Abstract
We investigated interactions between photosynthesis and nitrogen fixation in the non-heterocystous marine cyanobacterium Trichodesmium IMS101 at the single-cell level by two-dimensional (imaging) microscopic measurements of chlorophyll fluorescence kinetics. Nitrogen fixation was closely associated with the appearance of cells with high basic fluorescence yield (F0), termed bright cells. In cultures aerated with normal air, both nitrogen fixation and bright cells appeared in the middle of the light phase. In cultures aerated with 5% oxygen, both processes occurred at a low level throughout most of the day. Under 50% oxygen, nitrogen fixation commenced at the beginning of the light phase but declined soon afterwards. Rapid reversible switches between fluorescence levels were observed, which indicated that the elevated F0 of the bright cells originates from reversible uncoupling of the photosystem II (PSII) antenna from the PSII reaction center. Two physiologically distinct types of bright cells were observed. Type I had about double F0 compared to the normal F0 in the dark phase and a PSII activity, measured as variable fluorescence (Fv = Fm − F0), similar to normal non-diazotrophic cells. Correlation of type I cells with nitrogen fixation, oxygen concentration, and light suggests that this physiological state is connected to an up-regulation of the Mehler reaction, resulting in oxygen consumption despite functional PSII. Type II cells had more than three times the normal F0 and hardly any PSII activity measurable by variable fluorescence. They did not occur under low-oxygen concentrations, but appeared under high-oxygen levels outside the diazotrophic period, suggesting that this state represents a reaction to oxidative stress not necessarily connected to nitrogen fixation. In addition to the two high-fluorescence states, cells were observed to reversibly enter a low-fluorescence state. This occurred mainly after a cell went through its bright phase and may represent a fluorescence-quenching recovery phase.
Biological fixation of atmospheric nitrogen is performed by certain cyanobacteria when bioavailable forms of nitrogen (nitrate and ammonia) are limited. Nitrogen fixation is catalyzed by an essentially anaerobic enzyme, nitrogenase, which is irreversibly inhibited in vitro when exposed to molecular oxygen (for review, see Postgate, 1998). Diazotrophic cyanobacteria are the only nitrogen-fixing organisms that produce molecular oxygen as a by-product of photosynthesis and must prevent nitrogenase from being damaged by oxygenic photosynthesis (for review, see Gallon, 1992, 2001; Bergman et al., 1997; Berman-Frank et al., 2003). Most diazotrophic cyanobacteria achieve this by separating photosynthesis and nitrogen fixation either spatially, by differentiating highly specialized cells called heterocysts, or temporally, by fixing nitrogen at night (usually found in unicellular diazotrophic cyanobacteria). In contrast, filamentous non-heterocystous marine cyanobacteria of the genus Trichodesmium execute both processes during the light period without irreversible differentiation of specialized cells. Nitrogen fixation by Trichodesmium contributes a significant proportion to the total marine nitrogen fixation and to the addition of new nitrogen inputs in the tropical and subtropical oceans (Capone et al., 1997). Early studies on nitrogen fixation by Trichodesmium have shown light-dependent, DCMU-sensitive oxygen consumption by the Mehler reaction (Kana, 1993), as well as an unusually high dark respiration rate (Kana, 1993; Carpenter and Roenneberg, 1995), suggesting that oxygen-consuming mechanisms protect nitrogenase. Immunolocalization showed that nitrogenase is not simultaneously expressed in all cells of the filaments (called trichomes), showing that a partial differentiation is involved in the N2 fixation strategy of Trichodesmium (Lin et al., 1998). Moreover, nitrogenase genes in Trichodesmium are not significantly different from other cyanobacteria (Sroga et al., 1996; Zehr et al., 1997), indicating that the coexistence of photosynthesis and nitrogen fixation is not related to a special type of nitrogenase.
Measurements of chlorophyll (Chl) fluorescence kinetics yield comprehensive information about the regulation of photosynthesis in cyanobacteria (e.g. for review, see Campbell et al., 1998), and has led to the discovery and better understanding of diel activity cycles in Cyanothece sp. (Meunier et al., 1997, 1998; for review, see Sherman et al., 1998), Synechococcus sp. (Behrenfeld and Kolber, 1999), and Plectonema boryanum (Misra and Mahajan, 2000). We utilized the newly developed technique of Chl fluorescence kinetic microscopy (FKM; Küpper et al., 2000a) to resolve the spatial and temporal patterns of photosynthetic activity in Trichodesmium in relation to nitrogen fixation (Berman-Frank et al., 2001b). Thus, we have shown that Trichodesmium trichomes have a homogeneous high photosynthetic yield during most of the day, and perform a reversible partial differentiation of cells for the period of nitrogen fixation. This partial differentiation involves a decline in oxygen production by enhancing the Mehler reaction correlated to a reversible down-regulation of PSII activity (Berman-Frank et al., 2001b). Chl FKM (Küpper et al., 2000a) revealed that, during the period of high nitrogen fixation, some cells had a much higher Chl fluorescence yield than all the cells sampled when no nitrogen fixation occurs; these cells have been termed bright zones/cells (Berman-Frank et al., 2001b). Limitations in the detection system used at the time restricted our investigation to measuring the basic fluorescence yield (F0) and the maximum activity of PSII (Fv/Fm) of individual Trichodesmium cells. Hence, it remained unknown in which way Trichodesmium regulates the performance of PSII to allow nitrogen fixation in the middle of the photoperiod.
In this study, improvements to the FKM (Ferimazova et al., 2002) allowed a more detailed analysis of Chl fluorescence kinetics in nitrogen-fixing Trichodesmium cells, and revealed a complex pattern of photosynthetic regulation. The two-dimensional (imaging) fluorescence kinetic measurements were combined with traditional measurements of nitrogen fixation and counts of the percentage of the bright cells. These investigations were done in Trichodesmium cultures grown either at the normal (atmospheric) oxygen concentration or under stress caused by diminished or elevated oxygen levels.
RESULTS
Nitrogen Fixation, Chl Synthesis, and Percentage of Bright Cells
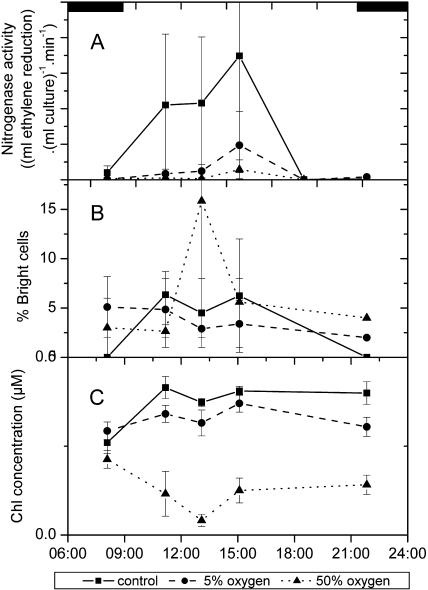
The cells of the control culture (air-grown cells) exhibited the typical diurnal activity pattern, i.e. a peak of nitrogen fixation after the first third of the light period (Fig. 1A) connected to the appearance of bright cells (Berman-Frank et al., 2001b). Now a quantitative correlation between the nitrogen fixation activity and the percentage of bright cells was found (Fig. 1, A and B). The beginning of nitrogen fixation was also associated with high activity of Chl synthesis (Fig. 1C). In cultures grown under low (5%) oxygen, nitrogen fixation rates were much lower than in the control culture, and the peak was less pronounced (Fig. 1A). Nitrogenase activity again positively correlated with the frequency of bright cells, which were much less abundant than in the control, but occurred throughout the whole cycle (Fig. 1B). Chl concentrations did not increase significantly (P = 0.05) over the time of analysis in this culture (Fig. 1C). In cultures grown under high (50%) oxygen, the nitrogen fixation over the day was lower than in the low-oxygen culture and much diminished compared to the control (Fig. 1A). This was accompanied by a decrease in Chl concentration over the day, with a pronounced Chl loss in the middle of the light period and a slight recovery towards its end (Fig. 1C). We always observed a higher percentage of bright cells in the high-oxygen culture during the scotoperiod as compared with the control (air) culture. The pattern of the percentage of bright cells in the 50% oxygen culture was less reproducible during the photoperiod (Fig. 1B).
