Abstract
Background
Recurrent infection with the hepatitis C virus (HCV) after liver transplantation is associated with decreased graft and patient survival. Achieving sustained virological response (SVR) with antiviral therapy improves survival. Because interferon-based therapy has limited efficacy and is poorly tolerated, there has been rapid transition to interferon-free direct-acting antiviral (DAA) regimens. Herein the experience with DAAs in the treatment of post-transplant genotype 1HCV from a consortium of community and academic centers (HCV TARGET) is described.
Methods
Twenty-one of the 54 centers contributing to the HCV TARGET consortium participated in this study. Enrollment criteria included positive post-transplant HCV RNA prior to treatment, HCV genotype 1, and documentation of use of a simeprevir/sofosbuvir (SMV/SOF) containing DAA regimen. Safety and efficacy were assessed. SVR was defined as undetectable HCV RNA 64 days or later after cessation of treatment.
Results
A total of 162 patients enrolled in HCV-TARGET started treatment with SMV/SOF with or without ribavirin following liver transplantation. The study population included 151 patients treated with these regimens for whom outcomes and safety data were available. The majority of the 151 patients were treated with sofosbuvir and simeprevir alone (n=119, 78%) or with ribavirin (n=32, 22%), The duration of therapy was 12 weeks for most patients, although 15 patients received 24 weeks of treatment. Of all patients receiving SOF/SIM +/− Ribavirin, 133/151 (88%) achieved SVR12 and 11 relapsed (7%). One patient had virologic breakthrough (n=1) and 6 patients were lost to post treatment follow up. Serious adverse events occurred in 12%; 3 patients (all cirrhotic) died due to aspiration pneumonia, suicide, and multi-organ failure. One experienced liver transplant rejection.
Conclusions
Interferon-free DAA treatment represents a major improvement over prior interferon-based therapy. Broader application of these and other emerging DAA regimens in the treatment of post-transplant hepatitis C is warranted.
INTRODUCTION
Recurrent hepatitis C virus (HCV) is the leading cause of graft loss and death in HCV-infected liver transplant (LT) recipients (1, 2). Achieving sustained virological response (SVR) with antiviral therapy improves post-LT survival (3–7), and may lead to stabilization or improvement in histology (8–15) and diminished risk of hepatic decompensation (4, 12).
Until very recently, interferon-based therapy was the only treatment option and rates of SVR in post-transplant patients with HCV GT1 infection were only 20 to 30%. Addition of either telaprevir or boceprevir doubled rates of SVR, but was associated with significant drug-drug interactions, profound anemia, rash, intolerability, complex management, and excessive resource utilization (3, 10, 13, 16–20). The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) approvals of sofosbuvir (SOF) and simeprevir (SMV) in 2013 heralded a new era in DAA therapy of HCV-related liver disease. The first, FDA-approved interferon-free regimen, SOF/RBV for HCV genotypes 2 and 3, was introduced and this combination was immediately tested in liver recipients with recurrent hepatitis C. The rate of SVR was 70%. At about the same time a Phase 2 study, COSMOS, achieved rates of SVR from 93% to 100% using SMV/SOF, even in treatment experienced patients with cirrhosis. Although only small numbers of patients were studied and SOF and SMV were initially FDA and EMA approved in combination with PEG/RBV for GT1, the COSMOS data influenced the American Association for the Study of Liver Diseases/Infectious Diseases Society of America (AASLD/IDSA) and the European Association for the Study of the Liver (EASL) to recommend SMV/SOF in combination for the treatment of patients with post-transplant recurrence of GT1 HCV. The FDA later approved the combination of SMV/SOF on November 6, 2014 for treatment of GT1 non-transplant patients. As a result, transplant physicians quickly adopted the use of not only SOF/RBV but also the SMV/SOF combination (21). Limited data with both SOF/ledipasvir as well as the combination of ombitasvir/paritaprevir/ritonavir and dasabuvir in post-transplant trials indicate high efficacy and tolerability of all oral regimens (22–24). However, real-world evaluation of the efficacy and safety of all-oral therapy for post-transplant patients is limited.
HCV-TARGET is a multicenter consortium of both academic and community liver centers that collect post-approval prospective treatment data on SVR rates and other clinical outcomes of new HCV regimens. Herein, we describe the safety and efficacy of simeprevir (SMV) and sofosbuvir (SOF) in post-LT patients infected with HCV genotype 1 from 21 transplant centers. This experience represents the most comprehensive real-world study in post-LT patients to date.
MATERIALS AND METHODS
Study population and design
HCV-TARGET is a longitudinal, observational study in chronic hepatitis C patients from a consortium of academic (n=39) and community (n=15) medical centers. Since 2011, consented patients prescribed HCV treatment as part of routine clinical practice at one of the participating medical centers have been enrolled in HCV-TARGET. The cohort of patients included in this analysis were liver transplant (LT) patients enrolled at 21 participating medical centers which were also transplant centers. The patients in the current study were infected with HCV genotype 1, were 18 or more years old, and started treatment with SMV+SOF with or without RBV prior to 10 October 2014.
Treatments
Treatment was chosen and administered per local standards at the study sites; the study protocol did not define specific treatment populations, regimens, dosing, duration, or safety management guidelines. The decision to initiate HCV treatment and the selection of the HCV treatment regimen (with or without ribavirin) was solely the responsibility of the treating clinician and his or her patient; this was a non-random process in which a regimen was selected for an individual patient.
Measurements
Data was captured from sequentially enrolled patients using a common database that utilized novel, standardized source data abstraction previously described (25). In brief, a centralized team of trained coders reviewed all redacted medical records obtained from participating sites for data entry. Throughout treatment and during post-treatment follow-up, demographic, clinical, adverse event, and virologic data were collected. The assay used for HCV RNA quantification/detection was specific to the treatment center. Independent data monitors systematically reviewed the data entries for completeness and accuracy. All records were screened for extreme or unlikely values and verified/resolved with additional queries. The choice of and management of immunosuppression and graft rejection was also at the discretion of the investigators. Pre- and end-treatment calcineurin inhibitor doses were recorded in addition to the use of mammalian target of rapamycin (mTOR) inhibitors, mycophenolate mofetil/mycophenolic acid and steroids. This study was approved by the local Institutional Review Boards at all participating sites. Participants provided written informed consent according to local IRB policies.
Primary Outcomes
The primary endpoint was sustained virological response 12 weeks post-therapy (SVR12) defined as HCV RNA below level of quantitation or undetected at least 64 days after treatment was discontinued. HCV RNA levels were measured according to local practice, usually prior to treatment initiation at weeks 4, 8, and at the end of treatment and 4 and 12 weeks after treatment discontinuation.
