Abstract
Background
Physicians and hospital systems often have relationships with biomedical manufacturers to develop new ideas, products, and further education. Because this relationship can influence medical research and practice, reporting disclosures is necessary to reveal any potential bias and inform consumers. The Sunshine Act was created to develop a new reporting system of these financial relationships called the Open Payments database. Currently, all disclosures submitted with research to scientific meetings are at the discretion of the physician. We hypothesized that financial relationships between authors and medical industry are underreported.
Objectives
We aimed to describe concordance between physicians’ financial disclosures listed in the abstract book from the 41st Annual Society of Gynecologic Surgeons’ (SGS) Scientific Meeting to physician payments reported to the Center for Medicaid and Medicare Services’ (CMS) Open Payments database for the same year.
Study Design
Authors and scientific committee members responsible for the content of the 41st SGS Scientific Meeting were identified from the published abstract book; each abstract listed disclosures for each author. Abstract disclosures were compared to transactions recorded on the CMS Open Payments database for concordance. Two authors reviewed each non-disclosed CMS listing to determine relatedness between the company listed on CMS and abstract content.
Results
Abstracts and disclosures of 335 physicians meeting inclusion criteria were reviewed. 209/335 (62%) physicians had transactions reported in CMS which totaled $1.99 million. 24/335 (7%) physicians listed companies with their abstracts; 5 of those 24 physicians were concordant with CMS. The total amount of all non-disclosed transactions was $1.3 million. Transactions reported in CMS associated with a single physician ranged from $11.72 to $405,903.36. Of the 209 physicians with CMS transactions that were not disclosed, the majority (68%) had at least one company listed in CMS that was determined after review to be related to the subject of their abstract.
Conclusion
Voluntary disclosure of financial relationships was poor, and the majority of unlisted disclosures in the abstract book were companies related to the scientific content of the abstract. Better transparency is needed by physicians responsible for the content presented at gynecologic scientific meetings.
Keywords: Financial Disclosures, Open Payments database, Sunshine Act
Condensation
Accuracy of self-reported financial disclosures by physicians at the Society of Gynecologic Surgeons' 2015 annual meeting was poor compared to the CMS Open Payments database.
Introduction
Physicians and hospital systems often have relationships with biomedical manufacturers. These relationships are established to promote and develop innovative ideas, products, and support physician education. Companies often provide funding for these efforts. Industry’s share of total investment in biomedical research and development increased substantially from 32% in 1980 to 62% in 2000 [1]. In 2001, US pharmaceutical companies spent more than $21 billion promoting prescription drugs, and 84% of the marketing was directed toward physicians [2]. In 2007, 94% of US physicians surveyed nationally reported that they had a relationship with industry [3].
Industry’s financial influence leaves a significant impact on physicians’ practice and research results. Physicians who have accepted money from pharmaceutical companies are more likely to request additions to the hospital formulary including drugs manufactured by those same companies [4]. Prescribing practices are also influenced by interactions between physicians and pharmaceutical companies. Physicians who receive financial support from industry are more likely to prescribe that industries’ medications [5–8]. Industry-sponsored studies are more likely to reach conclusions that were favorable to the sponsor than non-industry studies [1, 9]. Despite evidence that industry relationships influence physician decision making, the majority of physicians deny that their own industry relationships influence their practice [10–13]. While physician-industry relationships are valuable, transparency is key to maintaining a professionalism. Disclosing financial relationships or other invested interest in companies reveals potential biases in physician behavior. Currently, physician scientists are asked to divulge industry alliances when presenting scientific work, including presentations at scientific meetings and on publications to insure transparency and reveal potential biases. Until recently, all disclosures were at the discretion of the physician.
