Abstract
Bacillus mojavensis strains JF-2 (ATCC 39307), ROB2, and ABO21191T and Bacillus subtilis strains 168 (ATCC 23857) and ATCC 12332 required four deoxyribonucleosides or DNA for growth under strict anaerobic conditions. Bacillus licheniformis strains L89-11 and L87-11, Bacillus sonorensis strain TG8-8, and Bacillus cereus (ATCC 14579) did not require DNA for anaerobic growth. The requirement for the deoxyribonucleosides or DNA did not occur under aerobic growth conditions. The addition of a mixture of five nucleic acid bases, four ribonucleotides, or four ribonucleosides to the basal medium did not replace the requirement of B. mojavensis JF-2 for the four deoxyribonucleosides. However, the addition of salmon sperm DNA, herring sperm DNA, Escherichia coli DNA, or synthetic DNA (single or double stranded) to the basal medium supported anaerobic growth. The addition of four deoxyribonucleosides to the basal medium allowed aerobic growth of B. mojavensis JF-2 in the presence of hydroxyurea. B. mojavensis did not grow in DNA-supplemented basal medium that lacked sucrose as the energy source. These data provide strong evidence that externally supplied deoxyribonucleosides can be used to maintain a balanced deoxyribonucleotide pool for DNA synthesis and suggest that ribonucleotide reductases may not be essential to the bacterial cell cycle nor are they necessarily part of a minimal bacterial genome.
Ribonucleotide reductases make deoxyribonucleotides from ribonucleotides regardless of whether the nucleotide is made de novo or by the salvage pathways (25, 26, 31). The de novo pathways make nucleotides from metabolic precursors generated in central metabolic pathways. The salvage pathways use exogenously supplied bases and nucleosides or recycle endogenously produced bases and nucleosides (23). Ribonucleotide reductases are considered essential to all organisms to regulate and maintain the deoxyribonucleotide pool necessary for DNA synthesis (9, 18, 25, 29, 32, 33). Recently, the minimal essential genes of Bacillus subtilis were narrowed to only 192 “essential” genes out of 4,100 genes (19) and included the class I ribonucleotide reductase genes, nrdE and nrdF. In 1952, Lactobacillus acidophilus R-26 was reported to require at least one externally supplied deoxyribonucleoside and to lack ribonucleotide reductase activity (14). This finding suggests that ribonucleotide reductase activity may not be required for all microorganisms.
In order to use deoxyribonucleosides as DNA precursors, an organism must be able to phosphorylate the deoxyribonucleoside to a deoxyribonucleotide. Escherichia coli and Salmonella enterica serovar Typhimurium do not have the deoxyribonucleoside kinases necessary for converting deoxyribonucleosides into deoxyribonucleotides and therefore cannot utilize exogenously supplied deoxyribonucleosides as sole DNA precursors (25). However, B. subtilis and lactobacilli have all of the kinases necessary to phosphorylate the deoxyribonucleosides to deoxyribonucleotides and could potentially use them as sole DNA precursors without the need for ribonucleotide reductase activity (26).
Our work focuses on the use of microorganisms to recover petroleum hydrocarbons that remain entrapped after current recovery technologies reach their economic limit. A large energy resource (over 300 billion barrels of crude oil) exists in domestic reservoirs if a technology can be developed to recover this entrapped oil (21). Capillary forces between the hydrocarbon and aqueous phases are largely responsible for trapping the hydrocarbons in the pores of the rock, and large reductions in the interfacial tension between the hydrocarbon and aqueous phases are needed for hydrocarbon mobilization (1, 3, 4, 28, 35). Microorganisms produce a variety of biosurfactants (6), several of which generate the ultra-low interfacial tensions needed for hydrocarbon mobilization (6, 10, 20). In particular, the lipopeptide biosurfactant produced by Bacillus mojavensis strain JF-2 reduces the interfacial tension between hydrocarbon and aqueous phases to very low levels (<0.016 mN/m) (20, 22). B. mojavensis JF-2 grows under the environmental conditions found in many oil reservoirs, i.e., anaerobic, NaCl concentrations up to 80 g liter−1, and temperatures up to 45°C (16, 17), making it ideally suited for in situ applications. However, anaerobic growth of B. mojavensis JF-2 is inconsistent and difficult to replicate, which limits its use for in situ applications.
Here, we show that DNA or deoxyribonucleosides are required for the anaerobic growth of several B. mojavensis strains including strain JF-2 and two B. subtilis strains. This work suggests that other proteins, not solely ribonucleotide reductases, can regulate the intercellular balance of ribonucleotides and deoxyribonucleotides and that ribonucleotide reductases may not be essential to the cell cycle.