Secondary endpoints included rates of relapse, treatment discontinuation, and safety including episodes of rejection, rates of anemia, renal dysfunction, hepatic decompensation, and death. The efficacy and safety cohorts included all patients who received HCV therapy and for whom an outcome (SVR, virological failure, lost to follow-up) was recorded.
Definitions
Cirrhosis
The presence of cirrhosis was defined by biopsy and/or a combination of clinical, laboratory, and imaging criteria established a priori (27). Patients were determined to have cirrhosis if they had: 1) evidence of stage 4 fibrosis by liver biopsy at any time prior to therapy, or 2) evidence of stage 3 fibrosis by liver biopsy at any time prior to therapy plus any of the following criteria: platelets count <140,000 per µl, presence of esophageal varices on esophagogastroduodenoscopy, evidence of cirrhosis and/or portal hypertension and/or of ascites by imaging studies, FibroSure® or Fibrotest®, transient elastography, or equivalent compatible with stage 4 fibrosis, or 3) in the absence of liver biopsy, any two of the following criteria: platelets count <140,000 per µl, presence of esophageal varices on esophagogastroduodenoscopy, evidence of cirrhosis and/or portal hypertension and/or ascites by imaging studies, FibroSure® or Fibrotest®, transient elastography, or equivalent compatible with stage 4 fibrosis.
Adverse events (AE)
1) Any event that occurred on treatment was collected and reported regardless of the need or lack thereof for a prescription medication or a dose reduction or discontinuation of HCV treatment.
Anemia
Defined as the presence of at least one of the following: 1) Adverse Event of Anemia as reported by investigator; 2) administration of hematologic growth factors was documented; or 3) transfusion occurred.
Hepatic decompensation during therapy
Patient experienced one or more of the following AEs: 1) hepatic decompensation was listed as an AE by the healthcare practitioner; 2) new onset of hepatic encephalopathy; 3) new onset spontaneous bacterial peritonitis; 4) new onset variceal hemorrhage; 5) new onset ascites; 6) new onset hepatic hydrothorax or 7) patient received a new prescription for a medication to treat one of the above indications (1–5).
Serious adverse event (SAE)
An AE that required hospitalization or met criteria for expedited reporting per FDA form MEDWATCH 3500.
Statistical analyses
The unadjusted rate of SVR, relapse, treatment completion and frequency of AEs were calculated for the entire study population and for sub-populations. Confidence intervals of unadjusted rates were calculated using exact binomial methods. Measures of association between baseline covariates and SVR where estimated with conditional logistic regression models stratified on regimen. The set of baseline covariates were selected a priori based upon a consensus of clinical expertise. Relative risk estimates of SVR (SOF + SMV to SOF + SMV + RBV) were calculated using Mantel–Haenszel methods. Multiple imputation methods were used to account for missing data in both the conditional logistic regression models and the relative risk calculations. Analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, North Carolina) and R 3.0.2 (R Core Team, Vienna, Austria).
RESULTS
Patient Characteristics
A total of 151 post-LT patients with genotype 1 met the inclusion criteria. Baseline characteristics for all 151 patients are shown in Table 1. The mean age for all participants was 61 years (range 46–78 years) and 74% were male. Seventy six percent of the patients were Caucasian, 9% African American, 3% Asian and 13% were of other race or unreported. 15% were of Hispanic ethnicity. Mean total bilirubin was 1.6 mg/dl (range 0.3–21.0 mg/dl) with mean serum albumin of 3.8 g/dl (range 2.0–4.9 g/dl). Mean platelet count was 140,000µl (Range: 44,000 to 460,000/µl). The majority of patients had HCV genotype 1a (58%), were treatment-experienced (56%), and 7% had failed prior treatment with either telaprevir- or boceprevir-based therapy, either prior to or following transplant (timing of treatment was not collected). Allograft cirrhosis had developed in 64% of the patients (14 were biopsy proven) and 58% of the cirrhotic patients had evidence of clinical decompensation (Table 1). Child-Pugh score was not collected due to the subjective nature of ascites and encephalopathy grading and reporting.
Table 1.
Baseline characteristics of patients
| Characteristic | SMV/SOF (n=119) |
SMV/SOF/RBV (n=32) |
Total (n=151) |
|---|---|---|---|
| Mean age (range), y | 62 (49–78) | 60 (46–71) | 61 (46–78) |
| Male sex, n (%) | 86 (72.3) | 26 (81.3) | 112 (74.2) |
| Race, n (%) | |||
| White | 87 (73.1) | 27 (84.4) | 114 (75.5) |
| Black | 12 (10.1) | 2 (6.3) | 14 (9.3) |
| Asian | 3 (2.5) | 1 (3.1) | 4 (2.6) |
| Other/missing | 17 (14) | 2 (6) | 19 (13) |
| Ethnicity Hispanic,1 n (%) | 19 (16.0) | 3 (9.4) | 22 (14.6) |
| Mean years since LT (range) | 5 (0–23) | 4 (0–14) | 5 (0–23) |
| Mean ALT (range), mean IU/l | 107 (10–733) | 118 (26–461) | 109 (10–733) |
| Mean total bilirubin (range), mg/dl | 1.3 (0.3–21.0) | 2.5 (0.3–14.0) | 1.6 (0.3–21.0) |
| Mean albumin (range), g/dl | 3.8 (2.2–4.9) | 3.5 (2.0–4.3) | 3.8 (2.0–4.9) |
| Mean hemoglobin (range), g/dl | 13.3 (8.4–17.7) | 12.6 (7.9–16.7) | 13.2 (7.9–17.7) |
| Mean platelet count (range) (×103) per µl | 137 (44–460) | 151 (54–245) | 140 (44–460) |
| History of cirrhosis, n (%) | 74 (62.2) | 23 (71.9) | 97 (64.2) |
| History of liver decompensation, n (%) | 46 (38.7) | 14 (43.8) | 60 (39.7) |
| Presence of diabetes, n (%) | 48 (40.3) | 18 (56.3) | 66 (43.7) |
| HCV genotype, n (%) | |||
| 1a | 67 (56.3) | 20 (62.5) | 87 (57.6) |
| 1b | 32 (26.9) | 10 (31.3) | 42 (27.8) |
| 1, subtype unspecified | 20 (16.8) | 2 (6.3) | 22 (14.6) |
| Prior HCV treatment, n (%) | |||
| Treatment naïve | 57 (47.9) | 9 (28.1) | 66 (43.7) |
| Prior PI failure | 8 (6.7) | 3 (9.4) | 11 (7.3) |
| Immunosuppression, n (%) | |||
| Tacrolimus | 98 (82.4) | 24 (75.0) | 122 (80.8) |
| Cyclosporine | 15 (12.6) | 1 (3.1) | 16 (10.6) |
| Everolimus/ Sirolimus | 12 (10.1) | 11 (34.4) | 23 (15.2) |
| MMF/MPA | 51 (42.9) | 19 (59.4) | 70 (46.4) |
ALT, alanine aminotransferase; HCV, hepatitis C virus, MMF: mycophenolate mofetil; MPA: mycophenolic acid
Antiviral Regimen
The treatment duration was 12 weeks for the majority of the cohort, however 15/151 (10%) patients received 24 weeks. Only 5 patients (3%) had early treatment discontinuation, with one patient stopping for lack of efficacy, 4 for adverse events and/or death. Ribavirin dosing was per center preference with a median starting dose of 800 mg/day.