The Physician Payments Sunshine Act was created from the Patient Protection and Affordable Care Act passed in 2010. This national disclosure program requires public reporting of payments to physicians and teaching hospitals from applicable manufacturers and group purchasing organizations (GPOs). The Centers for Medicare and Medicaid Services (CMS) was assigned to collect information from these companies in a publicly available database called the Open Payments Program (http://www.cms.gov/openpayments). The purpose of this system is to improve transparency to consumers of possible biases in physician practice and research. Applicable manufacturers or GPOs are included in the CMS database if they are located and/or conduct activities within the United States and produce, purchase, arrange, and/or negotiate the purchase of pharmaceuticals, devices, biologics, or medical supplies. At least one product must be reimbursed by Medicare, Medicaid or Children’s Health Insurance Program, and either the product must require physician’s authorization/prescription to administer or the product requires pre-market approval/notification by the FDA [14]. All transactions including single payments greater than $10 and multiple payments exceeding $100 qualify for reporting. CMS lists all transactions with applicable companies including company names, number of transactions, physician payment amounts, and types of payments. Data collection began August 1, 2013 and 2014 was the first year this information became publically available.
One way to measure physician transparency is to compare industry relationships reported in the Open Payment Program with physician self-disclosure. We aimed to evaluate the concordance of authors’ self-reported disclosures at the 41st Annual Society of Gynecologic Surgeons (SGS) Scientific Meeting in 2015 compared to those published in the CMS Open Payments Program in the same year. We hypothesized that financial relationships between authors and medical industry are underreported at a scientific meeting, and many physician undisclosed associations with companies were directly related to the subject of the research being presented.
Materials and Methods
We performed a retrospective cohort study to compare the financial disclosures between those physicians responsible for the scientific content of the Society of Gynecologic Surgeons’ 41st Annual Scientific Meeting in 2015 and disclosures published on the online CMS database. This was an IRB exempt study, as all data were publically available. The Journal of Minimally Invasive Gynecology (JMIG), published in March/April 2015, listed the scientific program committee members as well as all abstracts presented at the annual meeting. Abstracts included oral presentations, oral posters, non-oral posters, video presentations, video fest, and the video café. The Society of Gynecologic Surgeons instructs all authors to disclose conflicts “whether or not this relationship is directly related to the material being presented”, and physician self-disclosures were published with their abstracts.
The authors and SGS scientific committee members responsible for the content at the meeting were identified from the abstract book. Demographic information including gender, specialty, level of training, type of institution (i.e. private practice, teaching hospital) was collected by searching publicly available internet sites using the authors’ name and institution. All presenters except for those who were identified as non-physicians and physicians practicing medicine outside the United States were included in this study. Physicians meeting eligibility criteria were searched in the CMS Open Payments database.
CMS payment information was compared to self-reported financial disclosures to determine the disclosure rate. If an author was listed on more than one abstract, disclosures were summed across abstracts and compared in total to the companies listed on CMS. Abstracts do not include a monetary value with the self-disclosed companies. To determine the monetary value of non-disclosed affiliations, the total amount of money from companies listed on CMS and self-disclosed by the authors in the abstracts was subtracted from the total amount reported in CMS. Others have proposed a “cut-off” value for associations, as associations of very low value may have been disclosed by a company without the author’s awareness and small amounts may not have as strong an influence as larger amounts on physician behavior. We performed a sub-analysis by excluding all CMS total values listings less than $100.
Relatedness of non-disclosed conflicts was also evaluated. Two authors each independently reviewed each non-disclosed CMS listing to determine whether the company’s product line was related to the content of the abstract. The companies were categorized by their products or services into pharmaceutical, surgical, or medical relevant to the treatment of pelvic floor dysfunction. Abstracts were similarly categorized. A disclosure was determined related to the content of the abstract if the company produced a product in the same category as the abstract. For example, a company involved with producing medications for overactive bladder was determined relevant to a study evaluating the outcomes of overactive bladder treatments. Both reviewers agreed on the relatedness assignment. If reviewers did not agree, discrepancies were adjudicated with a third party.
Study data were entered into REDCap [15]. Descriptive statistics were used to describe author characteristics, associations and disclosure rates.
