Abstract
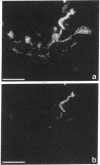
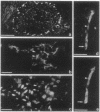
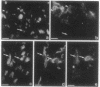
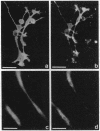
We produced earlier a monoclonal antibody against Schwann cell myelin protein (SMP), a glycoprotein expressed on Schwann cells (SC) but not on satellite cells of the ganglia or enteric glial cells. We now studied whether SMP expression is environmentally regulated in the different compartments of the peripheral nervous system. Quail neural-crest cells from either mes-metencephalic, vagal, or truncal levels of the neuraxis were heterochronically associated with gut wall, skin, or muscle tissues from embryonic day (E) 7 to E11 chickens. Coculture of these chimeric organs revealed that as in normal development glial cells, characterized by HNK1 immunoreactivity and the quail nuclear marker, expressed the SMP phenotype exclusively in skin and muscle, failing to do so in gut. However, when SMP+ SC from quail sciatic nerves were cocultured with chicken gut, these cells rapidly lost their initial SMP immunoreactivity. In contrast, when associated with muscle and skin, SC remained SMP+, even in the complete absence of neuronal cells. Enteric plexuses from E8 to E15 quail gut express SMP+ and laminin when withdrawn from the intestinal-mesenchyme environment. These results show that SMP can be expressed by enteric glial cells and that the SC SMP phenotype is strongly inhibited by the gut-wall environment. Moreover, these results strongly suggest that these two types of glial cells belong to the same lineage and that their terminal phenotype is modulated through cell-to-cell interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Aguayo A. J., Charron L., Bray G. M. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976 Oct;5(8):565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- Baetge G., Schneider K. A., Gershon M. D. Development and persistence of catecholaminergic neurons in cultured explants of fetal murine vagus nerves and bowel. Development. 1990 Nov;110(3):689–701. doi: 10.1242/dev.110.3.689. [DOI] [PubMed] [Google Scholar]
- Baroffio A., Dupin E., Le Douarin N. M. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5325–5329. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A. E., Carlei F., Lee V., Trojanowski J., Marangos P. J., Dahl D., Polak J. M. Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry. 1985;82(1):93–97. doi: 10.1007/BF00502095. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Ling N., Esch F., Böhlen P., Benoit R., Guillemin R. High biological activity of the synthetic replicates of somatostatin-28 and somatostatin-25. Regul Pept. 1981 Jan;1(4):255–264. doi: 10.1016/0167-0115(81)90048-3. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Todd M. S. Schwann cell myelination in a chemically defined medium: demonstration of a requirement for additives that promote Schwann cell extracellular matrix formation. Brain Res. 1987 Mar;429(1):95–102. doi: 10.1016/0165-3806(87)90142-8. [DOI] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. D., Burnstock G. The ultrastructure of Auerbach's plexus in the guinea-pig. II. Non-neuronal elements. J Neurocytol. 1976 Apr;5(2):195–206. doi: 10.1007/BF01181656. [DOI] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C., Cameron-Curry P., Ziller C., Le Douarin N. M. A surface protein expressed by avian myelinating and nonmyelinating Schwann cells but not by satellite or enteric glial cells. Neuron. 1988 May;1(3):211–220. doi: 10.1016/0896-6273(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Dupin E., Baroffio A., Dulac C., Cameron-Curry P., Le Douarin N. M. Schwann-cell differentiation in clonal cultures of the neural crest, as evidenced by the anti-Schwann cell myelin protein monoclonal antibody. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1119–1123. doi: 10.1073/pnas.87.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquet M., Smith J., Ziller C., Le Douarin N. M. Differentiation of autonomic neuron precursors in vitro: cholinergic and adrenergic traits in cultured neural crest cells. J Neurosci. 1981 May;1(5):478–492. doi: 10.1523/JNEUROSCI.01-05-00478.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri G. L., Probert L., Cocchia D., Michetti F., Marangos P. J., Polak J. M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982 Jun 3;297(5865):409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- GROBSTEIN C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature. 1953 Nov 7;172(4384):869–870. doi: 10.1038/172869a0. [DOI] [PubMed] [Google Scholar]
- Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6(3):425–436. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- Gershon M. D., Rothman T. P. Enteric glia. Glia. 1991;4(2):195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980 Aug 14;286(5774):736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Morgan L., Stewart H. J., Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c(Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990 May;109(1):91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Thorpe R., Mirsky R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J Neurocytol. 1984 Apr;13(2):187–200. doi: 10.1007/BF01148114. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974 Nov;41(1):162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973 Aug;30(1):31–48. [PubMed] [Google Scholar]
- Le Douarin N., Dulac C., Dupin E., Cameron-Curry P. Glial cell lineages in the neural crest. Glia. 1991;4(2):175–184. doi: 10.1002/glia.440040209. [DOI] [PubMed] [Google Scholar]
- Moya F., Bunge M. B., Bunge R. P. Schwann cells proliferate but fail to differentiate in defined medium. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6902–6906. doi: 10.1073/pnas.77.11.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S. L., Furcht L. T. Production of laminin and fibronectin by Schwannoma cells: cell-protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983 May;96(5):1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz H. D., Gershon M. D. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol. 1990 Feb;137(2):378–394. doi: 10.1016/0012-1606(90)90262-h. [DOI] [PubMed] [Google Scholar]
- Rothman T. P., Tennyson V. M., Gershon M. D. Colonization of the bowel by the precursors of enteric glia: studies of normal and congenitally aganglionic mutant mice. J Comp Neurol. 1986 Oct 22;252(4):493–506. doi: 10.1002/cne.902520406. [DOI] [PubMed] [Google Scholar]
- Smith J., Cochard P., Le Douarin N. M. Development of choline acetyltransferase and cholinesterase activities in enteric ganglia derives from presumptive adrenergic and cholinergic levels of the neural crest. Cell Differ. 1977 Oct;6(3-4):199–216. doi: 10.1016/0045-6039(77)90016-1. [DOI] [PubMed] [Google Scholar]
- Tucker G. C., Ciment G., Thiery J. P. Pathways of avian neural crest cell migration in the developing gut. Dev Biol. 1986 Aug;116(2):439–450. doi: 10.1016/0012-1606(86)90145-4. [DOI] [PubMed] [Google Scholar]
- YNTEMA C. L., HAMMOND W. S. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954 Oct;101(2):515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Ziller C., Dupin E., Brazeau P., Paulin D., Le Douarin N. M. Early segregation of a neuronal precursor cell line in the neural crest as revealed by culture in a chemically defined medium. Cell. 1983 Feb;32(2):627–638. doi: 10.1016/0092-8674(83)90482-8. [DOI] [PubMed] [Google Scholar]