Abstract
The role of a C4 pathway in photosynthetic carbon fixation by marine diatoms is presently debated. Previous labeling studies have shown the transfer of photosynthetically fixed carbon through a C4 pathway and recent genomic data provide evidence for the existence of key enzymes involved in C4 metabolism. Nonetheless, the importance of the C4 pathway in photosynthesis has been questioned and this pathway is seen as redundant to the known CO2 concentrating mechanism of diatoms. Here we show that the inhibition of phosphoenolpyruvate carboxylase (PEPCase) by 3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate resulted in a more than 90% decrease in whole cell photosynthesis in Thalassiosira weissflogii cells acclimated to low CO2 (10 μm), but had little effect on photosynthesis in the C3 marine Chlorophyte, Chlamydomonas sp. In 3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate-treated T. weissflogii cells, elevated CO2 (150 μm) or low O2 (80–180 μm) restored photosynthesis to the control rate linking PEPCase inhibition with CO2 supply in this diatom. In C4 organic carbon-inorganic carbon competition experiments, the 12C-labeled C4 products of PEPCase, oxaloacetic acid and its reduced form malic acid suppressed the fixation of 14C-labeled inorganic carbon by 40% to 50%, but had no effect on O2 evolution in photosynthesizing diatoms. Oxaloacetic acid-dependent O2 evolution in T. weissflogii was twice as high in cells acclimated to 10 μm rather than 22 μm CO2, indicating that the use of C4 compounds for photosynthesis is regulated over the range of CO2 concentrations observed in marine surface waters. Short-term 14C uptake (silicone oil centrifugation) and CO2 release (membrane inlet mass spectrometry) experiments that employed a protein denaturing cell extraction solution containing the PEPCKase inhibitor mercaptopicolinic acid revealed that much of the carbon taken up by diatoms during photosynthesis is stored as organic carbon before being fixed in the Calvin cycle, as expected if the C4 pathway functions as a CO2 concentrating mechanism. Together these results demonstrate that the C4 pathway is important in carbon accumulation and photosynthetic carbon fixation in diatoms at low (atmospheric) CO2.
Diatoms are important marine photoautotrophic protists that account for up to 25% of the primary production on Earth (Falkowski and Raven, 1997). They are also important fractionators of stable carbon isotopes that are used to evaluate trophic relationships in marine food webs (Checkley and Entzeroth, 1985; Fry and Wainright, 1991) and marine carbon cycling in modern and ancient oceans (Francois et al., 1993; Hayes, 1993). As a result, the mechanisms of uptake and fixation of inorganic carbon (Ci) in these organisms have been the topics of extensive research (Korb et al., 1997; Tortell et al., 2000; Rost et al., 2003; Tchernov et al., 2003). In particular, the existence and role of a C4 pathway for photosynthetic carbon fixation in marine diatoms has been the subject of research for over 25 years (Beardall et al., 1976; Mortain-Bertrand et al., 1987; Descolas-Gros and Oriol, 1992). Recently, Reinfelder et al. (2000) demonstrated short-term labeling of C4 compounds and transfer of carbon to PGA and sugars in the model marine diatom Thalassiosira weissflogii. But, the designation of diatoms as uni cellular C4 photoautotrophs has not been readily accepted (Riebesell, 2000; Johnston et al., 2001); the C4 pathway is seen as playing chiefly an anaplerotic role and its importance in photosynthesis has been questioned. Further a C4 pathway to support photosynthesis at low CO2 seems redundant with the known CO2 concentrating mechanism (CCM) of marine diatoms. In addition, the presence and localization of the necessary C4 enzymes have been questioned.
The question of the existence of enzymes necessary for a C4 pathway in diatoms has now been largely resolved by the recently sequenced genome of Thalassiosira pseudonana (E.V. Armbrust, J.A. Berges, C. Bowler, B.R. Green, D. Martinez, N.H. Putnam, S. Zhou, A.E. Allen, K.E. Apt, M. Bechner, M. Brzezinski, B.K. Chaal, A. Chiovitti, A.K. Davis, M.S. Demarest, J.C. Detter, T. Glavina, D. Goodstein, M.Z. Hadi, U. Hellsten, M. Hildebrand, B.D. Jenkins, J. Jurka, V.V. Kapitonov, N. Kröger, W.W.Y. Lau, T.W. Lane, F.W. Larimer, J.C. Lippmeier, S. Lucas, M. Medina, A. Montsant, M. Obornik, M.S. Parker, B. Palenik, G.J. Pazour, P.M. Richardson, T.A. Rynearson, M.A. Saito, D.C. Schwartz, K. Thamatrakoln, K. Valentin, A. Vardi, F.P. Wilkerson, and D.S. Rokhsar, unpublished data). Genes coding for phosphoenolpyruvate carboxylase (PEPCase), phosphoenolpyruvate carboxykinase (PEPCKase), and pyruvate orthophosphate dikinase (PPDK, which catalyzes the synthesis of PEP in many C4 plants) have been identified in the genome of this diatom. The intracellular localizations of all of these enzymes in diatoms, which are critical to a complete understanding of carbon metabolism in these organisms, are uncertain. The absence of upstream targeting sequences adjacent to the genes for PEPCase and PPDK in T. pseudonana is consistent with cytoplasmic localizations. The localization of PEPCase in the cytoplasm provides the necessary intracellular compartmentalization for simultaneous carbon fixation by PEPCase and Rubisco in a single cell (Magnin et al., 1997; Voznesenskaya et al., 2002). PEPCKase localization cannot be evaluated with certainty from the gene targeting sequence of T. pseudonana, but its activity was found to follow that of Rubisco in crude cell fractions of T. weissflogii (Reinfelder et al., 2000) and PEPCKase protein was immunolocalized within the chloroplast of the marine diatom Skeletonema costatum (Cabello-Pasini et al., 2001).