Figure 1.
Comparison of nitrogen fixation activity and the percentage of bright cells in Trichodesmium IMS101 cultures grown at different oxygen concentrations. A, Nitrogen fixation per volume of culture. The data are not normalized to the Chl concentration (C), as the stress-induced Chl bleaching in the high-oxygen culture would cause misleading values of the total nitrogen fixation under these conditions. B, Percentage of bright cells (defined as more than double the basic fluorescence yield of the average in the dark period). C, Chl concentration per volume of culture.
FKM Measurements
Control Culture
Basic Fluorescence Yield
Dramatic changes in the basic fluorescence (F0) of individual cells characterized the fluorescence kinetics of Trichodesmium. The time course of these changes in F0 was correlated with that of the other events during the diurnal activity cycle.
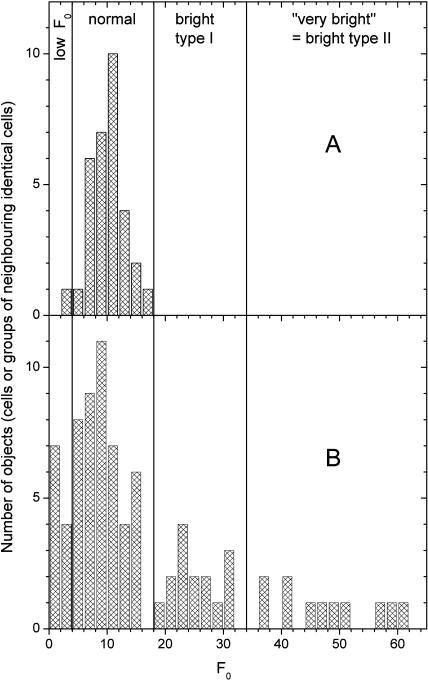
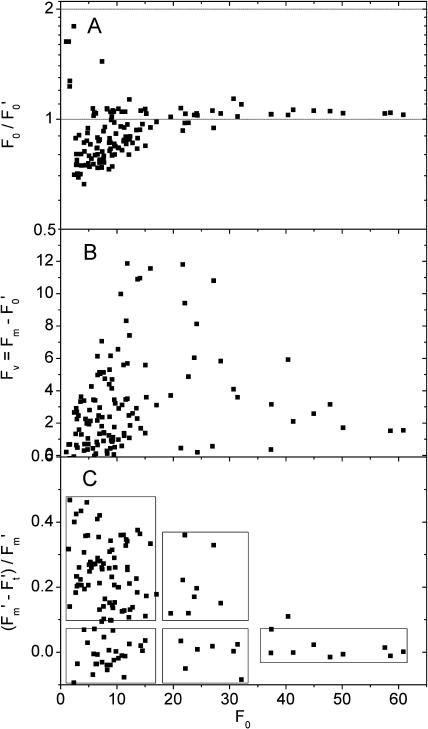
Figure 2 shows the changes of the basic fluorescence yield (F0) in the diazotrophic versus the non-diazotrophic period of the diurnal activity cycle. As seen in Figure 2A, during the scotophase the cell population of the control culture was rather homogeneous, i.e. the distribution of F0 values formed a single, rather narrow, approximately Gaussian peak. The remaining variability was mainly between filaments, not between cells of the same filament (see Berman-Frank et al., 2001b), and was caused partially by the inherent noise of the measurement. In contrast, at the peak of nitrogen fixation (see above), a large number of bright cells occurred with F0 values several times higher than the average of normal cells (Fig. 2B). The bright cells consisted of two groups, which were different both in F0 and physiologically (see below). Additionally, cells with less than half the F0 compared to the normal non-diazotrophic state were observed during this period, leading a peak close to zero in the histogram of F0. The basic fluorescence states (low, normal, high, and very high F0) are defined in Figure 2B.
Figure 2.
Distribution of intrinsic fluorescence values in the control culture during the diazotrophic phase compared with the dark phase and early light phase. Each data point in the graphs represents one cell or one group of neighboring cells in the same trichome that was identical in all fluorescence kinetic parameters. The same cells/objects were analyzed also for all parameters shown in Figures 5, 6 (for the respective time periods as defined below), and 7 (all data). The lines separate the fluorescence states. Cells with F0 < 5 were regarded as low F0, 5 < F0 < 18 as normal, 19 < F0 < 35 as bright type I, and F0 > 35 as very bright (bright type II). A, Distribution of fluorescence values between 9:00 pm and 10:30 am, i.e. during the night and the early light phase. B, Distribution of fluorescence values between 12:30 pm and 6:30 pm, i.e. during the diazotrophic phase.
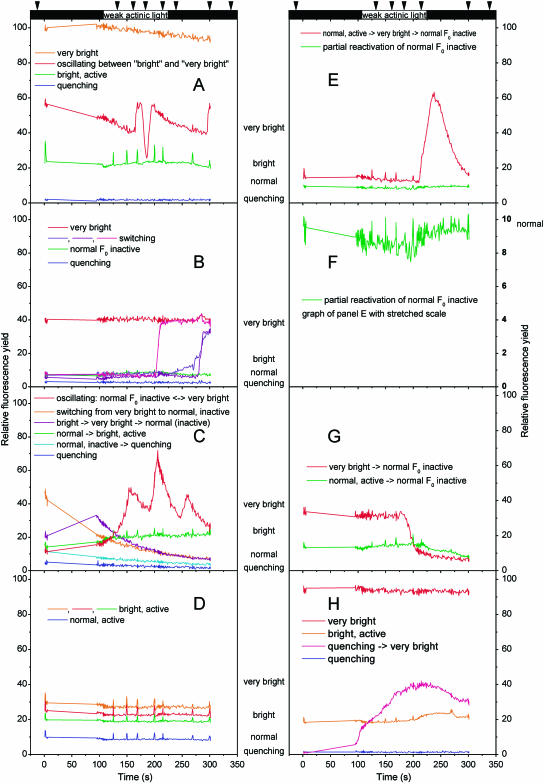
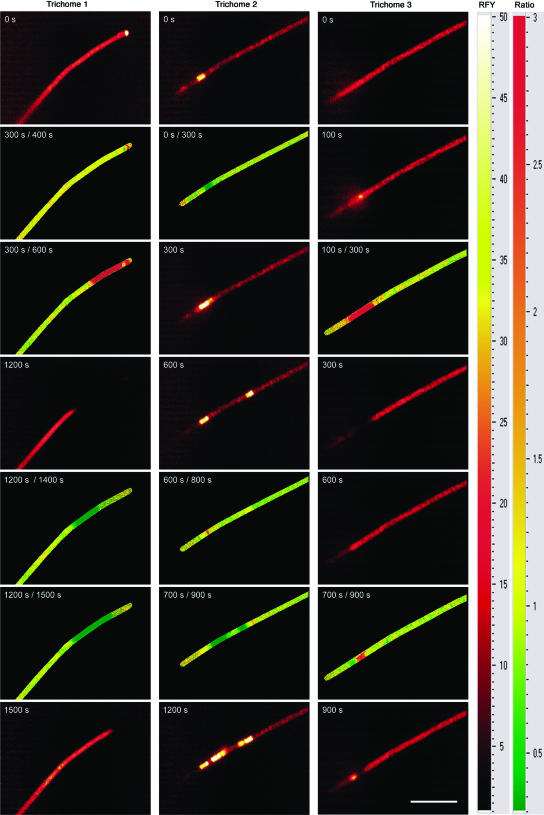
Reversible switches between all four basic fluorescence states often occurred within minutes, sometimes even faster (Figs. 3 and 4). Cells often switched to high fluorescence yield immediately after the beginning or the end of actinic light exposure (Fig. 3). If several cells in a filament switched, they often did not do so randomly, but sequentially, reminiscent of traffic lights (Fig. 4). Changes of F0 during the measurement were related to the fluorescence state at its beginning, as demonstrated by correlating the basic fluorescence yield immediately before (F0) and after the actinic light period (F′0; Fig. 5A). Most of the bright cells (F0 > 20) returned to lower fluorescence yields, so that their F0/F′0 was above one. In contrast, the low- and normal fluorescence cells tended to switch to the normal or the bright state, respectively, so that F0/F′0 was below one. A disparity in the different rates of transitions between the activity states caused a difference in F0/F′0. In the low-fluorescence cells, this ratio was usually much further below one (down to 0.65), than it was above one for the bright cells (max. 1.08). The fastest observed switches were those from normal to higher fluorescence levels. A complete switch from the normal to the very bright state sometimes took less than 10 s (Fig. 3, A–C and E). Switches down from the bright or very bright states to normal or low F0 were usually in the range of 1 to 5 min (Fig. 3, A, C, and E). Most of the switches occurred several minutes after the end of the actinic light, so that they were not manifested in the F0/F′0 value. However, we did observe several cases of faster switches (Fig. 3, A and G), with the cell shown in Figure 3G switching before the termination of the actinic light.
