Abstract
It is widely accepted that brassinosteroids (BRs) are important regulators of plant growth and development. However, in comparison to the other classical plant hormones, such as auxin, relatively little is known about BR transport and its potential role in the regulation of endogenous BR levels in plants. Here, we show that end-pathway BRs in pea (Pisum sativum) occur in a wide range of plant tissues, with the greatest accumulation of these substances generally occurring in the young, actively growing tissues, such as the apical bud and young internodes. However, despite the widespread distribution of BRs throughout the plant, we found no evidence of long-distance transport of these substances between different plant tissues. For instance, we show that the maintenance of steady-state BR levels in the stem does not depend on their transport from the apical bud or mature leaves. Similarly, reciprocal grafting between the wild type and the BR-deficient lkb mutants demonstrates that the maintenance of steady-state BR levels in whole shoots and roots does not depend on either basipetal or acropetal transport of BRs between these tissues. Together, with results from 3H-BR feeding studies, these results demonstrate that BRs do not undergo long-distance transport in pea. The widespread distribution of end-pathway BRs and the absence of long-distance BR transport between different plant tissues provide significant insight into the mechanisms that regulate BR homeostasis in plants.
Brassinosteroids (BRs) are steroidal plant hormones, which are essential for normal plant growth and development. Extensive research over the past two decades has revealed the importance of BRs in a wide variety of processes, including cell elongation, cell division, vascular differentiation, reproductive development, and pathogen and abiotic tolerance (Clouse, 2002). As a consequence, BRs are now widely recognized as an important class of plant growth regulators, alongside the classical plant hormones, such as auxin, GA, cytokinin, abscisic acid (ABA), and ethylene (Clouse, 2002).
The importance of BRs in such a diverse range of developmental processes implies the existence of mechanisms that strictly control the endogenous BR levels and their distribution in the target cells or tissues. It is widely accepted that the hormone level at any given site might be affected by the relative rates of its synthesis, destruction, inactivation, and transport within the plant. Each of these factors can be considered in terms of their input to, or output from, the level of free hormone (Bandurski et al., 1995). Inputs into the free hormone pool in a given tissue include de novo synthesis, conjugate hydrolysis, and transport from one site in the plant to another site. Outputs from the free hormone pool include catabolism, conjugate synthesis, and transport away from a given site (Bandurski et al., 1995). It is the coordinated regulation of these processes that determines the endogenous levels of bioactive BRs, which, when coupled with the regulation of BR responsiveness of the target cell or tissue, allows for the appropriate BR-mediated effect on growth and development. It follows, therefore, that an integrated understanding of the role of BRs in plant development requires a detailed knowledge of the way plants regulate their levels of, and response to, endogenous BRs.
Intensive research efforts, particularly through the use of BR mutants, have provided us with significant insights into BR biosynthesis (Fujioka and Yokota, 2003) and, more recently, BR perception and signaling (Bishop and Koncz, 2002; Clouse, 2002). For instance, a pathway for the biosynthesis of BRs is now well established, many of the genes involved in this pathway have been characterized, and several key points for the control of BR levels via feedback regulation of the pathway have been identified (Fujioka and Yokota, 2003). Similarly, the BR receptor has been characterized, and rapid recent progress has been achieved in unraveling components of the BR signal transduction pathway (Bishop and Koncz, 2002; Clouse, 2002; Tichtinsky et al., 2003). However, despite our increasing knowledge of the BR biosynthetic pathway and BR perception/signal transduction, our understanding of the mechanisms used to regulate endogenous BR levels is far from complete.
This issue was recently highlighted by results that show clear spatial and temporal patterns of distribution of BRs in plants (Bancos et al., 2002; Shimada et al., 2003). For instance, Bancos et al. (2002) published information on the spatial distribution of BRs by detailing the relative levels of BRs in whole-shoot and whole-root tissues of pea (Pisum sativum), Arabidopsis, and tomato (Lycopersicon esculentum) plants. This study showed that, in all three species, the levels of the early intermediates in the BR biosynthesis pathway were higher in the roots, while the end-pathway BRs, castasterone (CS) and 6-DeoxoCS, were more abundant in the shoots of all three species. In addition, it was shown that a number of confirmed and putative BR biosynthesis genes were differentially expressed in the roots and shoots of Arabidopsis, as well as temporally during Arabidopsis seedling development (Bancos et al., 2002). Furthermore, Shimada et al. (2003) published the first comprehensive report of both endogenous BR levels and BR biosynthesis gene expression in a range of different tissues from Arabidopsis seedlings. These studies demonstrate a widespread distribution of endogenous BRs throughout the plant, with the greatest accumulation of end-pathway BRs occurring in the young, actively growing tissues. Significantly, there was a good correlation between the tissues that show the highest BR concentrations and those that show the highest expression levels of the BR biosynthesis genes BR6ox1, BR6ox2, and DWF4 (Shimada et al., 2003).
While these studies by Shimada et al. (2003) and Bancos et al. (2002) demonstrate a clear temporal and spatial distribution of endogenous BRs, the mechanisms utilized to maintain differences in BR levels throughout the plant are not yet clear. The close correlation between BR levels and the levels of BR biosynthesis gene expression (Shimada et al., 2003) suggests that in situ BR biosynthesis plays a significant role in regulating the endogenous BR pool size. However, the widespread distribution of endogenous BRs reported by Shimada et al. (2003) also raises the possibility of long-distance transport of BRs between different plant tissues and of a role for this process in the regulation of endogenous BR levels. Indeed, this was recently highlighted by Sasse (2003), who posed the question: Is long-distance transport of endogenous BRs important?
In comparison to plant hormones such as auxin, relatively little is known about the transport of BRs between different sites within the plant. Indeed, the classification of plant growth-regulatory substances, such as the BRs, as hormones has created several conceptual problems by implying a similarity to animal endocrine systems (Davies, 1995). This has led to the assumption that a plant hormone must be synthesized by one organ before being transported to other tissues where it is perceived (Bishop and Yokota, 2001). Although this may be true for auxin (Davies, 1995), preliminary evidence suggests that BRs may be produced and then act in the same tissues, or possibly even in the same cells. For instance, in both Arabidopsis and rice (Oryza sativa), the gene that encodes the BR receptor, BRI1, is most highly expressed in young growing tissues such as the shoot apex (Friedrichsen and Chory, 2001). These tissues also show the highest expression levels of genes involved in BR biosynthesis (see above), suggesting similar sites of biosynthesis and action of BRs in plants. Consistent with this idea is the variegated, revertant phenotype of the transposon-mutagenized dwarf mutants in tomato (Bishop et al., 1996), which suggests that BRs are synthesized in the same tissues in which they function (Shimada et al., 2003).
