Abstract
Rising atmospheric carbon dioxide concentration ([CO2]) is widely recognized, but less appreciated is a concomitant rise in tropospheric ozone concentration ([O3]). In industrialized countries, [O3] has risen by 0.5% to 2.5% per year. Tropospheric [O3] is predicted to reach a global mean of >60 nL L−1 by 2050 with greater averages locally. Previous studies in enclosures suggest that this level of [O3] will decrease leaf photosynthesis, thereby limiting growth and yield of Glycine max L. Merr. SoyFACE (Soybean Free Air gas Concentration Enrichment) is the first facility to elevate atmospheric [O3] (approximately 1.2× current) in replicated plots under completely open-air conditions within an agricultural field. Measurements of gas exchange (assimilation versus light and assimilation versus intercellular [CO2]) were made on excised leaves from control and treatment plots (n = 4). In contrast to expectations from previous chamber studies, elevated [O3] did not alter light-saturated photosynthesis (Asat, P = 0.09), carboxylation capacity (Vc,max, P = 0.82), or maximum electron transport (Jmax, P = 0.66) for the topmost most recently fully expanded leaf at any stage of crop development. Leaves formed during the vegetative growth stage did not show a significant ozone-induced loss of photosynthetic capacity as they aged. Leaves formed during flowering did show a more rapid loss of photosynthetic capacity as they aged in elevated [O3]. Asat, Vc,max, and Jmax (P = 0.04, 0.004, and 0.002, respectively) were decreased 20% to 30% by treatment with ozone. This is noteworthy since these leaves provide photosynthate to the developing grain. In conclusion, a small (approximately 20%) increase in tropospheric [O3] did not significantly alter photosynthetic capacity of newly expanded leaves, but as these leaves aged, losses in photosynthetic carbon assimilation occurred.
In the northern mid-latitudes (36°–59°N), surface ozone has risen from an estimated preindustrial 10 nL L−1 to an average regional concentration of almost 60 nL L−1 today (1%–2% per year; Chameides et al., 1994; Prather et al., 2001). Although once considered a problem of conurbations, long-distance and even intercontinental ozone transport has resulted in a steady increase in rural areas hundreds and thousands of kilometers from the original sources of pollution (Prather et al., 2001; Gregg et al., 2003; Prather et al., 2003). Nearly one-quarter of the Earth's surface is currently at risk from mean tropospheric ozone in excess of 60 nL L−1 during midsummer with even greater local concentrations occurring (Fowler et al., 1999a, 1999b). A compilation of 10 different models projects that July surface ozone concentration ([O3]) in the northern mid-latitudes is likely to rise by a further 20% over the next 50 years (Prather et al., 2001, 2003). The current mean global concentration of 60 nL L−1 is well above the mean concentration of 40 nL L−1 that has been determined as a threshold for damage to sensitive plant species (Fuhrer et al., 1997; Mills et al., 2000). For plants grown at chronic background [O3] concentrations (40–80 nL L−1), substantial invisible damage, i.e. decreased photosynthetic rates, occurs in many plants before visible symptoms typically manifest at higher levels of ozone (>200 nL L−1; for review, see Ashmore, 2002; Long and Naidu, 2002). The world's most widely planted dicotyledonous crop (FAO-UN, 2002), soybean (Glycine max L. Merr.), is among the more ozone sensitive species (for review, see Ashmore, 2002). Mean July ozone levels are projected to increase over this century by 30 nL L−1 in the midwest U.S. and by 50 nL L−1 in eastern China (Prather et al., 2003), two of the largest soybean production areas of the globe (FAO-UN, 2002).
In a meta-analysis of 53 peer-reviewed studies of the effects of elevated [O3] (approximately 70 nL L−1 compared to ozone-free air) on soybean photosynthesis and production, elevated [O3] was found to significantly decrease leaf net carbon assimilation (A) by 23% in soybean (Morgan et al., 2003). Parallel large decreases in leaf total nonstructural carbohydrates were observed with decreased photosynthesis, suggesting that production was limited by photoassimilate supply (Morgan et al., 2003). Therefore, decreased photosynthesis appears to be the key change driving yield losses in soybean subjected to elevated [O3]. For soybean grown in open-top chambers, elevated [O3] decreased A in newly expanded leaves over the growing season, with the greatest decreases occurring during pod filling (Mulchi et al., 1992; Reid and Fiscus, 1998). Additionally, previous studies with soybean (Mulchi et al., 1992; Booker et al., 1997; Fiscus et al., 1997) and other crops (McKee et al., 1995) have shown that as leaves age, cumulative exposure to ozone impairs photosynthesis.
Typically, in field-grown plants, photosynthetic assimilation in saturating light (Asat) is limited by the maximum rate of carboxylation (Vc,max), which reflects the in vivo activity of Rubisco (Sage, 1994; Rogers and Humphries, 2000). Decreases in the activity and amount of Rubisco, rather than in electron transport and capacity for ribulose 1,5-bisphosphate (RuBP) regeneration, are primarily responsible for the deleterious effects of ozone on A (Farage et al., 1991; McKee et al., 1995; Pell et al., 1997; Farage and Long, 1999; Noormets et al., 2001; Zheng et al., 2002). Elevated [O3] has been shown to decrease Vc,max in soybean, and these decreases become progressively larger over the growing season (Reid and Fiscus, 1998). The maximum rate of electron transport (Jmax) for RuBP regeneration is the other major photosynthetic rate-limiting process (Sage, 1990). Ozone-induced declines in Vc,max were not reflected in concomitant losses in Jmax, suggesting that the thylakoid membrane capacity for electron transport was not impaired enough to detect via gas-exchange methods (Farage and Long, 1999).
In addition to effects on Rubisco and RuBP regeneration, other factors that could potentially affect A are decreased stomatal aperture (Zheng et al., 2002) and conductance (McKee et al., 1995; Fiscus et al., 1997; Farage and Long, 1999; McKee et al., 2000). Although stomatal aperture and conductance were decreased by elevated [O3] treatments, the stomatal limitation to A (l) was not affected (Fiscus et al., 1997; Zheng et al., 2002) or did not decrease photosynthesis (Farage and Long, 1999). These findings, however, are based primarily on controlled environment experiments, which can substantially modify the plant responses to environmental conditions (McLeod and Long, 1999; Ainsworth et al., 2002; Jablonski et al., 2002).
