Abstract
The contribution of fungi and bacteria to the decomposition of alder leaves was examined at two reference and two polluted sites in the Ave River (northwestern Portugal). Leaf mass loss, microbial production from incorporation rates of radiolabeled compounds into biomolecules, fungal biomass from ergosterol concentration, sporulation rates, and diversity of aquatic hyphomycetes associated with decomposing leaves were determined. The concentrations of organic nutrients and of inorganic nitrogen and phosphorus in the stream water was elevated and increased at downstream sites. Leaf decomposition rates were high (0.013 day−1 < k < 0.042 day−1), and the highest value was estimated at the most downstream polluted site, where maximum values of microbial production and fungal biomass and sporulation were found. The slowest decomposition occurred at the other polluted site, where, along with the nutrient enrichment, the lowest current velocity and dissolved-oxygen concentration in water were observed. At this site, fungal production, biomass, and sporulation were depressed, suggesting that stimulation of fungal activity by increased nutrient concentrations might be offset by other factors. Although bacterial production was higher at polluted sites, fungi accounted for more than 94% of the total microbial net production. Fungal yield coefficients varied from 10.2 to 13.6%, while those of bacteria were less than 1%. The contribution of fungi to overall leaf carbon loss (29.0 to 38.8%) greatly exceeded that of bacteria (4.2 to 13.9%).
Leaf litter decomposition in streams is an important ecosystem-level process (49), which depends on the activity of invertebrates and microorganisms (4). Both fungi and bacteria convert leaf carbon into microbial biomass, enhancing leaf palatability for shredding invertebrates (19).
Streams are naturally subjected to great spatial and temporal variability, which is likely to affect the distribution and activity of organisms. Several studies demonstrate that microbial activity and leaf decomposition in streams are regulated by leaf litter quality (39) and environmental factors such as temperature (10), concentration of dissolved nutrients (22, 41, 46), and pH (12).
Leaf litter decomposition in streams under stress has been a focus of interest over the last decades. A strong reduction in leaf mass loss as a result of the presence of either heavy metals (13, 33) or stream acidification (12) has been observed. Conversely, leaf decomposition tends to be faster at nutrient-enriched sites (23, 36, 37, 46), and increased concentrations of nitrogen and/or phosphorus have been reported to stimulate both fungal and bacterial activities on decomposing leaves (23). However, elevated nitrogen and phosphorus concentrations are often accompanied by oxygen depletion in aquatic systems with anthropogenic disturbances from urbanization and agriculture (9). Eutrophication can alter the relative abundance of species and the rates of processes affecting the community structure and/or ecosystem functioning (30, 49). Recently, Niyogi et al. (34) proposed a model in which biodiversity has a low threshold of response to anthropogenic stress whereas biomass and function are stable or increase under low to moderate stress and decrease only under high-stress conditions. However, the nature of the ecosystem response to stress may differ for different stressors, and further research is needed to better understand the ecological responses to multiple environmental stressors.
Fungi, in particular aquatic hyphomycetes, have been recognized as playing a dominant role in microbial decomposition of leaf litter in streams, whereas bacteria are though to increase their importance only after leaf material has been partially broken down (3, 50). A predominance of fungi in microbial decomposer assemblages has been found in streams (50), in large rivers (2, 3), and in nutrient enrichment experiments in either streams (23) or microcosms (24, 25). Much less is known about the relative contribution of fungi and bacteria to leaf decomposition in polluted streams.
Comparisons of fungal and bacterial biomass on leaves have been used to evaluate the decomposing activity of these microorganisms (3, 16, 23-26). However, biomass measurements may not give reliable information if microorganisms have high turnover rates and/or substantial losses of biomass occur, as detached bacterial cells or fungal sporulation and fragmentation (15). This limitation can be overcome by estimating the microbial production rate, which is a dynamic measure that reflects the specific microbial growth rate on leaf litter. Instantaneous fungal growth rates, determined from rates of [14C]acetate incorporation into ergosterol, have been used to estimate fungal production on decomposing leaves (2, 32, 47, 50) and can be directly compared with bacterial production estimated from either rates of [3H]leucine incorporation into protein (47, 50) or rates of [3H]thymidine incorporation into DNA (2, 15).