Figure 3.
FKM traces of cells that exhibited particularly interesting switches in their basic fluorescence and/or activity. The curves were recorded on samples taken from the culture during the period of nitrogen fixation. A uniform measuring protocol was applied: a 2-s saturation flash at the start followed by a 3-s measurement of relaxation, a 95-s dark period without measurement for complete relaxation of the sample in the absence of measuring light, a 5-s F0 measurement, a 100-s actinic irradiance (60 μmol m−2 s−1, 400–490 nm), and a dark period until the end of record. The arrows above the figure indicate the time points of additional 1-s saturating flashes; the black boxes indicate periods without actinic light. Note that all cells in each panel have been recorded simultaneously as a film, excluding that the drastic switches are caused by a measuring artifact. Each panel represents one example of an FKM record (two-dimensional data set = film), and each kinetic trace represents either one cell or a group of cells that displayed identical fluorescence kinetics within that FKM record. In sample A, all shown cells except bright active were on the same trichome; in the other samples, all cells were in the same trichome.
Figure 4.
FKM parameter images showing the spatial and temporal distribution of reversible switches between different fluorescence states. The data were recorded under measuring light during the period of nitrogen fixation. The images in the glow color scale show the actual fluorescence yield (white = high → yellow → red → black = low fluorescence yield). The images in red-yellow-green color scale show changes in fluorescence yield as a ratio of the first divided by the second image (red = decrease, yellow = constant, green = increase; areas without cells are labeled black). The scale bar in the lower right image represents 50 μm.
Figure 5.
Correlation between basic fluorescence yield (F0) and photosynthetic parameters in the control culture at an actinic irradiance of 60 μmol m−2 s−1 blue (400–490 nm) light. As in Figures 2 and 6, each data point in the graphs represents one cell or one group of neighboring cells in the same trichome that was identical in all fluorescence kinetic parameters. The boxes indicate the groups as shown also in Figures 2 and 6. The data points (kinetics of selected objects) shown here are the same as shown in Figure 6. A, F′0 versus F0. One cell, with an F0 of 30 and an F0/F′0 of 4.5, is not shown in order to make the distribution of the other values more visible. B, Variable fluorescence versus F0. C, Efficiency of PSII versus F0.
Photochemistry
Variable fluorescence parameters, characterizing photochemical activity, also varied over the course of the daily activity cycle, yet their patterns differed from that of the changes in basic fluorescence yield. A decline in the dark-adapted maximal variable fluorescence (Fv = Fm − F0; Fig. 6D) was observed during the diazotrophic period. This indicates a decline in the maximal photochemical yield of PSII, even without the normalization to Fm that would yield the standard parameter for the maximal photochemical yield of PSII, Fv/Fm (e.g. Maxwell and Johnson, 2000; Roháček, 2002). We assessed the variable fluorescence without the conventional normalization to Fm in order to separate the dramatic changes in F0 (Figs. 2 and 6A) from changes in the variable fluorescence proper; this was also done by Behrenfeld and Kolber (1999). The response to saturating flashes during actinic irradiance displayed a trend similar to that of the Fv values, both in its non-normalized (F′m − F′t; not shown) and in its more conventional, normalized form (F′m − F′t)/F′m (Fig. 6G). The latter is believed to indicate the rate of electron transport through the electron transport chain (Genty et al., 1989).
Figure 6.
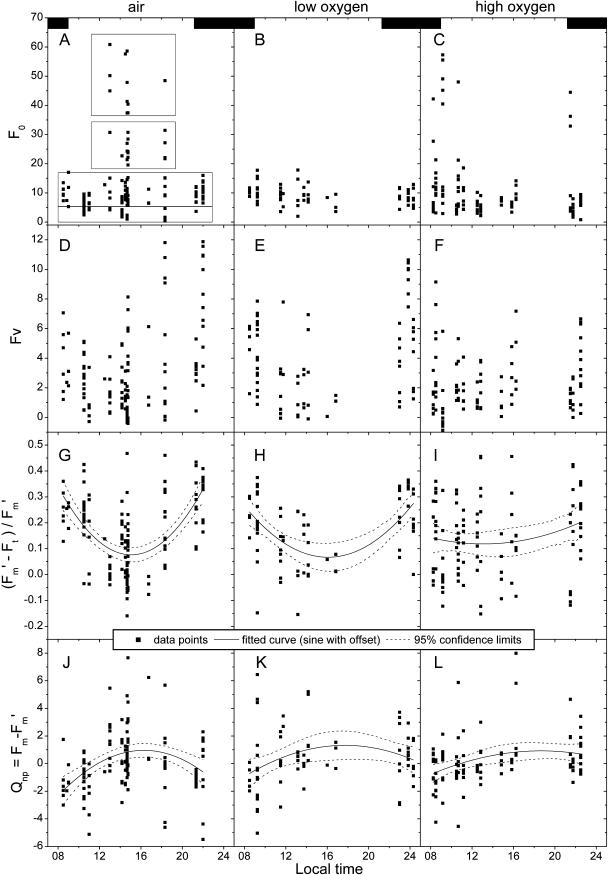
Diurnal cycle of photosynthetic activity and influence of variations in oxygen concentration. As in Figures 2 and 5, each data point in the graphs represents one cell or one group of neighboring cells in the same trichome that was identical in all fluorescence kinetic parameters. Usually several data points were recorded at the same time because several cells were selected in the same two-dimensional measurement (film) and several films were recorded from the same cell preparation. The kinetic records of the same cells/objects were analyzed not only for all panels within one column of this figure, but also for all parameters shown in Figures 2, 5, and 7. The dashed boxes in A indicate the classification of the physiological states correlating to F0. The black boxes in the first row of graphs indicate the dark period (9 pm–9 am). All data were measured at an actinic irradiance of 60 μmol m−2 s−1 blue (400–490 nm) light. The parameter (F′m − F′t)/F′m is mainly shown for comparison with previous studies; because of the variations in F0 studied here, it cannot be used in its conventional sense, i.e. as a measure of PSII efficiency. For the latter, the non-normalized parameters should be used instead. First row, F0; second row, Fv; third row, (F′m − Ft)/F′m; fourth row, Qnp = Fm − F′m. Left column, Control (air); middle column, 5% oxygen; right column, 50% oxygen.