MATERIALS AND METHODS
Bacterial strains.
B. mojavensis JF-2 (ATCC 39307), B. mojavensisT ABO21191, B. subtilis 168 (ATCC 23857), and Bacillus cereus (ATCC 14579) were obtained from the American Type Culture Collection (ATCC). B. mojavensis strain ROB2, Bacillus licheniformis strains L87-11 and L89-11, and Bacillus sonorensis TG8-8 were obtained from the stock culture collection of Kathleen Duncan at the University of Oklahoma (8, 15).
Cultivation.
Basal medium contained the following: TES buffer [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] (29 g/liter), NaCl (50 g/liter), sucrose (10 g/liter), yeast extract (DF 0127-17-9) (1 g/liter), NaNO3 (1 g/liter), K2HPO4 (1.0 g/liter), (NH4)2SO4 (1 g/liter), MgSO4 (0.25 g/liter), and 10 ml of a metal solution. The metal solution was a modification of Wolin's metal solution and contained the following components: EDTA (1 g/liter), MnSO4 · H2O (3 g/liter), FeSO4 · 7H2O (0.1 g/liter), CaCl2 · 2H2O (0.1 g/liter), CoCl2 · 2H2O (0.1 g/liter), ZnSO4 · 7H2O (0.1 g/liter), CuSO4 · 7H2O (0.01 g/liter), H3BO4 (0.01 g/liter), and Na2MoO4 · 2H2O (0.01 g/liter). Cysteine hydrochloride was added to the basal medium after degassing in the concentration of 0.25 g/liter. The pH was adjusted to 6.8.
Basal medium was also supplemented with amino acids, nucleic acid bases, vitamins, and fatty acids as described by Tanner (34). The amino acid supplement consisted of the following (final concentration): Casamino Acids (DF 0230-15-5) (4 g/liter), glutamine (Sigma G7029) (0.1 g/liter), tryptophan (Sigma T0254) (0.1 g/liter), asparagine (Sigma 4284) (0.02 g/liter), and methionine (Sigma M9625) (0.02 g/liter). The nucleic acid base supplement consisted of adenine (Sigma A8626), cytosine (Sigma C3506), guanine (Sigma G0381), thymine (Sigma T0895), and uracil (Sigma U0750) with a final concentration of 0.1 g/liter of each base. The vitamin supplement consisted of the following (final concentration): biotin (Sigma B4501) (0.02 mg/liter), folic acid (Sigma F7876) (0.02 mg/liter), pyridoxine-HCl (Sigma P9755) (0.1 mg/liter), thiamine-HCl (Sigma T4625) (0.05 mg/liter), riboflavin (Sigma R4500) (0.05 mg/liter), nicotinic acid (Sigma N3376) (0.05 mg/liter), calcium pantothenate (Sigma P2250) (0.05 mg/liter), p-aminobenzoic acid (Sigma A9878) (0.05 mg/liter), lipoic acid (Sigma T5625) (0.05 mg/liter), and vitamin B12 (Sigma V2876) (0.001 mg/liter). The fatty acid supplement consisted of the following (final concentration): sodium formate (Fisher S-301) (2.5 g/liter), sodium acetate (Fisher S-220-1) (2.5 g/liter), propionic acid (Aldrich 10979-1) (1.0 g/liter), butyric acid (Fisher A-81) (0.6 g/liter), isobutyric acid (Fisher A-80), valeric acid (Sigma V9759) (0.2 g/liter), isovaleric acid (Sigma I7128) (0.2 g/liter), and 2-methylbutyric acid (Aldrich 19307-0) (0.2 g/liter).
Alternatively, yeast extract, peptone (DF 0118-15-2), Soytone (DF 0436-17-5), neopeptone (DF 0119-17-9), tryptone (DF 0123-15-5), Proteose Peptone (DF 0120-17-6), Proteose Peptone 2 (DF 0121-17-5), Proteose Peptone 3 (DF 0122-17-4), and Casamino Acids were individually added to the medium to give a final concentration of 1 to 30 g/liter. Rumen fluid was obtained from a fistulated cow fed a high roughage diet (>60%) without antibiotics at the Animal Sciences Center of Oklahoma State University. The rumen fluid added to basal medium ranged in concentration from 5 to 50%. Hemin and inositol were added to give a final concentration of 0.01 g/liter.
Individual amino acids glutamate (Sigma G5513), glutamine (Sigma G3126), phenylalanine (Sigma P4905), tyrosine (Sigma T1145), tryptophan, and methionine and polyamino acids such as polyglutamate (Sigma P4636), polytyrosine (Sigma P1800), polyarginine (Sigma P4663), polyasparagine (Sigma P8137), and polytryptophan (Sigma P0644) were added individually at a concentration of 0.1 g/liter.