The aim of this work was to examine how stress from urbanization and industrial activities affects microbial decomposer assemblages and leaf decomposition process in the Ave River (northwestern Portugal). Two reference and two polluted sites were selected along a gradient of pollution characterized previously (37). Compared to reference sites, polluted sites had higher concentrations of organic and inorganic nutrients and differed with respect to current velocity and dissolved-oxygen concentration in water. It is expected that increased nutrient concentrations would stimulate microbial decomposing activity while low current velocity and oxygen depletion might cause the opposite effect. In addition, the relative contribution of fungi and bacteria to leaf decomposition could vary if they respond differently to the stress conditions. Leaf mass loss in fine-mesh bags, bacterial and fungal production, fungal biomass and sporulation, and diversity of aquatic hyphomycetes associated with decomposing leaves were determined during a 6-week study.
MATERIALS AND METHODS
Study sites and water analyses.
The Ave River is located in northwestern Portugal, in a region with high demographic density and several industrial units. The riparian vegetation is markedly affected by human activity, and exotic species such as Acacia spp. coexist with the dominant native species Alnus glutinosa (L.) Gaertn. Additional information about the river can be found in reference 37. Four sampling sites were selected along a 30-km stretch of the Ave River. The locations of three of them (L1, L2, and L7) was given previously (Fig. 1 in reference 37), and the fourth site (L6) is located ca. 1 km below the discharge of a tributary of the Ave River, the Vizela River, and ca. 7 km above L7.
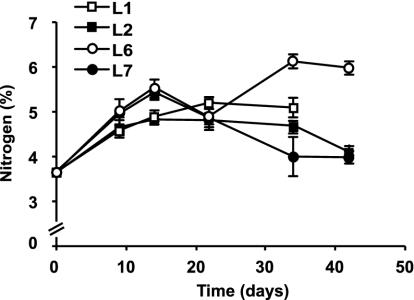
FIG. 1.
Nitrogen concentrations (percentage of leaf detrital AFDM) in alder leaves during decomposition at four sites (L1, L2, L6, and L7) in the Ave River. Values are means and SEM (n = 4).
At each sampling site, the temperature, pH, conductivity, and dissolved-oxygen concentration were measured in situ with field probes (Multiline F; WTW). The current velocity was determined with a flow meter (model 2030R; General Oceanics Inc.). Samples of stream water were collected into sterile glass bottles and kept cold (4°C) until analyzed; analyses were done within 24 h. The fecal coliform population was quantified by standard methods (1). The biochemical oxygen demand in 5 days (BOD5) was quantified by the manometer method (BSB controller model 1020T; WTW). A DR/2000 photometer (HACH Co., Loveland, Colo.) was used to estimate the chemical oxygen demand (COD) by dichromate digestion, nitrate concentration by cadmium reduction, ammonium concentration by the Nessler method, and orthophosphate concentration by the molybdate reagent, as described in the HACH procedures manual.
Leaf bags.
The study started on 16 May 2000 and continued for 6 weeks. Leaves of A. glutinosa were collected just before abscission, stored air dried, weighed into 6-g groups, and placed in fine-mesh bags (16 by 20 cm; 0.5-mm mesh). A total of 96 leaf bags were sealed and distributed among the four sampling sites. From each sampling site, four randomly selected replicates were retrieved after 9, 14, 22, 34, and 42 days of immersion and transported to the laboratory in a cool box. At the beginning of the study, an additional four leaf bags were retrieved from each site after 30 min of immersion to determine the initial mass of the leaves. In the laboratory, the leaves were rinsed in deionized water to remove debris and invertebrates when present and were cut into disks (12 mm in diameter). Leaf disks from three or four replicate bags were used to determine fungal and bacterial parameters or mass loss and nitrogen content of leaves, respectively.
Fungal sporulation, biomass, and production on decomposing leaves.
