other Middle Eastern, African and Asian countries. The total population of women of child-bearing age was reported as 447,298; of which 382,067 represent Arabic women (Qatari and non-Qatari) according to the Ministry of Development Planning and Statistics, Qatar [6]. The economic and demographic transition and the resulting dynamic socio-economic and socio-cultural environment, may affect the social and sexual behaviours in the country. However, how these changes affected the behavioral risk factors and impacts on the prevalence of HPV infection among Arab women is yet to be determined.
Therefore, in the present study, we extended our analysis to identify the potential risk factors for HPV acquisition along with HPV seroprevalence among Arab women, with normal or abnormal cytology, residing in the State of Qatar. The association of risk factors with HPV-DNA positivity and HPV-IgG seropositivity was also analyzed. In addition, HPV prevalence and genotypes were correlated with cytology results. It is hoped that the outcomes of this study will inform the evaluation of the relevance of HPV vaccination and HPV screening in the State of Qatar and the development of policies and guidelines for cervical cancer prevention.
Materials and Methods
Study population and sample collections
This cross-sectional study was conducted according to the principles expressed in the Declaration of Helsinki. This study was reviewed and approved by Research Committee of Hamad Medical Corporation, Weill Cornell Medicine-Qatar and Primary Health Care Corporation (PHCC), Doha, Qatar. All subjects and/or significant others provided written informed consent prior to their participation.
The sample was convenient sample of 406 Arab women, nationals of any of the 22 countries in the League of Arab States, attending the Women’s Hospital at HMC and PHCC. A sample size of 430 was calculated to estimate an HPV prevalence of 4% (precision rate = 2%; a reasonable precision given the expected low HPV prevalence) and a significance level of 5% (type I error α = 0.05). The refusal rate was 4% and mainly to time availability of the patients. Cervical samples were collected from all the subjects (406), of which only 292 individuals provided matched blood samples. Subjects refused to give a blood sample for several reasons such as they do not want to give blood, do not want to spend more time in the clinic, and also because of reluctance to undergo an extra medical procedure. Cervical samples were collected in ThinPrep vials (BD SurePath™) for Pap smear assay and molecular HPV typing. ThinPrep cytological smears were screened and evaluated at HMC and reported according to the Bethesda system for reporting of cervical cytology [19]. Whole blood samples were collected in plane tubes and sera was obtained by centrifuging the blood samples at 1902 g for 5 minutes and stored at -80°C for further use. Inclusion criteria for the study comprised married or previously married, non-pregnant women. Never married women were excluded from participating in this study since their participation was deemed culturally unacceptable. In addition, women with known diagnosis of cervical cancer and immunocompromised patients were also excluded from the study.
HPV risk factor survey
All subjects were interviewed using a structured questionnaire to collect socio-demographic and behavioral information on possible risk factors for HPV infection such as marital status, education, household income, smoking, contraceptive practices, knowledge about HPV, HPV vaccine and cervical cancer. The questionnaire was prepared on the basis of a previous study conducted in Qatar (S1 Questionnaire) [20]. Other data from medical records include age, nationality, clinical history, and cytological diagnosis were collected for each subject.
DNA isolation and HPV testing by real time PCR and sequencing
Viral DNA was extracted from cervical samples using QIAamp MinElute virus spin kit (Qiagen, CA, USA) as previously described [10]. To detect HPV-DNA, real-time polymerase chain reaction (RT-PCR) assay was carried out in ABI 7500 (Applied Biosystems, CA, USA) and PCR reaction mixture and amplification conditions for GP5+/GP6+ primers (HPV, L1 consensus primers) and PCO3/PCO4 primers (human β globin gene) have been previously described [10]. A positive control (cloned HPV-DNA) and a negative control (nuclease free water) were included in each amplification reaction. HPV DNA positivity among clinical samples was detected as previously described [10].
Real Time PCR-based kits (Sacace Biotechnology, Como, Italy) and Sanger method (Genewiz, NJ, USA) were used to identify the genotype(s) in the samples which tested positive for HPV DNA, as described previously [10, 21].
Detection of HPV IgG and IgM antibodies by Enzyme Linked Immunosorbent Assay (ELISA)
Human Papillomavirus IgG (HPV-IgG) and IgM (HPV-IgM) were quantified by double-antibody sandwich ELISA kits (Novateinbio, Cambridge, USA) according to the manufacturer's instructions. Briefly, 50 μl serum samples and 100 μl HRP conjugated antibody was added to pre coated 96 flat-bottomed plates and incubated for 1h at 37°C. Following incubation, unbound substances were removed by washing solution (5X) and finally, HRP substrate (50 μl of each Chromogen A and Chromogen B solution) was added and plate was incubated for 15 min at 37°C. After appearance of yellow color in negative wells, the reaction was stopped with 50 μl stop solution. The optical density (OD) was measured at 450 nm using an ELISA plate reader and results were analyzed. The cutoff value was calculated using the manufacturer’s instructions. The average OD of the negative control wells plus 0.15 was taken as the cutoff critical value. A negative HPV result was interpreted as sample OD less than the calculated cutoff value, and samples with an OD greater than the calculated cutoff value were reported as positive for HPV IgG.