Similarly, grafting studies in pea provided little evidence of BR transport (Reid and Ross, 1989). In this study, young lkb scions were grafted onto mature, leafy wild-type stocks (see Reid, 1979; Reid and Ross, 1989). In this case, the presence of wild-type root and shoot tissues did not restore internode elongation in the BR-deficient lkb scion, indicating that BRs may not be transported acropetally (upwards) in pea shoots (Bishop and Yokota, 2001). However, such grafting studies reveal little about the possibility of basipetal transport within the shoot (e.g. from the apical bud to the internodes), or from the shoot to the root.
In contrast, evidence from other species suggests that BRs may be transported acropetally from the roots to the shoots (Arteca, 1995; Sasse, 1999; Bishop and Yokota, 2001). For instance, studies in rice showed that a small percentage of 3H-brassinolide (BL) or 3H-CS applied to the roots was translocated to the shoots (Yokota et al., 1992). Similarly, when 14C-epiBL was applied to the roots of cucumber (Cucumis sativus) and wheat (Triticum aestivum) seedlings, 14C was soon detected throughout both plant species (Nishikawa et al., 1994).
Markovic-Housley et al. (2003) recently proposed a mechanism for the transport of BRs. This mechanism involves binding of BRs to a specific pathogenesis-related protein (PR-10) to form a complex that allows the water-insoluble, hydrophobic BRs to be transported from the cytosol to their receptors. However, while this mechanism may be involved in the short-distance transport of BRs within and between individual cells, it is unknown whether it could facilitate the coordinated long-distance transport of BRs between different tissue types. Thus, the crucial question of whether BRs are transported around the plant remains largely unanswered, and clearly requires more attention.
The aim of the research presented here was to determine whether long-distance transport of BRs occurs in pea and to examine its role (if any) in the regulation of endogenous BR levels. Consistent with the situation in Arabidopsis, we show that end-pathway BRs in pea also exist in a wide range of tissue types, and the highest levels of these substances were shown to occur in young, actively growing tissues. However, despite this widespread distribution of BRs, we found no evidence for their long-distance transport between different plant organs and no evidence that this process is important for the regulation of endogenous BR levels.
RESULTS
Spatial Distribution of BRs in Wild-Type Plants
BRs were detected in all tissue types tested, including the apical bud, mature leaves, stem, and roots (Fig. 1), although BR levels varied greatly between different tissue types (Table I; Fig. 2). For instance, in the shoot, the levels of CS, 6-DeoxoCS, and Typha were higher in young, actively growing tissues, such as the apical bud, and lowest in the mature leaves (Table I; Fig. 2). This spatial distribution of end-pathway BRs in the shoot follows a similar pattern to the observed distribution of indole-3-acetic acid (IAA), GA1, and ABA, which were also generally higher in young, actively growing tissues of the apical bud than in mature leaves (Table II). In the roots, BR levels were significantly lower than in shoot tissues, as CS and Typha levels were below detection limits and 6-DeoxoCS levels were between 5- and 18-fold lower than in the shoot tissues (Table I; Fig. 2). As was the case in previous studies in pea (Nomura et al., 1999; Symons and Reid, 2003a) and Arabidopsis (Shimada et al., 2003), BL levels were below detection limits in all of the tissue types studied (Tables I and III–V; Fig. 2).
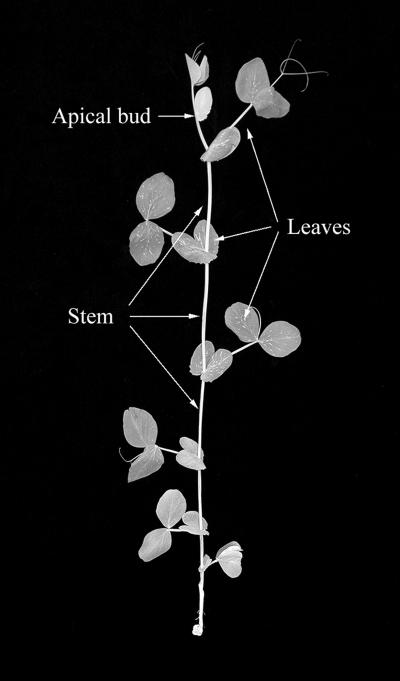
Figure 1.
Separation of a pea shoot into individual tissue types. The apical bud consisted of all tissue above the youngest expanded leaf (node 8). Leaves consisted of all leaf material (stipules, petioles, leaflets, and tendrils) from the three youngest expanded leaves (at nodes 8, 7, and 6). Stems consisted of both node and internode tissues from above node 5 and below node 8. Root tissue (not shown) consisted of all tissue below the cotyledonary node (node 0).
Table I.
Distribution of endogenous brassinosteroids in tissues of wild-type pea plants
| Tissue Type | Brassinosteroid
|
|||
|---|---|---|---|---|
| BL | CS | 6-DeoxoCS | Typha | |
| Apical bud | nd | 0.26 ± 0.05 | 5.29 ± 0.46 | 0.09 |
| Stem | nd | 0.21 ± 0.01 | 2.97 ± 0.20 | 0.04 |
| Leaves | nd | 0.17 ± 0.01 | 2.06 ± 0.04 | nd |
| Roots | nd | nd | 0.30 | nd |
Definitions of the various tissue types are outlined in Figure 1. All plants had eight fully expanded leaves at the time of harvest. Values represent the mean endogenous hormone levels ± the se, obtained from three separate replicate experiments. nd, Not detected. Typha levels in the apical bud and stem and 6-DeoxoCS levels in the roots were detected in one replicate only.
Figure 2.
Effect of reciprocal grafting between wild-type and lkb mutant plants on the phenotype of the shoot in 45-d-old plants (A) and endogenous BR levels in shoot and root tissues of 30-d-old plants (B). The grafts were made epicotyl to epicotyl using 7-d-old seedlings.