Projected ozone vulnerability of soybean and other crops is based on extensive and detailed studies conducted in controlled environments and in open-top chambers (for review, see Ashmore, 2002; Morgan et al., 2003). Lacking is confirmation that effects observed in these modified environments will also be evident in plants grown in the open-air field conditions. In open-top chambers, air is continuously stirred and forced through the plant canopy, thereby possibly increasing the coupling of lower leaves to the bulk atmosphere and exposing leaves to higher [O3] than they normally receive in undisturbed canopies. Similarly, in the natural environment when the air is still, [O3] within or near the canopy is likely depleted; a condition that could not develop in the stirred air of a chamber. While enclosure studies have provided important insights into mechanisms, meaningful extrapolation of experimental results to real-world agriculture may require validation in systems where perturbations of the soil-plant-atmosphere continuum are minimal, such as Free-Air gas Concentration Enrichment (FACE) systems (McLeod, 1995; McLeod and Long, 1999).
We have used such an open-air treatment facility (Ainsworth et al., 2004; Rogers et al., 2004; www.soyface.uiuc.edu) to determine how photosynthetic capacity in soybean responded to elevated [O3] exposure in the field and to further expand on previous chamber studies. Specifically, we predicted that (1) with open-air [O3] elevation, photosynthetic capacity losses would be less severe than observed in prior open-top and controlled environment chamber studies, (2) decrease in Asat would result primarily from decreased Vc,max, (3) ozone-induced decrease in photosynthesis would increase with leaf age due to cumulative exposure, and (4) elevated [O3] would not alter l. Over the 2002 growing season, leaf photosynthetic parameters were measured to assess these potential photosynthetic alterations caused by elevated [O3].
RESULTS
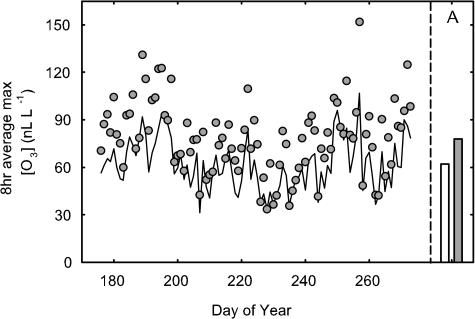
The ozone fumigation system provided a consistent treatment across the growing season. The season mean of the maximum daily 8-h average [O3] was 62 nL L−1 for the control plots and 75 nL L−1 for the elevated [O3]. Daily 8-h average concentrations for both the ambient and elevated [O3] treatments are shown in Figure 1. The effective treatment over the season was 1.2× ambient [O3]. Based on 1-min average concentrations, the achieved elevation was within ±10% of the set point concentration 74% of the time and within ±20% of the set point 90% of the time.
Figure 1.
Maximum 8-h average [O3] for ambient (black line) and elevated [O3] treatments (gray symbol) and seasonal average [O3] in control (62 nL L−1; white bar) and elevated (75 nL L−1; gray bar) treatments. On some dates, 225 and following, some ambient symbols are occluded by elevated treatment symbols due to shutdown of the treatment system (see “Materials and Methods”).
Photosynthesis Was Not Inhibited by Ozone in Newly Expanded Leaves
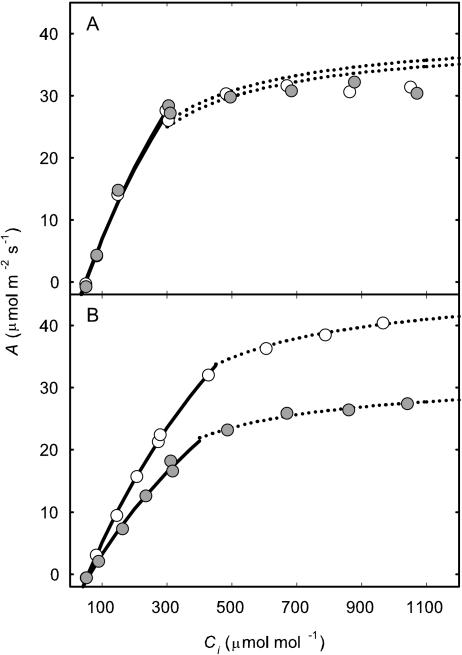
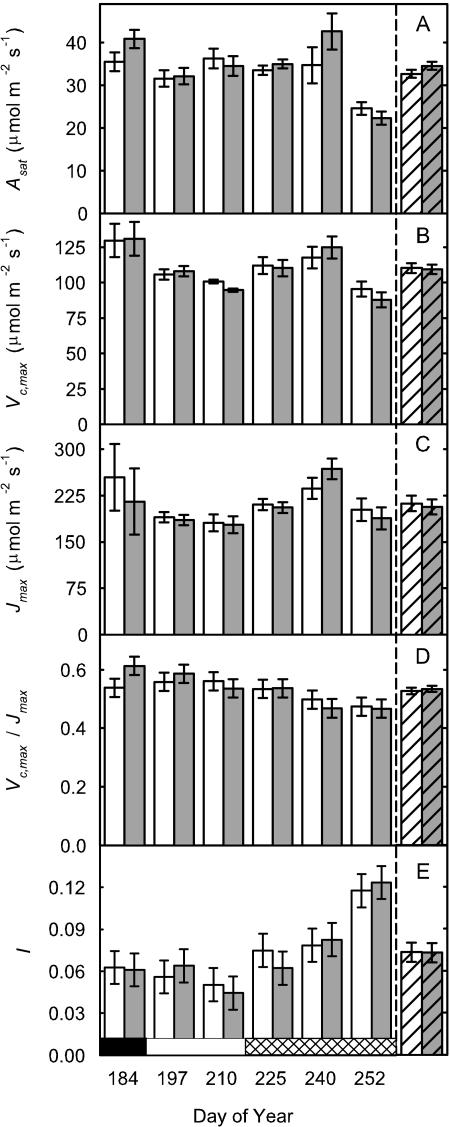
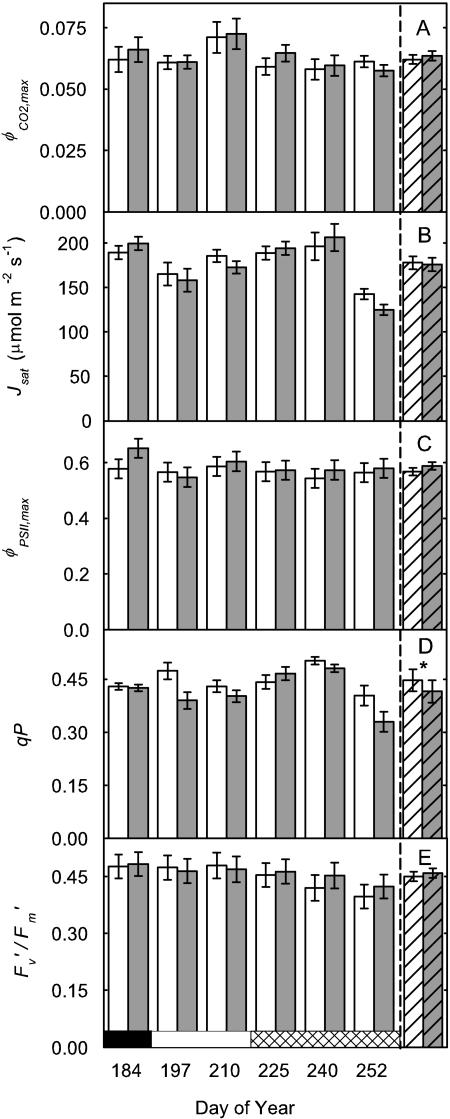