Statistical analysis
Statistical analyses were performed using SPSS (IBM SPSS version 23.0). Sample characteristics including age, nationality, marital status, education level, household monthly income, smoking habits, current method of contraception, knowledge about HPV, HPV vaccine and related cervical cancer, cytology results, and clinical findings were briefed using frequency distributions. Crude association between HPV positivity and each of the previously mentioned characteristics was assessed using the chi-squared test. Significance level was considered at α = 0.05 and unadjusted odds ratios (OR) were reported with their corresponding 95% confidence intervals (CI).
Results
Demographic and clinical profiles of subjects
All enrolled Arab women were categorized into 5 broad age groups (21–30, 31–40, 41–50, 51–60, ≥61 years), in which 34.5% of women were aged 41–50 years, 29.8% were aged 31–40 years, 17.5% were aged 51–60 years, 13.3% were aged 21–30 years, and only 4.9% of women were aged 61 years or more. The age range across the sample was 59 years with a mean age of 42.8 years (SD = 10.5 years). Additionally, 55.4% of women (n = 225, 95% CI: 50.7–60.6%) who participated in this study were Qatari nationals, followed by 26.8% (n = 109, 95% CI: 22.7–31.3%) from Fertile Crescent, 10.6% (n = 43, 95% CI: 7.9–13.8%) from Arabian Peninsula, and only 7.1% (n = 29, 95% CI: 4.7–9.9%) from North and East Africa (Table 1). This is reflective of the make-up of the general Arab women population in Qatar. Other demographic and behavioral characteristics are described in Table 1.
Table 1. Unadjusted odd ratios (ORs) for HPV DNA positivity and their corresponding 95% confidence intervals (CIs) according to socio-demographic and related characteristics among 406 Arab women in Qatar.
| Total Sample N (%) | HPV patient N (%) | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Nationality | |||||
| Qatar | 225 (55.4) | 22 (9.8) | REF | ||
| Arabian Peninsula$ | 43 (10.6) | 3 (7.0) | 0.692 | 0.198–2.423 | 0.565 |
| Fertile Crescent£ | 109 (26.8) | 7 (6.4) | 0.633 | 0.262–1.532 | 0.311 |
| North and East Africa# | 29 (7.1) | 1 (3.4) | 0.33 | 0.043–2.541 | 0.287 |
| Age | |||||
| 21–30 | 54 (13.3) | 2 (3.7) | REF | ||
| 31–40 | 121 (29.8) | 9 (7.4) | 2.089 | 0.436–10.013 | 0.357 |
| 41–50 | 140 (34.5) | 12 (8.6) | 2.437 | 0.527–11.271 | 0.254 |
| 51–60 | 71 (17.5) | 8 (11.3) | 3.302 | 0.672–16.229 | 0.242 |
| ≥61 | 20 (4.9) | 2 (1.0) | 2.889 | 0.379–22.039 | 0.306 |
| Marital Status | |||||
| Married | 352 (88.2) | 26 (7.4) | REF | ||
| Separated/Divorced | 23 (5.8) | 1 (4.3) | 0.57 | 0.074–4.398 | 0.59 |
| Widowed | 24 (6.0) | 4 (16.7) | 2.508 | 0.798–7.884 | 0.116 |
| Education | |||||
| No Schooling | 43 (10.7) | 5 (11.6) | REF | ||
| Elementary—Intermediate | 83 (20.7) | 9 (10.8) | 0.924 | 0.289–2.952 | 0.894 |
| Secondary/High school | 96 (23.9) | 7 (7.3) | 0.598 | 0.178–2.002 | 0.404 |
| College/University Degree | 179 (44.6) | 12 (6.7) | 0.546 | 0.182–1.642 | 0.282 |
| Household Monthly Income | |||||
| <5,000 QAR | 11 (3.1) | 3 (27.3) | REF | ||
| 5,000–20,000 QAR | 146 (40.9) | 11 (7.5) | 0.217 | 0.050–0.938 | 0.041 |
| > 20,000 QAR | 149 (41.7) | 13 (8.7) | 0.255 | 0.060–1.080 | 0.064 |
| Don't know | 51 (14.3) | 5 (9.8) | 0.29 | 0.058–1.459 | 0.133 |
| Smoking | |||||
| No | 371 (96.9) | 27 (7.3) | REF | ||
| Yes | 12 (3.1) | 2 (16.7) | 2.548 | 0.531–12.222 | 0.242 |
| Current Method of Contraception | |||||
| No | 287 (71.9) | 24 (8.4) | REF | ||
| Yes | 112 (28.1) | 8 (7.1) | 0.843 | 0.367–1.937 | 0.687 |
| Birth Control Pills | |||||
| No | 359 (91.1) | 26 (7.2) | REF | ||
| Yes | 35 (8.9) | 4 (11.4) | 1.653 | 0.542–5.041 | 0.377 |
| Condom | |||||
| No | 393 (99.7) | 30 (7.6) | REF | ||
| Yes | 1 (0.3) | 0 (0.0) | a/n | a/n | 1- |
| Intrauterine Device | |||||
| No | 352 (89.8) | 29 (8.2) | REF | ||
| Yes | 40 (10.2) | 1 (2.5) | 0.286 | 0.038–2.155 | 0.224 |
| Rhythm Method | |||||
| No | 388 (98.7) | 30 (7.7) | REF | ||
| Yes | 5 (1.3) | 0 (0.0) | a/n | a/n | 1- |
| Abstinence | |||||
| No | 390 (99.2) | 30 (7.7) | REF | ||
| Yes | 3 (0.8) | 0 (0.0) | a/n | a/n | 1- |
| Withdrawal | |||||
| No | 387 (98.5) | 28 (7.2) | REF | ||