Treatment response
Of 151 patients in the current analysis, 119 (78%) were treated with SMV+SOF without RBV and 32 (22%) were treated with SMV+SOF+RBV (Table 2). SVR12 was achieved by 88% (133/151) of those treated with SMV/SOF + RBV. SVR12 results for subgroups of patients are shown in Table 3. Patients with genotype 1a treated with SMV/SOF achieved SVR12 in 57/67 (85%) compared to 30/32 (94%) of those with genotype 1b. In general, patients with cirrhosis had numerically lower rates of SVR compared to non-cirrhotic patients (Table 3). Because post-transplant patients may have low platelet counts for reasons other than cirrhosis, the unadjusted SVR was calculated excluding 19 subjects who would be reclassified if low platelet count was not a criteria for determining cirrhosis and there were no substantial differences (data not shown). Treatment failures were mostly due to relapse (7%) although viral breakthrough did occur in one patient.
Table 2.
Patient disposition
| Disposition, n (%) | SMV/SOF (n=119) |
SMV/SOF/RBV (n=32) |
Total (n=151) |
|---|---|---|---|
| Completed therapy | 115 (96.6) | 31 (96.9) | 146 (96.7) |
| Discontinued earlya | 4 (3.4)b | 1 (3.1)c | 5 (3.3) |
| Adverse event | 3 (2.5) | 1 (3.1) | 4 (2.6) |
| Lack of efficacy | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Lost to post treatment follow-upd | 4 (3.4) | 2 (6.2) | 6 (4.0) |
Includes 3 patients who died
Cause of death: Multiorgan failure, aspiration pneumonia
Cause of death: Suicide
Lost to follow-up were included in SVR12 assessment and counted as treatment failures
Table 3.
Crude SVR12 among patients treated with SOF+SMV or SOF+SMV+RBV.
| Population | SOF+SMV | SOF+SMV+RBV | ALL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N | SVR12 | 95% CI | n/N | SVR12 | 95% CI | n/N | SVR12 | 95% CI | ||
| Overall | 105/119 | 88% | (81, 93) | 28/32 | 88% | (71, 96) | 133/151 | 88% | (82, 93) | |
| Tx Experience | Naïve | 51/57 | 89% | (78, 96) | 9/9 | 100% | (66, 100) | 60/66 | 91% | (81, 97) |
| Experienced | 54/62 | 87% | (76, 94) | 19/23 | 83% | (61, 95) | 73/85 | 86% | (77, 92) | |
| Prior PI Failure | 4/8 | 50% | (16, 84) | 3/3 | 100% | (29, 100) | 7/11 | 64% | (31, 89) | |
| Genotype | 1a | 57/67 | 85% | (74, 93) | 17/20 | 85% | (62, 97) | 74/87 | 85% | (76, 92) |
| 1b | 30/32 | 94% | (79, 99) | 9/10 | 90% | (55, 100) | 39/42 | 93% | (81, 99) | |
| Cirrhotic | Yes | 63/74 | 85% | (75, 92) | 20/23 | 87% | (66, 97) | 83/97 | 86% | (77, 92) |
| No | 42/45 | 93% | (82, 99) | 8/9 | 89% | (52, 100) | 50/54 | 93% | (82, 98) | |
| MELD | < 10 | 19/20 | 95% | (75, 100) | 2/3 | 67% | (9, 99) | 21/23 | 91% | (72, 99) |
| ≥ 10 | 6/10 | 60% | (26, 88) | 4/5 | 80% | (28, 99) | 10/15 | 67% | (38, 88) | |
| Genotype: ALL | Cirrhotic Experienced | 33/38 | 87% | (72, 96) | 15/18 | 83% | (59, 96) | 48/56 | 86% | (74, 94) |
| Non-cirrhotic Experienced | 21/24 | 88% | (68, 97) | 4/5 | 80% | (28, 99) | 25/29 | 86% | (68, 96) | |
| Cirrhotic Naive | 30/36 | 83% | (67, 94) | 5/5 | 100% | (48, 100) | 35/41 | 85% | (71, 94) | |
| Non-cirrhotic Naive | 21/21 | 100% | (84, 100) | 4/4 | 100% | (40, 100) | 25/25 | 100% | (86, 100) | |
| Genotype: 1a | Cirrhotic Experienced | 18/22 | 82% | (60, 95) | 9/11 | 82% | (48, 98) | 27/33 | 82% | (65, 93) |
| Non-cirrhotic Experienced | 9/11 | 82% | (48, 98) | 2/3 | 67% | (9, 99) | 11/14 | 79% | (49, 95) | |
| Cirrhotic Naive | 16/20 | 80% | (56, 94) | 4/4 | 100% | (40, 100) | 20/24 | 83% | (63, 95) | |
| Non-cirrhotic Naive | 14/14 | 100% | (77, 100) | 2/2 | 100% | (16, 100) | 16/16 | 100% | (79, 100) | |
| Genotype: 1b | Cirrhotic Experienced | 10/10 | 100% | (69, 100) | 5/6 | 83% | (36, 100) | 15/16 | 94% | (70, 100) |