Sporulation of aquatic hyphomycetes was induced by aeration of 15 leaf disks from each replicate bag in 40 ml of filtered (pore size, 0.2 μm) stream water for 48 ± 4 h at 18°C. The suspension was mixed with 50 μl of 0.5% Tween 80 and filtered (pore size, 5 μm; Millipore). The retained spores were stained with cotton blue in lactic acid, identified, and counted at a magnification between ×160 and ×250. When possible, about 300 spores were counted per filter. Sporulation rates were converted to conidial carbon production based on values of conidial mass either available in the literature (10) or estimated by the method of Baldy et al. (2) as M = −0.058V2 + 641V where M is the conidial mass in femtograms and V is the conidial volume in cubic micrometers, and assuming a 50% carbon content in the conidial mass. Conidial volumes were calculated by the method of Bärlocher and Schweizer (6).
Fungal biomass was estimated from ergosterol concentration by using a conversion factor of 5.5 μg of ergosterol mg of fungal mycelium−1 (17) and assuming a fungal carbon content of 50% (47). Sets of six disks from each replicate bag were refluxed in 10 ml of 0.8% KOH-methanol for 30 min at 80°C. The resulting lipid extract was purified by solid-phase extraction (21) followed by high-performance liquid chromatography (HPLC; Beckman Golden System), using a LiChrospher RP-18 column (25 by 0.40 cm; Merck). The system was run isocratically with HPLC-grade methanol as the mobile phase (33°C; 1.4 ml min−1). Ergosterol was detected at 282 nm and quantified based on a standard curve of ergosterol (Sigma) in isopropanol.
Fungal production was determined from rates of [1-14C]acetate incorporation into ergosterol (20, 47), using a conversion factor of 19.3 μg of fungal biomass nmol of acetate incorporated−1 (47). Two sets of six leaf disks from each replicate bag were put into 25-ml Erlenmeyer flasks containing 4 ml of filter-sterilized stream water. In one set, microorganisms were killed by the addition of 200 μl of 37% formaldehyde 30 min before the incubation with [1-14C]acetate to determine the background level of radioactivity. The reaction was started by the addition of sodium [1-14C]acetate to a final concentration of 2.5 mM (specific activity, 48 MBq mmol−1 [Amersham]). Incubations were carried out for 2 h at 18°C on a shaker (100 rpm, 25-mm path; Certomat HK, B. Braun Biotech International), and the uptake was stopped by adding 200 μl of 37% formaldehyde. The flask contents were filtered (glass microfiber filters; GF/C Whatman), and the leaf disks washed twice with 4 ml of deionized water and placed in 0.8% KOH-methanol. Ergosterol was extracted and quantified as described above. The ergosterol fractions of two HPLC injections (100-μl sample loop) from each replicate were pooled into a vial containing 10 ml of scintillation fluid (Optiphase Hisafe 2; Perkin-Elmer) and stored overnight before the radioactivity was measured (Packard Tri-Carb 2200 CA).
Preliminary experiments showed that the isotope dilution was negligible, incorporation rates of [1-14C]acetate were linear for at least 4 h, and saturation of radiolabeled acetate had been achieved (data not shown).
Bacterial production on decomposing leaves.
Bacterial production was determined from the incorporation rates of l-[4,5-3H]leucine into protein (47). Three sets of four leaf disks per replicate were put into screw-top tubes containing 4 ml of filter-sterilized stream water. In one set, microorganisms were killed by the addition of 500 μl of 40% trichloroacetic acid (TCA) to determine the background level of radioactivity. In another set, the potential eukaryotic incorporation of leucine was controlled by the addition of 8% cycloheximide (10 μl) and 8% colchicine (5 μl) 1 h before the addition of radiolabeled leucine. The tubes were equilibrated for 10 min prior the addition of l-[4,5-3H]leucine (final concentration, 400 nM; specific activity, 142 GBq mmol−1; Amersham) and incubated at 18°C for 30 min with gentle mixing every 10 min. The reaction was stopped by the addition of 500 μl of 40% TCA. Bacterial cells were dislodged from the leaves by sonication (model 2510 sonication bath; Branson) for 5 min, and bacterial protein was extracted by heating in a water bath at 95°C for 30 min. The contents of the tubes were filtered (pore size, 0.2 μm; GTTP Millipore), and the leaf disks were washed three times with 4 ml of cold 5% TCA before discarded. The filters were washed twice with 4 ml of deionized cold water before being placed into scintillation vials, and the radioactivity was counted as indicated above. Bacterial production (BP) was calculated by the method of Kirchman (27) as BP = (L × F × C)/(P × D), where L is the incorporation rate of l-[4,5-3H]leucine, F is the formula weight of leucine, C is the ratio of cellular carbon to protein (0.86), P is the fraction of leucine in protein (0.073), and D is the ash-free dry mass (AFDM) of leaf disks.