| Yes | 6 (15) | 2 (33.3) | 6.411 | 1.125–36.539 | 0.036 |
| Female Sterilization | |||||
| No | 373 (94.9) | 29 (10.5) | REF | ||
| Yes | 20 (5.1) | 1 (5.0) | 0.624 | 0.081–4.832 | 0.652 |
| Knowledge about HPV | |||||
| No | 349 (88.1) | 26 (7.4) | REF | ||
| Yes | 47 (11.9) | 5 (10.6) | 1.479 | 0.539–4.059 | 0.447 |
| Knowledge about cervical cancer | |||||
| No | 29 (7.4) | 2 (6.9) | REF | ||
| Yes | 363 (92.6) | 30 (8.3) | 1.216 | 0.276–5.365 | 0.796 |
| Knowledge about HPV Vaccine | |||||
| No | 320 (93.0) | 24 (7.5) | REF | ||
| Yes | 24 (7.0) | 4 (16.7) | 2.467 | 0.780–7.800 | 0.124 |
| Cytology results | |||||
| Normal | 382 (94.1) | 29 (7.6) | REF | ||
| Abnormal^ | 24 (5.9) | 4 (16.7) | 2.434 | 0.780–7.599 | 0.126 |
| Clinical findings | |||||
| Routine smear test | 291 (71.7) | 23 (7.9) | REF | ||
| Symptomatic results* | 115 (28.3) | 10 (8.6) | 1.11 | 0.511–2.411 | 0.793 |
^Abnormal cytology includes LGSIL + HGSIL.
*Symptomatic results include: cervical erosion, genital warts, lower abdominal pain, menorrhagia, pelvic pain, post coital bleeding, primary infertility, secondary infertility, vaginal bleeding, vaginal spotting and vulva itching.
$Arabian Peninsula excluding Qatar (KSA, Kuwait, Bahrain, UAE, Yemen).
£Fertile Crescent (Egypt, Iraq, Jordan, Lebanon, Palestine, Syria).
#North & East Africa (Morocco, Algeria, Tunisia, Sudan).
Furthermore, data on Table 1 shows that 71.7% (n = 291, 95% CI: 67.5–76.1%) of women underwent routine gynecologic care and only 28.3% (n = 115, 95% CI: 23.9–32.5%) had different clinical symptoms such as vaginal discharge, vaginal bleeding, genital warts, vulva itching, lower abdominal pain, infertility, dyspareunia, polymenorrhagia/menorrhagia, amenorrhea and others. Cytology results show 94.1% (n = 382, 95% CI: 91.6–96.3%) of women with normal cytology with no lesions, and 5.9% (n = 24, 95% CI: 3.7–8.4%) of women with abnormal cytology results, including either low-grade squamous intraepithelial lesion (LGSIL) indicating mild dysplasia; or high-grade squamous intraepithelial lesion (HGSIL) indicating severe intraepithelial neoplasia and atypical cells of undetermined significance (ASCUS).
Risk factors associated with HPV DNA and type-specific prevalence
A univariate analysis of the association between HPV DNA positivity / type distribution and socio-demographic/behavioural variables are shown in Table 1. The overall HPV prevalence in all Arab women was 8.1% (n = 33, 95% CI: 5.4–11.1%) with a higher prevalence (9.8%, n = 22, 95% CI: 6.2–13.8%) among Qatari women and 6.1% (n = 11, 95% CI: 2.8–9.9%) in non-Qatari women. The results in Table 1 show that there was no statistically significant relationship between HPV infection and nationality (Qatari and Non-Qatari) (p = 0.673). Among non-Qatari Arab women, HPV prevalence was highest in women originating from Arabian Peninsula 7.0% (n = 3, 95% CI: 0.0–16.3%), followed by women from Fertile Crescent countries 6.4% (n = 7, 95% CI: 2.8–11.9%). The lowest prevalence of 3.4% (n = 1, 95% CI: 0.0–10.3%) was found in women originating from North and East Africa (Table 1).
HPV prevalence was found to be highest (11.3%) in the age group 51–60 (n = 8, 95% CI: 5.6–18.3%) and lowest (3.7%) in the 21–30 age group (n = 2, 95% CI: 0.0–9.3%) (Table 1). However, no significant difference was found between HPV prevalence vs. age groups. From the cytology results, prevalence of HPV was 7.6% (n = 29, 95% CI: 5.0–10.2%) among women with normal cytology and 16.7% (n = 4, 95% CI: 4.2–33.3%) among those with abnormal cytology. The latter included all the 24 abnormal samples with LGSIL of which four were HPV positive. Of the four samples, three were Qatari women diagnosed with LGSIL (n = 2) and ASCUS (n = 1) and one women was Egyptian diagnosed with LGSIL. Among the Qatari nationals, the most frequent genotypes were LR HPV 11, 90 and 81 along with the HR HPV 16, while only HR HPV 16 was found in the Egyptian national. No HGSIL cases were detected. No significant difference was observed between HPV prevalence and cytology results (OR: 2.43, 95% CI: 0.78–7.60) and HPV prevalence and clinical findings (OR: 1.11, 95% CI: 0.51–2.41) (Table 1).