Adenosine (Sigma A4036), cytidine (Sigma C4654), guanosine (Sigma G6264), and thymidine (Sigma T1895) were combined to provide a ribonucleoside stock solution and AMP (ICN 100080), CMP (Sigma C1006), GMP (Sigma G8577), and dTMP (Sigma T7004) were combined to provide a nucleotide stock solution. The deoxyribonucleoside solution consisted of deoxyadenosine (Sigma D8668), deoxyguanosine (BioChemika 31070), deoxycytidine (Sigma D0776), and thymidine (Sigma T1895). The final concentration of each ribonucleoside, deoxyribonucleoside, and ribonucleotide in the medium was 1.0 g/liter. Salmon sperm DNA (Sigma D-1626), herring sperm DNA (D-3159), and E. coli DNA (D-2001) and RNA (R-6625) were added directly to the media at the concentration of 1.0 g/liter.
All stock solutions were filter sterilized, degassed by repeated evacuation and repressurization with 100% N2, and added after autoclaving. Anaerobic media and solutions were prepared by the procedure of Balch and Wolfe (2). The gas phase was 100% N2. Additions and sampling were done with sterile, degassed syringes and needles (2).
Agarose gel electrophoresis.
E. coli and herring sperm DNA were run on a 30-ml, 1.0% agarose gel, with 2 μl of ethidium bromide and PCR markers (Invitrogen) (1,000 to 50 bp) to determine the size of the oligonucleotides. The gel was run for 30 min at 96 V. The gel was viewed under UV light and recorded using a Nucleocam by Nucleotech Imaging (San Mateo, Calif.).
Synthetic DNA.
A random sequence of 50 nucleotide bases was generated and then tested for hairpin turns and self-annealing sequences with the oligonucleotide properties calculator found at http://www.basic.nwu.edu/biotools/oligocalc.html. Selected bases were changed until a sequence was generated that did not contain hairpin turns, or self-annealing areas, and was about 50% GC. The final sequence, named JF-2 SS, was TGG CGA AGG ATG CTG GCT ACA CTG CAG TTA TCT CTC ACC GTT CTG GCG AA. A DNA sequence that was complementary to JF-2 SS, named JF-2 COM, was also generated and tested. DNA was obtained from Integrated DNA Technologies. To determine if single-stranded or double-stranded DNA supported anaerobic growth, three tubes of basal medium with 0.05% each of JF-2 SS, JF-2 COM, and JF-2 SS plus JF-2 COM were used.
Inoculation protocol.
A sealed serum bottle with 100 ml of unreduced, anaerobic basal medium was inoculated directly from a well-isolated colony of B. mojavensis strain JF-2 on a basal medium agar plate that had been incubated for 24 h. The serum bottle was incubated for 24 to 48 h at 37°C and then used as an inoculum. One-half milliliter of the culture was inoculated into triplicate sealed serum tubes (10 ml each) for each condition. Each experiment was repeated at least once. An uninoculated control and an unamended control (basal medium) were used for each of the above treatments. All tubes and serum bottles were incubated under static conditions at 37°C. Growth was measured as absorbance at 600 nm. The cultures were diluted prior to measurement when the culture absorbance exceeded 0.3.
Purification of the growth-enhancing factor in Proteose Peptone.
Proteose Peptone (300 g) was stirred for 24 h with 500 ml of methanol. The methanol-insoluble fraction was filtered, dissolved in 100 ml of nanopure water, boiled for 5 min to remove traces of methanol, and then lyophilized. The 500-ml filtrate was combined with 500 ml of nanopure water and boiled until less than 400 ml of liquid remained. The remaining liquid was then lyophilized. The dry fractions were added to basal medium to give a final concentration of 0.03 g (dry weight)/ml.
Sephadex G-25 gel (Pharmacia, Uppsala, Sweden) was hydrated, degassed, and poured as recommended by the manufacturer. The column was equilibrated for 2 h with nanopure water at a flow rate of 2 ml/min. The void volume was determined with blue dextran. A 2-ml sample of a 30% solution of methanol-insoluble Proteose Peptone fraction in nanopure water was injected at 1.5 ml/min. After complete elution, this procedure was repeated three times. Thirty-two 5-ml fractions were collected each time. The fractions were pooled and lyophilized in the following manner: 7 to 11, 12 to 18, 19 to 22, and 23 to 32. The dry fractions were added to basal medium to give a final concentration of approximately 0.01 g (dry weight)/ml, except for the final fraction pool, which was added at about 0.001 g (dry weight)/ml due to the very small amount of material collected. Cyanocobalamin (molecular weight [MW], 1,355), tryptophan (MW, 204), and aprotinin (MW, 6,500) were used as standards to determine the average MW of the Proteose Peptone fractions. The protein content of the size fraction that best supported growth (12 to 18) was measured by the Lowry and the Bradford assays (11).