Table II.
Effect of removing the apical bud (decapitation) on the endogenous IAA, GA1, and ABA levels in apical, stem, and leaf tissues of wild-type pea plants
| Tissue | Treatment | Hormone Level
|
||
|---|---|---|---|---|
| IAA | GA1 | ABA | ||
| ng g−1 FW | ||||
| Apical bud | Intact | 82.6 ± 71 | 6.8 ± 0.3 | 4.2 ± 0.2 |
| Decapitated | – | – | – | |
| Stem | Intact | 100.4 ± 4.8 | 1.2 ± 0.04 | 1.7 ± 0.1 |
| Decapitated | 9.8 ± 2.5 | 0.08 ± 0.003 | 0.8 ± 0.02 | |
| Leaf | Intact | 16.9 ± 3.2 | 0.7 ± 0.1 | 2.2 ± 0.2 |
| Decapitated | 8.2 ± 0.1 | 0.5 ± 0.2 | 2.5 ± 0.2 | |
Table III.
Comparative distribution of endogenous brassinosteroids in apical, stem, and leaf tissues of wild-type and BR mutant plants
| Tissue | Genotype | Brassinosteroid Level
|
||||
|---|---|---|---|---|---|---|
| BL | CS | 6-DeoxoCS | Typha | Ratio of 6-DeoxoCS to CS | ||
| ng g−1 FW | ||||||
| Apical bud | Wild type | nd | 0.62 | 4.10 | 0.20 | 6.6 |
| lka | nd | 4.40 | 9.12 | 0.83 | 2.1 | |
| lkb | nd | 0.17 | 0.20 | nd | 1.2 | |
| Stem | Wild type | nd | 0.41 | 2.62 | 0.11 | 6.4 |
| lka | nd | 2.52 | 5.52 | 0.15 | 2.2 | |
| lkb | nd | 0.16 | 0.07 | nd | 0.4 | |
| Leaf | Wild type | nd | 0.21 | 1.20 | 0.03 | 5.7 |
| lka | nd | 1.49 | 2.26 | 0.08 | 1.5 | |
| lkb | nd | 0.06 | 0.05 | nd | 0.8 | |
Definitions of the various tissue types are outlined in Figure 1. All plants had eight fully expanded leaves at the time of harvest. Values were obtained from a single replicate consisting of between 50 and 80 plants of each genotype. nd, Not detected.
Table IV.
Effect of removing the apical bud (decapitation) on the levels of endogenous brassinosteroids in stem and leaf tissues of wild-type pea plants
| Tissue | Treatment | Brassinosteroid Level
|
|||
|---|---|---|---|---|---|
| BL | CS | 6-DeoxoCS | Typha | ||
| ng g−1 FW | |||||
| Stem | Intact | nd | 0.21 ± 0.02 | 2.78 ± 0.05 | nd |
| Decapitated | nd | 0.31 ± 0.01 | 2.92 ± 0.19 | nd | |
| Leaf | Intact | nd | 0.13 ± 0.02 | 2.10 ± 0 | nd |
| Decapitated | nd | 0.14 ± 0.01 | 2.14 ± 0.18 | nd | |
Definitions of the various tissue types are outlined in Figure 1. All plants had 8 fully expanded leaves at the time of harvest. Plants were decapitated by removing the apical bud via a cut made directly above node 8. Values represent the mean endogenous hormone levels ± the se, 48 h after decapitation. Values were derived from two separate replicate experiments. nd, Not detected.
Table V.
Effect of removing mature leaves (defoliation) on the distribution of endogenous brassinosteroids in the apical and stem tissues of wild-type pea plants
| Tissue | Treatment | Brassinosteroid Level
|
|||
|---|---|---|---|---|---|
| BL | CS | 6-DeoxoCS | Typha | ||
| ng g−1 FW | |||||
| Apical Bud | Intact | nd | 0.55 ± 0.06 | 3.25 ± 0.02 | 0.039 ± 0.008 |
| Defoliated | nd | 0.43 ± 0 | 3.01 ± 0.01 | 0.023 ± 0.003 | |
| Stem | Intact | nd | 0.43 ± 0.01 | 2.50 ± 0.28 | 0.037 |
| Defoliated | nd | 0.43 ± 0.03 | 2.49 ± 0.05 | 0.022 ± 0.001 | |
Definitions of the various tissue types are outlined in Figure 1. All plants had eight fully expanded leaves at the time of harvest. Plants were defoliated by separating the petiole from the stem at nodes 6, 7, and 8. Stipules were left intact. Values represent the mean endogenous BR levels ± the se, 48 h after defoliation. Values were derived from two separate replicate experiments. nd, Not detected.
Comparative Distribution of BRs in Wild-Type, lka, and lkb Plants
Nomura et al. (1999) have previously reported that BR levels are elevated in whole shoots of lka mutant plants and reduced in whole shoots of lkb mutant plants, in comparison with the wild type. This is consistent with the finding that the lkb mutation blocks the biosynthesis of BRs, while the lka mutation causes impaired perception of these substances (Nomura et al., 1999, 2003; Schultz et al., 2001). In this study, we show that these changes in endogenous BRs in lka and lkb mutant plants (compared to the wild type) are consistent across a wide range of different shoot tissues and are not restricted to any specific location (Table III). For instance, BR levels were consistently reduced (2.5- to 3.5-fold for CS, 20- to 37-fold for 6-DeoxoCS) in the apical buds, shoots, and leaves of lkb plants, in comparison to the wild type. Similarly, BR levels were consistently increased (6- to 7-fold for CS, 1.8- to 2.2-fold for 6-DeoxoCS, and 1.4- to 2.6-fold for Typha in the apical buds, shoots, and leaves of lka plants, in comparison to the wild type (Table III). Interestingly, even in lka plants, where CS levels were increased up to 7-fold in comparison with the wild type, BL was still below detection limits, estimated to be approximately 0.005 ng g−1 of fresh weight (FW).