The risk factors such as marital status (widowed), level of education (no schooling and intermediate), household monthly income (<5000.00 QAR) and smoking tended to have increase of HPV infection risk (16.7%, 11.1%, 27.3% and 16.7%, respectively), however, did not observe any statistical significance. The other risk factors like reproductive factor (contraceptive methods), knowledge about HPV, cervical cancer and HPV vaccine have no influence in HPV infection risk and had no significant association.
Of the total of 406 samples collected in this study population, 33 samples (8.13%) were positive for HPV DNA. These positive samples were further screened to identify the HPV genotypes and were classified based on their oncogenic potential to high-risk (HR) and low-risk (LR) as shown in Table 2. At least one of 8 different genotypes (HR or LR) was detected in the 33 positive samples by HPV genotyping kits and/or DNA based sequencing (Table 2).
Table 2. The distribution of HPV types among HPV DNA positive cervical samples among Arab women in Qatar.
| Normal Cytology (N = 382) | Abnormal Cytology (N = 24) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single | Double | Unknown | Total | Percent | Single | Double | Unknown | Total | Percent | |
| HPV positive | 18 | 3 | 8 | 29 | 7.59 | 3 | 1 | 0 | 4 | 16.67 |
| High-risk | ||||||||||
| 16 | 0 | 0 | - | 0 | 0.00 | 1 | 0 | - | 1 | 25.00 |
| 33 | 0 | 1 | - | 1 | 3.45 | 0 | 0 | - | 0 | 0.00 |
| 35 | 1 | 1 | - | 2 | 6.90 | 0 | 0 | - | 0 | 0.00 |
| 39 | 1 | 0 | - | 1 | 3.45 | 0 | 0 | - | 0 | 0.00 |
| 59 | 0 | 1 | - | 1 | 3.45 | 0 | 1 | - | 1 | 25.00 |
| Low-risk | ||||||||||
| 11 | 8 | 1 | - | 9 | 31.03 | 1 | 0 | - | 1 | 25.00 |
| 81 | 8 | 2 | - | 10 | 34.48 | 0 | 1 | - | 1 | 25.00 |
| 90 | 0 | 0 | - | 0 | 0.00 | 1 | 0 | - | 1 | 25.00 |
Among HPV positive women, taking into account the total frequency of occurrence of each specific genotype occurring either as single/multiple infection (sum of frequency of genotypes belonging to a specific risk category/total number of women (33)), 63.6% (n = 21, 95% CI: 45.5–78.8%) had at least one LR HPV genotype and 18.2% (n = 6, 95% CI: 6.1–33.3%) had at least one HR HPV genotype (Table 2). The most frequent HR genotype among women with normal cytology was HPV 35 (6.9%), while HPV 16 and HPV 59 were the most frequent genotypes among women with abnormal cytology (25%) (Table 2). Furthermore, HPV 81 (34.5%) was the most frequent LR genotype among women with normal cytology; while HPV 11, HPV 81, and HPV 90 had equal distribution among women with abnormal cytology (Table 2). Eight samples (24.2%) remained uncharacterized and all of them were with normal cytology (Table 2). Moreover, single infection and double infections (infection with two different HPV genotypes) were noted. Among women with normal cytology, 18 women (62.1%; 95% CI: 44.8–79.3%) had single infection, and 3 (10.3%; 95% CI: 0.0–24.1%) had double infections (Table 2). However, in women with abnormal cytology, 3 (75.0%; 95% CI: 25.0–100.0%) had single infection, and 1 (25.0%; 95% CI: 0.0–75.0%) had double infections (Table 2).
HPV type-specific was not significantly associated with any of potential risk factors, assessed in the present study, such as marital status, education level, economic status, usage of contraception, smoking habits and knowledge about HPV, HPV vaccine and cervical cancer (Table 1).
Seroprevalence and associated risk factors in Arab women
We assessed the levels of IgG and IgM in the sera of 292 Arab women residing in the State of Qatar. The seroprevalence of HPV according to different variables is shown in Table 3. The overall HPV-IgG prevalence was 4.5% (n = 13, 95% CI: 2.4–6.8%). The prevalence of HPV-IgG in Qatari women was higher (5.3%, n = 9, 95% CI: 2.3–8.8%) than non-Qatari women 3.3% (n = 4, 95% CI: 0.0–6.6%) (Table 3). Among non-Qatari Arab women, HPV-IgG antibodies were detected higher (9.1%) in women originating from North and East Africa (n = 2, 95% CI: 0.0–22.7%) followed by Fertile Crescent countries 2.8% (n = 2, 95% CI: 0.0–6.9%).