| Non-cirrhotic Experienced | 8/8 | 100% | (63, 100) | 1/1 | 100% | (3, 100) | 9/9 | 100% | (66, 100) | |
| Cirrhotic Naive | 7/9 | 78% | (40, 97) | 1/1 | 100% | (3, 100) | 8/10 | 80% | (44, 97) | |
| Non-cirrhotic Naive | 5/5 | 100% | (48, 100) | 2/2 | 100% | (16, 100) | 7/7 | 100% | (59, 100) | |
Predictors of Treatment Response
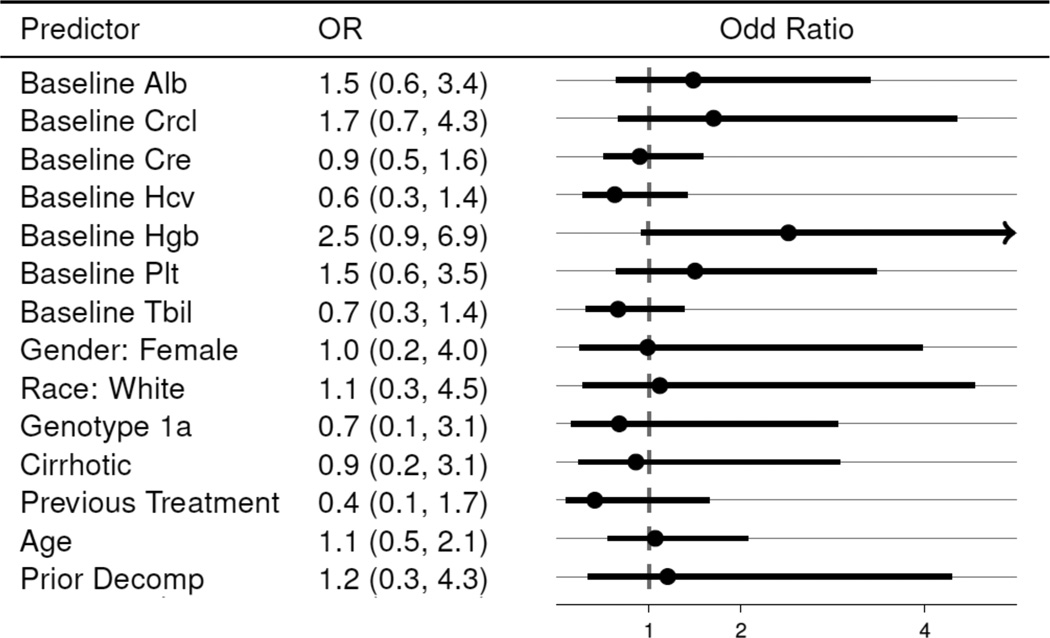
Multivariable models were limited to the 129 patients that (a) were treated with SMV/SOF ± RBV for a duration of 14 or fewer weeks (to capture those with intended duration of therapy of 12 weeks) and (b) completed treatment or discontinued due to virological failure. Conditional logistic regression models of SVR12 stratified by regimen did not identify any factors with statistically significant association with SVR12. While not reaching the level of statistical significance, the estimated association between SVR12 and some factors such as higher levels of baseline hemoglobin (OR 2.5, 95% CI 0.9–6.9 suggest possible correlation with higher rates of SVR (Figure 1).
Figure 1.
Odds Ratio Estimates of SVR12 Controlling for Regimen Choice. (Excludes non-virological failures.)
Odds ratios are calculated from conditional logistic regression models stratified on regimen. Each line represents an estimate from a distinct model in which SVR is the outcome, the predictor is the covariate, and regimen is the stratum.
Comparative Effectiveness of SMV/SOF + RBV and SMV/SOF
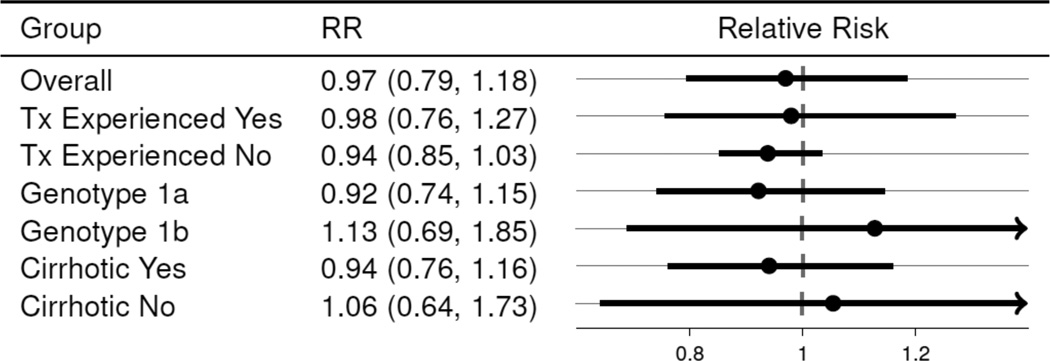
The sample size constraints prohibit a comparative effectiveness analysis that controls for all factors that affect regimen choice. As such, the comparative effectiveness estimates in the 129 patients who (a) were treated with SMV/SOF with and without RBV for a duration of 14 or fewer weeks and (b) completed treatment or discontinued due to virological failure are reported. Further, an adjustment for previous treatment status was made because of its strong association with regimen choice. Overall, the estimated SVR rate was very similar between the two regimen choices when controlling for previous treatment experience. The probability of SVR in SMV+SOF was 0.97 (95% CI: 0.79, 1.18) that of SMV+SOF+RBV. The relative risk (RR) estimates comparing SMV+SOF+RBV to SMV+SOF in various subgroups are reported in Figure 2; the estimates of RR when controlling for previous treatment indicate that the addition of RBV had no detectable impact on SVR.
Figure 2.
Relative Risk of SVR12 (SOF+SMV+RBV reference). (Excludes non-virological failures.)
Relative risks are Mantel-Haenszel estimates stratified on previous treatment (experienced v. naive). Previous treatment is a predictor of regimen choice (SOF+SMV v. SOF+SMV+RBV) among patients which completed treatment or discontinued due to virological reasons. (Relative risk estimates for Tx experienced and Tx Naive are not stratified.)
Safety
The most common adverse event was fatigue (25%), followed by headache (19%), any infection (15%), rash/pruritus (14%), influenza like illness (12%), nausea/vomiting (11%), and anemia (11%); (Table 4). The mean hemoglobin at treatment initiation was 13.0 g/dL (range 7.9 – 17.7). Anemia was reported as an AE in 11% of the total patients, which varied dramatically by treatment regimen: 38% of SMV/SOF plus RBV, and 3% of SMV/SOF treated patients. Only 1 SAE for anemia was reported (SMV/SOF). The mean change in hemoglobin in the SMV+SOF+RBV group was −1.2 g/dL, with −0.1 g/dL mean change reported in the SMV/SOF group (Table 5). The use of erythropoietin and transfusion for anemia was low at 3% for both. RBV dose reduction was required in 8 (5%) patients, and RBV discontinuation was only necessary in 1 patient (Table 5).
Table 4.