Results
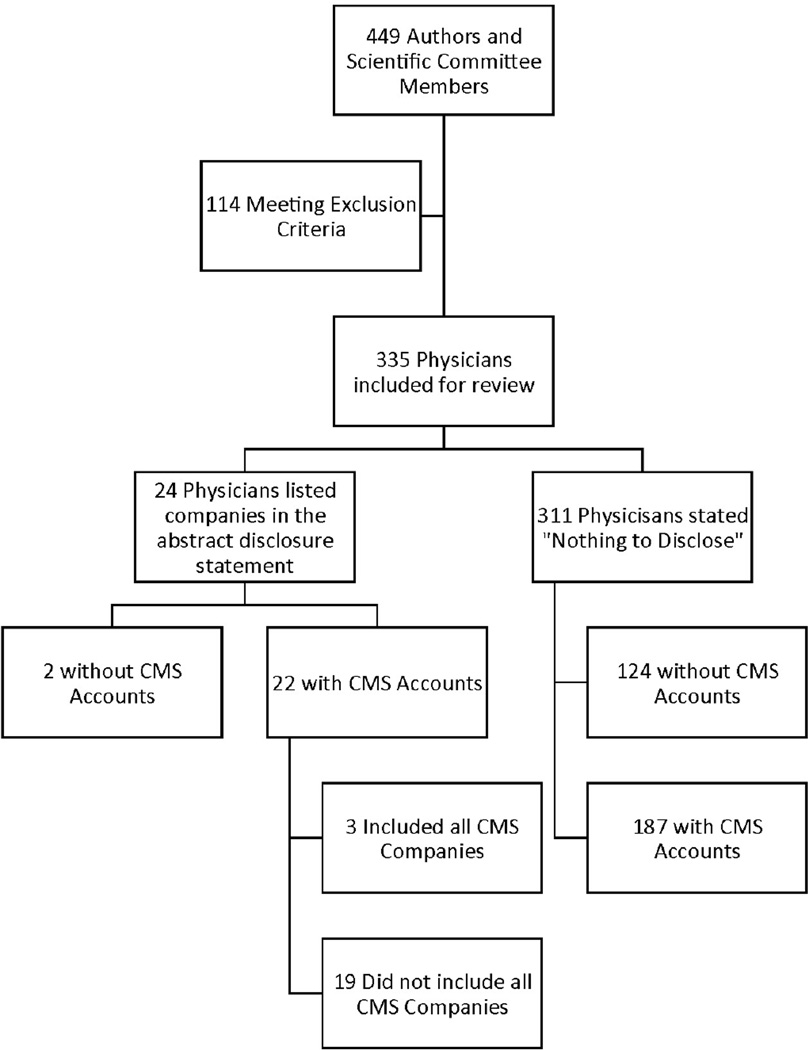
The authors and SGS scientific committee members from the 41st Scientific Meeting totaled 449 people. After excluding non-physicians and those working outside the United States, 335 were included for review. Scientific committee members did not have separate disclosures included in the JMIG abstract book unless they were authors listed in the abstract book. Those SGS scientific committee members without abstracts were still included due to their responsibility for the scientific content of the meeting.
Most presenters were female, attending physicians working at teaching hospitals and specialized in Female Pelvic Medicine and Reconstructive Surgery (Table 1).
Table 1.