On day 225 (early pod-filling stage), there was no difference in the A to intercellular carbon dioxide concentration ([CO2]) ratio (A/Ci) response curves of a recently expanded leaf grown in current ambient compared with elevated [O3] (Fig. 2A). Photosynthesis of newly expanded leaves was measured on six dates across the growing season from vegetative growth through flowering to the late stages of pod filling. At all stages, except final pod filling, photosynthetic rates (Asat) were high and photosynthetic capacities, as indicated by Vc,max and Jmax values, were at the upper end of reported ranges for these variables (Wullschleger, 1993). However, there was no evidence of any effect of elevated [O3] on Asat, Vc,max, or Jmax on any of these six dates covering all major growth stages of the crop (Fig. 3; Table I). This confirms the inference from the single A/Ci responses illustrated (Fig. 2A). Although l rose on the final sampling date, during late pod filling, there was again no effect of growth in elevated ozone (Fig. 3E; Table I). A very small but significant decrease in photochemical quenching (qP) was observed (Fig. 4D; Table I) but was not reflected in Asat. The maximum apparent quantum efficiency (φCO2,max) averaged approximately 0.06 throughout, which is close to the maximal value expected in normal air (Long et al., 1993), and was completely unaffected by elevated [O3] (Fig. 4A; Table I). The maximum light-adapted apparent quantum efficiency of PSII (φPSII,max,l), electron transport at growth [CO2] and saturating light (Jsat), and probability of an absorbed photon reaching an open PSII reaction center (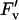 /
/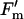 ) were all similarly unaffected by growth in elevated [O3] (Fig. 4; Table I).
) were all similarly unaffected by growth in elevated [O3] (Fig. 4; Table I).
Figure 2.
Example responses of A/Ci from soybean grown in current (62 nL L−1 8-h daily average; white symbol) or elevated (approximately 1.2× current; gray symbol) [O3]. Assimilation rates are for the topmost fully expanded trifoliate on DOY 225 (A) and leaves from that same cohort after 26 d of elevated [O3] treatment (DOY 251; B). The Vc,max (solid lines) was fit to the initial portion of the response and Jmax (dotted lines) was fit using the photosynthetic model of Farquhar et al. (1980) with temperature corrections by Bernacchi et al. (2001, 2003) as described previously (Long and Bernacchi, 2003).
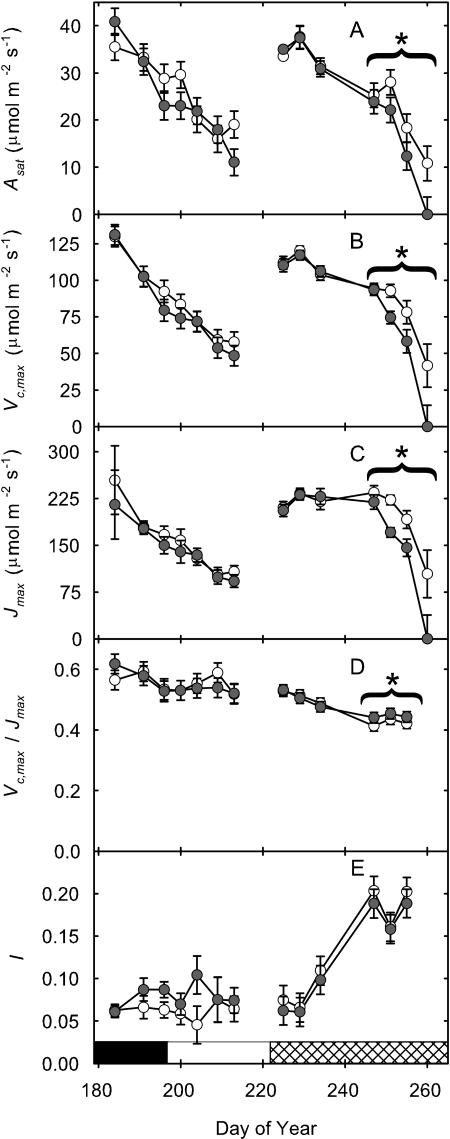
Figure 3.
Photosynthetic parameters from the topmost fully expanded trifoliate from soybean grown in current (62 nL L−1 8-h daily average; white bar) or elevated (approximately 1.2× current; gray bars) [O3] over the growing season. Parameters are Asat (A), Vc,max (B), Jmax (C), Vc,max/Jmax (D), and l (which was calculated as percentage difference between potential photosynthesis at growth [CO2] and actual photosynthesis; E). Season averages are reported as the striped pair of bars at right of each figure. Each bar is the least squared mean (n = 4), and the error bars are ± the se of the difference in least square means. Significant differences (P value ≤ 0.05) are starred. Developmental stages are represented by the horizontal bar: vegetative (black), flowering (white), and pod filling (cross-hatched).
Table I.