Macro-Prep High S cation exchange support and Macro-Prep High Q anion exchange support (Bio-Rad) were hydrated, degassed, and washed as recommended by the manufacturer. A 10-ml sample of 30% Proteose Peptone was pulled through 100 ml of hydrated beads by vacuum filtration. The filtrate was lyophilized and added to basal medium at the concentration of 0.03 g (dry weight)/ml.
RESULTS
B. mojavensis JF-2 grew well aerobically in basal medium (Amax of 0.6 to 1.3) but grew poorly anaerobically (Amax of 0 to 0.10). We attempted to improve the anaerobic growth of B. mojavensis JF-2 with the addition of common medium supplements. Vitamins, hemin, inositol, amino acids, Soytone, peptone, and rumen fluid (up to 50%) were added individually and in combination to the basal medium, at various concentrations, but none of these additions were effective in supporting anaerobic growth (Table 1). Eventually we found that 30-g/liter concentrations of tryptone, neopeptone, or Proteose Peptone (grades 1, 2, or 3) all supported anaerobic growth of B. mojavensis JF-2 (Table 1). The addition of Proteose Peptone 3 consistently gave the highest absorbance and was used for all subsequent experiments.
TABLE 1.
The effect of various additions to basal medium on the anaerobic growth of B. mojavensis JF-2
| Addition to sucrose-based basal mediuma | Growth (Amax) |
|---|---|
| Basal medium with no addition | <0.1 |
| Vitamins | <0.1 |
| Hemin | <0.1 |
| Inositol | <0.1 |
| Casamino Acids (up to 30 g/liter) | <0.1 |
| Rumen fluid (up to 50%) | <0.1 |
| Peptone (up to 30 g/liter) | <0.1 |
| Soytone (up to 30 g/liter) | <0.1 |
| Tryptone (30 g/liter) | 0.6-0.8 |
| Neopeptone (30 g/liter) | 0.6-0.8 |
| Proteose Peptone (1, 2, and 3) (30 g/liter) | 0.6-0.8 |
| Salmon sperm DNA (1 g/liter) | 0.6-0.8 |
| E. coli DNA (1 g/liter) | 0.6-0.8 |
| Synthetic DNA (SS and DS) (1 g/liter) | 0.6-0.8 |
| Adenine, cytosine, guanine, thymine, and uracil (combined at 1 g/liter each) | <0.1 |
| Deoxyadenosine, deoxycytidine, deoxyguanosine, and thymidine (individually added at 1 g/liter each) | 0.1-0.2 |
| AMP, CMP, GMP, and TMP (combined at 1 g/liter each) | <0.1 |
| 2-Deoxyribose, adenine, cytosine, guanine, thymine, and uracil (combined at 1 g/liter each) | <0.1 |
| RNA (1 g/liter) | <0.1 |
See Materials and Methods for a description of the basal medium and medium additions. All experiments were performed in triplicate and each treatment was repeated at least once.
In basal medium or in basal medium with nucleic acid bases, amino acids, vitamins, and fatty acids, anaerobic growth was very poor (Amax less than 0.1) (Fig. 1). With Proteose Peptone, anaerobic growth was consistently above an Amax of 0.6.
FIG. 1.
The effect of the addition of Proteose Peptone to basal medium and basal medium with nucleic acid bases, amino acids, fatty acids, and vitamins on the anaerobic growth of B. mojavensis JF-2. Symbols: squares, basal medium; circles, basal medium with nucleic acid bases, amino acids, fatty acids, and vitamins; open symbols, each medium supplemented with 30 g of Proteose Peptone/liter.
It was initially assumed that the growth factor consisted of an amino acid or peptide since Proteose Peptone is an enzymatic digest of protein. However, individual amino acids such as glutamate, glutamine, phenylalanine, tyrosine, tryptophan, and methionine and polyamino acids such as polyglutamate, polytyrosine, polyarginine, polyasparagine, and polytryptophan did not replace the requirement for Proteose Peptone. We also found that growth did not occur in basal medium supplemented alone or in combination with adenine, guanine, thymine, cytosine, uracil, a mixture of amino acids, vitamins (including folate), and fatty acids, nor did growth occur in basal medium supplemented with a combination of adenosine, guanosine, cytosine, and thymidine or with the corresponding monophosphates.