Physician Characteristics
| Demographic Characteristics |
N | Percentage | |
|---|---|---|---|
| Gender | Female | 191 | 57 |
| Male | 144 | 43 | |
| Medical Specialty |
FPMRS* | 173 | 51.6 |
| Ob/Gyn | 114 | 34.0 | |
| Gynecologic Oncology | 11 | 3.3 | |
| MIS* | 8 | 2.4 | |
| Urology | 6 | 1.8 | |
| REI* | 5 | 1.5 | |
| General Surgery | 4 | 1.2 | |
| Colorectal Surgery | 3 | 0.9 | |
| Internal Medicine | 3 | 0.9 | |
| Plastic Surgery | 2 | 0.6 | |
| Cardiology | 2 | 0.6 | |
| Radiology | 2 | 0.6 | |
| Neurosurgery | 1 | 0.3 | |
| Anesthesiology | 1 | 0.3 | |
| Type of Institution of physician practice |
Teaching Hospital | 318 | 94.9 |
| Private Practice | 8 | 2.4 | |
| Military | 7 | 2.1 | |
| Other | 2 | 0.6 | |
| Level of Training | Attending Physician | 233 | 69.6 |
| Resident | 55 | 16.4 | |
| Fellow | 47 | 14.0 | |
FPMRS = Female Pelvic Medicine and Reconstructive Surgery, MIS = Minimally Invasive Surgery, REI = Reproductive Endocrinology and Infertility
Of the 335 physicians, 209/335 (62%) had disclosures listed in the CMS database but only 24/335 (7.2%) authors listed at least one company in the financial disclosure section of their abstract. Of the 24 authors with self-disclosures, only 5/24 (20.8%) physicians accurately included all of the companies listed on CMS. The remaining 311/335 (92.8%) physicians stated in their abstract “nothing to disclose”. Overall, 129 (38.5%) people reported accurate financial disclosures, meaning that they disclosed nothing with their abstract which was confirmed with review of CMS listings, or they disclosed everything listed in CMS. Reporting disclosures was consistent for the 85 authors with more than one abstract; four presenters varied in their disclosures between abstracts.
The total monetary value of the 2014 CMS disclosures associated with the authors and scientific committee members was nearly 2 million dollars. (Table 2) These transactions were categorized within the CMS database into general payments (56.6%), research (3.6%), associated research (39.7%), and investments (0.1%). General payments included honoraria, education, travel/lodging, food/beverage, gifts, grants, entertainment, royalties/license, consulting fees, and compensation for other services (Table 2). While the majority (2463/3283, 75%) of transactions were labeled as food/beverage, the most money in general payments was spent on education and consulting fees. The number of transactions listed for a physician ranged from 1 to 366 (median 6 transactions). The value of these transactions per physician ranged from $11.72 to $405,903.36 (Interquartile ranges: $106.95; $321.01; $2,069.14; $403,394.54). Nearly half (47.8%) of those with CMS accounts had recorded values greater than $500.00.
Table 2.
CMS Categories of Transactions
| Type of Transaction | Total Amount | Number of Transactions |
Min | Max | Median |
|---|---|---|---|---|---|
| General Payments | $1,130,683.19 | 3283 | $0.26 | $31,000.00 | $23.61 |
| Education | $313,709.80 | 129 | $1.26 | $31,000.00 | $99.00 |
| Consulting | $195,833.60 | 84 | $1.26 | $8,312.50 | $2,000.00 |
| Compensation* | $193,839.13 | 81 | $10.33 | $6,800.00 | $2,400.00 |
| Travel/Lodging | $170,626.68 | 486 | $1.14 | $10,890.26 | $133.53 |
| Honoraria | $122,180.00 | 28 | $80.00 | $8,000.00 | $4,000.00 |
| Food/Beverage | $99,606.88 | 2463 | $0.26 | $6,798.56 | $18.44 |
| Royalties/License | $32,405.79 | 5 | $698.53 | $25,000.00 | $1,093.20 |
| Gift | $1,815.01 | 3 | $12.08 | $1,483.00 | $319.93 |
| Grant | $622.22 | 2 | $22.22 | $600.00 | $311.11 |
| Entertainment | $44.06 | 2 | $15.21 | $28.85 | $22.03 |
| Research | $ 71,946.34 | 36 | $24.69 | $10,000.00 | $1,750.00 |
| Associated Research | $792,549.44 | 67 | $150.00 | $105,360.00 | $2,500.00 |
| Investments | $ 2,000.00 | 1 | |||
| Total | $1,997,178.97 | 3387 |
“Compensation for services other than consulting, including serving as faculty or as a speaker at a venue other than continuing education program”.
The value of disclosures from the abstracts that were also listed on the CMS database was $674,007.25. The total value of CMS reports that were not disclosed on abstracts was $1,323,171.72 accounting for 66.3% of all CMS payments to authors and committee members at the meeting. Before any transactions are made public, CMS posts a 45 day review period which allows physicians to dispute any of the transactions. None of the transactions were disputed in CMS by any of the physicians.