Analysis of variance of fixed effects on photosynthetic and gas-exchange parameters of topmost fully expanded leaves taken from field-grown soybean in current and elevated [O3]
| Day of Year
|
Treatment
|
Day of Year by Treatment
|
||||
|---|---|---|---|---|---|---|
| F | P Value | F | P Value | F | P Value | |
| Asat | 56.42 | <0.0001 | 3.96 | 0.0881 | 3.20 | 0.0531 |
| Vc,max | 22.77 | <0.0001 | 0.06 | 0.8151 | 2.06 | 0.0959 |
| Jmax | 14.32 | <0.0001 | 0.21 | 0.6593 | 1.27 | 0.3257 |
| Ci inflection | 26.01 | <0.0001 | 2.26 | 0.1832 | 1.94 | 0.1164 |
| Vc,max/Jmax | 10.79 | <0.0001 | 0.47 | 0.4972 | 1.84 | 0.1323 |
| l | 25.13 | <0.0001 | 0.00 | 0.9530 | 0.57 | 0.7219 |
| φCO2,max | 3.26 | 0.0382 | 0.65 | 0.4341 | 1.19 | 0.3632 |
| θ | 7.56 | 0.0106 | 1.74 | 0.2256 | 2.07 | 0.1847 |
| Jsat | 5.67 | 0.0039 | 0.07 | 0.8028 | 13.07 | <0.0001 |
| φPSII,max,l | 1.02 | 0.4422 | 2.52 | 0.2106 | 0.74 | 0.6034 |
| qP | 8.74 | 0.0005 | 8.15 | 0.0290 | 9.83 | 0.0003 |
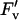 / /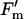
|
4.00 | 0.0177 | 0.56 | 0.4882 | 0.47 | 0.7928 |
The F statistic and associated P values were calculated from a mixed model of a repeated measure analysis in a randomized complete block design (n = 4).
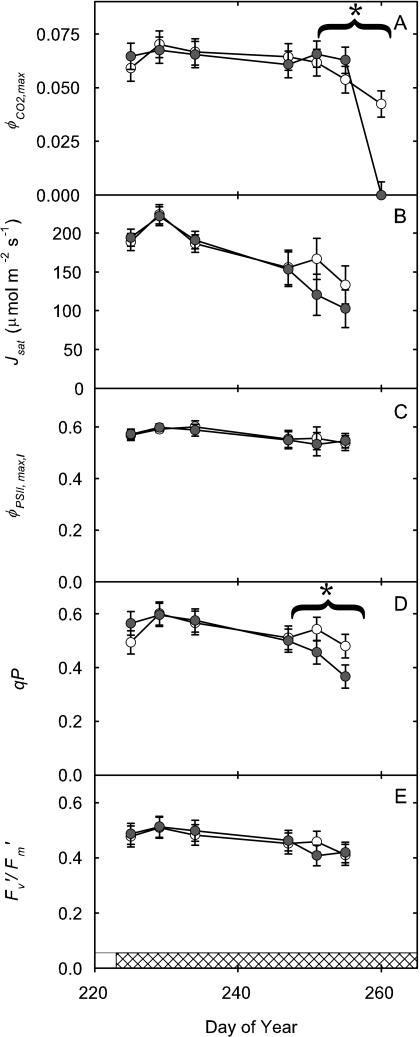
Figure 4.
Electron transport and fluorescence parameters of the leaves described in Figure 3. Parameters are φCO2,max (calculated as the initial slope of the photosynthetic carbon assimilation response to light; A), Jsat (B), φPSII,max,l (C), qP (D), and 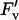 /
/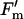 at 2,000 μmol m−2 s−1 (E). All parameters were calculated from fluorescence measurements of electron transport rates in increasing light. Data presented as in Figure 3.
at 2,000 μmol m−2 s−1 (E). All parameters were calculated from fluorescence measurements of electron transport rates in increasing light. Data presented as in Figure 3.
Prolonged Ozone Exposure Resulted in Significant Inhibition of Photosynthesis in Older Leaves during Pod Filling
Two leaf cohorts were tracked from completion of expansion to senescence; the first was formed during vegetative development (V5, day of year [DOY] 185) and the second during pod filling (R4, DOY 225). In the vegetative leaf cohort, there was no significant effect of ozone on any photosynthetic parameter, except l (Fig. 5; Table II). But significant losses due to elevated ozone were apparent in Asat, Vc,max, and Jmax as this second cohort of leaves aged (Figs. 2B and 5, A–C), and this was confirmed by significant treatment by age interactions (Table II). There was also a small but significant decrease in Vc,max/Jmax (Fig. 5D), implying a slightly greater effect of the treatment on in vivo Rubisco activity relative to capacity for RuBP regeneration. There was no significant effect of ozone on l in this cohort, but the significant decrease in Asat with age was paralleled by significant losses in Jsat and qP (Fig. 6, B and D; Table II). Capacity for light-limited photosynthesis, as indicated by φCO2,max, was decreased significantly by elevated ozone only on the final date on which this cohort was examined (Fig. 6A), suggesting that impairment of light-limited photosynthesis occurred later than impairment of processes determining light-saturated photosynthetic rate.
Figure 5.
Photosynthetic parameters of two leaf cohorts grown in current (62 nL L−1 8-h daily average; white symbol) or elevated (approximately 1.2×; gray symbol) [O3] over the growing season. The first cohort was initiated in the vegetative phase of growth (V5), the second during flowering (R4) and persisted to late pod filling (R7). Parameters are Asat (A), Vc,max (B), Jmax (C), Vc,max/Jmax (D), and l (E). Each point is the mean (n = 4), and the error bars are ± the se of the difference in least square means. Significant linear contrasts over the last 3 or 4 d are noted with a starred bracket. Values of 0 indicate the absence of leaves at that time point. Vegetative (black), flowering (white), and pod-filling (cross-hatched) developmental stages are represented by the horizontal bar.
Table II.