In this study, we examine the importance of the C4 pathway in diatom photosynthesis by measuring the effects of PEPCase inhibition and C4 organic carbon-Ci competition on whole cell O2 evolution and inorganic carbon fixation in T. weissflogii. We also examine the form of carbon concentrated during short-term 14C uptake (silicone oil centrifugation) and CO2 release (membrane inlet mass spectrometry) experiments to evaluate the role of the C4 pathway in the diatom CCM. To provide a benchmark to differentiate C4 and C3 pathways, we conduct parallel experiments with the marine Chlorophyte Chlamydomonas sp.
RESULTS AND DISCUSSION
The Importance of C4 Carbon Fixation in Diatom Photosynthesis
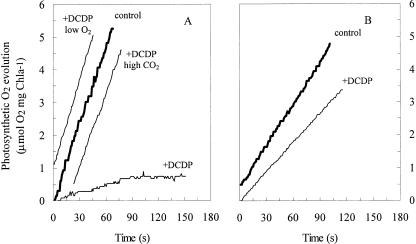
If the C4 pathway is quantitatively important to photosynthesis in diatoms, then the inhibition of PEPCase should have a major effect on photosynthetic O2 evolution. To test the role of PEPCase in diatom photosynthesis, we used the PEPCase-specific inhibitor 3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate (DCDP), an analog of PEP that inhibits PEPCase from a range of C4 and C3 plants, but does not inhibit enzymes that catalyze other reactions in which PEP is a substrate (Jenkins et al., 1987). The addition of DCDP to suspensions of T. weissflogii cells grown in air-equilibrated medium caused a more than 90% decrease in photosynthetic O2 evolution compared with uninhibited cells (Fig. 1). Thus, in diatoms acclimated to 10 μm CO2, a major fraction of net carbon fixation depends on the synthesis of C4 organic carbon by PEPCase. In Chlamydomonas sp. grown under identical conditions as T. weissflogii, DCDP had a small effect on whole cell photosynthetic O2 evolution (Fig. 1). Chlamydomonas sp. are C3 photoautotrophs in which PEPCase serves an anaplerotic role replacing TCA cycle carbohydrates used in amino acid synthesis (Huppe and Turpin, 1994; Rivoal et al., 1998). The minimal effect in this organism provides confidence that the major depression of photosynthesis by DCDP observed in the diatom was not due to the inhibition of anaplerotic PEPCase activity. If the inhibition of PEPCase in T. weissflogii impairs the ability of the diatom cells to concentrate CO2, but not carbon fixation, then the addition of a high concentration of CO2 to the external medium should restore photosynthesis in DCDP-inhibited diatoms to the level of uninhibited cells. Indeed the addition of 150 μm CO2 to DCDP-inhibited T. weissflogii cells restored photosynthesis to the level of the control (Fig. 1), demonstrating that DCDP did not interfere with the Calvin cycle and that the shutdown of photosynthesis resulting from PEPCase inhibition in T. weissflogii is linked to the supply of inorganic carbon to the cell. Thus DCDP-inhibited cells behave as C3 autotrophs lacking a mechanism to actively concentrate inorganic carbon for photosynthesis. Such cells should be particularly susceptible to the negative effects of O2 on photosynthesis as a consequence of photorespiration and show increased photosynthesis rates when the concentration of O2 is lowered. Decreasing the O2 concentration in the medium did in fact result in an increase in photosynthetic O2 production of DCDP-inhibited diatom cells to the levels of the uninhibited control (Fig. 1). The complete recovery of photosynthesis in response to lowering the O2 concentration in these experiments is likely due to the high carboxylation to oxygenation ratio of diatom Rubisco compared with that from other eukaryotic microalgae (Badger et al., 1998). This effect may have been pronounced due to the somewhat elevated CO2 concentrations (15–30 μm) at the experimental pH.