Since the growth-enhancing factor was not one of the more commonly known growth supplements, we proceeded to purify it for the purposes of identification. Crude purification of the growth-enhancing factor revealed that it was methanol insoluble, had an average MW of 3,900 g/mol, was retained by an anion exchange column, was acid and base stable, was low in protein content, and exhibited a maximum absorbance at 260 nm. These results suggested that the growth-enhancing factor consisted of nucleic acids. However, neither the addition of nucleic acid bases alone nor the combination of nucleic acid bases with peptides in the basal medium replaced the requirement for Proteose Peptone.
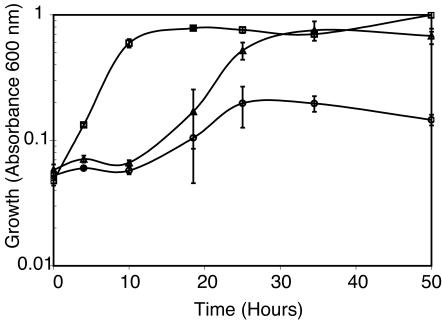
Ultimately, we found that various sources of DNA replaced the requirement for Proteose Peptone for anaerobic growth of B. mojavensis JF-2 in reduced basal medium. The addition of E. coli DNA (1,000 to 750 bp), salmon sperm DNA (Table 1), or herring sperm DNA (∼50 bp) (Fig. 2) to basal medium supported anaerobic growth of B. mojavensis JF-2. The addition of single-stranded or double-stranded synthetic DNA (50 bp) to the basal medium also supported anaerobic growth of B. mojavensis JF-2. The addition of RNA or 2-deoxyribose combined with nucleic acid bases did not support growth (Table 1). However, a mixture of deoxyribonucleosides consisting of deoxyadenosine, deoxyguanosine, deoxycytidine, and deoxythymidine replaced the requirement for DNA, whereas the ribonucleosides did not (Fig. 3). Subsequent studies showed that even an unbalanced external pool (0.1-g/liter concentrations of three deoxyribonucleosides and a 0.2-g/liter concentration of the fourth) of deoxyribonucleosides supported anaerobic growth in a manner indistinguishable from that of a balanced pool of deoxyribonucleosides, where each deoxyribonucleoside is added at an identical concentration (Fig. 4). The individual addition of each deoxyribonucleoside alone supported growth to an Amax of less than 0.2 (Table 1).
FIG. 2.
The effect of the addition of DNA to the basal medium on the anaerobic growth of B. mojavensis JF-2. Symbols: squares, basal medium; circles, basal medium with 30 g of Proteose Peptone/liter; triangles, basal medium with 1 μg of herring sperm DNA/ml.
FIG. 3.
The effect of the addition of deoxyribonucleosides and ribonucleosides to basal medium on the anaerobic growth of B. mojavensis JF-2. Symbols: squares, basal medium; circles, basal medium with deoxyribonucleosides (deoxycytidine, deoxyguanosine, deoxyadenosine, and deoxythymidine; each at 1 μg/ml); triangles, basal medium with ribonucleosides (cytidine, guanosine, and adenosine) and thymidine, each at 1 μg/ml.
FIG. 4.
The effect of an unbalanced pool of deoxyribonucleosides on the anaerobic growth of B. mojavensis JF-2 in basal medium. Symbols: squares, basal medium; circles, basal medium with deoxyribonucleosides (deoxycytidine, deoxyguanosine, deoxyadenosine, and deoxythymidine; at 1 μg/ml each); triangles, basal medium with unbalanced deoxyribonucleosides. For unbalanced deoxyribonucleosides, three of the deoxyribonucleosides were added at 1 μg/ml and the fourth at 4 μg/ml. Each of the four deoxyribonucleosides was tested in excess separately. The results were similar and the results of all four tests were averaged, resulting in a single data set.
Aerobic growth of B. mojavensis JF-2 in basal medium was greatly reduced in the presence of 15 mM hydroxyurea (Amax of less than 0.2) compared to the control without hydroxyurea (Amax of 1 to 1.3). With the addition of all four of the deoxyribonucleosides to basal medium containing hydroxyurea, aerobic growth was similar to the control without hydroxyurea (Fig. 5).
FIG. 5.
The effect of the addition of hydroxyurea and deoxyribonucleosides to aerobic basal medium on the aerobic growth of B. mojavensis JF-2. Symbols: squares, basal medium; circles, basal medium with 15 mM hydroxyurea; triangles, basal medium with deoxyribonucleosides (deoxycytidine, deoxyguanosine, deoxyadenosine, and deoxythymidine; each at 1 μg/ml) and 15 mM hydroxyurea.