A sub-analysis was performed that excluded accounts with total CMS values of less than $100, which left 168 accounts with total values greater than or equal to $100. The overall total value of the 41 accounts with values less than $100 was 2007.06. The accurate disclosure rate excluding those accounts less than $100 is then 177/335 (52.8%).One hundred twenty-two different companies were identified from the CMS accounts and were affiliated with the authors and committee members. Ten of these companies were responsible for 63% of the total payments, or $1,266,402. (Table 3) Payments included all categories of transactions (general payments, research, associated research, and investments). While Medtronic, American Medical Systems, and Astellas Pharma Inc were the companies most commonly listed with the authors’ CMS accounts, Boston Scientific, Coloplast, and Intuitive Surgical reported the most money.
Table 3.
CMS Companies
| Company | Percentage of Accounts Listing the Company |
Number of Transactions |
Total Amount Paid |
Minimum Payment |
Maximum Payment |
Median Payment |
|---|---|---|---|---|---|---|
| Medtronic | 34.9 | 347 | $64704.23 | $0.86 | $6800.00 | $31.25 |
| AMS | 30.1 | 311 | $91774.00 | $1.14 | $7658.00 | $50.00 |
| Astellas | 30.1 | 316 | $96028.22 | $0.26 | $27461.00 | $18.44 |
| Allergan | 23.9 | 232 | $85470.85 | $1.48 | $21025.60 | $23.96 |
| Boston Scientific |
22.9 | 132 | $242870.30 | $5.05 | $65000.00 | $96.35 |
| Pfizer | 22.5 | 182 | $81231.67 | $0.38 | $18718.50 | $11.97 |
| Coloplast | 20.6 | 345 | $395773.40 | $0.85 | $31000.00 | $73.06 |
| Ethicon | 17.7 | 89 | $25774.37 | $0.77 | $8312.50 | $30.59 |
| Intuitive | 15.8 | 296 | $180791.20 | $0.98 | $8000.00 | $40.23 |
| Actavis | 15.3 | 150 | $1983.26 | $0.98 | $99.99 | $13.12 |
| Total | 2268 | $1266402.00 |
Relatedness between CMS companies and abstract subject was determined concordant in all cases by the two reviewers. Of the 209 physicians with CMS transactions, the majority (68%) had at least one company listed in CMS that was determined to be related to the subject of their abstract. After excluding the self-disclosures listed with the abstracts, 139/209 (66.5%) physicians still had at least one CMS company related to the abstract that was not disclosed. The range of companies related per physician was from 1 to 13 (median 2 companies).
Comment
We found that the accuracy of self-reported financial disclosures at the Society of Gynecologic Surgeons’ annual meeting in 2015 was poor. Despite instructions to disclose all financial relationships, the majority of authors and committee members with company affiliations did not include all transactions documented in the CMS Open Payments database. Furthermore, the majority of non-disclosed companies were deemed relevant to the scientific content of the abstract presented. We also found that the amount of money from companies associated with physicians presenting their scientific work varied greatly as did the number of affiliations with companies. Finally, we found that financial associations with physicians were concentrated in a few companies that produce gynecologic products.
Research in conflicts of interest is limited since the creation of the CMS Open Payments database. In 2007, payments made by manufacturers of joint prostheses to authors of presentations, committee or board members at the annual meeting of the American Academy of Orthopaedic Surgeons were compared to disclosure of possible conflicts in the abstract book of the meeting. The authors of this paper found that the overall disclosure rate for possible conflicts was 245/344 (71.2%) of payments, disclosure rates higher than those in our study. In the orthopedic study, physicians were surveyed for reasons why they did not disclose all industry affiliations. Physicians reported the most common reasons for non-disclosure was that they felt that the payment was unrelated to the presentation topic, and that the physician had misunderstood the disclosure requirements [16]. Under-reporting in our study may be for similar reasons; physicians may have felt that the association with industry was not related to the content of the abstract or they may have misunderstood the directions to authors for abstract presentation. Nonetheless, a significant number of affiliations remained undisclosed.