Table 3. Unadjusted odd ratios (ORs) for HPV IgG positivity and their corresponding 95% confidence intervals (CIs) according to selected descriptive variables among 292 Arab women in Qatar.
| Total Sample N (%) | IgG Positivity N (%) | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Nationality | |||||
| Qatar | 171 (58.6) | 9 (5.3) | REF | ||
| Arabian Peninsula$ | 27 (9.2) | 0 (0.0) | n/a | n/a | n/a |
| Fertile Crescent£ | 72 (24.6) | 2 (2.8) | 0.514 | 0.108–2.442 | 0.403 |
| North and East Africa# | 22 (7.5) | 2 (9.1) | 1.800 | 0.363–8.925 | 0.472 |
| Age | |||||
| 21–30 | 38 (13.0) | 2 (5.3) | REF | ||
| 31–40 | 81 (27.7) | 6 (7.4) | 1.440 | 0.277–7.490 | 0.665 |
| 41–50 | 99 (33.9) | 2 (2.0) | 0.371 | 0.050–2.734 | 0.331 |
| 51–60 | 58 (19.9) | 3 (5.2) | 0.982 | 0.156–6.169 | 0.984 |
| ≥61 | 16 (5.5) | 0 (0.0) | a/n | a/n | 0.999 |
| Marital Status | |||||
| Married | 253 (87.5) | 9 (3.6) | REF | ||
| Separated/Divorced | 17 (5.9) | 3 (17.6) | 5.810 | 1.414–23.874 | 0.015 |
| Widowed | 19 (6.6) | 1 (5.3) | 1.506 | 0.181–12.557 | 0.705 |
| Education | |||||
| No Schooling | 28 (9.7) | 1 (3.6) | REF | ||
| Elementary—Intermediate | 64 (22.1) | 3 (4.7) | 1.328 | 0.132–13.352 | 0.81 |
| Secondary/High School | 66 (22.6) | 2 (3.0) | 0.844 | 0.073–9.702 | 0.892 |
| College/University Degree | 132 (45.2) | 7 (5.3) | 1.512 | 0.179–12.802 | 0.704 |
| Household Monthly Income | |||||
| <5,000 QAR | 8 (3.1) | 0 (0.0) | a/n | a/n | 0.999 |
| 5,000–20,000 QAR | 104 (35.6) | 8 (7.7) | REF | ||
| > 20,000 QAR | 109 (37.3) | 3 (2.8) | 0.340 | 0.088–1.317 | 0.118 |
| Don't know | 41 (15.6) | 2 (4.9) | 0.615 | 0.125–3.028 | 0.550 |
| Knowledge about HPV | |||||
| No | 246 (86.0) | 12 (4.9) | REF | ||
| Yes | 40 (14.0) | 1 (2.5) | 0.5 | 0.063–3.954 | 0.511 |
| Knowledge about Cervical Cancer | |||||
| No | 18 (6.3) | 2 (1.1) | REF | ||
| Yes | 268 (93.7) | 11 (4.1) | 0.342 | 0.070–1.677 | 0.186 |
| Knowledge about HPV Vaccine | |||||
| No | 250 (92.6) | 10 (4.0) | REF | ||
| Yes | 20 (7.4) | 2 (1.0) | 2.667 | 0.543–13.102 | 0.227 |
| Cytology Results | |||||
| Normal | 274 (93.8) | 9 (3.3) | REF | ||
| Abnormal^ | 18 (6.2) | 4 (2.2) | 8.413 | 2.305–30.704 | 0.001 |
| Clinical Findings | |||||
| Routine Smear Test | 217 (74.3) | 13 (6.0) | REF | ||
| Symptomatic Results* | 75 (25.7) | 0 (0.0) | n/a | n/a | n/a |
^Abnormal cytology includes LGSIL + HGSIL.
*Symptomatic results include: cervical erosion, genital warts, lower abdominal pain, menorrhagia, pelvic pain, post coital bleeding, primary infertility, secondary infertility, vaginal bleeding, vaginal spotting and vulva itching.
$Arabian Peninsula excluding Qatar (KSA, Kuwait, Bahrain, UAE, Yemen).
£Fertile Crescent (Egypt, Iraq, Jordan, Lebanon, Palestine, Syria).
#North & East Africa (Morocco, Algeria, Tunisia, Sudan).
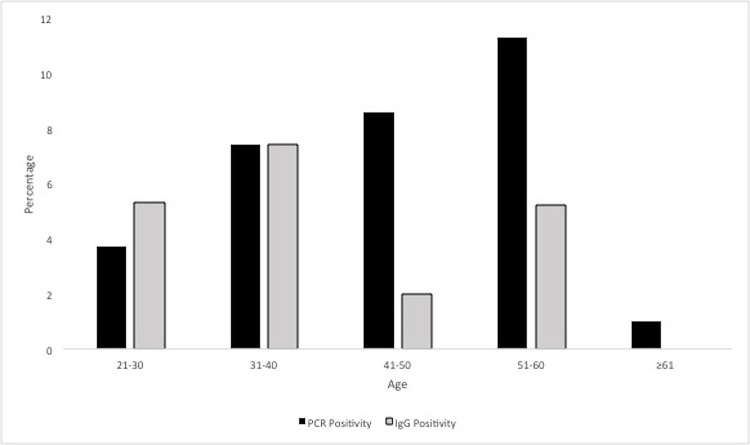
According to age groups, the prevalence of HPV-IgG was highest (7.4%, n = 6, 95% CI: 2.4–13.6%) in the age group 31–40, however, did not differ significantly according to patient age (P = 0.615). Additionally, HPV DNA positivity was also correlated with seropositivity by age group, but no significant difference was observed. However, the prevlence of HPV-DNA was higher in the age group 41–60 as compared to the HPV-IgG positive women (Fig 1). Furthermore, the seroprevalence of HPV-IgG was highest among separated/divorced women (17.6%, n = 3) and lowest among married women (3.6%, n = 9), but the difference was not statistically significant (P = 0.705). The HPV-IgG seropositivity was slightly higher among women with college/university education, monthly income 5000.00–20,000.00 QAR, knowledge about cervical cancer but not HPV vaccine, no smoking, no use of any contraceptive methods, normal cytology and underwent routine health care, however, had no significant associations with HPV infection. It is noteworthy that all samples were negative for HPV-IgM.