With the addition of up to 1 g of DNA/liter to basal medium, it was possible that the DNA was used as a carbon or energy source and not simply as a growth factor. However, Fig. 6 shows that DNA did not serve as a sole carbon or energy source for B. mojavensis JF-2. No growth occurred when the basal medium lacked sucrose but had 1 g of DNA/liter.
FIG. 6.
The effect of sucrose on anaerobic growth of B. mojavensis JF-2 in basal medium supplemented with 1 μg of DNA/ml. Symbols: squares, no sucrose added; circles, 5 mM sucrose; triangles, 20 mM sucrose.
Since the requirement for DNA for anaerobic growth has not been reported in any other organisms, other strains of Bacillus were tested to see if they also required DNA for growth in basal medium under strict anaerobic conditions. The type strain B. mojavensisT (ABO21191), B. mojavensis strain ROB2, B. subtilis strain 168, and B. subtilis strain ATCC 12332 all required DNA for anaerobic growth (data not shown). B. licheniformis strains L89-11 and L87-11, B. sonorensis strain T68-8, and B. cereus strain 14579 did not require DNA for anaerobic growth (data not shown). It should be noted that DNA was not the only growth factor that these organisms required. B. mojavensis JF-2 also required amino acids and vitamins under both aerobic and anaerobic growth conditions. All the nutritional requirements for anaerobic growth of the other DNA-requiring strains have not yet been determined.
DISCUSSION
The requirement for DNA as a growth factor for anaerobic growth is unusual, especially since this requirement does not exist during aerobic growth of B. mojavensis or of the B. subtilis strains tested. This novel physiology is probably not due to the selection of a mutant during routine laboratory cultivation since it is found in several independent isolates. In addition, L. acidophilus R-26 has been reported to require deoxyribonucleosides (13). B. subtilis and lactobacilli have the necessary kinases to phosphorylate the deoxyribonucleosides to deoxyribonucleotides and could potentially use them as sole DNA precursors without the need for ribonucleotide reductase activity (26). The fact that B. subtilis 168 required deoxyribonucleosides for anaerobic growth was surprising since it has been reported to grow anaerobically in minimal (24, 27) or defined media without DNA (5). However, the anaerobic procedures involved the replacement of the headspace with nitrogen without the addition of a reductant (22) or the use of static incubations (2) or were not specified (27). Thus, it is possible that small amounts of oxygen could have been present, which allowed growth of B. subtilis strain 168 under these conditions.
The requirement of some Bacillus strains for DNA or deoxyribonucleosides for anaerobic growth should not be unexpected given their ability to uptake DNA by way of the competence system (7). Also, B. subtilis can cannibalize sibling cells (12), which prevents sporulation during transient periods of nutrient starvation. The nutrients released from lysed cells would include DNA and/or deoxyribonucleosides that could be used as nutrients. Thus, it is likely that some bacilli would have evolved the ability to utilize DNA or deoxynucleosides as a nutrient source.
The type of ribonucleotide reductase present in the cell may explain why some bacilli require deoxyribonucleosides or DNA for growth. Three main classes of ribonucleotide reductases have been identified. Class I requires oxygen to generate the tyrosyl radical needed to reduce the ribonucleotide to the deoxyribonucleotide (18). This class of ribonucleotide reductases is inhibited by hydroxyurea since hydroxyurea inactivates the tyrosyl radical (18). Class II neither uses oxygen nor is sensitive to oxygen and functions under both aerobic and anaerobic conditions (18). The class III has a glycyl radical that is destroyed by oxygen and functions only under anaerobic conditions (18). Each class of ribonucleotide reductase generates all four deoxyribonucleotides from the respective ribonucleotides. If the B. mojavensis and B. subtilis strains that require deoxyribonucleosides for anaerobic growth have only the class I ribonucleotide reductase and not one of the other classes, then they would be unable to convert ribonucleotides into deoxyribonucleotides under anaerobic conditions and would require exogenously supplied deoxyribonucleosides.