Leaf mass loss and nitrogen content.
The remaining leaf material from each replicate bag was dried at 60°C to constant weight. Fifty leaf disks per replicate were ignited at 500°C to determine the AFDM (7). Leaf portions (ca. 500 mg) from each replicate bag were ground and used to determine the nitrogen content by the Kjedahl method (31).
Statistical analysis.
Rates of leaf decomposition (k) were estimated by linear regression after log-normal transformation as follows: ln (Wt/W0) = −kt + b, where Wt is the leaf AFDM remaining at time t, W0 is the initial AFDM, t is the time in days, and b is the y intercept. Regression lines were compared by analysis of covariance (ANCOVA) followed by a multiple-comparison Tukey test (51).
Differences in bacterial production, fungal biomass and production, aquatic hyphomycete sporulation rates, and stream water variables among sites were examined by either randomized-block analysis of variance (ANOVA) (51), with time as a block and sites as treatment factor, or one-way ANOVA (51) when peak values were considered. When differences were significant (P < 0.05), Tukey's test was used to determine where the differences occurred. Data were log-normal transformed whenever necessary to achieve normal distribution. For graphic presentation, nontransformed data (mean ± standard error of the mean (SEM)) were used.
ANCOVA and ANOVA were performed with the statistical package Prism 4.0 for Macintosh (GraphPad Software Inc., San Diego; Calif.).
Pearson correlation with Bonferroni adjustment for multiple comparisons (51) was used to examine the relationship between stream water variables, leaf decomposition rate, peak fungal biomass, production and sporulation rate, and peak bacterial production. Correlations were done with the statistical package SYSTAT 5.2.1 for Macintosh (SYSTAT Software Inc).
The ordination of sampling sites and dates was performed by correspondence analysis (CA) (29), based on average values (log-normal transformed data) of sporulation rates of aquatic hyphomycetes. The analysis was done with the statistical package ADE-4 for Macintosh (48).
RESULTS
Water parameters, leaf mass loss, and nitrogen dynamic.
The water of the Ave River had a high load of inorganic and organic nutrients (Table 1). Upstream sites (L1 and L2) differed from downstream sites (L6 and L7) with respect to temperature, conductivity, nitrate, ammonium and phosphate concentrations, COD, and fecal coliform density, with the highest values associated with the downstream sites (randomized-block ANOVA, P = 0.023 to <0.0001). The dissolved-oxygen concentration in the stream water was lowest at L6 (randomized-block ANOVA, P = 0.003), where the current flow was extremely low (<10 cm s−1). No statistical differences for pH and BOD5 were found among sites (randomized-block ANOVA, P = 0.73 and 0.08, respectively).
TABLE 1.
Physical, chemical, and microbial characteristics of stream water at four sites in the Ave River during the study period
| Characteristic | Value obtained at sitea:
|
|||
|---|---|---|---|---|
| L1 | L2 | L6 | L7 | |
| Temp (°C) | 17.6 (17.3-20.5) | 17.4 (16.1-18.1) | 20.3 (18.6-21.3) | 20.3 (18.6-21.3) |
| pH | 7.7 (6.0-8.1) | 7.4 (6.6-7.9) | 7.1 (6.9-7.3) | 7.2 (7.1-7.4) |
| Current velocity (cm s−1) | 22 (18-24) | <10 | <10 | 48 (28-80) |
| Conductivity (μS cm−1) | 72 (40-76) | 68 (48-91) | 286 (180-366) | 376 (215-482) |
| Dissolved O2 (mg liter−1) | 8.4 (8.1-8.7) | 6.9 (6.1-7.5) | 4.1 (2.9-5.3) | 7.4 (6.9-9.0) |
| COD (mg of O2 liter−1) | 14 (8-20) | 15 (9-20) | 36 (24-47) | 67 (33-100) |
| BOD5 (mg of O2 liter−1) | 3 (2-3) | 3 (2-4) | 9 (4-13) | 9 (4-14) |
| N-NH4+ (μg liter−1) | 54 (31-78) | 47 (23-70) | 420 (148-684) | 443 (264-622) |
| N-NO3 (μg liter−1) | 960 (790-1129) | 1,242 (677-1,355) | 2,552 (2,484-2,598) | 2,645 (2,597-2,710) |
| P-PO43− (μg liter−1) | 42 (29-52) | 29 (23-36) | 333 (150-512) | 408 (248-565) |
| Fecal coliforms (CFU ml−1) | 35 (10-60) | 13 (10-15) | 460 (240-680) | 580 (340-820) |
Data represent means (n = 4), with ranges in parentheses.