Figure 1.
Effects of PEPCase inhibition on net photosynthetic O2 evolution in (A) the marine diatom T. weissflogii and (B) the marine chlorophyte Chlamydomonas sp. Graphs depict whole cell O2 evolution in control incubations (thick lines; 15–30 μm CO2 and 300–400 μm O2) or in the presence (thin lines) of the PEPCase inhibitor DCDP (750 μm). For T. weissflogii, photosynthesis rates of PEPCase-inhibited cells with 150 μm CO2 plus 300 to 400 μm O2 (+DCDP high CO2) and 80 to 180 μm O2 plus 15 to 30 μm CO2 (+DCDP low O2) are also shown.
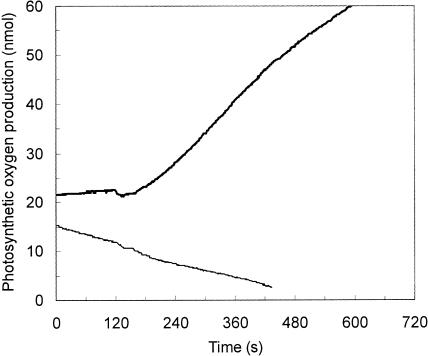
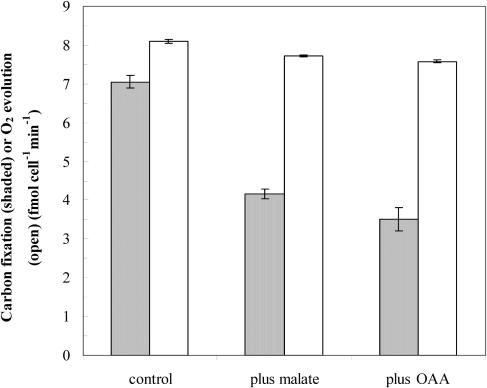
A complementary test of the importance of C4 organic carbon in diatom photosynthesis is provided by the measurement of the effects of C4 compounds on photosynthetic O2 evolution and inorganic carbon fixation. In diatoms at the inorganic carbon (Ci) compensation point, the C4 compound oxaloacetic acid stimulated O2 evolution, but not respiratory O2 consumption (Fig. 2), indicating that the C4 compound was decarboxylated by a nonrespiratory pathway to supply carbon-depleted cells with CO2. In photosynthesizing T. weissflogii cells given sufficient inorganic carbon (1.2 mm) to maintain photosynthesis at its maximum carbon-saturated rate, the addition of 2 mm oxaloacetic acid (OAA) or malic acid suppressed inorganic carbon fixation by 40% to 50%, but had no effect on photosynthetic O2 evolution (Fig. 3). This confirms that the C4 compounds can provide a large fraction of the photosynthetically fixed carbon in this diatom.
Figure 2.
Oxaloacetic acid-dependent O2 evolution (thick line) and dark O2 consumption (thin line) in T. weissflogii cells at the Ci compensation point given 1 mm OAA at 120 s.
Figure 3.
Effects of C4 organic acids on carbon fixation (shaded bars) and O2 evolution (open bars) in the marine diatom T. weissflogii. Measurements were made in the presence or absence of 2 mm malic acid or OAA. Error bars represent propagated 14C counting errors for carbon fixation rates and ses for O2 evolution rates.
If C4 carbon fixation is the primary mechanism to concentrate CO2 in diatoms, then the ability to use C4 compounds for photosynthesis should be modulated by the concentration of CO2 to which the diatoms have been acclimated. PEPCase activity in T. weissflogii was previously found to increase in cells acclimated to low CO2 concentrations (Reinfelder et al., 2000). As an extension of this observation, we compared OAA-dependent O2 evolution rates in cells acclimated to 10 and 22 μm CO2. In diatoms brought to the Ci compensation point (Ci < 2 μm), the rate of OAA-dependent O2 evolution was twice as high in diatoms acclimated to 10 μm CO2 as in cells grown with 22 μm CO2 (Table I). Since the concentration of CO2 to which the diatoms were acclimated had no effect on Ci-saturated (1 mm Ci, 170 μm CO2) photosynthesis rates (Table I), these results indicate that the capacity of the diatoms to decarboxylate C4 organic acids and provide carbon for photosynthesis is greater in cells acclimated to low CO2.
Table I.