A BLAST (National Center for Biotechnology Information) search using the protein sequence of the class III ribonucleotide reductase, nrdD, from E. coli (AE000495) was performed against the genomic sequences of Bacillus anthracis strain A2012, B. anthracis strain Ames, B. cereus ATCC 14579, Bacillus halodurans, and B. subtilis subsp. subtilis strain 168 (data not shown). All the bacilli except for B. subtilis subsp. subtilis strain 168 and B. halodurans had at least a putative nrdD gene (at least 32% identical and 49% similar) and were annotated as anaerobic ribonucleotide-triphosphate reductases. It is possible that B. halodurans also has an nrdD, but the match in this case was not strong enough (28% identical and 47% similar) to make a definitive conclusion and was not annotated as such. Similar results were obtained when the search was performed with the nrdD gene of B. cereus (AAP10536). In addition, nrdD was not among the list of annotated genes in the genome of B. subtilis strain 168 (http://genolist.pasteur.fr/SubtiList/). This in silico analysis is consistent with the hypothesis that deoxyribonucleosides or DNA is required for anaerobic growth due to the lack of the appropriate ribonucleotide reductase. B. subtilis has a class I ribonucleotide reductase (30). Inhibition of aerobic growth by hydroxyurea and the restoration of aerobic growth in the presence of hydroxyurea by the addition of the four deoxyribonucleosides indicates that B. mojavensis JF-2 has a class I ribonucleotide reductase but lacks a class II ribonucleotide reductase that would allow it to grow in the presence of hydroxyurea. The hypothesis is also supported by the fact that the addition of deoxyribonucleosides and not ribonucleosides to basal medium supported anaerobic growth.
It is possible that the requirement for DNA or deoxyribonucleosides for anaerobic growth may not be limited to the bacilli and lactobacilli. Most common bacteriological growth media do not contain deoxyribonucleosides as indicated by typical analysis reports. As a result, bacteria that require deoxyribonucleosides would be unlikely to grow on most laboratory media and may represent an important class of uncultured microorganisms. Our data further suggest that currently unidentified proteins may be involved in regulating the ribonucleotide and deoxyribonucleotide pools.
Acknowledgments
We thank K. Duncan, J. Ballard, and M. Whiteley for helpful suggestions.
This work was supported by U.S. Department of Energy grants DE-FG03-96ER20214 and DE-FC26-02NT15321.
REFERENCES
- 1.Austad, T., and K. Taugbol. 1995. Chemical flooding of oil reservoirs. 1. Low tension polymer flood using a polymer gradient in three-phase region. Colloids Surf. A Physiochem. Eng. Asp. 101:87-97. [Google Scholar]
- 2.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy, D. P., and A. J. Hudak. 2001. Microorganism selection and biosurfactant production in a continuously and periodically operated bioslurry reactor. J. Hazard. Mater. 84:253-264. [DOI] [PubMed] [Google Scholar]
- 4.Chiu, Y. C., and P. R. Kuo. 1999. An empirical correlation between low interfacial tension and micellar size and solubilization for petroleum sulfonates in enhanced oil recovery. Colloids Surf. A Physiochem. Eng. Asp. 152:235-244. [Google Scholar]
- 5.Clements, L. D., U. N. Streips, and B. S. Miller. 2002. Differential proteomic analysis of Bacillus subtilis nitrate respiration and fermentation in defined medium. Proteomics 2:1724-1734. [DOI] [PubMed] [Google Scholar]
- 6.Desai, J., and I. M. Banat. 1997. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 61:47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubnau, D. 1991. Genetic competence in Bacillus subtilis. Microbiol. Rev. 55:395-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan, K. E., N. Ferguson, K. Kimura, X. Zhou, and C. A. Istock. 1994. Fine-scale genetic and phenotypic structures in natural populations of Bacillus subtilis and Bacillus licheniformis: important implications for bacterial evolution and speciation. Evolution 48:2002-2025. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson, M., U. Uhlin, S. Ramaswamy, M. Ekberg, K. Regnstrom, B.-M. Sjoberg, and H. Eklund. 1997. Binding of allosteric effectors to ribonucleotide reductase protein R1: reduction of active-site cysteines promotes substrate binding. Structure 5:1077-1092. [DOI] [PubMed] [Google Scholar]
- 10.Georgiou, G., S. C. Lin, and M. M. Sharma. 1992. Surface-active compounds from microorganisms. Biotechnology 10:60-65. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. 1994. Methods for general and molecular bacteriology. ASM Press, Washington, D.C.