Analysis of variance of fixed effects on photosynthetic and gas-exchange parameters of two leaf cohorts taken from field-grown soybean in current and elevated [O3]
| Cohort 1 (V5, DOY 185) | Day of Year
|
Treatment
|
Day of Year by Treatment
|
|||
|---|---|---|---|---|---|---|
| F | P Value | F | P Value | F | P Value | |
| Asat | 23.81 | <0.0001 | 1.20 | 0.3175 | 3.98 | 0.0268 |
| Vc,max | 65.46 | <0.0001 | 4.21 | 0.0566 | 0.63 | 0.7022 |
| Jmax | 52.28 | <0.0001 | 1.29 | 0.2852 | 1.08 | 0.4947 |
| Ci inflection | 33.93 | <0.0001 | 1.71 | 0.2288 | 1.76 | 0.2185 |
| Vc,max/Jmax | 4.54 | 0.0044 | 0.24 | 0.6269 | 1.10 | 0.3946 |
| l | 1.29 | 0.3377 | 12.65 | 0.0034 | 1.76 | 0.1952 |
| φCO2,max | 1.57 | 0.3001 | 2.80 | 0.2969 | 0.76 | 0.6334 |
| θ | 0.54 | 0.7676 | 0.78 | 0.4060 | 0.45 | 0.8222 |
| Cohort 2 (R4, DOY 225) | ||||||
| Asata | 66.93 | <0.0001 | 6.14 | 0.0412 | 2.88 | 0.0293 |
| Vc,maxa | 76.45 | <0.0001 | 4.65 | 0.0037 | 22.07 | <0.0001 |
| Jmaxa | 24.95 | <0.0001 | 27.36 | 0.0020 | 9.11 | 0.0001 |
| Ci inflectionb | 51.68 | <0.0001 | 0.01 | 0.9196 | 0.77 | 0.5876 |
| Vc,max/Jmaxb | 13.52 | <0.0001 | 1.25 | 0.3056 | 0.88 | 0.5163 |
| lb | 13.23 | <0.0001 | 1.69 | 0.2419 | 0.11 | 0.9884 |
| φCO2,maxa | 29.25 | <0.0001 | 2.61 | 0.2221 | 8.62 | 0.0005 |
| θb | 2.71 | 0.0606 | 0.05 | 0.8279 | 2.04 | 0.1303 |
| Jsatb | 19.73 | <0.0001 | 2.94 | 0.1370 | 1.15 | 0.3541 |
| φPSII,max,lb | 6.38 | 0.0014 | 0.17 | 0.6854 | 0.25 | 0.9332 |
| qPb | 7.94 | <0.0001 | 1.62 | 0.2114 | 2.36 | 0.0613 |
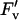 / /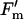 b b
|
5.13 | 0.0028 | 0.00 | 0.9777 | 0.42 | 0.8270 |
The F statistic and associated P values were calculated from a repeated measures, mixed model analysis of a randomized complete block design (n = 4).
Value of 0 used for elevated [O3] treatment on DOY = 260, when leaves had senesced.
Calculations through day 255 since plants grown in elevated [O3] did not have leaves.
Figure 6.
Electron transport and fluorescence parameters of the leaves described in Figure 5. φCO2,max (A), Jsat (B), φPSII,max,l (C), qP (D), and 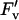 /
/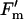 at 1,500 μmol m−2 s−1 (E). Data are presented as in Figure 5.
at 1,500 μmol m−2 s−1 (E). Data are presented as in Figure 5.
DISCUSSION
To our knowledge, this is the first study to examine the effects of elevated [O3] on photosynthesis of soybean, or of any arable crop, under fully open-air conditions. A statistical review of 53 independent published reports from enclosure studies of elevated [O3] effects on Asat of newly expanded leaves suggested that growth at approximately 70 nL L−1 ozone caused a 20% decrease in Asat relative to photosynthetic rates in plants grown under ozone-free air (Morgan et al., 2003). A practical disadvantage of FACE is that the control is the current [O3], and, unlike chamber studies, it is not possible to deplete the gas concentration in the control, making direct comparisons with these prior studies impossible. Therefore, FACE is useful in providing realistic field comparisons between current and future projected [O3], but cannot reveal the effects of an ozone-free atmosphere. However, given that 40 nL L−1 is the threshold for any ozone effects on plants (Fuhrer et al., 1997) and that above this threshold, damage increases linearly with concentration (Mills et al., 2000; Ashmore, 2002), we would expect that physiological effects would follow these whole-plant responses. Therefore, the [O3] increase from 62 nL L−1 in the control plots to 75 nL L−1 in the treated plots here should have decreased Asat by 12% (derived from Ashmore, 2002). Based on the expected 12% loss, our experiment would have detected a significant (α = 0.05) effect over the growing season 99.6 times out of 100 (power = 0.9962, see “Materials and Methods” for description). However, there was no evidence of any effect on Asat of recently expanded leaves at any growth stage in this crop; in fact, a nonsignificant, slight increase was observed, contrasting sharply to expectations from previous chamber studies (e.g. Miller et al., 1991; Mulchi et al., 1992; Vozzo et al., 1995; Booker et al., 1997; Reid and Fiscus, 1998).
Further Asat, stomatal conductance (gs), Vc,max, and Jmax of the control and treated plants equaled or exceeded the highest rates of the prior chamber studies (for review, see Morgan et al., 2003), suggesting other differences between open-air and enclosure-grown plants. Another possibility is that the cultivar used in this study is exceptionally resistant to ozone, but by comparison to 22 different lines representing a broad range of soybean germplasm that were scored for accelerated senescence, this cultivar appeared among the more sensitive lines (R.L. Nelson, personal communication). Therefore, these results suggest a substantial difference between the effects of elevated ozone on the photosynthesis of recently expanded leaves when examined in open-air treatment conditions versus chambers. This might be attributed either to the substantially altered environment of chambers and/or to the continuous delivery of ozone to the leaf caused by continual stirring and pumping of air within chambers.
A number of prior studies with soybean (Mulchi et al., 1992) and other plants (McKee et al., 1995, 2000; Zheng et al., 2002) have suggested that the effects of elevated [O3] on photosynthesis accumulate with leaf age and that this reflects cumulative uptake of ozone. Two cohorts of leaves were followed from completion of expansion through senescence, a period of approximately 35 d. The first cohort of leaves was formed during the vegetative stage of growth and remained green until about halfway through the flowering phase. At complete leaf expansion, both control and elevated [O3] treated leaves showed a high Asat (approximately 40 μmol m−2 s−1), which subsequently declined to 15 μmol m−2 s−1 over their lifetime. However, at no point in the lifetime of these leaves was there evidence of any elevated ozone effect relative to the controls (Fig. 5; Table II). The second cohort completed expansion near the beginning of pod filling and persisted throughout the pod-filling developmental stage. Again, at completion of expansion, Asat was high (35 μmol m−2 s−1). In contrast to the previous cohort, there was a highly significant treatment effect resulting from the accumulation of damage such that by the time the treated leaves reached an average Asat of 0 (leaf senescence), control leaves still maintained an Asat of 11 μmol m−2 s−1 (Fig. 5; Table II). This accelerated decline in the elevated [O3] treated leaves was underlain by accelerated losses in Vc,max and Jmax but with a greater decline in Vc,max. In a study of elevated [O3] on Plantago major, Vc,max and Jmax were also found to decrease as leaves aged (Zheng et al., 2002). These results are consistent with prior observations that Rubisco activity appears one of the most vulnerable parts of the photosynthesis apparatus (Farage et al., 1991; Pell et al., 1994; Long and Naidu, 2002). Beside decreased capacity for light-saturated photosynthesis, light-limited photosynthesis, as indicated by φCO2,max, was also significantly decreased but only on the final measurement date (Fig. 6; Table II). This later emergence of an effect on light-limited photosynthesis is consistent with earlier studies of the kinetics of ozone damage to the photosynthetic apparatus in vivo, which demonstrated that a decline in Rubisco activity precedes any effect on the photosynthetic membrane (for review, see Pell et al., 1994). Decrease in φCO2,max probably represents photoinhibition resulting from decreased capacity for carbon metabolism, causing decreased JPSII and qP (Farage et al., 1991).