Patient safety profile
| SMV/SOF (n=119) |
SMV/SOF/RBV (n=32) |
Total (n=151) |
|
|---|---|---|---|
| Most common Adverse Events, n (%) | |||
| Fatigue | 31 (26.1) | 7 (21.9) | 38 (25.2) |
| Headache | 20 (16.8) | 8 (25.0) | 28 (18.5) |
| Infections and infestations | 20 (16.8) | 2 (6.2) | 22 (14.6) |
| Rash/Pruritus | 17 (14.3) | 4 (12.5) | 21 (13.9) |
| Influenza like illness | 12 (10.1) | 6 (18.8) | 18 (11.9) |
| Nausea/Vomiting | 13 (10.9) | 4 (12.5) | 17 (11.3) |
| Anemia | 4 (3.4) | 12 (37.5) | 16 (10.6) |
| Total patients with reported AEs | 86 (72.3) | 28 (87.5) | 114 (75.5) |
| All Serious Adverse Events, n (%) | |||
| Renal failure acute | 3 (2.5) | 1 (3.1) | 4 (2.7) |
| Abdominal pain upper | 2 (1.7) | 0 (0.0) | 2 (1.3) |
| Infections | 2 (1.7) | 0 (0.0) | 2 (1.3) |
| Hepatic decompensation | 1 (0.8) | 1 (3.1) | 2 (1.3) |
| Anemia | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Hepatic arteriovenous malformation | 0 (0.0) | 1 (3.1) | 1 (0.7) |
| Nausea | 0 (0.0) | 1 (3.1) | 1 (0.7) |
| Esophageal stenosis | 0 (0.0) | 1 (3.1) | 1 (0.7) |
| Edema | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Pyrexia | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Cholangiolitis | 0 (0.0) | 1 (3.1) | 1 (0.7) |
| Hepatic enzyme increased | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Dehydration | 0 (0.0) | 1 (3.1) | 1 (0.7) |
| Hyperglycemia | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Hypervolemia | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Suicide | 0 (0.0) | 1 (3.1) | 1 (0.7) |
| Pneumonia aspiration | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Pulmonary embolism | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Hematoma | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Total patients with reported SAEs | 12 (10.1) | 6 (18.8) | 18 (11.9) |
| Mean change in laboratory values, baseline to end of treatment | |||
| Creatinine change, mean (range) | 0.01 (−0.84,+0.68) | −0.06 (−0.85,+0.33) | −0.01 (−0.85,+0.68) |
| Total Bilirubin change, mean (range) | −0.2 (−5.3,+2.5) | −1.2 (−8.0,+0.9) | −0.4 (−8.0,+2.5) |
| Immunosuppression dose change, mean (range) | |||
| TAC | 0 (−6,+2) | 0 (−2,+4) | 0 (−6,+4) |
| CSA | −18 (−275,+125) | −17 (−275,+125) | |
| Everolimus/ Sirolimus | 0 (−2,+0.5) | 0 (−1, +2) | 0 (−2,+2) |
| MMF/MPA | −20 (−1000,+1000) | 104 (0–750) | 14 (−1000,+1000) |
Table 5.
Anemia management
| SMV/SOF (n=119) |
SMV/SOF/RBV (n=32) |
Total (n=151) |
|
|---|---|---|---|
| AE Anemia, n (%) | 4 (3.4) | 13 (40.6) | 17 (11.3) |
| SAE Anemia, n (%) | 1 (0.8) | 0 (0.0) | 1 (0.7) |
| Hemoglobin change, delta mean (range) | −0.1 (−4.9,+3.1) | −1.2 (−6.9,+4.1) | −0.4 (−6.9,+4.1) |
| Anemia management, n (%) | |||
| Ribavirin dose reduction/interruption | 0 (0.0) | 8 (25.0) | 8 (5.3) |
| Epoietin use | 0 (0.0) | 5 (15.6) | 5 (3.3) |
| Blood transfusion | 2 (1.7) | 3 (9.4) | 5 (3.3) |
| Ribavirin discontinuation | 0 (0.0) | 1 (3.1) | 1 (0.7) |
Hepatic decompensation occurred in 8 patients. All had known cirrhosis at baseline and 5 had a baseline MELD of 10 or greater (of 8 patients with known baseline MELD). Three patients died, 2 of which had cirrhosis (pre-treatment MELD of 8 and 26). Deaths were attributed to hepatic and renal failure in one, suicide in another (occurred during treatment) and aspiration pneumonia (occurred post-treatment) in third patient- all with cirrhosis. Renal failure and aspiration pneumonia were indicated as being related to treatment by the treating physician. There was only one episode of acute rejection reported. Acute kidney injury or failure occurred in 3 patients taking SMV+SOF+RBV, and in 8 patients taking SMV+SOF. Overall mean creatinine change was small, 0.0 mg/dL (range −0.9 to 0.7 mg/dL). Mean total bilirubin changes were small −0.4 mg/dL, though there was a broad range −8.0 to 2.5 mg/dL. In the 16 patients treated with cyclosporine on SMV/SOF the mean increase in creatinine was −0.01 mg/dL, with a range from −0.84 to 0.31 mg/dL.
Immunosuppression
Of 151 patients, 137 (91%) patients were on calcineurin inhibitors [121 tacrolimus, 15 cyclosporine and one received both] and 23 (15%) on mTOR inhibitors at the start of antiviral therapy. In addition, 70 patients (46%) were on mycophenolate mofetil or mycophenolic acid. One patient was on maintenance steroids (not shown in table). Changes in immunosuppression dosages on average were small, with delta mean changes in tacrolimus, cyclosporine, and everolimus/sirolimus of respectively 0 mg, −17 mg, and 0 mg (Table 4). There were no safety signals for the concomitant use of cyclosporine and simeprevir, though the number of patients is small.
DISCUSSION
Patients with recurrent HCV after liver transplantation have the most urgent need for effective antiviral therapy due to their potential for rapidly progressive fibrosis, graft loss, and death. However, treatment with interferon-based regimens has been difficult due to safety concerns and inadequate efficacy. Potent, well-tolerated, IFN-free therapy promises to greatly improve efficacy and expand treatment options for these patients in great need for cure of their HCV infection. Preliminary reports from phase 2 clinical trials suggest that all-oral regimens are effective in liver transplant recipients (23, 26). The present study reports the safety and efficacy of interferon-free all oral treatment in a real world cohort of post-LT patients with recurrent genotype 1 HCV, many of whom had more severe disease than in the clinical trials or other reported studies and had failed prior PI based triple therapy regimens.