Carbon-dependent O2 evolution rates in T. weissflogii cells acclimated to 10 or 22 μm CO2 (360 or 700 μmol mol−1 CO2, respectively)
| Acclimation [CO2] | P1 mm Ci | P1 mm OAA |
|---|---|---|
| μm | fmol cell−1 min−1 | fmol cell−1 min−1 |
| 10 | 10.5 (0.11) | 6.3 (0.03) |
| 22 | 9.6 (0.03) | 3.1 (0.03) |
Photosynthesis was measured in cells given either 1 mm Ci (170 μm CO2) or 1 mm OAA at pH 7. Values in parentheses are ses of the rate estimates.
The Nature of CO2 Concentration in Marine Diatoms
Unequivocal evidence that marine diatoms possess a CCM has been obtained by a number of researchers (Rotatore et al., 1995; Burkhardt et al., 2001; Tortell and Morel, 2002), but the biochemical mechanism of the diatom CCM is unknown. The production and decarboxylation of C4 organic carbon to supply Rubisco with CO2 represents a carbon storage and delivery mechanism that may function as a CCM to support diatom photosynthesis in low CO2 surface waters. Thus, the CCM and the C4 pathway in diatoms may be one and the same. This is supported indirectly by the observation that C4 carbon fixation (PEPCase activity) in T. weissflogii increases over the range of CO2 concentrations (10 μm and lower; Reinfelder et al., 2000) where CCM activity increases in marine diatoms (Rotatore et al., 1995; Burkhardt et al., 2001; Tortell and Morel, 2002).
If the C4 pathway serves as the CCM in diatoms, transported carbon should be stored as an organic (C4) rather than inorganic (presumably 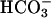 ) compound before fixation in the Calvin cycle in these organisms. In this case, the inorganic carbon that has been measured as intracellular in previous studies of the CCM in diatoms should have resulted from rapid decarboxylation of a C4 compound. We thus attempted to prevent such decarboxylation in the course of the same types of experiments—using the silicon oil centrifugation or the membrane inlet mass spectrometry (MIMS) methods—that have been used to demonstrate the existence of a CCM in diatoms.
) compound before fixation in the Calvin cycle in these organisms. In this case, the inorganic carbon that has been measured as intracellular in previous studies of the CCM in diatoms should have resulted from rapid decarboxylation of a C4 compound. We thus attempted to prevent such decarboxylation in the course of the same types of experiments—using the silicon oil centrifugation or the membrane inlet mass spectrometry (MIMS) methods—that have been used to demonstrate the existence of a CCM in diatoms.
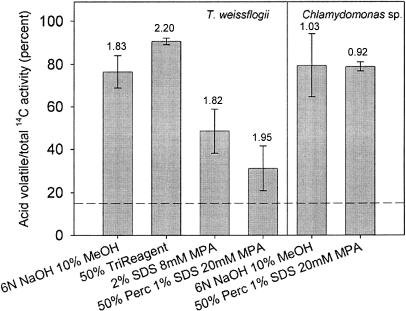
In the silicone oil centrifugation method, inorganic carbon is measured in cells that have been spiked with inorganic 14C and then collected within seconds by centrifugation into a lower layer of an organic extraction and inorganic carbon trapping solution containing methanol and NaOH. In an attempt to inactivate all intracellular enzymes after centrifugation, we tried various extraction-trapping solutions containing cell membrane and protein denaturants (TriReagent, SDS) at low osmotic strength. In experiments run with SDS in the trapping solution, the amount of intracellular 14C measured as inorganic carbon (acid volatile) in T. weissflogii during short-term uptake was lower than that obtained with the normal trapping solution of methanol and NaOH (Fig. 4). The lowest amount of short-term carbon accumulation measured as inorganic carbon was observed with a low osmotic strength trapping solution containing 1% SDS and 20 mm mercaptopicolinic acid (MPA). MPA is an inhibitor of PEPCKase that catalyzes the decarboxylation of OAA in some C4 plants (Rathnam and Edwards, 1977) and is hypothesized to catalyze C4 decarboxylation in the chloroplast of the marine diatom T. weissflogii (Reinfelder et al., 2000). With the SDS-MPA metabolic inactivation solution, approximately 70% of the 14C taken up by T. weissflogii in 10 s was present as acid-stable organic carbon compared to only 24% with the standard methanol-NaOH trapping solution (Fig. 4). Since total 14C activities—organic plus inorganic—were the same (average residual within 6% of the mean) in all treatments with T. weissflogii, this result indicates that, in experiments with the standard trapping solution, a substantial portion of newly formed organic carbon was rapidly converted to inorganic carbon and that the trapping solution routinely used in the silicone oil centrifugation method does not immediately stop all metabolic activity after cells are removed from 14C-Ci. The most potent trapping solution for T. weissflogii (SDS-MPA) had no effect on the amount of intracellular 14C measured as inorganic carbon during short-term 14C exposures in Chlamydomonas sp. (Fig. 4). Thus a large fraction of the carbon taken up in 10 s is stored as organic carbon prior to fixation in the Calvin cycle in the diatom T. weissflogii, but not in the C3 microalga Chamydomonas sp.