What might underlie the striking difference between these two cohorts of leaves? Unlike the first cohort, which was overlain by new layers of leaves as more nodes were added to the stem, the second cohort was formed close to completion of node formation and therefore remained close to the top of the canopy throughout its life. This could explain the differences in response as the two cohorts aged since under open-air conditions ozone reaches the lower canopy only by diffusion down through the upper canopy. Since leaves are an infinite sink for ozone, the [O3] is likely to decline rapidly with canopy depth. This contrasts to increasing [O3] in chambers where stirring is likely to deliver a similar [O3] to all leaves. Thus, the first cohort of leaves may have been protected from ozone exposure as they aged, in contrast to the second cohort. However, the significant effect of elevated [O3] on the second cohort is of particular importance, since these are the leaves that provide photoassimilate to the filling pods. A similar effect was observed in wheat, where damage was most pronounced with aging in the flag leaf, which provides most photoassimilate to the developing grain (McKee et al., 1995).
In summary, this first investigation (to our knowledge) of elevation of [O3] to anticipated 2050 levels under fully open-air field conditions suggests that the effects on photosynthesis in a sensitive plant, such as soybean, are substantially less than those predicted by prior chamber studies—particularly during vegetative growth. This raises doubts about the efficacy of screens for resistance germplasm based on growth stages prior to grain filling. However, the results do confirm that elevated [O3] accelerates loss of light-saturated photosynthetic capacity in the late-season cohort of leaves, which are critical in providing assimilate to the developing seeds, and that the most significant loss is in apparent in vivo Rubisco activity.
MATERIALS AND METHODS
Site Description
The soybean FACE (SoyFACE) facility is contained within a 32 ha field (80 acre; South Farms, University of Illinois at Urbana-Champaign; 40°03′21.3″N, 88°12′3.4″W, 230 m elevation). The soil is a Drummer-Flanagan soil series (fine-silty, mixed, mesic Typic Endoaquoll) typically very deep and formed from loess and silt parent material deposited on the till and outwash plains. This site has been in continuous cultivation of arable crops for more than 100 years. Soybean (Glycine max L. Merr. cv Pana, 2001, and cv Pioneer 93B15, 2002 and 2003) and corn each occupied one-half of the field and follow an annual rotation. After the fall corn harvest, the stubble was chopped with a mower followed by tillage with a rip chisel, and conventional single-pass cultivator tillage was used in the spring. Soybean row spacing was 0.38 m (15 in), and cultivation followed typical Illinois agricultural practices (Ainsworth et al., 2004; Rogers et al., 2004).
Within the SoyFACE site, four blocks, each containing one control and one elevated [O3] treatment plot, were nested within the 16 ha planted with soybean. The plots were separated by at least 100 m to avoid cross-contamination. Treatments were control (current [O3]) and elevated (current [O3]× 1.2) arranged in a randomized complete block design (n = 4) to control for topographic and soil variation across the field. The target of 1.2× current concentration was based on projected future mean global tropospheric concentrations, which suggest a 20% increase by 2050 (Prather et al., 2001, 2003).
Elevation of [O3] was based on the method of Miglietta et al. (2001), using compressed air enriched in ozone replacing compressed CO2 in the earlier design. In this system, each plot is defined by an octagon (21 m diameter) of horizontal pipes that release ozone to provide a constant elevation above the current level. Ozone release was maintained at approximately 10 cm above the top of the crop canopy throughout the growing season by raising the horizontal pipes as the crop grew taller. As described previously for CO2 by Miglietta et al. (2001), the quantity and duration of the ozone release was controlled by a proportional integral derivative algorithm for computer feedback that compares achieved [O3] to the desired target [O3] of 1.2× current with a gas concentration monitor (model 49C O3 analyzer; Thermo Environmental Instruments, Franklin, MA; calibration USA EPA Equivalent Method EQQA-0880-047, range 0–0.05–1.0 ppm), anemometer, and wind direction vane (12005; R.M. Young, Traverse City, MI) mounted in the center of each ring. Ozone was generated by passing pure oxygen through a high-voltage electrical field generating a composite gas consisting of approximately 10% ozone and 90% oxygen (GSO-40; Wedeco Environmental Technologies, Herford, Germany). Using a mass flow controller, the ozone/oxygen mixture was added to a compressed air stream through a Venturi bypass system. Ozone-enriched air was injected through supersonic velocity jets into the wind, thus entraining surrounding air in a turbulent motion that diluted the ozone before it was carried back across the plot by the wind, as described previously for CO2 enrichment (Miglietta et al., 2001). Fumigation began 20 d after planting in 2002 and continued throughout the daylight hours until harvest. Sampling plots were located a minimum of 2 m internal to the octagon of horizontal pipes to minimize any residual effect of the injection system.
Measurements on Newly Expanded Leaves
Predawn every 2 weeks from crop emergence until senescence, two uppermost fully expanded soybean leaves per plot were randomly selected, and the petioles were cut under water. The petioles of the excised leaves were placed in deionized water throughout the duration of the measurements. Gas exchange and fluorescence measurements were made on two leaves per replicate plot. Based on previous studies, cutting leaves predawn ensured that leaves were not water stressed, photoinhibited, or triose-phosphate limited (Ainsworth et al., 2004); this allowed measurement of potential rates of the tissue, independent of transient diurnal changes that could depress apparent photosynthetic capacity. Furthermore, photosynthetic rates achieved previously with leaves cut from the site in this way (Ainsworth et al., 2004) equaled or exceeded maximum rates of attached leaves measured in the field (Rogers et al., 2004). The excised leaves were kept in low light (<10 μmol m−2 s−1) until 15 min prior to when measurements were to be taken, at which time they were light acclimated (approximately 1,000 μmol m−2 s−1 white light) outside the gas exchange chamber. Leaf gas-exchange measurements were coupled with measurements of chlorophyll fluorescence using an open gas-exchange system (LI-6400; LI-COR, Lincoln, NE) with an integrated fluorescence chamber head (LI-6400-40 leaf chamber fluorometer; LI-COR). Responses of A to Ci were measured and Vc,max and Jmax calculated as described previously (Bernacchi et al., 2001, 2003; Long and Bernacchi, 2003). The two highest Ci values were not used to calculate Jmax, thereby avoiding photosynthetic assimilation rates that could possibly be triose-phosphate limited. Stomatal limitation to photosynthesis (l) was determined following the method of Farquhar and Sharkey (1982), as described previously (Long and Bernacchi, 2003).