The decrease in PSII activity, measured as Fv, occurred only in one type of bright cells, as revealed by a comparison of F0 and Fv (Figs. 5 and 7). Figure 5B shows the highest values of Fv in cells with normal F0 and, unexpectedly, in bright cells with an F0 up to 30. However, for the very bright cells (F0 > 35; Fig. 2), the Fv values became low. The distribution of the (F′m − F′t)/F′m values (Fig. 5C) shows a similar distinction.
Figure 7.
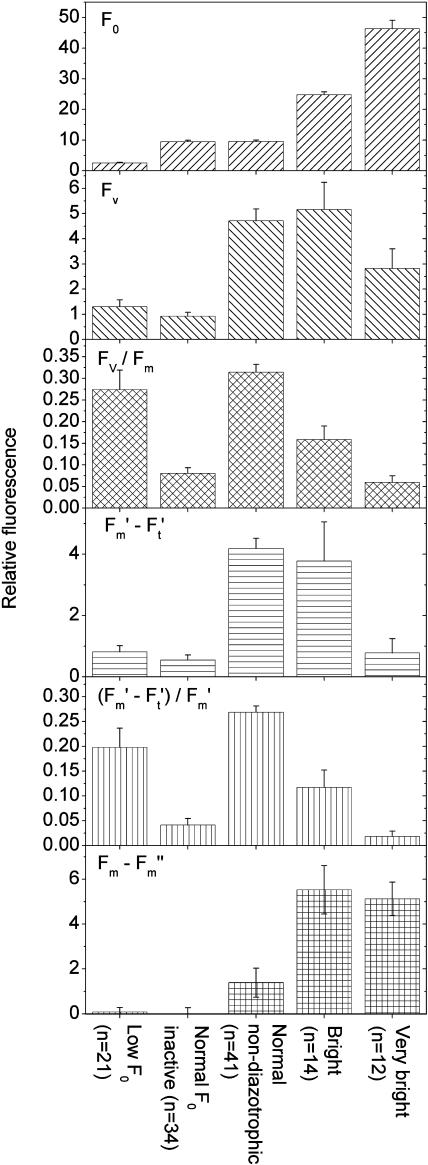
Fluorescence kinetic parameters of the different physiological states of Trichodesmium cells. All data were taken from the control culture and measured at an actinic irradiance of 60 μmol m−2 s−1 blue (400–490 nm) light. The definition of the groups by the level of basic fluorescence was used as shown in Figures 2, 5, and 6, i.e. cells with F0 < 5 were regarded as low F0 (quenching), 5 < F0 < 18 as normal, 19 < F0 < 35 as bright type I, and F0 > 35 as very bright (bright type II). The group normal non-diazotrophic refers to all cells with normal fluorescence measured between 12:00 am to 10:30 am and between 9:00 pm to 12:00 am, and was defined this way to exclude those cells that had normal F0 but occurred during the photosynthetically inactive recovery phase after nitrogen fixation. The group normal F0 inactive includes all cells with normal F0 (see above) with an Fv/Fm lower than the average of the normal non-diazotrophic group and measured during the diazotrophic period. As in Figure 2, the parameters (F′m − F′t)/F′m and Fv/Fm are mainly shown for comparison with previous studies; because of the variations in F0 studied here, they cannot be used in their conventional sense, i.e. as a measure of PSII efficiency. For the latter, the non-normalized parameters should be used instead. The number of samples (n) refers to the number of objects within the group. As in all other figures, each object represents a cell or a group of neighboring cells that was identical in fluorescence kinetics; the objects shown here are the same as shown in Figures 2, 5, and 6. All groups were highly significantly (t tests, P < 0.01) different for all tested parameters (F0, Fv, Fv/Fm, F′m − F′t, (F′m − F′t)/F′m, Fm − F′m), except for the following t tests (Q, quenching; NI, normal F0 inactive; NA, normal non-diazotrophic; B, bright type I; V, very bright [bright type II]): (1) F0 was not significantly different between NI and NA (P = 0.96). (2) Fv was not significantly different between Q and NI (P = 0.20) and between NA and B (P = 0.66). (3) Fv/Fm was not significantly different between Q and NA (P = 0.33), between Q and B (P = 0.07), and between NI and V (P = 0.38). (4) F′m − F′t was not significantly different between NI and V (P = 0.55), between Q and NI (P = 0.31), between Q and V (P = 0.94), and between NA and B (P = 0.66). (5) (F′m − F′t)/F′m was not significantly different between Q and B (P = 0.17) and between NI and V (P = 0.25). (6) Qnp = Fm − F′m was not significantly different between Q and NI (P = 0.80), between Q and NA (P = 0.16), and between B and V (P = 0.77). (7) The P value of the differences in Fv was 0.03 for Q versus V, and 0.05 for NA versus V and B versus V. The P value of the difference in Fv/Fm was 0.01 for B versus V. The P value of the F′m − F′t comparison was 0.04 for B versus V. The P value of the difference in Qnp = Fm − F′m was 0.07 for NI versus NA. The P value of the difference in (F′m − F′t)/F′m was 0.03 for NI versus B and 0.02 for B versus V.
Using the activity data combined with the groups of basic fluorescence values, we devised a general classification of cells with different fluorescence properties related to the various metabolic phases of the Trichodesmium diurnal cycle (Fig. 7). The individual cell types differ in their fluorescence characteristics in the following way. The normal, non-diazotrophic cells had the highest dark-adapted efficiency of PSII, measured as Fv/Fm, and also the highest efficiency of PSII under actinic irradiance (F′m − F′t)/F′m. However, calculated as Fv without normalization to Fm, the dark-adapted efficiency of PSII of bright cells was not different from that of normal cells. Only the very bright cells had about a 40% lower Fv (and, consequently, a drastically lower Fv/Fm). And much of their Fv, shown in Figure 7, was actually due to their tendency to return to lower fluorescence levels (Fig. 5A), leading to a decrease in F0 between the Fm and F0 measurement. The high noise in the Fv measurement of the very bright cells rendered the Fv differences between this group and the more active cell types (i.e. the normal and bright cells) only marginally statistically significant (P values of 0.051 and 0.054; see legend, Fig. 7). Measurements during actinic illumination accentuated the difference in PSII activity between bright and very bright cells. Under these conditions, the non-normalized PSII activity (F′m − F′t) of the very bright cells was only about 25% of the activity of the bright and normal cells. This is more significant because, in this study, the F′m − F′t values are more reliable than the Fv (Fv = Fm − F0) values. F′m was measured about a second after F′t and was, therefore, unaffected by the slow decrease in basic fluorescence that distorted Fv in the very bright cells because F0 was measured 95 s after Fm.