Decomposition rates of alder leaves (Table 2) were high, although they were markedly different among sites (ANCOVA, P < 0.0001). Decomposition was significantly faster at L7 (k = 0.042 day−1), intermediate at L1 and L2, and significantly slower at L6 (k = 0.013 day−1).
TABLE 2.
Decomposition rates of alder leaves at four sites in the Ave River
| Site | k (day−1)a | W0 (%)b | r2c | nd |
|---|---|---|---|---|
| L1 | 0.022 ± 0.0021a | 97.7 | 0.85 | 20 |
| L2 | 0.020 ± 0.0011a | 99.0 | 0.94 | 24 |
| L6 | 0.013 ± 0.0018b | 87.4 | 0.70 | 24 |
| L7 | 0.042 ± 0.0024c | 104.3 | 0.93 | 24 |
Values are means ± SEM. Similar letters indicate no significant differences (P ≥ 0.05) between leaf decomposition rates (ANCOVA, Tukey's test).
W0, initial AFDM of leaves.
r2, coefficient of determination.
n, number of samples.
The initial nitrogen concentration in alder leaves was 3.8% of leaf detrital AFDM (Fig. 1). The nitrogen concentration increased during the first 14 days at all sites and then declined, except at L6, where it continued to increase until day 34. Leaf nitrogen concentration was significantly higher at L6 than at the other sites (randomized-block ANOVA, P = 0.017; Tukey's test, P < 0.05).
Fungal biomass, production, and sporulation on decomposing leaves.
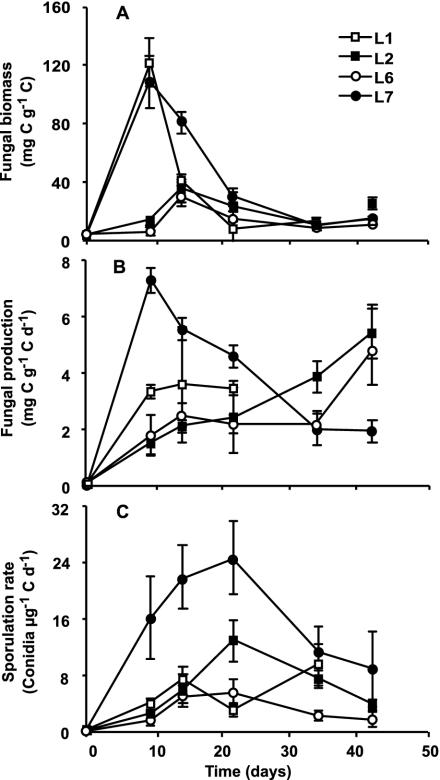
Fungal biomass on leaves in the Ave River reached a maximum after 9 days of decomposition at L1 and L7, corresponding to 120 and 108 mg of fungal C g of leaf C−1, respectively (Fig. 2A). The peaks of fungal biomass at L2 and L6 occurred later (14 days) and were significantly lower than those at the other sites (one-way ANOVA, P < 0.01; Tukey's test, P < 0.05 for all cases). Overall, leaves from L7 had significantly greater fungal biomass than those from L2 and L6 (randomized-block ANOVA, P = 0.004; Tukey's test, P < 0.05 for both cases) and no differences between either L2 and L6 or L1 and all the other sites were found (Tukey's test, P > 0.05 for all comparisons). Maximum fungal biomass was positively correlated with both dissolved-oxygen concentration in the stream water (r = 0.61, P = 0.05) and leaf decomposition rate (r = 0.63, P = 0.04).