Fig 1. Prevalence of HPV-DNA and HPV-IgG positivity according to age group among Arab women residing in Qatar.
Discussion
Cervical cancer is a leading cause of cancer death among women worldwide, and approximately 85% of these deaths occur in developing countries [22,23]. It has been shown that genital HPV is the main causal agent for cervical cancer [2]. The high burden of cervical cancer in developing countries reflects the insufficiency and poor capacity of existing screening programs to detect precursor for or early stage of cervical carcinoma [24,25]. Recently, we have shown lower rate of HPV prevalence and heterogeneous distribution of HPV type among Arab women with normal or abnormal cytology [10]. In the present study, we sought to determine the HPV seroprevalence and potential risk factors associated with HPV infection among Arab women living in the State of Qatar.
In this study, HPV DNA prevalence among Arab women in Qatar was found to be slightly higher (8.1%) than we previously report (6.1%) in the large cohort [10]. However, this finding is still consistent with the low incidence of cervical cancer in the EMENA and both measres are nearly equal [26,27]. On the other hand, no statistical differences in the demographic data and HPV DNA positivity between Qatari and non-Qatari Arab women were observed, which is consistent with our previous report [10]. Refelctive of the overall pattern of sexually transmitted infections in EMENA [28,29], it is likely that the infection is transmitted sexually to the infected women from their spouses. Moreover, the rising tourism and the high frequency of expatriates (75%) residing in Qatar [30], hinders drawing inferences about the local dynamics of the infection and prevailing norms of sexual partenring.
In the present study, the high-risk HPV genotypes 16 and 59 were the most common among women with abnormal cytology, while HPV 35, 33, 39, 59 were found among those with normal cytology. HPV 81, 90, 11 were the most prevalent low-risk genotypes among women with both normal and abnormal cytology, a finding that is consistent with our previous study [10]. HPV 81 was also the most frequent LR genotype among women with normal (34.5%) and abnormal cytology (25%), and this also corroborates our previous findings in this population [10].
The findings on HPV DNA prevalence and genotype distribution, as well as HPV seroprevalence and potential risk factors for HPV infection, have the clinical potential to improve cervical cancer screening through identifying women at high risk for cervical dysplasia/cancer. Furthermore, they inform the development of genotype-specific vaccines for trials in population-level programs. It has been shown that HPV serology may underestimate infection exposure, as many women do not develop an HPV antibody response [31]. Though, seroprevalence studies has their limitations, such studies, such as the present study, provide a useful tool to understand the dynamics of HPV infection, thereby providing a baseline assessment for the incorporation of HPV vaccination programs in the State of Qatar. In the present study, we found a lower seroprevalence of 4.5% for HPV-IgG antibodies in Arab women than globally [32,33]. This low seroprevalence supports lower intensity of HPV transmission in the Arab female population, possibly due to poorly connected and sparse sexual networks, a result of the more conservative sexual norms in this part of the world [28].
Interestingly, none of the women were found positive for HPV IgM antibodies and there was no evidence of an association between behavioral risk factors and HPV seropositivity. Furthermore, it has been reported that once seroconversion occurs, anti-HPV antibody levels remain detectable for years [34,35], but in the present study, all the HPV DNA positive women were HPV seronegative. The reason could be that these women never seroconverted, despite acquiring HPV infection previously [34,36]. Additionally, there was no evidence of higher HPV DNA prevalence and seroprevalence among young women in this study, further supporting a flat distribution for HPV prevalence by age. This finding is consistent with our previous findings [10] and those reported in other limited-resource countries in Africa, Asia and globally [32,37]. This finding however contrasts with the common sharp peak in prevalece of HPV infection among young women following their sexual debut in most studies globally [27,38]. Lastly, many potential risk factors have been established for HPV infections but none of these (marital status, education level, economic status, smoking, usage of contraception and awareness of HPV, cervical cancer and HPV vaccine) were associated with HPV DNA positivity or antibody positivity in the present study.
The strengths of the present study lie in the use of a standardized and sensitive molecular assay for HPV detection, rendering our findings amenable to a detailed analysis of HPV prevalence, distribution of HPV genotypes, seroprevalence and risk factors among general population of Arab women residing in the State of Qatar, and comparison to global patterns. In the present study, we aimed for such analysis to be relevant for planning of health service provision and development of appropriate interventions based on the current characteristics of the infection burden and implied future trends for squamous intraepithelial lesions and cervical cancer. The indications that HPV infection burden might be increasing in EMEMNA, adds further importance to this investigation.
The limitations of this study include the fact that, because of socio-cultural context, we were unable to collect detailed data on sexual behaviour such as number of sexual partners, age at first sexual intercourse and extramarital relationships. Additionally, the present study was based on a convenient sample from women attending the Women hospital and Gynecology clinic at PHCC. Therefore, it is not known how representative is this sample of the wider Arab women population residing in the State of Qatar.