The low-fluorescence cells showed both a much lower basic fluorescence yield (F0) as well as a proportionally diminished response to actinic (Ft) and saturating light (Fm, F′m; Fig. 7). The latter effect represents a diminished photochemical activity as seen in the non-normalized variable fluorescence (Fm − F0 or F′m − F′t). Because of the almost proportional decrease of all fluorescence signals, however, normalization to Fm (yielding Fv/Fm) or F′m (yielding (F′m − F′t)/F′m) results in values that would normally indicate a relatively high photochemical activity.
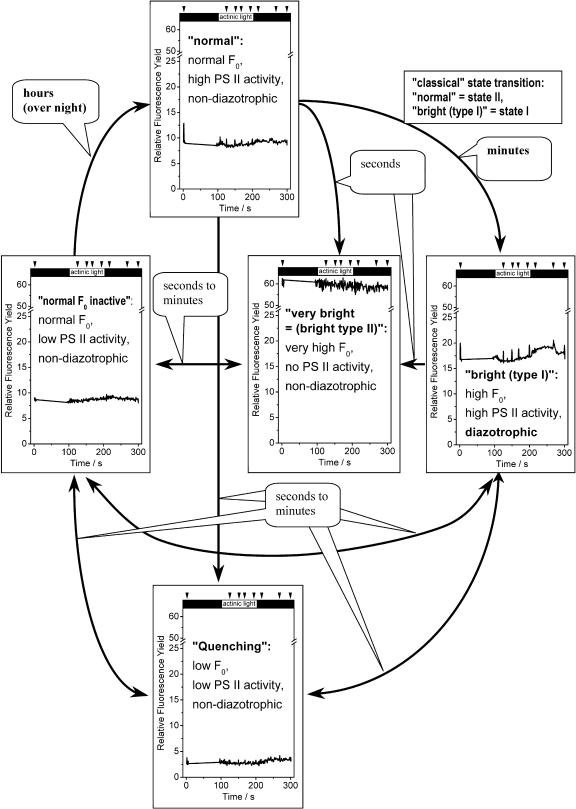
The switches between active and inactive states (changes in Fv) were generally much slower than those between low and high basic fluorescence yield (changes in F0). The transitions from high to low variable fluorescence were at least in the range of minutes (Fig. 3, C, E, and G), and the recovery from low to normal variable fluorescence usually in the range of hours (in the evening and night). In contrast, changes between the four states of basic fluorescence yield were often in the range of seconds. Because of this, low-fluorescence cells returned to normal F0 faster than to normal Fv; this state is shown in Figure 7 as normal F0 inactive. The correlation of low variable with normal basic fluorescence led to very low Fv/Fm and (F′m − F′t)/F′m values (Fig. 7). Only on rare occasions was a partial transition from normal F0 inactive to normal non-diazotrophic observed within a single measurement (Fig. 3, E and F). Normally, this transition could only be concluded from the comparison of samples taken at different times after the end of nitrogen fixation. Figure 8 presents a schematic overview of the observed transitions between the various states of basic fluorescence yield and activity, including examples of typical fluorescence kinetics.
Figure 8.
Scheme illustrating the proposed roles of the different physiological activity states observed in this study, and approximate range of the observed interconversions between the activity states. The definition of the activity states is the same as in Figure 7. The graphs in each panel show examples of the fluorescence kinetics of cells in these activity states, measured at an actinic irradiance of about 1,300 μmol m−2 s−1 blue (400–490 nm) light. The arrows above the figure indicate the time points of saturating flashes; the black boxes indicate periods without actinic light.
Nonphotochemical Quenching
Changes in F0 levels (Figs. 5A and 8) strongly influenced the difference Fm − F′m, which is conventionally used (after normalization) to estimate the nonphotochemical quenching (Qnp) known from green plants (Schreiber et al., 1986; Bilger and Björkman, 1990; van Kooten and Snel, 1990). Qnp showed an opposite and weaker diurnal trend than that measured for photochemical quenching (i.e. PSII activity). Qnp increased during the period of nitrogen fixation (Fig. 6, fourth row) due to the tendency of the bright cells to return to lower F0 levels. Comparison of the activity states, however, showed that the increased Qnp during the diazotrophic period was due to a drastically increased Qnp of both the bright and the very bright cells compared to the cells with normal F0 (Fig. 7). The low-fluorescence cells displayed no significant (P = 0.05) Qnp.
Effect of Oxygen Concentrations
Fluorescence Yield
Cultures exposed to high-oxygen stress displayed about 30% reduction of the normal F0 (Fig. 6C) and a completely different pattern in the appearance of bright cells as compared to the control cultures (Figs. 1C and C). Two peaks in the number of bright cells were measured; the first directly after the onset of the photoperiod (correlating with nitrogen fixation; Fig. 1B) and the second in the afternoon/evening when nitrogen fixation was low or absent (Fig. 6, A and 6C). Analysis of basic fluorescence yield (Fig. 6) showed that all the bright cells that appeared in the evening belonged to the very bright type as described above, i.e. they had an F0 > 35 (despite the diminished normal F0 of this culture) and low PSII activity. In contrast, some of the bright cells in the morning belonged to the type that had an F0 only about double the normal state (Fig. 6C) and an active PSII as shown for the bright cells of the control culture (Fig. 7).
For cultures adapted to low-oxygen stress (> 24 h) the basic fluorescence (F0) was not significantly (P = 0.9) different from that of the control (Fig. 6B). The major difference between the low-oxygen and the air-control treatments was a much lower percentage of bright cells in the former. Low oxygen also yielded the total absence of very bright cells with an F0 > 35. The few bright cells that did occur all belonged to those with an F0 not more than double the normal level (Fig. 6B) and the relatively high variable fluorescence characteristic of this physiological state (see above).
Photochemical and Nonphotochemical Quenching
In contrast to the pronounced diurnal cycle of photochemical activity of the control culture, the high-oxygen culture did not display any clear pattern (Fig. 6, F and I) and contained fewer photochemically active cells [i.e. with high (F′m − F′t)/F′m] throughout the diurnal cycle (compare points with high Fv values in Fig. 6, D and F). The low-oxygen culture showed a daily cycle of photochemical activity, but less pronounced than the control (Fig. 6, E and H), because of the absence of very bright cells. There was no obvious difference between the average photochemical activity of the low-oxygen compared to the control culture (Fig. 6, G and H). Similar to the observed pattern of photochemical quenching, the daily cycle of Qnp (mainly related to transitions of activity state, not conventional Qnp; see above) was much weaker for the low-oxygen culture compared to the control culture, and almost absent in the high-oxygen culture (Fig. 6, fourth row).
DISCUSSION
Comparing the nitrogenase measurements with the FKM measurements, it is obvious that nitrogenase activity only occurs when there are cells with elevated basic fluorescence yield (F0), termed bright cells, confirming our earlier results (Berman-Frank et al., 2001b). Yet, this study also shows that bright cells may occur without the presence of nitrogenase activity. While in the control culture the percentage of bright cells directly correlated with the nitrogenase activity, in the high-oxygen culture, bright cells also occurred in the evening and night in the absence of nitrogenase activity. A closer look revealed that this was related to the occurrence of two distinct types of bright cells—one with active and one with inactive PSII.