A comparison of the CS and 6-DeoxoCS levels in wild-type, lka, and lkb mutant plants from this study also provides an insight into the possible regulation of the BR pathway. For instance, in wild-type pea plants, the ratio of 6-DeoxoCS to CS is consistently around 6:1 in all tissue types tested, while in the lka and lkb mutants this ratio is consistently around 2:1 or less (Table III). This may suggest that the conversion of 6-DeoxoCS to CS is increased in the lkb and lka mutants, most likely in response to the perceived or real deficiency of active BRs in these plants, respectively.
Influence of the Apical Bud on BR Levels in the Shoots of Wild-Type Plants
Removal of the apical bud (decapitation) did not cause a reduction in BR levels in either stem or leaf tissues of wild-type plants 48 h after the apical bud was removed (Table IV). Indeed, CS and 6-DeoxoCS levels were actually slightly increased in the internodes and leaves of decapitated plants. However, with the exception of the slight increase in CS levels (P < 0.05) in the stem tissues of decapitated plants, the changes were not statistically significant.
In contrast, the levels of the other classical plant hormones, IAA, GA1, and ABA, were all dramatically reduced in stem tissues after decapitation (in comparison with levels in intact plants; see Table II). For instance, IAA, GA1, and ABA levels were reduced (10-, 15-, and 2-fold, respectively, compared to intact plants) in stem tissues 48 h after decapitation (Table II). Decapitation also resulted in a reduction in IAA levels (2-fold compared to intact plants) in the mature leaves, but did not cause any significant change in GA1 or ABA levels in these tissues (Table II).
Influence of Mature Leaves on BR Levels in the Shoots of Wild-Type Plants
Removal of the three youngest expanded leaves (defoliation) also did not result in a dramatic change in BR levels in either internode or apical tissues of wild-type plants 48 h after the leaves were removed (Table V). CS, 6-DeoxoCS, and Typha levels in the apical bud were slightly decreased in response to defoliation (Table V). In contrast, CS and 6-DeoxoCS levels in the stem tissue remained unchanged, while Typha levels were again decreased slightly after defoliation (Table V).
Influence of the Roots on BR Levels in the Shoots
Grafting an lkb shoot onto a wild-type rootstock did not restore either the endogenous BR levels in, or the phenotype of, the lkb shoot to that of the wild type (Fig. 2). In this case, the endogenous BR levels and the shoot phenotype were both comparable to the shoots of self-grafted lkb plants (Fig. 2). Similarly, when a wild-type shoot was grafted onto an lkb rootstock, the presence of the lkb rootstock did not alter BR levels in the wild-type shoot (Fig. 2). In this case, BR levels in the shoot were comparable to those in the shoots of self-grafted wild-type plants (Fig. 2). This suggests that the maintenance of steady-state BR levels in the shoot is not dependent on BR synthesis in, or acropetal (root to shoot) BR transport from, the roots.
Influence of the Shoots on BR Levels in the Roots
Grafting a wild-type shoot onto an lkb rootstock did not restore BR levels in the lkb root to that of the wild type (Fig. 2). Indeed, BR levels in the root were below detection limits, which is similar to the situation in the roots of self-grafted lkb plants (Fig. 2). This suggests that the maintenance of steady-state BR levels in the roots is not dependent on BR synthesis in, or basipetal (shoot to root) transport from, the shoots.
Transport of Exogenously Applied, Radiolabeled BRs
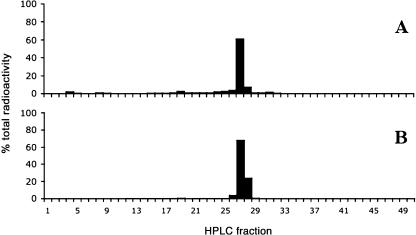
Radiolabeled BRs (1 × 106 dpm in 5 μL ethanol of 3H-BL or 3H-CS; 0.78 Ci mm−1), applied either to the youngest expanded leaf or directly to the apical bud of wild-type plants, were not detected in adjacent stem or leaf tissues 48 h later (data not shown). Recovery of the radioactivity at the site of application was consistent with the amount of substrate applied to the plants (data not shown), and HPLC radiocounting confirmed that the majority of radioactivity recovered remained in its original form (3H-BL or 3H-CS) and was not broken down or metabolized during the experiment (Fig. 3).
Figure 3.
A, HPLC chromatogram showing the retention time for authentic 3H-CS. Peak height was determined by HPLC radiocounting. B, HPLC chromatogram showing the metabolism of 3H-CS 48 h after application to mature leaf tissues of wild-type plants. A total of 1 × 106 dpm of 3H-CS was applied to the leaflets at node 9 of intact wild-type plants. After 48 h, all leaf tissue (including the stipules, tendrils, and petioles) at node 9 was harvested, homogenized, and extracted in 80% methanol. Metabolism of 3H-CS was analyzed by HPLC radiocounting. Similar results were obtained when 3H-CS was applied to the apical bud and when 3H-BL was applied to either leaves or the apical bud (data not shown).
Application of unlabeled BL (200 ng in 5 μL of ethanol) to the youngest expanded leaf of BR-deficient lkb plants caused a localized increase in leaflet elongation and lightening of the leaf color, both of which were confined to the site of application (Fig. 4). The localized nature of this growth response resulted in an abnormal leaf shape and curling of the leaflet (Fig. 4). However, application of BL to the youngest expanded leaf did not alter the growth of adjacent expanding stem tissue, suggesting that there was little or no transport of the exogenous BL from these tissues into the stem (data not shown).
Figure 4.
Changes in leaflet morphology of the BR-deficient lkb mutant in response to BL application. A total of 200 ng of BL in 2 μL of ethanol was applied to the upper surface of the leaflet only. The treated leaflet is indicated by the arrow. Control plants were treated with 2 μL of ethanol only. BL-treated and untreated leaflets were photographed 4 d after BL application.
DISCUSSION
If BRs act as important regulators of plant growth, then they must not only be present in the growing tissues but also their endogenous levels must be strictly regulated. This study provides an insight into both aspects of BR biology by examining both the spatial distribution of BRs in various plant tissues and the role of long-distance BR transport in the regulation of endogenous BR levels.