To determine the response of A to light (Q), chamber [CO2] was set to 370 μmol mol−1 and leaf temperature to 25°C. The initial slope of the A versus Q curves was fit with a linear function to estimate φCO2,max. In addition, entire curves were fit to a nonrectangular empirical function to estimate Asat and the convexity of the light response (θ, as described previously [Long and Drake, 1991; Long and Hällgren, 1993]). Simultaneous measurements of modulated chlorophyll fluorescence were made with the A versus Q response. The rate of electron transport (J), qP, and 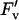 /
/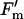 were determined as described previously (Bernacchi et al., 2002).
were determined as described previously (Bernacchi et al., 2002).
Effects of Leaf Age
Measurements of photosynthesis were made on leaf cohorts from their initial expansion until they were visibly senescenced (i.e. 75% yellow). Twice during the growing season (DOY 184 and 225), newly expanded leaves were marked with plastic flagging to aid in identification. For each leaf cohort, one leaf from two different plants was excised predawn, as described above, every 3 to 5 d from emergence until senescence. Measurements of the A versus Ci, A versus Q, and A versus J were made, and analysis of the curves were as described above except that qP and 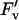 /
/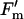 were calculated from measurements at 1,500 μmol m−2 s−1 to compensate for lower light saturation as the leaves aged.
were calculated from measurements at 1,500 μmol m−2 s−1 to compensate for lower light saturation as the leaves aged.
Statistical Analysis
For all parameter comparisons, a repeated measures, mixed model ANOVA (PROC MIXED version 8.02; SAS Institute, Cary, NC) was used with DOY, treatment, and the DOY by treatment interaction as fixed effects. The best fit variance/covariance matrices were chosen for each variable using Akaike's information criterion (Keselman et al., 1998; Littell et al., 1998, 2000). A priori pairwise linear comparisons were made between treatments across days and between treatments within days (α = 0.05). The sensitivity of the experiment was tested by calculation of the power of the test (power = 1 − β), where β is the probability of committing a Type II error (Kuehl, 2000). Calculation of β was based on the experimental replication (r = 4), Type I error rate of 5% (α = 0.05), estimated experimental variance from our measurements, and an expected 12% difference between the estimated means (derived from Ashmore, 2002).
Acknowledgments
We thank Timothy A. Mies and Frank G. Dohleman for their invaluable support in operating and maintaining the SoyFACE experimental facility such that season long treatment was possible. Frank G. Dohleman also graciously provided the developmental data, and Randall L. Nelson of USDA/ARS, Soybean Germplasm Collection, National Plant Germplasm System, provided the relative ozone sensitivity of cultivars. We also thank Andrew D.B. Leakey, Shawna L. Naidu, and Emily A. Heaton for helpful comments on this manuscript. Experimental Station: Soybean Free Air gas Concentration Enrichment (SoyFACE) plots at the South Farms, University of Illinois at Urbana-Champaign, IL.
This work was supported by the Illinois Council for Food and Agricultural Research (C-FAR), by the Archer Daniels Midland Company (ADM), by the Argonne National Laboratory, and by the USDA-ARS.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043968.
References
- Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Ra HY, Zhu X, et al (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob Change Biol 8: 1–15 [Google Scholar]
- Ainsworth EA, Rogers A, Nelson RN, Long SP (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122: 85–94 [Google Scholar]
- Ashmore MR (2002) Effects of oxidants at the whole plant and community level. In JNB Bell, M Treshow, eds, Air Pollution and Plants, Ed 2. J. Wiley, London
- Bernacchi CJ, Pimentel C, Long SP (2003) In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ 26: 1419–1430 [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130: 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24: 253–259 [Google Scholar]
- Booker SL, Reid CD, Brunschon-Harti S, Fiscus EL, Miller JE (1997) Photosynthesis and photorespiration in soybean [Glycine max (L.) Merr.] chronically exposed to elevated carbon dioxide and ozone. J Exp Bot 48: 1843–1852 [Google Scholar]
- Chameides WL, Kasibhatla PS, Yienger J, Levy HL (1994) Growth of continental-scale metro-agro-plexes, regional ozone pollution, and world food production. Science 264: 74–77 [DOI] [PubMed] [Google Scholar]
- FAO-UN (2002) FAO Trade Yearbook, Vol 165. Publishing Management Service, Information Division FAO, Rome
- Farage PK, Long SP (1999) The effects of O3 fumigation during leaf development on photosynthesis of wheat and pea: an in vivo analysis. Photosynth Res 59: 1–7 [Google Scholar]
- Farage PK, Long SP, Lechner EG, Baker NR (1991) The sequence of change within the photosynthetic apparatus of wheat following short-term exposure to ozone. Plant Physiol 95: 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Fiscus EL, Reid CD, Miller JE, Heagle AS (1997) Elevated CO2 reduces O3 flux and O3-induced yield losses in soybeans: possible implications for elevated CO2 studies. J Exp Bot 48: 307–313 [Google Scholar]
- Fowler D, Cape JN, Coyle M, Flechard C, Kuylenstierna J, Hicks K, Derwent D, Johnson C, Stevenson D (1999. a) The global exposure of forests to air pollutants. Water Air Soil Pollut 116: 5–32 [Google Scholar]
- Fowler D, Cape JN, Coyle M, Smith RI, Hjellbrekke AG, Simpson D, Derwent RG, Johnson CE (1999. b) Modeling photochemical oxidant formation, transport, deposition and exposure of terrestrial ecosystems. Environ Pollut 100: 43–55 [DOI] [PubMed] [Google Scholar]
- Fuhrer J, Skarby L, Ashmore MR (1997) Critical levels for ozone effects on vegetation in Europe. Environ Pollut 97: 91–106 [DOI] [PubMed] [Google Scholar]
- Gregg JW, Jones CG, Dawson TE (2003) Urbanization effects on tree growth in the vicinity of New York City. Nature 424: 183–187 [DOI] [PubMed] [Google Scholar]