Disclosures may also vary between the abstract and oral presentation of scientific work. A comparison of disclosures on published abstracts and oral presentations at the Annual Scientific Meeting of the American Urogynecologic Society found that 13% of presentations did not include a disclosure slide, and the discordance in disclosures between the printed abstract and oral presentation was present in nearly half [18]. The presentation slides were not available for this study to compare the difference in disclosure practices.
Self-disclosure rates vary widely between physicians. We found a significant number of authors failed to disclose companies that were pertinent to the content of the abstract. The effect of industry affiliations on physician behavior has been previously reported although many physicians believing that their affiliations would not affect their practice of medicine or scientific interpretation of results [1, 4–13]. This belief that the affiliation was not related or would not influence the author may have similarly influenced the low disclosure rates of physicians in our study. Physicians’ limited knowledge regarding the Open Payments database may have greatly influenced our study results. None of the physicians in this study disputed any of the transactions reported in 2014—the first year of complete data in the Open Payments database. While applicable companies are required by law to submit their disclosures to CMS, no other safeguards are in place to review these reports besides the physicians or hospital systems involved. To dispute a transaction, a physician must first register with CMS’s Enterprise Portal and Open Payments and explain why the transaction is deemed inaccurate. The company is then contacted, and a consensus must be made within 15 days between the physician and company involved [14]. Although some company disclosure may have been made in error and were not disputed, these errors are unlikely to explain the high non-disclosure rate we observed.
Strengths of this study include the use of public databases that are mandated to report financial affiliations. In addition, the use of a publically available record of disclosures as published in the Journal of Minimally Invasive Surgery replicates the information available to attendees of the annual SGS meeting. Weaknesses include that not all companies with financial relationships with physicians are included in CMS, and affiliations with companies may exist for some authors which remain undisclosed. For example, companies that pay physicians royalties for scientific writings are not disclosed on CMS but are often reported as self-disclosures. In addition, we do not know if the disclosure slide presented with the abstract at the annual meeting more accurately included all financial affiliations than those printed in the abstract book. We chose to compare disclosures between the March 2015 SGS annual meeting with the year in which the CMS payments were made publically available in 2014, and the overlap in timeframes may have led to discrepancies that are explained because of the timing of the affiliation. However, abstracts were due to SGS in October, 2014. Nonetheless, at the time of presentation, it is reasonable to expect that recent disclosures would be made available to attendees. Perhaps better instructions to authors regarding the timeframe of the disclosures would improve concordance between CMS and meeting disclosures. While we chose to examine disclosures of a single society meeting, it is probable that the same discrepancies would be found at other gynecologic meetings.
The purpose of the CMS database is to increase transparency regarding the extent and nature of relationships between physicians, teaching hospitals, and industry manufacturers so patients can make better informed decisions when choosing healthcare professionals and treatment decisions. In addition CMS was designed to help and deter inappropriate financial relationships between industry and physicians [14]. Currently, it is unclear if the CMS database is reaching those goals. Further studies are required to determine if there is any appreciable change in self-disclosures after the CMS Open Payments database has become more widely utilized and publicized. From our study, we determined that physicians must take a greater responsibility for accurate disclosure practices and improve their awareness of the Sunshine Act including the Open Payments database and disputing process.
Figure 1. Study Participants.
This figure describes the participants included for further review in the CMS database. The participants are categorized by those with CMS accounts, those with companies listed in their abstract disclosure statement, and those who included all CMS companies in the disclosure statement.
Acknowledgments
Sources of Support: None
This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000041. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
GC Dunivan receives research support from Pelvalon, Inc. unrelated to the submitted work
PC Jeppson received a general payment from American Medical Systems listed on CMS unrelated to the submitted work.
YM Komesu reports grants from NIH funded grant (grant #PA11-260), non-financial support from National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number 8UL1TR000041, The University of New Mexico Clinical and Translational Science Center, grants from NIH: U grant funding the Pelvic Floor Support Disorders Network via the Eunice Kennedy Shriver NICHD (institute): general payment through Uroplasty listed on CMS; all of which are unrelated the submitted work.