Figure 4.
The fraction of short-term carbon accumulation measured as inorganic carbon (acid volatile 14C activity) by the silicon oil centrifugation technique in the marine diatom T. weissflogii and the marine chlorophyte Chlamydomonas sp. Treatments correspond to various extraction-trapping solutions. Numbers above bars are total (organic plus inorganic) 14C activities (kBq) collected after the cells were transferred to the trapping solutions by centrifugation. Error bars are sds of means (n = 3). Note that in such experiments the entrainment of medium with the cells during centrifugation results in a background inorganic carbon in the trapping solution of up to 15% (dashed line) of the total (Tortell et al., 2000).
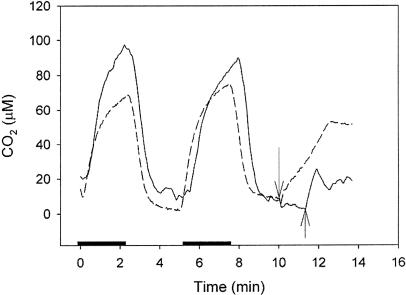
In the MIMS method, the accumulation of carbon by microalgae is quantified by measuring the rate of increase in the concentration of CO2 in cell suspensions in which photosynthesis is stopped by turning off all light (Rotatore et al., 1995; Burkhardt et al., 2001). Such an increase of CO2 concentration (Fig. 5) results from the sum of three processes: (1) dehydration of 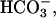 (2) dark respiration, and (3) release of stored carbon by the cells (Burkhardt et al., 2001). Note that the hydration of CO2 released by dense cell suspensions is slow in the presence of added inhibitors of carbonic anhydrase resulting in a CO2 concentration in the medium that is higher than its equilibrium value. If the third process, which is often dominant, results from the rapid decarboxylation of a C4 compound, we would expect the increase in extracellular CO2 observed following the addition (in the light) of our protein denaturing extraction-trapping solution (SDS-MPA), which would stop both C4 decarboxylation and respiration, to be markedly lower than that observed upon turning off the light. Indeed the amount of CO2 released from T. weissflogii cells following the addition of SDS-MPA (Fig. 5, arrow on solid line) was nearly 90% lower than the amount of CO2 released from diatom cells after the light was turned off (Fig. 5, dark periods beginning at 0 and 5.2 min), indicating the presence of a major pool of stored organic carbon. In Chlamydomonas sp. (Fig. 5, dashed line), the amount of CO2 release after the addition of SDS-MPA was only 30% lower than the amount of CO2 released from Chlamydomonas cells after turning off the light. For the marine diatom T. weissflogii, stopping all metabolic activity and inhibiting PEPCKase dramatically decreased CO2 release, while for Chlamydomonas, metabolic inactivation resulted in a smaller decrease in CO2 release, perhaps corresponding to that associated with respiration. The results of the MIMS experiments are thus wholly consistent with the silicone oil centrifugation experiments and with the storage of an organic carbon compound in the diatom and of inorganic carbon in Chlamydomonas sp. prior to ultimate fixation.
(2) dark respiration, and (3) release of stored carbon by the cells (Burkhardt et al., 2001). Note that the hydration of CO2 released by dense cell suspensions is slow in the presence of added inhibitors of carbonic anhydrase resulting in a CO2 concentration in the medium that is higher than its equilibrium value. If the third process, which is often dominant, results from the rapid decarboxylation of a C4 compound, we would expect the increase in extracellular CO2 observed following the addition (in the light) of our protein denaturing extraction-trapping solution (SDS-MPA), which would stop both C4 decarboxylation and respiration, to be markedly lower than that observed upon turning off the light. Indeed the amount of CO2 released from T. weissflogii cells following the addition of SDS-MPA (Fig. 5, arrow on solid line) was nearly 90% lower than the amount of CO2 released from diatom cells after the light was turned off (Fig. 5, dark periods beginning at 0 and 5.2 min), indicating the presence of a major pool of stored organic carbon. In Chlamydomonas sp. (Fig. 5, dashed line), the amount of CO2 release after the addition of SDS-MPA was only 30% lower than the amount of CO2 released from Chlamydomonas cells after turning off the light. For the marine diatom T. weissflogii, stopping all metabolic activity and inhibiting PEPCKase dramatically decreased CO2 release, while for Chlamydomonas, metabolic inactivation resulted in a smaller decrease in CO2 release, perhaps corresponding to that associated with respiration. The results of the MIMS experiments are thus wholly consistent with the silicone oil centrifugation experiments and with the storage of an organic carbon compound in the diatom and of inorganic carbon in Chlamydomonas sp. prior to ultimate fixation.
Figure 5.