Spatial Distribution of BRs in Pea Shoots
In light of results obtained by Shimada et al. (2003), we examined the spatial distribution of BRs in pea in order to determine whether the distribution pattern of BRs in Arabidopsis is similar in a species with a different growth habit. Indeed, this proved to be the case because BRs in pea were also detected (although at different levels) in all tissue types tested, including the apical bud, stem, mature leaves, and roots (Tables I and III–V; Fig. 2). As was the case in Arabidopsis, the highest levels of BRs in pea were found in the young, actively growing tissues such as the apical bud, while the lowest BR levels were found in the mature leaves and the roots (Tables I and III–V; Fig. 2; Shimada et al., 2003). This pattern is consistent with results from the northern analysis of the LKB (a BR biosynthesis gene) expression in pea shoots, which showed that LKB transcript levels were also highest in the apical bud and unexpanded internodes and lowest in the leaves (Schultz et al., 2001). Together, the presence of endogenous BRs and the expression of a BR biosynthesis gene at a wide range of sites around the pea plant suggest that these tissues may all be capable of BR biosynthesis.
Furthermore, the presence of high BR levels in young, actively growing tissues in two different species (Tables I and III–V; Fig. 2; Shimada et al., 2003) and the similarity of this pattern to the spatial distribution of other classical plant hormones, such as IAA and GA1 (Table II), is consistent with an important role for BRs in plant growth and development.
BRs Do Not Undergo Long-Distance Transport between Different Shoot Tissues
Despite the widespread distribution of BRs throughout the plant, we found no evidence to suggest that these compounds undergo long-distance transport between different shoot tissues. For instance, decapitation (removal of the apical bud) did not result in any significant changes in endogenous BR levels in either the stem or leaf tissues of the wild-type plants (Table IV). This suggests that maintenance of steady-state BR levels in the stem and leaves is not dependent on BR synthesis in, or transport from, the apical bud. Similarly, defoliation (removal of the three youngest expanded leaves) did not dramatically alter endogenous BR levels in the stem or apical tissues of wild-type plants (Table V). This suggests that the maintenance of steady-state BR levels in the stem and apical bud is also not dependent on BR synthesis in, or transport from, the mature leaves.
These results are in stark contrast to the dramatic and well-characterized reductions in endogenous IAA and GA1 levels observed in stem tissues after decapitation (Table II; Ross et al., 2000). In this case, the reduction in IAA is likely to be a direct consequence of the removal of the apical bud (the presumed site of IAA biosynthesis) and the disruption of the basipetal transport of IAA from these tissues. In contrast to the BRs, this demonstrates that the maintenance of steady-state IAA levels in the stem and leaves is dependent on IAA synthesis in, and transport from, the apical bud. Furthermore, the low IAA level in decapitated plants in turn leads to a reduction in GA1 levels, since IAA is required for the promotion of GA1 biosynthesis in pea stems (Ross et al., 2000). Interestingly, these results also demonstrate that BR homeostasis is not affected by the decapitation-induced reduction in IAA or GA1 levels. This suggests that there may be no clear interaction between IAA or GA1 levels and endogenous BR levels.
BRs Do Not Undergo Long-Distance Transport between the Shoot and Root
Grafting studies provide further support for the idea that bioactive BRs do not undergo long-distance transport in pea. For instance, grafting a BR-deficient lkb shoot onto a wild-type rootstock did not restore either the endogenous BR levels in, or the phenotype of, the lkb shoot to that of the wild type (Fig. 2). This is consistent with previous grafting studies in which young lkb scions were grafted onto mature, leafy wild-type stocks (see Reid, 1979; Reid and Ross, 1989). In this study, the presence of wild-type root and shoot tissues did not restore internode elongation in the BR-deficient lkb scion (Reid and Ross, 1989). Assuming that grafting does not disrupt normal BR transport pathways, these results suggest that the maintenance of steady-state BR levels in the pea shoot is not dependent on BR synthesis in, or acropetal BR transport from, the roots. Indeed, this is further supported by results showing that when a wild-type shoot was grafted onto an lkb rootstock, the presence of the lkb rootstock did not alter BR levels in the wild-type shoot (Fig. 2). In this same graft combination, the presence of the wild-type shoot did not increase BR levels in the BR-deficient lkb root (Fig. 2). Assuming that grafting does not disrupt normal BR transport pathways, this suggests that the maintenance of steady-state BR levels in pea roots is not dependent on BR synthesis in, or basipetal BR transport from, the shoots.
These conclusions clearly contradict the findings of previous studies in rice, which showed that a small percentage of 3H-BL or 3H-CS, applied to the roots, was translocated to the shoots (Yokota et al., 1992). Similarly, when 14C-epiBL was applied to the roots of cucumber and wheat seedlings, 14C was soon detected throughout both plant species (Nishikawa et al., 1994). However, in this case, it is not clear if the 14C detected was 14C-epiBL itself or a labeled metabolite of 14C-epiBL. Interpretation of these results is therefore inherently difficult and, as a consequence, the observed movement of exogenous radiolabeled substances may not accurately reflect transport of endogenous hormones (see Hoad, 1995). Indeed, in the current study, the grafting results provide no evidence for the acropetal transport of BRs from root to shoot in pea and clearly show that BR levels in the shoot are not dependent on BR transport from the roots in this species. Thus, unless grafting disrupts normal BR transport pathways or there are dramatic species-specific differences in the transport of BRs in plants, it is possible that the movement of exogenously applied BRs from the root (Yokota et al., 1992; Nishikawa et al., 1994) may not accurately reflect the transport of endogenous BRs in plants.
A number of further implications also arise from the results of these grafting studies. For instance, Bancos et al. (2002) and Shimada et al. (2003) have shown that the levels of midstream intermediates in the BR biosynthesis pathway are higher in roots than in shoots of pea, tomato, and Arabidopsis plants. This is in contrast with levels of end-pathway (or downstream) BRs, such as CS and 6-DeoxoCS, which are higher in the shoots than in the roots of these species (Bancos et al., 2002; Shimada et al., 2003; Table I; Fig. 2). Together with the molecular data on the distribution of BR biosynthesis gene expression, this suggests that the roots actively participate in BR biosynthesis. However, the reason for the differences between the relative levels of early and late-pathway BRs in the roots and shoots is not clear. Bancos et al. (2002) suggest that a suppression of metabolic flow to biologically active BRs might be a mechanism to protect the roots from BR-induced growth inhibition. Similarly, Shimada et al. (2003) also propose that the lower CS and 6-DeoxoCS levels are maintained so as to counteract the increased BR responsiveness of the root tissues to BR, and suggest that this is achieved by an increase in BAS1-mediated catabolism of end-pathway BRs (Shimada et al., 2003).