- 12.Gonzales-Pastor, J. E., E. C. Hobbs, and R. Losick. 2003. Cannibalism by sporulating bacteria. Science 301:510-513. [DOI] [PubMed] [Google Scholar]
- 13.Hoff-Jorgensen, E. 1957. Microbiological assay method for deoxyribonucleosides, deoxyribonucleotides and deoxyribonucleic acid. Methods Enzymol. 3:781-785. [Google Scholar]
- 14.Hoff-Jorgensen, E. 1952. Microbiological assay of desoxyribonucleosides and desoxyribonucleic acid. Biochem. J. 50:400-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Istock, C. A., N. Ferguson, N. L. Istock, and K. E. Duncan. 2001. Geographical diversity of genomic lineages in Bacillus subtilis (Ehrenberg) Cohn sensu lato. Org. Divers. Evol. 1:179-191. [Google Scholar]
- 16.Javaheri, M., G. E. Jenneman, M. J. McInerney, and R. M. Knapp. 1985. Anaerobic production of a biosurfactant by Bacillus licheniformis JF-2. Appl. Environ. Microbiol. 50:698-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenneman, G. E., M. J. McInerney, R. M. Knapp, J. B. Clark, J. M. Feero, D. E. Revus, and D. E. Menzie. 1983. A halotolerant, biosurfactant-producing Bacillus species potentially useful for enhanced oil recovery. Dev. Ind. Microbiol. 24:485-492. [Google Scholar]
- 18.Jordan, A., and P. Reichard. 1998. Ribonucleotide Reductases. Annu. Rev. Biochem. 67:71-98. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. M. L. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, S. C., M. A. Minton, M. M. Sharma, and G. Georgiou. 1994. Structural and immunological characterization of a biosurfactant produced by Bacillus licheniformis JF-2. Appl. Environ. Micro. 60:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundquist, A., D. Cheney, C. L. Powell, P. O'Niell, G. Norton, A. M. Veneman, D. L. Evans, N. Y. Minda, S. Abraham, J. M. Allbaugh, C. T. Whitman, J. A. Bolten, M. E. Daniels, L. B. Lindsey, and R. Barrales. 2001. Energy for a new century: increasing domestic energy production, p. 70-90. National energy policy report of the National Energy Policy Development Group. U.S. Government Printing Office, Washington, D.C.
- 22.McInerney, M. J., and D. W. S. Westlake. 1990. Microbial enhanced oil recovery, p. 409-445. In H. J. Ehrlich and C. Brierly (ed.), Microbial mineral recovery. MacMillan Publishing Co., New York, N.Y.
- 23.Munch-Petersen, A. 1983. Metabolism of nucleotides, nucleosides, and nucleobases in microorganisms. Academic Press, New York, N.Y.
- 24.Nakano, M. M., Y. P. Dailly, P. Zuber, and D. P. Clark. 1997. Characterization of anaerobic fermentative growth of Bacillus subtilis: identification of fermentation end products and genes required for growth. J. Bacteriol. 179:6749-6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhard, J., and P. Nygaard. 1987. Purines and pyrimidines, p. 445-473. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for microbiology, Washington, D.C.
- 26.Nygaard, P. 1993. Purine and pyrimidine salvage pathways, p. 359-378. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.
- 27.Ogawa, K.-I., E. Akagawa, K. Yamane, Z.-W. Sun, M. LaCelle, P. Zuber, and M. M. Nakano. 1995. The nasB operon and nasA gene are required for nitrate/nitrite assimilation in Bacillus subtilis. J. Bacteriol. 177:1409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed, R. L., and R. N. Healy. 1977. Some physical aspects of microemulsion flooding: a review, p. 383-437. In D. O. Shah and R. S. Scheckter (ed.), Improved oil recovery by surfactant and polymer flooding. Academic Press, Inc., New York, N.Y.
- 29.Reichard, P. 1993. From RNA to DNA, why so many ribonucleotide reductases? Science (Washington, D.C.) 260:1773-1777. [DOI] [PubMed] [Google Scholar]
- 30.Scotti, C., A. Valbuzzi, M. Prego, A. Galizzi, and A. M. Albertinni. 1996. Bacillus subtilis genes for ribonucleotide reductase are similar to the genes for the second class I NrdE/NrdF enzymes of Enterobacteriaceae. Microbiology 142:2995-3004. [DOI] [PubMed] [Google Scholar]
- 31.Sonenshein, A. L., J. A. Hoch, and R. Losick. 1993. Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.
- 32.Stubbe, J. 1998. Ribonucleotide reductases in the twenty-first century. Proc. Natl. Acad. Sci. USA 95:2723-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stubbe, J., J. Ge, and C. S. Yee. 2001. The evolution of ribonucleotide reduction revisited. Trends Biochem. Sci. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 34.Tanner, R. S. 1989. Monitoring sulfate-reducing bacteria: comparison of enumeration media. J. Microbiol. Methods 10:83-89. [Google Scholar]
- 35.West, C. C., and J. H. Harwell. 1992. Surfactants and subsurface remediation. Environ. Sci. Technol. 26:2324-2330. [Google Scholar]