Uptake and release of CO2 by T. weissflogii (solid line) and Chlamydomonas sp. (dashed line) during alternating periods of light and dark (bar on x axis) and following the addition (in the light) of 20 mm MPA in 1% SDS (arrows) at constant pH (7.5) recorded in a membrane inlet mass spectrometer. Note that in the presence of the carbonic anhydrase inhibitor acetazolamide, the production of CO2 by the cells in these concentrated suspensions may lead to CO2 concentrations that are higher than that at equilibrium with 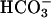 .
.
In conclusion, our results indicate that the C4 pathway plays a central role in photosynthesis in diatoms acclimated to low (e.g. atmospheric) CO2 concentrations. In addition, the carbon accumulated intracellularly by the CCM of these organisms is chiefly organic rather than inorganic, consistent with the formation of C4 intermediates. As reflected in their high 13C content (Fry and Wainright, 1991), diatoms have a photosynthetic mechanism that is clearly unlike that of green algae. On the basis of these and earlier results (Reinfelder et al., 2000), it appears that diatoms utilize a type of unicellular C4 photosythesis under low ambient CO2 that is analogous to that observed in some aquatic plants (Bowes et al., 2002).
MATERIALS AND METHODS
Phytoplankton Cultures
Axenic cultures of the marine diatom Thalassiosira weissflogii (CCMP 1336) and the marine chlorophyte Chlamydomonas sp. (CCMP 222) were maintained in air-equilibrated synthetic ocean water (Aquil; Price et al., 1988, 1989) in continuous light (200 μmol photon m−2 s−1) at 18°C. Stock cultures were maintained in glass tubes and experimental cultures were grown in acid-soaked (1 n HCl) clear polycarbonate bottles. Cell growth was monitored by measuring in vivo fluorescence and microscope cell counts. Cells for all experiments were harvested in mid-exponential growth phase (μ = 1 d−1).
PEPCase Inhibition Experiments
The inhibition of whole cell photosynthesis by DCDP, a specific, competitive (with PEP) inhibitor of PEPCase (Jenkins et al., 1987), was measured in T. weissflogii and Chlamydomonas sp. Photosynthesis was measured as oxygen evolution using an oxygen electrode cell (Hansatech, King's Lynn, UK) with 400 μmol photon m−2 s−1 photosynthetically active radiation at 22°C. For PEPCase inhibition treatments, DCDP was added to a final concentration of 750 μm from a 10-mm stock solution in 10 mm HCl. Cells were concentrated to 2 × 106 (T. weissflogii) and 5.6 × 106 (Chlamydomonas) cells mL−1 in seawater culture media with 2 mm bicine (pH 7.4), 15 to 30 μm CO2, and 300 to 400 μm O2. Photosynthesis rates of DCDP-inhibited cells with excess CO2 (150 μm CO2, 300–400 μm O2) and low O2 (80–180 μm O2, 15–30 μm CO2) were also measured. DCDP inhibition experiments were also tried at pH 8 to maintain lower CO2, but DCDP was not taken up above pH 7.4.
C4 Acid-Dependent Oxygen Evolution Experiments
The stimulation of O2 evolution by C4 compounds was studied in T. weissflogii cells brought to the Ci compensation point (photosynthesis is equal to respiration) in an oxygen electrode. Cells were concentrated to 106 cells mL−1 in buffer (25 mm HEPES, 350 mm sorbitol, pH 7) and incubated in the light until nearly all inorganic carbon was consumed and the concentration of O2 remained constant. Based on the photosynthesis-Ci relationship and respiration rates of this diatom, the total Ci at the compensation point was estimated to be <2 μm. Once the cells were brought to the Ci compensation point, 1 mm OAA was added. Maximum OAA-dependent O2 evolution rates were compared with maximum rates in cells resuspended in buffer with 1 mm Ci. Cells were grown as described above and for at least two transfers (9–10 generations) in media bubbled with either air (10 μm CO2) or air containing 700 μmol mol−1 CO2 (22 μm CO2).