An alternative explanation for differences between the relative levels of early and late-pathway BRs is that the root may be a major site of production for early and midstream BR intermediates, which are then transported to the shoot to be converted to the bioactive compounds. However, this idea is not supported by results from the current study. For instance, grafting an lkb shoot onto a wild-type rootstock did not restore either endogenous levels of late-pathway (or downstream) BRs or the phenotype of the shoot (Fig. 2). Furthermore, Nomura et al. (1999) showed that sterols such as 2H7 24-methylenecholesterol, when supplied in media, were taken up by pea roots and metabolized by them but were not transported into the shoot. Together, these results suggest that the root does not serve as a source of early pathway BRs for the shoot.
Transport of Exogenously Applied Radiolabeled BRs
Decapitation, defoliation, and grafting studies indicate that BRs do not undergo long-distance transport between the shoot and root or between different tissues within the shoot. This conclusion is further supported by the apparent immobility of exogenously applied 3H-BL and 3H-CS. Indeed, results show that the radiolabeled BRs applied to the mature leaves or apical buds of intact wild-type pea plants did not move beyond the application site. It could be argued that the radiolabeled BRs were not transported because they did not adequately enter the plant tissues after application. However, this is unlikely because 200 ng of BL applied in an identical manner readily enters the leaf tissue as shown by the localized promotion of growth in these treated tissues (Fig. 4).
These results are consistent with studies that show that the majority of radiolabeled BL and CS incorporated into leaves of rice remained in the treated leaves 24 h after it was applied (Yokota et al., 1992). In this study, a small amount of radioactivity was shown to accumulate in the roots after 72 h, but this was largely in the form of water-soluble BR metabolites (Yokota et al., 1992). Similarly, in wheat, exogenously applied 14C-epiBL was not transported from the treated leaf even after 7 d (Nishikawa et al., 1994).
In contrast, it has previously been reported that 100 ng of BL in 10 μL of ethanol, applied to the third leaf (including leaflets, stipules, and petioles) of various pea genotypes, results in increased growth of the fourth internode (Nomura et al., 1997, 1999, 2003), thus implying some movement of exogenous BRs from the leaf to the stem. However, due to the relatively large volume of ethanol used, we cannot exclude the possibility that the increase in growth came about because some of the applied BL came into contact with the surface of the fourth internode itself. Indeed, we have found that the precise placement of 100 ng of BL in 2 μL of ethanol to the stipules, petioles, or leaflets of the third leaf (avoiding any contact with the stem tissues) does not result in an increase in growth of the fourth internode (data not shown).
Insights into the Regulation of Bioactive BR Levels
If BRs are acting as a true hormone signal, then they must have mechanisms that control their endogenous levels. However, it seems that one mechanism that may not have a significant role in regulating BR levels is BR transport or, more specifically, long-distance BR transport. Instead, it appears more likely that endogenous BR levels may be regulated through the strict control of BR biosynthesis and metabolism. This suggestion is consistent with studies that have shown that several steps in the BR biosynthesis pathway undergo feedback regulation in response to BR levels (see Fujioka and Yokota, 2003).
The conversion of 6-DeoxoCS to CS is thought to be one such step. For instance, in wild-type Arabidopsis, pea, and tomato plants, the level of 6-DeoxoCS was shown to be an order of magnitude higher than the level of CS, suggesting that the conversion of 6-DeoxoCS to CS is an important rate-limiting step in all three species (Nomura et al., 2001). This implies that the levels or activity of the enzyme that catalyzes this step might be strictly regulated (Nomura et al., 2001). Indeed, this idea is supported by molecular evidence (Bancos et al., 2002; Goda et al., 2002; Shimada et al., 2003) for the feedback regulation of the Arabidopsis BR6ox1 and BR6ox2 genes (homologs of the tomato DWARF gene that convert 6-DeoxoCS to CS; see Bishop et al., 1999) by bioactive BR levels. Further evidence for this phenomenon can be seen by examining the ratio of 6-DeoxoCS to its direct metabolite CS in pea BR mutants. For instance, the ratio of 6-DeoxoCS to CS in wild-type pea plants is consistently around 6:1, while in the lka and lkb mutants this ratio is around 2:1, or less, in a wide range of tissue types (Table II; Nomura et al., 1999). This suggests that the conversion of 6-DeoxCS to CS is increased in the lkb and lka mutants, most likely in response to the real or perceived deficiency of active BRs in these plants, respectively. The apparent widespread occurrence of feedback regulation of BR biosynthesis is in contrast to the more specific locations of the feedback regulation of the GA biosynthesis pathway in pea. Indeed, feedback regulation of key biosynthesis genes PsGA20ox1 and PsGA3ox1 has been shown to occur in the apical and root tissues but not in leaflets or internodes (Elliott et al., 2001).
In addition to BR biosynthesis, it is clear that BR metabolism also plays an important role in the regulation of endogenous BR levels. Considerable progress has been made in our understanding of BR metabolism, and more than 30 BR metabolites have now been identified (Adam and Schneider, 1999; Fujioka and Yokota, 2003). Recently, Turk et al. (2003) reported the characterization of one of the key genes involved in BR metabolism in Arabidopsis, CYP72B1. CYP72B1 was shown to encode a cytochrome P450 monooxygenase, which catalyzes the hydroxylation of the bioactive BRs, BL and CS, to form the inactive products 26-hydroxybrassinolide and 26-hydroxycastasterone, respectively (Turk et al., 2003). Significantly, it was shown that CYP72B1 transcript accumulation in dark-grown seedlings was rapidly down-regulated after exposure to light (Turk et al., 2003). It is likely that this light-induced down-regulation of BR inactivation may, at least in part, facilitate the increase in endogenous BR levels observed in light-grown plants in a number of different species (Symons and Reid, 2003b).