The combination of sofosbuvir and simeprevir for the treatment of HCV recurrence after liver transplantation is attractive due to the low potential for drug-drug interactions (27, 28). In our study, there did not appear to be any safety consideration for the use of simeprevir with either cyclosporine or decompensated cirrhosis, though the small numbers limit conclusions and in the absence of a control group it is impossible to exclude a potential drug effect in the patients who had further hepatic decompensation on therapy. Nevertheless, it should be noted that the use of simeprevir is not recommended in patients with decompensated cirrhosis (CP-B and C cirrhosis) (29).
SVR12 was achieved in 88% of this study cohort. This is substantially higher than the rates reported for peginterferon and RBV dual therapy or protease inhibitor-based triple therapy (19, 20, 30). Additionally the safety profile was excellent with a low rate of AE and SAE and extremely low rate of treatment discontinuation (<5%). This is similar to the rates of SVR seen in the Mayo Clinic experience with SOF/SMV of 90% in 105 patients and the University of Miami experience, which achieved SVR12 of 93% in 61 patients (31–33). It is important to note that our series included a more advanced population with a higher rate of cirrhosis (64% versus 30% or less in those 2 studies) with more patients with decompensated cirrhosis). We did see one episode of rejection with IFN-free therapy, previously a concern with IFN-based therapy (34).
Of the baseline covariates selected a priori as potential predictors of SVR12, none exhibited a statistically significant association with the outcome. This is likely a result of high observed SVR rates that requires a larger sample to detect differences. However, among many of the potential predictors, the direction of the estimated association is consistent with prior expectations. As one example, the estimated association of SVR12 and cirrhosis status indicates that non-cirrhotic patients have higher SVR rates than their cirrhotic counterparts. Unfortunately Q80K mutation data was only available for 19 patients (not shown in table) and RAV data was not collected, so the impact of Q80K or baseline RAV’s on treatment response could not be assessed, however the response rate was higher in GT 1b than GT1a patients (93% vs 85%), similar to what was seen in the single center studies, neither of which collected data on Q80K mutations (31–33).
The current study also represents the largest report of safety and efficacy in a diverse population of real-world patients treated with all-oral therapy post-LT outside of a tightly controlled clinical trial. Several smaller studies of post-LT patients treated with dual combinations of sofosbuvir, a NS5A replication complex inhibitor (ledipasvir or daclatasvir) and simeprevir, have been reported (23, 35, 36). These studies used 12 or 24 weeks of therapy and included RBV. In the present study patients treated with or without RBV had similar rates of SVR. Of note, recent FDA approval of SMV/SOF for treatment of genotype 1 HCV was for 24 weeks in patients with cirrhosis, although too few patients in our study were treated for 24 weeks to determine if extended duration therapy improved outcome. Higher rates of relapse in advanced fibrosis patients (F3 and F4) were also seen with 12 weeks of SMV+SOF+RBV in other studies (31, 32). It is anticipated that, pre-emptive or early post-LT HCV therapy will lead to most patients being treated before they develop advanced fibrosis/cirrhosis and thus obviate the future need for extended duration of therapy.
Serious adverse events occurred in 12% of the present cohort, and included anemia, renal dysfunction, liver decompensation and death. Much of this may represent progression of disease despite antiviral therapy as all treated patients were included and there were no exclusions based on severity of disease. This has been seen in the compassionate use programs for SOF and daclatasvir (22, 35). However, whether clinical decompensation can occur with antiviral clearance in the absence of peginterferon remains to be determined.
In a phase 2 open-label study of LT recipients who received SOF+RBV for 24 weeks, 100% of patients achieved RVR and EOTR, while only 70% achieved SVR12 (26). This study included patients with genotypes 2–4, and 40% had recurrent cirrhosis but no patients had evidence of hepatic decompensation. In the SOF compassionate use program, patients with either severe early recurrence within the first year or recurrent cirrhosis were treated with 24–48 weeks of SOF/RBV (22). Of the patients who did not undergo retransplantation (n=12), SVR rate was 59%, although it was higher (73%) in the 48 patients with severe cholestatic hepatitis. Fortunately, the majority (57%) experienced clinical improvement, but SAE’s occurred in 47%; 18% had hepatic decompensation and 13% died.
Another all oral regimen, ombitasvir/paritaprevir/ritonavir plus dasabuvir plus RBV for 24 weeks, was studied in a Phase 2 open-label study in stable noncirrhotic (F0-F2) post-LT patients >1 year after transplant (23). The regimen was extremely effective with a SVR rate of 97% in 34 patients with a low rate of adverse events other than RBV induced anemia. However, the significant drug-drug interactions seen secondary to ritonavir boosting required close monitoring and adjustment of tacrolimus and cyclosporine levels. In addition, efficacy and safety was not established for shorter duration therapy, more advanced fibrosis/cirrhosis, or in a real world setting. Another all oral regimen under study is simeprevir in combination with daclatasvir and ribavirin. This study is limited to patients on tacrolimus-based immunosuppression due to the known drug interaction between simeprevir and cyclosporine. Preliminary results indicate a 90–93% SVR4 rate and good tolerability, though SVR 12 is not yet available and the number of patients with advanced liver disease (F3–4) is limited (37).
SOF/LDV plus RBV has been studied in the post-LT population across a broader range of liver disease severity including cirrhosis and decompensation (36). Patients with earlier stage disease and those with cirrhosis had similar SVR12 rates of 96–98% regardless of treatment duration (12 vs. 24 weeks). SVR12 decreased to 83–85% in Child class B cirrhotic patients and 60–67% in Child class C cirrhosis, although the number of patients in the latter group was very small. All regimens studied to date used RBV in post-LT patients and the development of anemia was more profound and frequent compared to the non-transplant setting.
Although our study benefits from its large, multicenter, prospective, real world experience, it has some methodological limitations. The inclusion and exclusion of patients and treatment regimen chosen was not standardized. However the safety profile in this real world cohort, where there were expanded indications of advanced liver disease, was excellent and coupled to high efficacy. The immunosuppression and HCV treatment regimens also varied between patients and centers likely leading to heterogeneity, which is compounded by a relatively small sample size in the subgroups; yet there were no significant issues with rejection. Rare renal events potentially related to HCV therapy were reported but are difficult to interpret in the absence of an untreated control group. In this study cohort, 137 patients (91%) had been treated with a calcineurin inhibitor (CNI) of tacrolimus (122, 80%) or cyclosporine (16, 10%). (One patient recorded treatment with both) The SVR rate among patients who received tacrolimus was 91% while the rate among patients receiving cyclosporine was 80%. Because the selection of a CNI drug is influenced by factors that may also influence SVR, we do not provide a statistical comparison of the SVR rates in the two groups as such a comparison may be biased by treatment choice. Only 16 patients were treated with cyclosporine, and given the 4.81-fold increase in SMV levels expected in cyclosporine treated patients, this combination remains not recommended (38). Nevertheless, in aggregate this represents a real world experience in the treatment of diverse post-transplant patients including those with decompensated cirrhosis, and this heterogeneity enhances the study’s generalizability. Finally, follow up for the patients was generally limited to SVR12, thus, the long-term benefits of SVR such as histological stabilization or regression of fibrosis, prevention of hepatic decompensation and improved survival remains under investigation.