FIG. 2.
Fungi associated with alder leaves during decomposition at four sites (L1, L2, L6, and L7) in the Ave River. (A) Fungal biomass estimated from ergosterol concentrations (milligrams of fungal carbon per gram of leaf detrital carbon). (B) Fungal production determined from rates of [1-14C]acetate incorporation into ergosterol (milligrams of fungal carbon per gram of leaf detrital carbon per day). (C) Sporulation rate of aquatic hyphomycetes (number of conidia per microgram of leaf detrital carbon per day). Values are means and SEM (n = 3).
Peaks of fungal production (Fig. 2B), estimated from rates of [1-14C]acetate incorporation into ergosterol, were significantly higher at L7 (7.2 mg of C g of C−1 day−1) than at L1 and L6 (one-way ANOVA, P = 0.012; Tukey's test, P < 0.05 for both comparisons). As a whole, fungal production was higher at L7, intermediate at L1, and lower at L2 and L6 (randomized-block ANOVA, P < 0.0001). A positive correlation was found between peak fungal production and phosphate concentration in the stream water (r = 0.73, P = 0.04).
Sporulation rates of aquatic hyphomycetes reached a peak after 22 days of leaf decomposition at all sites except L1 (Fig. 2C). Maximum sporulation was significantly higher at L7 (24 conidia μg of C−1 day−1), intermediate at L1 and L2, and lower at L6 (one-way ANOVA, P = 0.023). Overall, sporulation rates of aquatic hyphomycetes were significantly higher at L7 than at the other sites (randomized-block ANOVA, P < 0.0001; Tukey's test, P < 0.01). The maximum sporulation rate was significantly correlated with both leaf decomposition rate (r = 0.78, P = 0.03) and peak fungal production (r = 0.63, P = 0.04).
Total conidial net production ranged from 25.3 to 106.9 mg of C g of initial leaf C−1, while total fungal net production, determined from acetate incorporation rates into ergosterol, varied from 42.7 to 85.1 mg of C g of initial leaf C−1 (Table 3). Total fungal net production represented between 10.2% (L7) and 13.6% (L6) of the leaf carbon loss. The contribution of fungal assimilation to overall leaf carbon loss corresponded to 29.0 and 38.8% at L7 and L6, respectively (Table 3).
TABLE 3.
Microbial production and contribution of fungi and bacteria to alder leaf decomposition at four sites in the Ave Rivera
| Characteristicb | Value obtained at site:
|
|||
|---|---|---|---|---|
| L1c | L2 | L6 | L7 | |
| Leaf mass loss excluding microbial biomass (%) | 33.5 | 60.2 | 43.2 | 83.8 |
| Total conidial net production (mg of C g of initial leaf C−1) | 27.5 | 64.6 | 25.3 | 106.9 |
| Total fungal net production (mg of C g of initial leaf C−1) | 42.7 | 63.2 | 58.7 | 85.1 |
| Fungal yield coefficient (%) | 12.7 | 10.5 | 13.6 | 10.2 |
| Initial leaf C assimilated by fungi (%) | 12.2 | 18.1 | 16.8 | 24.3 |
| Contribution of fungi to overall leaf C loss (%) | 36.4 | 30.0 | 38.8 | 29.0 |
| Total bacterial net production (mg of C g of initial leaf C−1) | 0.8 | 2.0 | 3.5 | 3.0 |
| Bacterial yield coefficient (%) | 0.2 | 0.3 | 0.8 | 0.4 |
| Initial leaf C assimilated by bacteria (%) | 1.4 | 3.4 | 6.0 | 5.2 |
| Contribution of bacteria to overall leaf C loss (%) | 4.2 | 5.6 | 13.9 | 6.2 |
| Microbial contribution to overall leaf C loss (%) | 40.6 | 35.6 | 52.7 | 35.2 |
The total net production of conidia, fungi, and bacteria is the sum of daily production rates over the study period, assuming the mean daily rate between sampling dates.