CONCLUSIONS
While our understanding is far from complete, it does appear that many of the classical plant hormones undergo some form of long-distance transport around the plant. For instance, the basipetal transport of IAA is well established (Friml and Palme, 2002). It is also clear that cytokinins move from the root to the shoot in the xylem sap (Haberer and Keiber, 2002), and ABA can move rapidly through the plant in both the xylem and the phloem (Sauter et al., 2001). The situation regarding GA transport is less clear. For instance, GA-deficient dwarf1 sectors in genetic mosaic maize plants did not show altered growth relative to the surrounding tissues, suggesting that endogenous GA1 is mobile (Winkler and Freeling, 1994). However, in pea, endogenous GA1 is not mobile, although its precursor, GA20, is thought to be transported in some circumstances (Reid et al., 1983).
In contrast, the results from this study provide no evidence for the long-distance transport of endogenous BRs. This is consistent with previous findings such as the variegated, revertant phenotype of the transposon-mutagenized dwarf mutants in tomato (Bishop et al., 1996), which is suggested to indicate that BRs are synthesized in tissues adjacent to where they function (Shimada et al., 2003).
It is important to note that results from the current study do not rule out the short-distance transport of BRs between cells or within tissues. This is a particularly important consideration in light of the recent suggestion that BRs could be transported by binding to a specific pathogenesis-related protein, PR-10 (see Markovic-Housley et al., 2003). The possible involvement of this mechanism in the short-distance transport of BRs within or between individual cells may have an important role in the microregulation of BR levels and clearly warrants further investigation.
However, the available evidence, including the occurrence of late-pathway BRs in a wide range of plant tissues, the apparent lack of BR transport between these different tissue types, and the expression of genes involved in BR biosynthesis and perception throughout the plant suggest that BRs may be synthesized and act at least in the same tissues, or perhaps even in the same cells. Consistent with this view are results from tomato, which show that transcripts of the DWARF gene (which is responsible for the conversion of 6-DeoxoCS to CS; see Bishop et al., 1999) accumulate in the meristem, specifically within cells initiating the new leaf (Pien et al., 2001). This suggests the occurrence of cell-specific regulation of BR biosynthesis in the leaf primordium (Clouse, 2002). Indeed, it seems likely that endogenous BR levels are primarily regulated through such site-specific control of BR biosynthesis and metabolism rather than through long-distance transport.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The pure lines of garden pea (Pisum sativum) used in this study were Hobart line 107 (cv Torsdag, wild type) and the single-gene BR mutant lines NGB5862 (lkb, semi-erectoides) and NGB5865 (lka, semi-erectoides). NGB5862 and NGB5865 were both derived from Torsdag by mutagenesis with ethyl methanesulfonate by Dr. K.K. Sidorova (Reid and Ross, 1989; Nomura et al., 1999). Mutant lkb has been shown to be a lesion in the gene homologous to DIM/DWF1 in Arabidopsis (Schultz et al., 2001), while lka has been shown to be a lesion in the pea homolog of the Arabidopsis BRI1 gene (Nomura et al., 2003).
Seeds were sown 2 to 3 cm deep in 14-cm slim-line pots containing a 1:1 (v/v) mixture of vermiculite and 10-mm dolerite chips topped with 4 cm of pasteurized peat/sand potting mixture. All plants were grown under an 18-h photoperiod in a heated greenhouse, with the natural daylength extended at its beginning and end with light from 40-W cool-white fluorescent tubes and 100-W incandescent bulbs, providing about 25 μm m−2 s−1 at pot top. Nutrient was applied weekly in the form of Aquasol (Hortico, Melbourne, Australia). Node counts commenced from the first scale leaf as node 1; internode 1 was the internode between nodes 1 and 2.
Harvest Procedure
All plants utilized for hormone quantification were harvested after the leaf at node 8 was fully expanded (approximately 30 d old). In these experiments, plant shoots and roots were separated at node 0. Shoots were either left whole or separated into individual tissue types (see Fig. 1), while the roots were left whole and washed free of excess soil. All plant tissues used for hormone analysis were weighed and then immediately immersed in cold (−20°C) 80% v/v methanol.
Extraction, Purification, and Gas Chromatography-Mass Spectrometry Quantification of BRs
Procedures for the extraction, purification, and gas chromatography-mass spectrometry quantification of endogenous BRs, IAA, GA1, and ABA have been previously outlined in Symons and Reid (2003a).
Metabolism and Transport of 3H-BL and 3H-CS
A total of 1 × 106 dpm of radiolabeled BRs (3H-BL and 3H-CS; 0.78 Ci mm−1) was applied in 5 μL of ethanol to either the youngest expanded leaf (at node 9) or directly to the apical bud of intact, 33-d-old wild-type plants. Forty-eight hours later, the site of BR application and all adjacent shoot tissues (including individual leaves, internodes, and the apical bud) were harvested separately, frozen in liquid nitrogen, and stored at −20°C. Tissue samples were homogenized and BRs were extracted in a mixture of 80% methanol and 20% distilled water. The level of radioactivity in the individual tissue samples was determined by radiocounting (using a Beckman LS 6500 scintillation counter; Beckman Instruments, Fullerton, CA). Metabolism of the radiolabeled BRs was analyzed by subjecting extracts from the treated tissues to HPLC radiocounting. Details of the HPLC system and the solvent program were as outlined in Symons and Reid (2003a).
Acknowledgments
We thank Tracey Jackson, Ian Cummings, and Noel Davies for technical assistance, Dr. Suguru Takatsuto (Department of Chemistry, Joetsu University of Education, Joetsu-shi, Niigata, Japan), and Professor Takao Yokota (Department of Biosciences, Teikyo University, Utsunomiya, Japan) for 2H6- and 3H-labeled BRs, Dr. S. Neil (University of Bristol, UK) for labeled ABA, Professor L.N. Mander (Australian National University, Canberra, Australia) for labeled GA1, Professor Peter Davies (Cornell University, Ithaca, NY) for labeled IAA, and Dr. John Ross for assistance with the preparation of the manuscript.
This work was supported by the Australian Research Council.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043034.