Bright cells with values of F0 two- to threefold higher than normal non-diazotrophic cells (type I bright cells) had fluorescence characteristics indicating that PSII still retains an activity similar to the nondiazotrophic state. Such cells occurred exclusively during the period of nitrogen fixation and their frequency was proportional to the rate of nitrogen fixation. Due to its inherent sensitivity to oxygen, nitrogenase can only coexist with a fully functional PSII if the latter does not lead to a net production of oxygen. This can be achieved if the activity of PSII is used to transport electrons ultimately used through photosystem I (PSI) for oxygen-consuming processes. The most likely scenario for high F0 of type I bright cells seems to be a change of the association of the phycobilisome antenna from a coupling with the generally less fluorescent PSI to a coupling with the more fluorescent PSII, thus representing a classical state II → state I transition, as observed in other species (Bonaventura and Myers, 1969; Biggins and Bruce, 1989; Campbell et al., 1998; Meunier et al., 1998). The elevated F0 we measured during state I was most likely caused by the very low ratio of PSII to PSI that is characteristic for Trichodesmium (Berman-Frank et al., 2001a) and will thus leave surplus, free phycobilisomes upon a state II → state I transition. Nutrient deficiency, such as iron, has also been observed to bring about dramatic changes in F0 associated with uncoupled phycobilisomes (Behrenfeld and Kolber, 1999). Iron limitation inhibited the synthesis of the iron-rich PSI, so that a state I → state II transition led to surplus, uncoupled phycobilisomes. Our cultures were iron replete, with more PSI than PSII, as characteristic for Trichodesmium (Berman-Frank et al., 2001a), so that the elevated F0 would be caused by the opposite state transition. Funneling of excitation energy to PSII during state I would stimulate the activity of PSII, which is required for pseudocyclic electron transport from water via PSII and back to oxygen, via the Mehler reaction, in this way compensating for net oxygen production while increasing the energy supply to nitrogenase. Earlier studies on Trichodesmium indicated an enhanced Mehler reaction (Kana, 1993; Berman-Frank et al., 2001b) and an unusually high dark respiration (Carpenter and Roenneberg, 1995). Our results, demonstrating that the diazotrophic bright cells have an active PSII, provide further support for a role of the Mehler reaction in the nitrogen fixation strategy of Trichodesmium. The unicellular cyanobacterium Cyanothece also displays a state II → state I transition and a corresponding increase of F0 during nitrogen fixation (Meunier et al., 1997, 1998; for review, see Sherman et al., 1998). In Cyanothece, however, the state II → state I transition related to nitrogen fixation occurs in the middle of the scotoperiod, with nitrogen fixation fueled by respiratory exhaustion of carbohydrates, and therefore is not related to photosynthesis (Meunier et al., 1997). In Trichodesmium, PSII activity directly contributes to nitrogen fixation (Berman-Frank et al., 2001b). Despite these two fundamental differences, there may be an important similarity between the physiological mechanisms of these two organisms. In Cyanothece, the state II → state I transition during nitrogen fixation is caused by exhaustion of the carbohydrate storage, resulting in an oxidation of the plastoquinone pool (Meunier et al., 1997). A similar situation likely exists in Trichodesmium, which displays a much higher dark respiration than other cyanobacteria (Carpenter and Roenneberg, 1995), and it is further increased during the period of nitrogen fixation (Berman-Frank et al., 2001b). Running into this limitation of carbohydrates and the subsequent state II → state I transition in the light rather than the dark may be the main reason why Trichodesmium fixes more nitrogen per Chl than Cyanothece (Berman-Frank et al., 2003), because it can utilize the state I for carrying out the Mehler reaction and thereby overcome the limitation by carbohydrates. Revealing the differences in the genetic regulation of the circadian rhythms (for review, see Ditty et al., 2003) of Trichodesmium versus Cyanothece will be an interesting subject for future studies.
In contrast to type I bright cells, type II bright cells (very bright cells) with the highest fluorescence yield (up to 10 times higher than the average of non-diazotrophic cells, Fig. 3) had a much lower PSII activity than the normal non-diazotrophic cells, possibly indicating a complete uncoupling of the phycobilisome antenna from both PSII and PSI. With the antenna uncoupled, a maximum percentage of photons captured by the antenna would be re-emitted. In addition to the low PSII activity, the occurrence of type II bright cells, in the high-oxygen culture, independently of nitrogen fixation indicates that they have another role. Their absence in the low-oxygen culture clearly shows that they only occur under conditions of oxidative stress. Currently, we do not know whether the mechanism causing the high F0 of these cells is a defense against oxidative stress (shut down of oxygen production through PSII), or whether oxidative damage leads to damage of the PSII reaction center and through this to an uncoupling of the antenna, so that the cells can subsequently only switch between F0 levels, but never display the variable fluorescence that indicates PSII activity.
The high fluorescence of both type I and type II bright cells cannot be related to protein or pigment degradation or synthesis, as the cells reversibly switched between the different fluorescence states within a few seconds. In contrast, phycobilisomes are known to diffuse rapidly (in seconds) between PSII and PSI (e.g. Sarcina et al., 2001), leading to rapid changes in fluorescence yield as a result of their coupling/uncoupling with photosystems. The return to lower F0 within seconds also excludes photoinhibition from being the mechanism behind the type II bright cells, as recovery from photoinhibition is always at least in the range of minutes (Kyle et al., 1987). Moreover, photoinhibition has not been reported to cause a strong increase of F0, and is unlikely under the low growth and measuring irradiances we used, or in the dark when type II bright cells appeared under high-oxygen stress.
In addition to the two types of bright cells, cells with unusually low Chl fluorescence yield also appeared during the diazotrophic period. Their color did not indicate any change in pigment content, and also the return to normal fluorescence within a few minutes excluded pigment degradation from being the reason for their diminished fluorescence. It is therefore most likely that they represent another type of antenna coupling/uncoupling, one that leads to quenching of most of the captured excitation energy. This is not, however, Qnp in the conventional sense, i.e. a quenching that occurs in response to actinic light exposure and is therefore measured as a difference in the response to saturating flashes in the dark- and the light-adapted state (e.g. Maxwell and Johnson, 2000; Roháček, 2002). The latter parameter was zero in contrast to all other activity states. The much lower fluorescence yield of these cells affected the basic fluorescence (F0) and the variable fluorescence proportionally. Consequently, calculating the parameters of the remaining (very feeble) fluorescence resulted in relatively high values for Fv/Fm (the maximum photochemical activity of PSII) and (F′m − F′t)/F′m (the activity of electron transport through PSII). In these cells, the activity of PSII may be quenched in a way that leads to thermal relaxation of all excitons, so that they do not emit any fluorescence. The fluorescence quenching is likely due to an impaired donor side (e.g. oxygen-evolving complex) of the PSII reaction center; this would lead to a P680+ trap that dissipates excitons as heat (Meunier et al., 1998; Sherman et al., 1998). Alternatively, the quenching may also be caused by the coupling of antennae to an unknown quencher. The small percentage of normally working PSII yields the measured remaining fluorescence. Furthermore, the observed switches into the quenching state usually occurred immediately following the bright (type I or type II) state, and cells returning from the quenching state to normal F0 still displayed the low variable fluorescence of the quenching state (leading to very low Fv/Fm values; normal F0 inactive state). We did not observe a clear separation between the activities of the normal F0 inactive and the normal non-diazotrophic cells (Fig. 5). These two characteristics indicate that the low fluorescence state represents a period of recovery and reconfiguration of photosynthesis after nitrogen fixation, gradually returning to the normal non-diazotrophic state via the normal F0 inactive intermediate. Figure 8 summarizes the roles and interconversions of the physiological activity states as discussed above.