C4-Ci Competition Experiments
The inhibition of inorganic carbon fixation by OAA or its reduced form, malic acid, was measured in photosynthesizing T. weissflogii cells concentrated to 2.5 × 105 cells mL−1 in buffer (25 mm HEPES, 350 mm sorbitol, pH 7.5) and incubated in the light (400 μmol photon m−2 s−1) at 22°C with 1.2 mm 14C-Ci. C4 organic acids were added from freshly prepared stock solutions of the free acids (50 mm) to give final concentrations in the incubations of 2 mm OAA or malic acid. For the malic acid experiments, incubations were begun with the simultaneous addition of 14C-Ci (pH 9.5) and C4 acid dissolved in HEPES buffer (pH 7.5) to cells that had been photosynthesizing in the light for 5 min. In the OAA experiment, photosynthesizing cells were incubated with 2 mm OAA for 30 s prior to the addition of 14C-Ci. At various times during the 5-min incubations, 1-mL subsamples were transferred to 2 mL methanol plus 50 μL 6 n HCl. The liquid was evaporated and the residues resuspended in a small volume (0.2–0.3 mL) of ultra-pure water. The acid-stable (organic) 14C content of extracts was quantified by liquid scintillation counting. Carbon fixation rates were estimated from the slopes of the 14C fixation curves and the specific activity of the radiocarbon. The spontaneous decarboxylation rate of oxaloacetic acid in the HEPES/sorbitol buffer was sufficiently slow (<1 nmol min−1 over 5 min) so as not to be a significant source of CO2. The effects of OAA and malic acid on photosynthetic O2 evolution in T. weissflogii were also measured.
Silicone Oil Centrifugation
Short-term 14C carbon accumulation experiments were conducted using the silicone oil centrifugation technique (Badger et al., 1980; Tortell et al., 2000). Cells were resuspended in Ci-free 50 mm HEPES buffer with seawater salts (pH = 8). Concentrated cells (3.5 × 106 for T. weissflogii and 9 × 106 for Chlamydomonas sp. in 200 μL) were layered on top of silicon oil in 0.6-mL microcentrifuge tubes. Illumination was provided from the side at a photon flux density of 500 μmol m−2 s−1 using a tungsten-halogen bulb from a slide projector. To start the incubation 1 mm NaH14CO3 was added and mixed with the pipette. Incubations (duration 10 s) were terminated by centrifugation through the silicon oil layer into a basic cell extraction and inorganic carbon trapping solution (6 n NaOH with 10% methanol). Other trapping solutions (all at pH 8) included 50% TriReagent (Sigma, St. Louis), 2% SDS and 8 mm MPA (Toronto Research Chemicals), and 50% Percol (to provide density at low ionic strength) with 1% SDS and 20 mm MPA. The resulting pellet was immediately frozen in liquid nitrogen. The base of the centrifuge tube was cut off and retained quantified using liquid scintillation counting. The 14C counts (corrected for quench) for the unacidified samples were used to determine the total accumulated carbon (inorganic and organic) and those for the acidified samples were used to determine the amount of organic carbon fixed.
Membrane Inlet Mass Spectrometry
The short-term accumulation and release of carbon was followed using a membrane inlet system attached to a Prisma QMS-200 (Pfeiffer) quadrapole mass spectrometer with closed ion source recording at mass/charge (m/z) ratios of 40 and 44. The membrane inlet system was modified from a water-jacketed DW/2 oxygen electrode chamber (Hansatech Instruments) in which the electrode base plate was replaced by a stainless steel base plate with a gas port drilled through the center. The standard Teflon membrane (thickness 12.5 μm) supplied with the DW/2 system was used. Illumination was provided by a tungsten projector bulb at 300 μmol m−2 s−1. Temperature was maintained at 20°C. The mass spectrometer was calibrated for CO2 using buffer equilibrated with 100 and 750 μmol mol−1 CO2. Cells were concentrated to 9.4 × 106 (T. weissflogii) and 3.6 × 106 (Chlamydomonas) cells mL−1 in buffer (25 mm HEPES, 350 mm sorbitol, pH 7.5) with 100 mm acetazolamide to suppress extracellular carbonic anhydrase activity and an initial Ci concentration of 100 μm. Release of CO2 was measured in cells transferred to the dark and in cells treated with 1% SDS plus 20 mm MPA (pH = 7.5), an inhibitor of the C4 PEPCKase.
Acknowledgments
We thank Katsura Izui (Kyoto University) for kindly providing the DCDP.
This work was supported by the NSF-EMSI program through the Center for Environmental Bioinorganic Chemistry (CEBIC) and by a Hatch/McIntyre-Stennis grant through the New Jersey Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041319.