SB Cichowski received a general payment from American Medical Systems listed on CMS unrelated to the submitted work.
RG Rogers is DSMB chair for the TRANSFORM trial sponsored by American Medical Systems and receives royalties for scientific writings from UptoDate and McGraw Hill
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study conducted in Albuquerque, New Mexico, United States of America
Disclaimers: None
Disclosures:
JC Thompson reports no conflict of interest
KA Volpe reports no conflict of interest
LK Bridgewater reports no conflict of interest
F Qeadan reports no conflict of interest
References
- 1.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. Jama. 2003;289(4):454–465. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 2.A. Brichacek JLS. Flexing Their Budgets: Big Pharma Spending Trends. Pharmaceutical Executive. 2001:78–86. [Google Scholar]
- 3.Campbell EG, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356(17):1742–1750. doi: 10.1056/NEJMsa064508. [DOI] [PubMed] [Google Scholar]
- 4.Chren MM, Landefeld CS. Physicians' behavior and their interactions with drug companies. A controlled study of physicians who requested additions to a hospital drug formulary. Jama. 1994;271(9):684–689. [PubMed] [Google Scholar]
- 5.Lurie N, et al. Pharmaceutical representatives in academic medical centers: interaction with faculty and housestaff. J Gen Intern Med. 1990;5(3):240–243. doi: 10.1007/BF02600542. [DOI] [PubMed] [Google Scholar]
- 6.Bower AD, Burkett GL. Family physicians and generic drugs: a study of recognition, information sources, prescribing attitudes, and practices. J Fam Pract. 1987;24(6):612–616. [PubMed] [Google Scholar]
- 7.Peay MY, Peay ER. The role of commercial sources in the adoption of a new drug. Soc Sci Med. 1988;26(12):1183–1189. doi: 10.1016/0277-9536(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 8.Orlowski JP, Wateska L. The effects of pharmaceutical firm enticements on physician prescribing patterns. There's no such thing as a free lunch. Chest. 1992;102(1):270–273. doi: 10.1378/chest.102.1.270. [DOI] [PubMed] [Google Scholar]
- 9.Davidson RA. Source of funding and outcome of clinical trials. J Gen Intern Med. 1986;1(3):155–158. doi: 10.1007/BF02602327. [DOI] [PubMed] [Google Scholar]
- 10.Steinman MA, Shlipak MG, McPhee SJ. Of principles and pens: attitudes and practices of medicine housestaff toward pharmaceutical industry promotions. Am J Med. 2001;110(7):551–557. doi: 10.1016/s0002-9343(01)00660-x. [DOI] [PubMed] [Google Scholar]
- 11.Madhavan S, et al. The gift relationship between pharmaceutical companies and physicians: an exploratory survey of physicians. J Clin Pharm Ther. 1997;22(3):207–215. doi: 10.1046/j.1365-2710.1997.94975949.x. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons RV, et al. A comparison of physicians' and patients' attitudes toward pharmaceutical industry gifts. J Gen Intern Med. 1998;13(3):151–154. doi: 10.1046/j.1525-1497.1998.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks JW, 3rd, Mainous AG., 3rd Attitudes of medical school faculty toward gifts from the pharmaceutical industry. Acad Med. 1992;67(9):610–612. doi: 10.1097/00001888-199209000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Transparency Reports and Reporting of Physician Ownership of Investment Interests; Final Rule, Department of Health and Human Services. 2013:9458–9528. Federal Register. [PubMed] [Google Scholar]
- 15.Harris PA, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okike K, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361(15):1466–1474. doi: 10.1056/NEJMsa0807160. [DOI] [PubMed] [Google Scholar]
- 17.Alhamoud HA, et al. Author Self-disclosure Compared with Pharmaceutical Company Reporting of Physician Payments. Am J Med. 2015 doi: 10.1016/j.amjmed.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18(2):79–81. doi: 10.1097/SPV.0b013e3182436643. [DOI] [PubMed] [Google Scholar]