In summary, substantially higher SVR12 rates are achieved with SMV/SOF-based anti-HCV therapy post-LT than previously reported for IFN-based therapy. While the optimal choices of all oral DAA regimens for HCV treatment post-LT is likely to rapidly evolve in the coming years, SMV/SOF is an effective, well tolerated, all-oral and potentially RBV-free choice for recurrent HCV with minimal drug-drug interactions with common immunosuppressive agents. As more clinical trials with safe, all oral, short courses of DAA are performed in transplant patients, all post-LT patients will likely be treated either pre-, peri-, or shortly after liver transplantation.
Acknowledgments
Funding:
HCV-TARGET is an investigator-initiated study jointly sponsored by The University of Florida, Gainesville, FL (PI: Nelson), and The University of North Carolina at Chapel Hill, Chapel Hill, NC (PI: Fried). It was funded in part by AbbVie, BristolMyers Squibb, Gilead, Janssen, Kadmon, Merck, and Vertex. Funded in part by CTSA UF UL1TR000064 and UNC 1UL1TR001111. Dr. Fried is funded in part by NIH Mid-Career Mentoring Award K24 DK066144.
Abbreviations
- CNI
calcineurin inhibitor
- CTP
Child-Turcotte–Pugh
- EOTR
end of treatment response
- eRVR
extended rapid virologic response
- GCSF
granulocyte colony stimulating factor
- HCV
Hepatitis C virus
- LT
liver transplantation
- mTOR
mammalian target of rapamycin
- MMF
mycophenolate mofetil
- MPA
mycophenolate acid
- NPV
negative predictive value
- P-IFN
pegylated interferon
- PPV
positive predictive value
- PI
protease inhibitor
- RVR
rapid virologic response
- SVR
sustained virologic response
Footnotes
Conflicts of Interest:
Robert S. Brown, Jr., M.D., M.P.H.: Consulting, grant support from Gilead, Abbvie, Janssen
Jacqueline G. O’Leary M.D. M. P.H: Gilead, Abbvie, Jansen, Novartis, Astellas
K. Rajender Reddy M.D: Ad-Hoc Advisory Board and Grant Support: Gilead, Abbvie, BMS, Merck, Janssen, Vertex
Alexander Kuo, M.D.: Gilead grant funding
Giuseppe (Joseph) Morelli, M.D.: Grant funding from AbbVie, BMS, Gilead, Merck, Janssen, Vertex, Idenix, Conatus, and Salix
James R. Burton, Jr. M.D.: Research grant support with AbbVie, Gilead, and Janssen.
R. Todd Stravitz, M.D.: Gilead grant funding
Christine Durand, M.D.: Consultant and grant funding from Gilead
Adrian M. Di Bisceglie, M.D.: Grant funding from Gilead, AbbVie, Janssen, consultant funds from Gilead and AbbVie outside the submitted work.
Paul Kwo, M.D.: Reports consultant work from Advisory Board, AbbVie, BMS, Gilead, Merck, Janssen, grant funding from AbbVie, BMS, Gilead, Merck, Janssen, Conatus.
Catherine T. Frenette, M.D.: Gilead employment and stockholder
Thomas G. Stewart, PhD: Reports no disclosures during the conduct of the study.
David R. Nelson, M.D.: Reports grant funding from AbbVie, Gilead, BMS, Janssen, Merck, Vertex, and GSK during the conduct of the study.
Michael W. Fried, M.D.: Reports grant funding from AbbVie, Bristol-Myers Squibb, Gilead, Glaxo, Merck, Vertex, Genentech/Roche and consultant funding from Genentech/Roche, Tibotec/Janssen, Vertex, Merck, Glaxo, Novartis, AbbVie, Gilead, Bristol-Myers Squibb during the conduct of the study along with funding from the NIH for research.
Norah Terrault, MD: Grants: Gilead, AbbVie; Advisory board: Janssen, Merck, BMS, Achillion
References
- 1.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 2.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122(4):889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 3.Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, et al. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46(3):459–465. doi: 10.1016/j.jhep.2006.10.017. Epub 2007/01/02. [DOI] [PubMed] [Google Scholar]
- 4.Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8(3):679–687. doi: 10.1111/j.1600-6143.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 5.Bizollon TPP, Mabrut JY, Chevallier M, Adham M, Radenne S, Souquet JC, Ducerf C, Baulieux J, Zoulin F, Trepo C. Benefit of sustained virological response to combination therapy on graft survival of liver transplanted patients with recurrent chronic hepatitis C. Am J Transplant. 2005;5(8):1909–1913. doi: 10.1111/j.1600-6143.2005.00976.x. [DOI] [PubMed] [Google Scholar]
- 6.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8(11):2426–2433. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 7.Crespo G, Carrion JA, Coto-Llerena M, Marino Z, Lens S, Perez-Del-Pulgar S, et al. Combinations of simple baseline variables accurately predict sustained virological response in patients with recurrent hepatitis C after liver transplantation. J Gastroenterol. 2012 doi: 10.1007/s00535-012-0680-2. Epub 2012/09/27. [DOI] [PubMed] [Google Scholar]
- 8.Xirouchakis E, Triantos C, Manousou P, Sigalas A, Calvaruso V, Corbani A, et al. Pegylated-interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta-analysis of prospective controlled studies. J Viral Hepat. 2008;15(10):699–709. doi: 10.1111/j.1365-2893.2008.01019.x. Epub 2008/08/05. [DOI] [PubMed] [Google Scholar]
- 9.Angelico M, Petrolati A, Lionetti R, Lenci I, Burra P, Donato MF, et al. A randomized study on Peg-interferon alfa-2a with or without ribavirin in liver transplant recipients with recurrent hepatitis C. J Hepatol. 2007;46(6):1009–1017. doi: 10.1016/j.jhep.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Carrion JA, Navasa M, Garcia-Retortillo M, Garcia-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132(5):1746–1756. doi: 10.1053/j.gastro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Hanouneh IA, Miller C, Aucejo F, Lopez R, Quinn MK, Zein NN. Recurrent hepatitis C after liver transplantation: on-treatment prediction of response to peginterferon/ribavirin therapy. Liver Transpl. 2008;14(1):53–58. doi: 10.1002/lt.21312. [DOI] [PubMed] [Google Scholar]
- 12.Berenguer M, Schuppan D. Progression of liver fibrosis in post-transplant hepatitis C: mechanisms, assessment and treatment. J Hepatol. 2013;58(5):1028–1041. doi: 10.1016/j.jhep.2012.12.014. Epub 2012/12/25. [DOI] [PubMed] [Google Scholar]