References
- Badger MR, Kaplan A, Berry JA (1980) Internal inorganic carbon pool of Chlamydomonas-reinhardtii – evidence for a carbon-dioxide concentrating mechanism. Plant Physiol 66: 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot 76: 1052–1071 [Google Scholar]
- Beardall J, Mukerji D, Glover HE, Morris I (1976) The path of carbon in photosynthesis by marine phytoplankton. J Phycol 12: 409–417 [Google Scholar]
- Bowes G, Rao SK, Estavillo GM, Reiskind JB (2002) C4 mechanisms in aquatic angiosperms: comparisons with terrestrial C4 systems. Funct Plant Biol 29: 379–392 [DOI] [PubMed] [Google Scholar]
-
Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D (2001) CO2 and
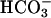 uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46: 1378–1391 [Google Scholar]
uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr 46: 1378–1391 [Google Scholar] - Cabello-Pasini A, Swift H, Smith GJ, Alberte RS (2001) Phosphoenolpyruvate carboxykinase from the marine diatom Skeletonema costatum and the phaeophyte Laminaria setchellii. II. Immunological characterization and subcellular localization. Bot Mar 44: 199–207 [Google Scholar]
- Checkley DM Jr, Entzeroth LC (1985) Elemental and isotopic fractionation of carbon and nitrogen by marine, planktonic copepods and implications to the marine nitrogen cycle. J Plankton Res 7: 553–568 [Google Scholar]
- Descolas-Gros C, Oriol L (1992) Variations in carboxylase activity in marine phytoplankton cultures. β-carboxylation in carbon flux studies. Mar Ecol Prog Ser 85: 163–169 [Google Scholar]
- Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell Science, Malden, MA
- Francois R, Altabet MA, Goericke R, McCorkle DC, Brunet C, Poisson A (1993) Changes in the δ13C of surface water particulate organic matter across the subtropical convergence in the SW Indian Ocean. Global Biogeochem Cycles 7: 627–644 [Google Scholar]
- Fry B, Wainright SC (1991) Diatom sources of 13C-rich carbon in marine food webs. Mar Ecol Prog Ser 76: 149–157 [Google Scholar]
- Hayes JM (1993) Factors controlling 13C contents of sedimentary organic compound: Principles and evidence. Mar Geol 113: 111–125 [Google Scholar]
- Huppe HC, Turpin DH (1994) Integration of carbon and nitrogen-metabolism in plant and algal cells. Annu Rev Plant Phys 45: 577–607 [Google Scholar]
- Jenkins CLD, Harris RLN, McFadden HG (1987) 3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate, a new specific inhibitor of phosphoenolpyruvate carboxylase. Biochem Int 14: 219–226 [Google Scholar]
- Johnston AM, Raven JA, Beardall J, Leegood RC (2001) Photosynthesis in a marine diatom. Nature 412: 40–41 [DOI] [PubMed] [Google Scholar]
- Korb RE, Saville PJ, Johnston AM, Raven JA (1997) Sources of inorganic carbon for photosynthesis by three species of marine diatom. J Phycol 33: 433–440 [Google Scholar]
- Magnin NC, Cooley BA, Reiskind JB, Bowes G (1997) Regulation and localization of key enzymes during the induction of Kranz-less, C-4-type photosynthesis in Hydrilla verticillata. Plant Physiol 115: 1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortain-Bertrand A, Descolas-Gros C, Jupin H (1987) Short-term 14C incorporation in Skeletonema costatum (Greville) Cleve (Bacillariophyceae) as a function of light regime. Phycologia 26: 262–269 [Google Scholar]
- Price NM, Harrison GI, Hering JG, Hudson RJ, Nirel PMV, Palenik B, Morel FMM ((1988) 89) Preparation and chemistry of the artificial algal culture medium Aquil. Biol Oceanogr 6: 443–461 [Google Scholar]
- Rathnam CKM, Edwards GE (1977) Use of inhibitors to distinguish between C4 acid decarboxylation mechanisms in bundle sheath cells of C4 plants. Plant Cell Physiol 18: 963–968 [Google Scholar]
- Reinfelder JR, Kraepiel AML, Morel FMM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407: 996–999 [DOI] [PubMed] [Google Scholar]
- Riebesell U (2000) Carbon fix for a diatom. Nature 407: 959–960 [DOI] [PubMed] [Google Scholar]
- Rivoal J, Plaxton WC, Turpin DH (1998) Purification and characterization of high- and low-molecular-mass isoforms of phophoenolpyruvate carboxylase from Chlamydomonas reinhardtii. Biochem J 331: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sultemeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48: 55–67 [Google Scholar]
- Rotatore C, Colman B, Kuzma M (1995) The active uptake of carbon dioxide by the marine diatom Phaeodactylum tricornutum and Cyclotella sp. Plant Cell Environ 18: 913–918 [Google Scholar]
- Tchernov D, Silverman J, Luz B, Reinhold L, Kaplan A (2003) Massive light-dependent cycling of inorganic carbon between oxygenic photosynthetic microorganisms and their surroundings. Photosynth Res 77: 95–103 [DOI] [PubMed] [Google Scholar]
- Tortell PD, Rau GH, Morel FMM (2000) Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnol Oceanogr 45: 1485–1500 [Google Scholar]
- Tortell PD, Morel FMM (2002) Sources of inorganic carbon for phytoplankton in the eastern subtropical and equatorial Pacific ocean. Limnol Oceanogr 47: 1012–1022 [Google Scholar]
- Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE (2002) Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J 31: 649–662 [DOI] [PubMed] [Google Scholar]