References
- Adam G, Schneider B (1999) Uptake, transport and metabolism. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids: Steroidal Plant Hormones. Springer, Tokyo, pp 113–136
- Arteca RN (1995) Brassinosteroids. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Press, Dordrecht, The Netherlands, pp 206–213
- Bancos S, Nomura T, Sato T, Molnar G, Bishop GJ, Koncz C, Yokota T, Nagy F, Szekeres M (2002) Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski RS, Cohen JD, Slovin JP, Reinecke DM (1995) Auxin biosynthesis and metabolism. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Press, Dordrecht, The Netherlands, pp 35–57
- Bishop GJ, Harrison K, Jones JDG (1996) The tomato Dwarf gene isolated by heterologous transposon tagging encodes the first member of a new cytochrome p450 family. Plant Cell 8: 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JD, Kamiya Y (1999) The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA 96: 1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T (2001) Plant steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol 42: 114–120 [DOI] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C (2002) Brassinosteroids and plant steroid hormone signalling. Plant Cell (Suppl) 14: S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD (2002) Brassinosteroids: plant counterparts to animal steroid hormones? Vitam Horm 65: 195–223 [DOI] [PubMed] [Google Scholar]
- Davies PJ (1995) The plant hormones, their nature, occurrence, and functions. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology. Kluwer Academic Press, Dordrecht, The Netherlands, pp 1–5
- Elliott RC, Ross JJ, Smith JJ, Lester DR, Reid JB (2001) Feed-forward regulation of gibberellin deactivation in pea. J Plant Growth Regul 20: 87–94 [Google Scholar]
- Friedrichsen D, Chory J (2001) Steroid signalling in plants: from the cell surface to the nucleus. Bioessays 23: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Friml J, Palme K (2002) Polar auxin transport – old questions and new concepts? Plant Mol Biol 49: 273–284 [PubMed] [Google Scholar]
- Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54: 137–164 [DOI] [PubMed] [Google Scholar]
- Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S (2002) Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 130: 1319–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer G, Keiber JJ (2002) Cytokinins. New insights into a classic phytohormone.Plant Physiol 128: 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoad GV (1995) Transport of hormones in the phloem of higher plants. J Plant Growth Regul 16: 173–182 [Google Scholar]
- Markovic-Housley Z, Degano M, Lamba D, von Roepenack-Lahaye E, Clemens S, Susani M, Ferreira F, Scheiner O, Breiteneder H (2003) Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. Plant Mol Biol 325: 123–133 [DOI] [PubMed] [Google Scholar]
- Nishikawa N, Toyama S, Shida A, Fatatsuya F (1994) The uptake and transport of 14C-labeled epibrassinolide in intact seedlings of cucumber and wheat. J Plant Res 107: 125–130 [Google Scholar]
- Nomura T, Bishop GJ, Kaneta T, Reid JB, Chory J, Yokota T (2003) The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J 36: 291–300 [DOI] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T (1999) Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol 119: 1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T (1997) Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol 113: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Sato T, Bishop GJ, Kamiya Y, Takatsuto S, Yokota T (2001) Accumulation of 6-deoxocathasterone and 6-deoxocastasterone in Arabidopsis, pea and tomato is suggestive of common rate-limiting steps in brassinosteroid biosynthesis. Phytochemistry 57: 171–178 [DOI] [PubMed] [Google Scholar]
- Pien S, Wyrzykowska J, Fleming AJ (2001) Novel marker genes for early leaf development indicate spatial regulation of carbohydrate metabolism within the apical meristem. Plant J 25: 663–674 [DOI] [PubMed] [Google Scholar]
- Reid JB (1979) Flowering in Pisum: the effect of age on the gene Sn and the site of action of gene Hr. Ann Bot (Lond) 44: 163–173 [Google Scholar]
- Reid JB, Murfet IC, Potts WC (1983) Internode length in Pisum. II. Additional information on the relationship and action of loci Le, La, Cry, Na and Lm. J Exp Bot 34: 349–364 [Google Scholar]
- Reid JB, Ross JJ (1989) Internode length in Pisum. Two further gibberellin insensitivity genes lka and lkb. Physiol Plant 75: 81–88 [Google Scholar]
- Ross JJ, O'Neill DP, Smith JJ, Kerckhoffs LHJ, Elliott RC (2000) Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J 21: 547–552 [DOI] [PubMed] [Google Scholar]
- Sasse J (1999) Physiological actions of brassinosteroids. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids: Steroidal Plant Hormones. Springer-Verlag, Tokyo, pp 137–161
- Sasse J (2003) Physiological action of brassinosteroids: an update. J Plant Growth Regul 22: 276–288 [DOI] [PubMed] [Google Scholar]
- Sauter A, Davies WJ, Hartung W (2001) The long-distance abscisic acid signal in the droughted plant: the fate of the hormone on its way from root to shoot. J Exp Bot 52: 1991–1997 [DOI] [PubMed] [Google Scholar]
- Schultz L, Kerckhoffs LHJ, Klahre U, Yokota T, Reid JB (2001) Molecular characterisation of the brassinosteroid-deficient lkb mutant in pea. Plant Mol Biol 47: 491–498 [DOI] [PubMed] [Google Scholar]
- Shimada Y, Goda H, Nakamura A, Takatsuto S, Fujioka S, Yoshida S (2003) Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol 131: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons GM, Reid JB (2003. a) Hormone levels and response during de-etiolation in pea. Planta 216: 422–431 [DOI] [PubMed] [Google Scholar]
- Symons GM, Reid JB (2003. b) Interactions between light and plant hormones during de-etiolation. J Plant Growth Regul 22: 3–14 [Google Scholar]
- Tichtinsky G, Vanoosthuyse V, Cock JM, Gaude T (2003) Making inroads into plant receptor kinase signalling pathways. Trends Plant Sci 8: 231–237 [DOI] [PubMed] [Google Scholar]
- Turk EM, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Denzel MA, Torres QI, Neff MM (2003) CYP72B1 inactivates brassinosteroid hormones: an intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol 133: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler RG, Freeling M (1994) Analysis of the autonomy of maize Dwarf1 action in genetic mosaics. J Hered 85: 377–380 [DOI] [PubMed] [Google Scholar]
- Yokota T, Higuchi K, Kosaka Y, Takahashi N (1992) Transport and metabolism of brassinosteroids in rice. In CM Karssen, LC van Loon, D. Vreugdenhil, eds, Progress in Plant Growth Regulation. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 298-305