- 13.Roche B, Sebagh M, Canfora ML, Antonini T, Roque-Afonso AM, Delvart V, et al. Hepatitis C virus therapy in liver transplant recipients: response predictors, effect on fibrosis progression, and importance of the initial stage of fibrosis. Liver Transpl. 2008;14(12):1766–1777. doi: 10.1002/lt.21635. [DOI] [PubMed] [Google Scholar]
- 14.Berenguer M, Aguilera V, Rubin A, Ortiz C, Jimenez M, Prieto M. Comparison of two non-contemporaneous HCV-liver transplant cohorts: strategies to improve the efficacy of antiviral therapy. J Hepatol. 2012;56(6):1310–1316. doi: 10.1016/j.jhep.2011.12.031. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 15.Oton E, Barcena R, Moreno-Planas JM, Cuervas-Mons V, Moreno-Zamora A, Barrios C, et al. Hepatitis C recurrence after liver transplantation: Viral and histologic response to full-dose PEG-interferon and ribavirin. Am J Transplant. 2006;6(10):2348–2355. doi: 10.1111/j.1600-6143.2006.01470.x. Epub 2006/07/28. [DOI] [PubMed] [Google Scholar]
- 16.Berenguer M, Aguilera V, Prieto M, Ortiz C, Rodriguez M, Gentili F, et al. Worse recent efficacy of antiviral therapy in liver transplant recipients with recurrent hepatitis C: impact of donor age and baseline cirrhosis. Liver Transpl. 2009;15(7):738–746. doi: 10.1002/lt.21707. Epub 2009/06/30. [DOI] [PubMed] [Google Scholar]
- 17.Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49(2):274–287. doi: 10.1016/j.jhep.2008.05.002. Epub 2008/06/24. [DOI] [PubMed] [Google Scholar]
- 18.Ponziani FR, Annicchiarico EB, Siciliano M, D’Aversa F, Pompili M, Gasbarrini A. Treatment of hepatitis C in compensated cirrhotic patients is equally effective before and after liver transplantation. World J Gastroenterol. 2013;19(21):3255–3262. doi: 10.3748/wjg.v19.i21.3255. Epub 2013/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verna EC, Saxena V, Burton JR, Jr, O’Leary JG, Dodge JL, Stravitz RT, et al. Telaprevir- and Boceprevir-Based Triple Therapy for Hepatitis C in Liver Transplant Recipients With Advanced Recurrent Disease: A Multicenter Study. Transplantation. 2015 doi: 10.1097/TP.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton JR, Jr, O’Leary JG, Verna EC, Saxena V, Dodge JL, Stravitz RT, et al. A US multicenter study of hepatitis C treatment of liver transplant recipients with protease-inhibitor triple therapy. J Hepatol. 2014;61(3):508–514. doi: 10.1016/j.jhep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 22.Forns X, Charlton M, Denning J, McHutchison JG, Symonds WT, Brainard D, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C following liver transplantation. Hepatology. 2014 doi: 10.1002/hep.27681. [DOI] [PubMed] [Google Scholar]
- 23.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Jr, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371(25):2375–2382. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 24.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Gordon SC, Muir AJ, Lim JK, Pearlman B, Argo CK, Ramani A, et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol. 2015;62(2):286–293. doi: 10.1016/j.jhep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlton M, Gane E, Manns MP, Brown RS, Jr, Curry MP, Kwo PY, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148(1):108–117. doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Ouwerkerk-Mahadevan S. No Clinically Significant Interaction Between the Investigational HCV Protease Inhibitor Simeprevir (TMC435) and the Immunosuppressive Agents Cyclosporine and Tacrolimus. Hepatology. 2012 Abstract. [Google Scholar]
- 28.Ouwerkerk-Mahadevan S. Significant drug-drug interaction between simeprevir and cyclosporine A but not tacrolimus in patients with recurrent chronic HCV infection after orthotopic liver transplantation: the SATURN study. J Hepatol; Vienna EASL, 2015. 2015:P0834. [Google Scholar]
- 29.2015 http://www.olysio.com/shared/product/olysio/prescribing-information.pdf.
- 30.Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver Transpl. 2006;12(8):1192–1204. doi: 10.1002/lt.20865. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez JA, Carrion AF, Avalos D, O’Brien C, Martin P, Bhamidimarri KR, et al. Sofosbuvir and Simeprevir for Treatment of Hepatitis C Virus Infection in Liver Transplant Recipients. Liver Transpl. 2015 doi: 10.1002/lt.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015 doi: 10.1002/hep.27770. [DOI] [PubMed] [Google Scholar]
- 33.Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015;61(6):1880–1886. doi: 10.1002/hep.27770. [DOI] [PubMed] [Google Scholar]
- 34.Feray C, Samuel D, Gigou M, Paradis V, David MF, Lemonnier C, et al. An open trial of interferon alfa recombinant for hepatitis C after liver transplantation: antiviral effects and risk of rejection. Hepatology. 1995;22(4 Pt 1):1084–1089. [PubMed] [Google Scholar]
- 35.Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, et al. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13(6):1601–1605. doi: 10.1111/ajt.12209. Epub 2013/04/19. [DOI] [PubMed] [Google Scholar]
- 36.Reddy KR, Everson GT, Flamm SL, Denning JM, Arterburn S, Brandt-Sarif T, et al. Ledipasvir/Sofosbuvir with ribavirin for the treatment of HCV in patients with post transpalnt recurrence: Preliminary resutls of a prosepcitve, multicenter study. Hepatology. 2014 AASLD Abstract 8. [Google Scholar]
- 37.Forns X, et al. On-treatment virologic response and tolerability of simeprevir, daclatasvir and ribavirin in patients with recurrent hepatitis C virus genotype 1b infection after orthotopic liver transplantation (OLT): Interim data from the Phase 2 SATURN Study. Vienna: EASL; 2015. [DOI] [PubMed] [Google Scholar]
- 38.Eyster ME, Sherman KE, Goedert JJ, Katsoulidou A, Hatzakis A. Prevalence and changes in hepatitis C virus genotypes among multitransfused persons with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis. 1999;179(5):1062–1069. doi: 10.1086/314708. [DOI] [PubMed] [Google Scholar]