Conclusion
The overall HPV DNA prevalence and seroprevalence among Arab women in the State of Qatar are rather low in comparison to other countries. Despite the low prevalence, there is a diverse distribution of HPV genotypes among Arab women living in Qatar, and there appears to be an increase in prevalence over the last decade. Our study suggests that these genotypes should also included in future vaccines targeting this specific population. Furthermore, the information about the molecular and sero-prevalence of HPV infection will be helpful for policy makers in making an informed decision regarding introduction and development of policies, guidelines and implementation of HPV vaccination in the State of Qatar. Contrary to our expectations, no statistically significant association was observed between HPV DNA positivity or antibody positivity and potential risk factors for HPV infection. Though, Arab women in Qatar reported knowledge of cervical cancer, there was limited knowledge of its link to HPV infection and HPV vaccination. Awareness programs of risk associated with HPV infection and HPV vaccination are warranted in Qatar. Finally, further observational studies of HPV infection levels and HPV incidence among different age groups may help elucidate several poorly understood aspects of HPV epidemiology in this part of the world.
Supporting Information
(PDF)
Acknowledgments
Disclaimer: The statements made herein are solely the responsibility of the author[s].
This work was supported by Weill Cornell Medicine-Qatar and publication was made possible by NPRP grant #09-344-3-082 from the Qatar National Research Fund (a member of Qatar Foundation). The authors would like to thank Dr. Afaf Al-Ansari, HMC, Doha and Dr. Juliet Ibrahim and health care staffs of PHCC for helping in sample collection.
Data Availability
Due to the patient's confidentiality and ethical restrictions and also because of the sensitive nature of the information collected from the subject such as sexual and behavioral history, the data are available upon request. The data will be available on request from the author. Requests for the data may be sent to Dr. Devendra Bansal and Dr. Ali A Sultan, Department of Microbiology and Immunology, Weill Cornell medicine-Qatar (WCM-Q) (deb2022@qatar-med.cornell.edu; als2026@qatar-med.cornell.edu).
Funding Statement
This work was supported by Weill Cornell Medicine – Qatar and publication was made possible by NPRP grant # 09-344-3-082 from the Qatar National Research Fund (a member of Qatar Foundation). Dr. Ali A Sultan received the grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.International Agency for Research on Cancer (IARC): IARC Monographs on the evaluation of carcinogenic risks of Human Papillomaviruses In Human Papillomaviruses, Volume 64 Lyon, France: IARC; 1995:35–86. [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–19 [DOI] [PubMed] [Google Scholar]
- 3.Hariri S, Dunne E, Saraiya M, Unger E, Markowitz L. Human Papillomavirus In VPD Surveillance Manual. 5th edition Atlanta, GA: Centers for Disease Control and Prevention; 2011: Chapter 5:1–11. [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–27. 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- 5.WHO. Comprehensive cervical cancer prevention and control: a healthier future for girls and women. 2014. Available at http://www.who.int/immunization/hpv/learn/comprehensive_cervical_cancer_who_2013.pdf. Accessed 15 Dec 2015.
- 6.ICO Information Centre on HPV and Cancer. Qatar. Human Papillomavirus and Related Cancers, Fact Sheet. 2016. Available at http://www.hpvcentre.net/statistics/reports/QAT_FS.pdf. Accessed 10 March 2016.
- 7.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. 2013. Available at http://globocan.iarc.fr. Accessed 25 January 2016.
- 8.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43:S5–25. 10.1016/j.jadohealth.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 9.Tiggelaar SM, Lin MJ, Viscidi RP, Ji J, Smith JS. Age-specific human papillomavirus antibody and deoxyribonucleic acid prevalence: a global review. J Adolesc Health. 2012;50:110–31. 10.1016/j.jadohealth.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal D, Elmi AA, Skariah S, Haddad P, Abu-Raddad LJ, Al Hamadi AH, et al. Molecular epidemiology and genotype distribution of Human Papillomavirus (HPV) among Arab women in the State of Qatar. J Transl Med. 2014; 12:300 10.1186/s12967-014-0300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler CM. Natural history of human papillomavirus infections, cytologic and histologic abnormalities, and cancer. Obstet Gynecol Clin North Am. 2008; 5:519–36 [DOI] [PubMed] [Google Scholar]
- 12.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines-immune responses. Vaccine. 2012; 30:F83–7. 10.1016/j.vaccine.2012.04.106 [DOI] [PubMed] [Google Scholar]
- 13.Deligeoroglou E, Giannouli A, Athanasopoulos N, Karountzos V, Vatopoulou A, Dimopoulos K, et al. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol. 2013; 2013:540850 10.1155/2013/540850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006; 24:S16–S22. 10.1016/j.vaccine.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 15.Newton R, Bousarghin L, Ziegler J, Casabonne D, Beral V, Mbidde E, et al. Human papillomaviruses and cancer in Uganda. Eur J Cancer Prev. 2004; 13:113–8. [DOI] [PubMed] [Google Scholar]