If several cells in a filament switched, they often did not do so randomly, but sequentially (like traffic lights). Nitrogenase is frequently localized in clusters of Trichodesmium cells (Lin et al., 1998; Berman-Frank et al., 2001b), so that a clustering of the switches between activity states might be expected. But it still would not exclude random switches within the cell cluster. The sequential switching between cells suggests that there is a direct interaction between neighboring cells, which operates to reduce oxygen diffusion into a nitrogen-fixing cell from its neighbors. This would be especially beneficial in an organism that does not permanently differentiate specialized cells for nitrogen fixation, so that its diazotrophic cells do not possess diffusion barriers such as those found in heterocysts.
Remarkably, not only the high-oxygen, but also the low-oxygen treatment resulted in strongly inhibited growth and nitrogen fixation in Trichodesmium. The low rates in the high-oxygen culture can easily be explained by an increased oxidative damage to the Fe4S4 clusters in one of the nitrogenase subunits (Burgess and Lowe, 1996), proteolysis (Durner et al., 1996), suppression of nitrogenase synthesis, and post-translational modification (Gallon, 1992; Bergman et al., 1997), as observed also in other diazotrophic cyanobacteria. In many cyanobacteria, however, lower than atmospheric oxygen concentrations result in normal or even enhanced nitrogen fixation due to the diminished inhibition of nitrogenase (Bergman et al., 1997). On time scales of minutes to hours in the photoperiod, also in Trichodesmium, diminished oxygen stress results in enhanced nitrogen fixation, with activity peaking between 2.5% to 5% oxygen (Ohki and Fujita, 1988; I. Berman-Frank, unpublished data). The diminished oxidative stress also leads to the absence of type II bright cells. However, during longer exposure to low oxygen, as in this study (cells in 5% oxygen for over 24 h), the unusually high dark respiration in Trichodesmium (Carpenter and Roenneberg, 1995) may cause a permanent over-reduction of the plastoquinone pool. This would lead to a permanent state I of photosynthesis, i.e. the observed almost constant number of type I bright cells throughout the diurnal cycle. But, at the same time, such reduced conditions most likely render the Mehler reaction and thereby the nitrogen fixation of Trichodesmium inefficient. This is probably exacerbated during the night, when the strong respiration cannot be balanced by oxygenic photosynthesis, leading to a further decline in intracellular oxygen concentrations. This likely induces damage by anaerobiosis, enhanced mortality, and a biomass crash after about 3 d of exposure to low-oxygen concentrations (I. Berman-Frank, unpublished data).
CONCLUSIONS
FKM allowed, for the first time, a detailed study on a single-cell level of the diurnal regulation of photosynthesis in a diazotrophic cyanobacterium. We show that, in Trichodesmium, the coordination of photosynthesis and nitrogen fixation involves a complicated spatial and temporal pattern of reversible alternation of photosynthetic activity states. These activity states provide the coordination and fine tuning required to utilize electron transport via PSII for nitrogen fixation, and to minimize the inhibitory effects of photosynthetic oxygen evolution on nitrogenase in a non-heterocystous organism fixing and assimilating nitrogen during the photoperiod. External oxygen concentrations lower or higher than that of air can modulate the coupling between nitrogen fixation and the photosynthetic activity states. On time scales longer than a few hours, however, this interactive modulation is not sufficient, so that processes like oxidative damage to nitrogenase or lack of respiratory substrates result in inhibited nitrogen fixation and growth. Thus, the traffic lights control on the coupling between photosynthetic activity and nitrogen fixation in Trichodesmium may provide an optimal strategy allowing Trichodesmium to thrive in the subtropical and tropical oceans at present atmospheric oxygen concentrations.
MATERIALS AND METHODS
Culture Media and Culture Conditions
Cultures of Trichodesmium IMS101 were grown in sterile filtered natural seawater, with the addition of 4 μm Fe-EDTA in cylindrical tubes of 3-cm diameter and 100-mL culture volume. The tubes were aerated at a flow rate of 100 mL min−1 with either air (approximately 20% O2), 50% O2 + 50% N2 + 0.035% CO2, or 5% O2 + 95% N2 + 0.035% CO2 for at least one complete light/dark cycle before the measurements were started, in order to adapt the cells to the gas conditions. The experiment was carried out twice; the cultures were diluted to OD750 = 0.1 at the start of each experiment. The cultures were maintained at a 12 h daylength (9:15 am–9:15 pm local time) and 26°C day/24°C night temperature. The photon flux density during the light period was 100 μm m−2 s−1 supplied by cool-white OSRAM (Munich) fluorescent tubes.
Preparation of Samples for FKM Measurements
About 2 mL of cell suspension were taken from the culture tubes, and most of the medium was removed by gentle filtration through a membrane filter. Cells were resuspended in 100 μL medium, transferred to the window of the measuring chamber (Küpper et al., 2000a), and a 2% solution of ultralow-melting (melting point 25°C) agarose in seawater was added before sealing the chamber with gas-permeable cellophane. Tightening the cellophane distributed the cells, embedded in the agarose, in a thin film between the cellophane and the chamber window. The chamber was temperature controlled and pumped through with 100 mL min−1 medium (25°C, saturated with the gas mixture also used for cultivating the cells). To minimize handling artifacts, fresh samples were prepared for each time point.
FKM Measurements and Data Analysis
Photosynthetic performance was measured using the fluorescence kinetic microscope described by Küpper et al. (2000a), with the following modifications that were introduced by Ferimazova et al. (2002).
A sensitive, nonintensified CCD camera (the camera of the FluorCam system [version 700M] from Photon Systems Instruments [Brno, Czech Republic]) was used instead of the intensified one. This substitution resulted in improved spatial resolution and decreased noise, as discussed in detail (at that time as a perspective for future developments) by Küpper et al. (2000a).
Nonactinic measuring light was produced by a xenon flash lamp equipped with a set of filters to produce a spectrum similar to the earlier used LED (blue light, about 400–490 nm). The resulting measuring light irradiance was about 14 μmol m−2 s−1. This substitution allowed for a better comparison of total fluorescence levels, because the LEDs used earlier had to be used at very high currents that caused it to age (leading to a drop of output) rather rapidly.
The applied spectrum of the excitation light (400–490 nm for actinic, measuring, and saturating light) almost exclusively excited Chl and the short-wavelength form of phycoerythrin (phycourobilin or CU phycoerythrin); compare optical properties of Trichodesmium (Subramaniam et al., 1999).
The measurements were performed with an automatic subtraction of background signals and a maximum time resolution of 80 ms. A lower time resolution was applied for the slower kinetics. Each image of the resulting fluorescence kinetic records had a resolution of 300 × 400 pixels at 256 gray values (8 bits). The FKM was also used as a regular epifluorescence microscope to observe the cultures and count the percentage of bright cells.
Analysis of Fluorescence Kinetic Records
The original two-dimensional data, i.e. films of Chl fluorescence kinetics, were analyzed using the FluorCam software (Photon Systems Instruments) in the following way: Images of Fm, F0, Fs, Fv/Fm, etc. were generated as described in detail earlier (Küpper et al., 2000a). The heterogeneity visible on these images was used to select cells for further analysis. The selected cells were passed again through the analysis routine, which resulted in fluorescence kinetic traces representing the average of the kinetics of all pixels within the chosen objects. The individual kinetic traces obtained in this way were exported for further numerical analysis.
In this study, both the variable fluorescence (Fv = Fm − F0 and F′m − F′t) and the nonphotochemical quenching (Qnp = Fm − F′m) were calculated without the conventional normalization to Fm, as the latter causes an artefactual decrease of PSII activity upon an increase of F0 at constant variable fluorescence (Behrenfeld and Kolber, 1999).