- Jablonski LM, Wang XZ, Curtis PS (2002) Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol 156: 9–26 [Google Scholar]
- Keselman HJ, Algina J, Kowalchuk RK, Wolfinger RD (1998) A comparison of two approaches for selecting covariance structures in the analysis of repeated measurements. Commun Stat Simulat 27: 591–604 [Google Scholar]
- Kuehl RO (2000) Designs of experiments: statistical principles of research design and analysis, Ed 2. Duxbury Press at Brooks/Cole Publishing, Pacific Grove, CA
- Littell RC, Henry PR, Ammerman CB (1998) Statistical analysis of repeated measures data using SAS procedures. J Anim Sci 76: 1216–1231 [DOI] [PubMed] [Google Scholar]
- Littell RC, Pendergast J, Natarajan R (2000) Modeling covariance structure in the analysis of repeated measures data. Stat Med 19: 1793–1819 [DOI] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54: 2393–2401 [DOI] [PubMed] [Google Scholar]
- Long SP, Drake BG (1991) Effect of the long-term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3 sedge, Scirpus olneyi. Plant Physiol 96: 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Hällgren JE (1993) Measurement of CO2 assimilation by plants in the field and the laboratory. In DO Hall, JMO Scurlock, HR Bolhar-Nordenkampf, RC Leegood, SP Long, eds, Photsynthesis and Production in a Changing Environment: A Field and Laboratory Manual. Chapman & Hall, London, pp 129–167
- Long SP, Naidu SL (2002) Effects of oxidants at the biochemical, cell and physiological levels, with particular reference to ozone. In JNB Bell, M Treshow, eds, Air Pollution and Plants, Ed 2. J. Wiley, London, pp 69–88
- Long SP, Postl WF, Bolhar NHR (1993) Quantum yields for uptake of carbon-dioxide in C3 vascular plants of contrasting habitats and taxonomic groupings. Planta 189: 226–234 [Google Scholar]
- McKee IF, Farage PK, Long SP (1995) The interactive effects of elevated CO2 and O3 concentration on photosynthesis in spring wheat. Photosynth Res 45: 111–119 [DOI] [PubMed] [Google Scholar]
- McKee IF, Mulholland BJ, Craigon J, Black CR, Long SP (2000) Elevated concentrations of atmospheric CO2 protect against and compensate for O3 damage to photosynthetic tissues of field-grown wheat. New Phytol 146: 427–435 [Google Scholar]
- McLeod AR (1995) An open-air system for exposure of young forest trees to sulfur-dioxide and ozone. Plant Cell Environ 18: 215–225 [Google Scholar]
- McLeod AR, Long SP (1999) Free-air carbon dioxide enrichment (FACE) in global change research: a review. Adv Ecol Res 28: 1–55 [Google Scholar]
- Miglietta F, Peressotti A, Vaccari FP, Zaldei A, deAngelis P, Scarascia-Mugnozza G (2001) Free-air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytol 150: 465–476 [Google Scholar]
- Miller JE, Pursley WA, Vozzo SF, Heagle AS (1991) Response of net carbon exchange rate of soybean to ozone at different stages of growth and its relation to yield. J Environ Qual 20: 571–575 [Google Scholar]
- Mills G, Hayes F, Buse A, Reynolds B (2000) Air pollution and vegetation. In Annual Report 1999/2000 of UN/ECE ICP Vegetation. Center for Ecology and Hydrology, Bangor, ME
- Morgan PB, Ainsworth EA, Long SP (2003) Elevated O3 impact on soybeans, a meta-analysis of photosynthetic, biomass, and yield responses. Plant Cell Environ 26: 1317–1328 [Google Scholar]
- Mulchi CL, Slaughter L, Saleem M, Lee EH, Pausch R, Rowland R (1992) Growth and physiological characteristics of soybean in open-top chambers in response to ozone and increased atmospheric CO2. Agr Ecosyst Environ 38: 107–118 [Google Scholar]
- Noormets A, Sober A, Pell EJ, Dickson RE, Podila GK, Sober J, Isebrands JG, Karnosky DF (2001) Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (Populus tremuloides Michx.) clones exposed to elevated CO2 and/or O3. Plant Cell Environ 24: 327–336 [Google Scholar]
- Pell EJ, Eckardt NA, Glick RE (1994) Biochemical and molecular basis for impairment of photosynthetic potential. Photosynth Res 39: 453–462 [DOI] [PubMed] [Google Scholar]
- Pell EJ, Schlagnhaufer CD, Arteca RN (1997) Ozone-induced oxidative stress: mechanisms of action and reaction. Physiol Plant 100: 264–273 [Google Scholar]
- Prather M, Ehhalt D, Dentener F, Derwent R, Dlugokencky E, Holland E, Isaksen I, Katima J, Kirchhoff V, Matson P, et al (2001) Atmospheric chemistry and greenhouse gases. In JT Houghton, Y Ding, DJ Griggs, M Noguer, PJ van der Linder, X Dai, K Maskell, CA Johnson, eds, Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK pp 239–287
- Prather M, Gauss M, Berntsen T, Isaksen I, Sundet J, Bey I, Brasseur G, Dentener F, Derwent R, Stevenson D, et al (2003) Fresh air in the 21st century? Geophys Res Lett 30: 1100–1104 [Google Scholar]
- Reid CD, Fiscus EL (1998) Effects of elevated [CO2] and/or ozone on limitations to CO2 assimilation in soybean (Glycine max). J Exp Bot 49: 885–895 [Google Scholar]
- Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody OC, Heaton EA, Mahoney J, et al (2004) Leaf photosynthesis and carbohydrate dynamics of soybean grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant Cell Environ 28: 449–458 [Google Scholar]
- Rogers A, Humphries SW (2000) A mechanistic evaluation of photosynthetic acclimation at elevated CO2. Glob Change Biol 6: 1005–1011 [Google Scholar]
- Sage RF (1990) A model describing the regulation of ribulose-1,5-bisphosphate carboxylase, electron-transport, and triose phosphate use in response to light-intensity and CO2 in C3 plants. Plant Physiol 94: 1728–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF (1994) Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynth Res 39: 351–368 [DOI] [PubMed] [Google Scholar]
- Vozzo SF, Miller JE, Pursley WA, Heagle AS (1995) Atmospheric pollutants and trace gases – effects of ozone and water deficit on field-grown soybean. 1. Leaf gas exchange. J Environ Qual 24: 663–670 [Google Scholar]
- Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants – a retrospective analysis of the A/Ci curves from 109 species. J Exp Bot 44: 907–920 [Google Scholar]
- Zheng Y, Shimizu H, Barnes JD (2002) Limitations to CO2 assimilation in ozone-exposed leaves of Plantago major. New Phytol 155: 67–78 [DOI] [PubMed] [Google Scholar]