- 16.Wang IJ, Viscidi R, Hwang Kc, Lin Ty, Chen Cj, Huang LM, et al. Seroprevalence and risk factors for human papillomavirus in Taiwan. J Trop Pediatr. 2008; 54:14–8. 10.1093/tropej/fmm062 [DOI] [PubMed] [Google Scholar]
- 17.Husaiyin S, Han L, Mamat H, Husaiyin K, Wang L, Niyazi M. A serological epidemiology survey of antibodies against four HPV subtypes in Uygur women in Xinjiang. Jpn J Infect Dis. 2015; 7:154. [DOI] [PubMed] [Google Scholar]
- 18.Butsashvili M, Abzianidze T, Kajaia M, Agladze D, Kldiashvili E, Bednarczyk R, et al. Seroprevalence and awareness of human papillomavirus infection and cervical cancer screening results among reproductive-aged Georgian women. J Fam Plann Reprod Health Care. 2015; 41:265–71. 10.1136/jfprhc-2013-100833 [DOI] [PubMed] [Google Scholar]
- 19.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. J Am Med Assoc 2002; 287:2114–9. [DOI] [PubMed] [Google Scholar]
- 20.Al-Thani AAJ, AIA-R, Afaf AA, Mandy A, Moza AK, Sabah AL. Prevalence of human papillomavirus infection in women attending a gynecology/oncology clinic in Qatar. Future Virol 2010, 5:513–519. [Google Scholar]
- 21.Feoli-Fonseca JC, Oligny LL, Filion M, Brochu P, Simard P, Russo PA, et al. A two-tier polymerase chain reaction direct sequencing method for detecting and typing human papillomaviruses in pathological specimens. Diagn Mol Pathol 1998; 7:317–23. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 23.Denny L. Screening for Cervical Cancer in Resource-Limited Settings In UpToDate. Edited by Section Editor Goff Barbara, Deputy Editor, Falk SJ. Waltham, MA: UpToDate; 2014. Available at http://www.uptodate.com/contents/screening-for-cervical-cancer. Accessed 05 March 2016. [Google Scholar]
- 24.Sankaranarayanan R. Screening for cancer in low- and middle-income countries. Ann Glob Health. 2014; 80:412–7. 10.1016/j.aogh.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 25.Denny L. Control of cancer of the cervix in low- and middle-income countries. Ann Surg Oncol. 2015; 22:728–33. 10.1245/s10434-014-4344-8 [DOI] [PubMed] [Google Scholar]
- 26.Vaccarella S, Bruni L, Seoud M. Burden of human papillomavirus infections and related diseases in the extended Middle East and North Africa region. Vaccine. 2013; 31:G32–44. 10.1016/j.vaccine.2012.06.098 [DOI] [PubMed] [Google Scholar]
- 27.Seoud M. Burden of human papillomavirus-related cervical disease in the extended middle East and north Africa-a comprehensive literature review. J Low Genit Tract Dis. 2012; 16:106–20. 10.1097/LGT.0b013e31823a0108 [DOI] [PubMed] [Google Scholar]
- 28.Abu-Raddad LJ, Hilmi N, Mumtaz G, Benkirane M, Akala FA, Riedner G, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS. 2010; 24:S5–23. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Raddad L, Akala FA, Semini I, Riedner G, Wilson D, Tawil O. Characterizing the HIV/AIDS Epidemic in the Middle East and North Africa: Time for Strategic Action In World Bank/UNAIDS/WHO Publication, Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. Washington DC: The World Bank Press; 2010:1–265. [Google Scholar]
- 30.World Migration 2005. Costs and Benefits of International Migration. Geneva, Switzerland: International Organization for Migration; 2005. Available at https://publications.iom.int/system/files/pdf/wmr_2005_3.pdf. Accessed 29 January 2016. [Google Scholar]
- 31.Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996; 174:927–36. [DOI] [PubMed] [Google Scholar]
- 32.Tiggelaar SM, Lin MJ, Viscidi RP, Ji J, Smith JS. Age-specific human papillomavirus antibody and deoxyribonucleic acid prevalence: a global review. J Adolesc Health. 2012; 50:110–31. 10.1016/j.jadohealth.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aminu M, Gwafan J, Inabo H, Oguntayo A, Ella E, Koledade A. Seroprevalence of human papillomavirus immunoglobulin G antibodies among women presenting at the reproductive health clinic of a university teaching hospital in Nigeria. Int J Womens Health. 2014; 6:479–87. 10.2147/IJWH.S56388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adekunle S, Sule WF, Oluwayelu DO. High negativity of IgG antibodies against human papillomavirus type 6, 11, 16 and 18 virus-like particles in healthy women of childbearing age. J Exp Integr Med. 2014; 4:37–41. [Google Scholar]
- 35.Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005; 15:727–46. 10.1111/j.1525-1438.2005.00246.x [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC). Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Mortality & Morbidity Weekly Report (MMWR). 2007; 56:RR-2. [PubMed] [Google Scholar]
- 37.Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Anh PTH, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. International J Cancer. 2006; 119:2677–84. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005; 353:2101–4. 10.1056/NEJMp058171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Due to the patient's confidentiality and ethical restrictions and also because of the sensitive nature of the information collected from the subject such as sexual and behavioral history, the data are available upon request. The data will be available on request from the author. Requests for the data may be sent to Dr. Devendra Bansal and Dr. Ali A Sultan, Department of Microbiology and Immunology, Weill Cornell medicine-Qatar (WCM-Q) (deb2022@qatar-med.cornell.edu; als2026@qatar-med.cornell.edu).