Measurement of Nitrogen Fixation
Nitrogenase activity was assayed by the acetylene reduction method. Samples of 10-mL volumes were taken from all three cultures at five time points throughout the daily cycle. Samples were incubated in gas-tight glass vials, in which 10 mL air were exchanged with 10 mL acetylene, for 1 h under the same light and temperature conditions as the cultures. Afterward, 5 mL of the gas phase in the vials were taken out and analyzed by gas chromatography.
Chl Concentration
Chl was assayed by filtration on 5-μm polycarbonate filters, extraction with 100% acetone, recording the absorbance spectra from 550 to 750 nm recorded, and analyzing the spectra using the Gauss-Peak-Spectra method according to Küpper et al. (2000b).
Acknowledgments
We are very grateful to Martin Trtílek (Photon Systems Instruments, Brno, Czech Republic) for continuous support and updates on the Fluorescence Kinetic Microscope and FluorCam Software, and to Ondrej Prášil (Institute of Microbiology, Academy of Sciences of the Czech Republic) for facilitating this study.
This work was supported by the Ministry of Education of the Czech Republic (grant nos. LN00A141 and MSM123100004), by the Academy of Sciences of the Czech Republic (grant no. AV0Z5020903), by a fellowship from the Alexander von Humboldt Foundation (to H.K.), and by a grant from Bar Ilan University (to I.B.-F.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045963.
References
- Behrenfeld MJ, Kolber ZS (1999) Widespread iron limitation of phytoplankton in the south pacific ocean. Science 283: 840–843 [DOI] [PubMed] [Google Scholar]
- Bergman B, Gallon JR, Rai AN, Stal LJ (1997) N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev 19: 139–185 [Google Scholar]
- Berman-Frank I, Cullen JT, Shaked Y, Sherrell RM, Falkowski PG (2001. a) Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol Oceanogr 46: 1249–1260 [Google Scholar]
- Berman-Frank I, Lundgren P, Chen Y-B, Küpper H, Kolber Z, Bergman B, Falkowski P (2001. b) Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294: 1534–1537 [DOI] [PubMed] [Google Scholar]
- Berman-Frank I, Lundgren P, Falkowski P (2003) Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res Microbiol 154: 157–164 [DOI] [PubMed] [Google Scholar]
- Biggins J, Bruce D (1989) Regulation of excitation energy transfer in organisms containing phycobilins. Photosynth Res 20: 1–34 [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25: 173–185 [DOI] [PubMed] [Google Scholar]
- Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189: 366–383 [DOI] [PubMed] [Google Scholar]
- Burgess BK, Lowe DJ (1996) Mechanism of molybdenum nitrogenase. Chem Rev 96: 2983–3011 [DOI] [PubMed] [Google Scholar]
- Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62: 667–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ (1997) Trichodesmium, a globally significant marine cyanobacterium. Science 276: 1221–1229 [Google Scholar]
- Carpenter EJ, Roenneberg T (1995) The marine planktonic cyanobacterium Trichodesmium spp.: photosynthetic rate measurements in the SW Atlantic Ocean. Mar Ecol Prog Ser 118: 267–273 [Google Scholar]
- Ditty JL, Williams SB, Golden SS (2003) A cyanobacterial circadian timing mechanism. Annu Rev Genet 37: 513–543 [DOI] [PubMed] [Google Scholar]
- Durner J, Bohm I, Knorzer OC, Böger P (1996) Proteolytic degradation of dinitrogenase reductase from Anabaena variabilis (ATCC 29413) as a consequence of ATP depletion and impact of oxygen. J Bacteriol 178: 606–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferimazova N, Küpper H, Nedbal L, Trtílek M (2002) New insights into photosynthetic oscillations revealed by two-dimensional microscopic measurements of chlorophyll fluorescence kinetics in intact leaves and isolated protoplasts. Photochem Photobiol 76: 501–508 [DOI] [PubMed] [Google Scholar]
- Gallon JR (1992) Reconciling the incompatible: N2 fixation and oxygen. New Phytol 122: 571–609 [Google Scholar]
- Gallon JR (2001) N2 fixation in phototrophs: adaptation to a specialized way of life. Plant Soil 230: 39–48 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Kana TM (1993) Rapid oxygen cycling in Trichodesmium thiebautii. Limnol Oceanogr 38: 18–24 [Google Scholar]
- Küpper H, Šetlík I, Trtílek M, Nedbal L (2000. a) A microscope for two-dimensional measurements of in vivo chlorophyll fluorescence kinetics using pulsed measuring light, continuous actinic light and saturating flashes. Photosynthetica 38: 553–570 [Google Scholar]
- Küpper H, Spiller M, Küpper F (2000. b) Photometric method for the quantification of chlorophylls and their derivatives in complex mixtures: fitting with gauss-peak-spectra. Anal Biochem 286: 247–256 [DOI] [PubMed] [Google Scholar]
- Kyle DJ, Osmond CB, Arntzen CJ (1987) Photoinhibition. In J Barber, ed, Topics in Photosynthesis, Vol 9. Elsevier, Amsterdam, 315 pp
- Lin S, Henze S, Lundgren P, Bergman B, Carpenter EJ (1998) Whole-cell immunolocalization of nitrogenase in marine diazotrophic cyanobacteria, Trichodesmium spp. Appl Environ Microbiol 64: 3052–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- Meunier PC, Colón-López MS, Sherman LA (1997) Temporal changes in state transitions and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Plant Physiol 115: 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier PC, Colón-López MS, Sherman LA (1998) Photosystem II cyclic heterogeneity and photoactivation in the diazotrophic, unicellular cyanobacterium Cyanothece Species ATCC 51142. Plant Physiol 116: 1551–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra HS, Mahajan SK (2000) Excitation energy transfer from phycobilisomes to photosystems: a phenomenon associated with the temporal separation of photosynthesis and nitrogen fixation in a cyanobacterium, Plectonema boryanum. Biochim Biophys Acta 1459: 139–147 [PubMed] [Google Scholar]
- Ohki K, Fujita Y (1988) Aerobic nitrogenase activity measured as acetylene reduction in the marine non-heterocystous cyanobacterium Trichodesmium spp. grown under artificial conditions. Mar Biol 91: 9–13 [Google Scholar]
- Postgate JR (1998) Nitrogen Fixation, Ed 3. Cambridge University Press, Cambridge, p112
- Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40: 13–29 [Google Scholar]
- Sarcina M, Tobins MJ, Mullineaux CW (2001) Diffusion of phycobilisomes on the thylakoid membranes of the cyanobacterium Synechococcus 7942. J Biol Chem 278: 46830–46834 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62 [DOI] [PubMed] [Google Scholar]
- Sherman LA, Meunier P, Colón-López MS (1998) Diurnal rhythms in metabolism: a day in the life of a unicellular, diazotrophic cyanobacterium. Photosynth Res 58: 25–42 [Google Scholar]
- Sroga GE, Landegren U, Bergman B, Lagerstrom-Fermer M (1996) Isolation of nifH and part of nifD by modified capture polymerase chain reaction from a natural population of the marine cyanobacterium Trichodesmium sp. FEMS Microbiol Lett 136: 137–145 [DOI] [PubMed] [Google Scholar]
- Subramaniam A, Carpenter EJ, Karentz D, Falkowski PG (1999) Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and action spectra. Limnol Oceanogr 44: 608–617 [Google Scholar]
- van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Zehr JP, Harris D, Dominic B, Salerno J (1997) Structural analysis of the Trichodesmium nitrogenase iron protein—implications for aerobic nitrogen fixation activity. FEMS Microbio Lett 153: 303–309 [DOI] [PubMed] [Google Scholar]