Yield coefficient was calculated by dividing the production of either fungi or bacteria by leaf C loss. The amount of initial leaf C assimilated was calculated by dividing the production of fungi or bacteria by the growth efficiency: 35% for fungi (43) and 5.8% for bacteria (25). The contribution of fungal and bacterial assimilation to overall leaf C loss was estimated by dividing the assimilation of fungi and bacteria by leaf C loss.
Values estimated based on 22 days of leaf decomposition due to sample losses.
A total of 30 species of aquatic hyphomycetes on alder leaves were observed (Table 4). The highest fungal richness was found at L1 and L7 (25 species), and the lowest was found at L6 (20 species). At the sporulation peak, Flagellospora curta was one of the dominant species at all sites. At L6 and L7, species that also exhibited high relative abundances were Anguillospora filiformis, Clavariopsis aquatica, and Clavatospora longibrachiata. Articulospora tetracladia was a codominant species at L1 and L2, together with C. longibrachiata or A. filiformis, respectively.
TABLE 4.
Relative abundance of aquatic hyphomycete species on decomposing alder leaves at four sites in the Ave River at the time of sporulation peaks
| Species | % of conidia at site:
|
|||
|---|---|---|---|---|
| L1 | L2 | L6 | L7 | |
| Alatospora acuminata Ingold | 2.1 | 1.4 | 1.7 | 2.4 |
| Alatospora pulchella Marvanová | *a | * | ||
| Anguillospora crassa Ingold | 1.2 | * | ||
| Anguillospora filiformis Greath. | 0.7 | 18.8 | 10.7 | 15.3 |
| Articulospora tetracladia Ingold | 13.6 | 20.7 | 6.0 | |
| Clavariopsis aquatica De Wild | 4.6 | 1.7 | 13.5 | 10.9 |
| Clavatospora longibrachiata (Ingold) Marvanová and Sv. Nilsson | 25.7 | 0.4 | 15.4 | 14.9 |
| Culicidospora aquatica R. H. Petersen | * | |||
| Cylindrocarpon sp. | 8.3 | 9.5 | 3.7 | 5.9 |
| Dimorphospora foliicola Tubaki | 9.5 | |||
| Flagellospora curta J. Webster | 10.4 | 32.8 | 23.4 | 11.3 |
| Heliscella stellata (Ingold and V.J. Cox) Marvanová | * | * | 0.4 | * |
| Heliscus lugdunensis Sacc. and Thérry | 0.3 | 7.0 | 8.4 | 4.4 |
| Heliscus submersus H. J. Huds. | 0.7 | 1.3 | ||
| Heliscus tentaculus Umphlett | * | * | * | 0.2 |
| Lemonniera aquatica De Wild. | 2.4 | 1.3 | * | 1.8 |
| Lemonniera sp. (cf. filiformis R. H. Petersen ex Dyko) | 1.0 | 0.7 | ||
| Lunulospora curvula Ingold | * | 0.4 | 8.0 | |
| Mycofalcella calcarata Marvanová | 1.7 | * | * | 2.9 |
| Tetrachaetum elegans Ingold | 7.8 | 0.9 | 5.3 | 3.1 |
| Tetracladium marchalianum De Wild. | 0.7 | 0.3 | ||
| Tricellula sp. | 0.7 | |||
| Tricladium chaetocladium Ingold | 9.0 | 1.8 | * | 1.3 |
| Tricladium splendens Ingold | 0.5 | * | ||
| Tripospermum myrti (Lind) S. Hughes | * | 0.8 | * | |
| Tripospermum camelopardus Ingold | * | 0.2 | ||
| Triscelophorus sp. | * | 0.3 | 1.9 | 0.3 |
| Varicosporium elodeae W. Kegel | 0.7 | 4.9 | 7.6 | |
| Sigmoid 1 (40-60 μm) | * | * | * | |
| Sigmoid 2 (15-25 μm) | 1.3 | 4.8 | 1.6 | |
| Sum | 100 | 100 | 100 | 100 |
| No. of species at sporulation peak | 18 | 16 | 14 | 20 |
| Total no. of species | 25 | 21 | 20 | 25 |
*, present on other sampling dates.
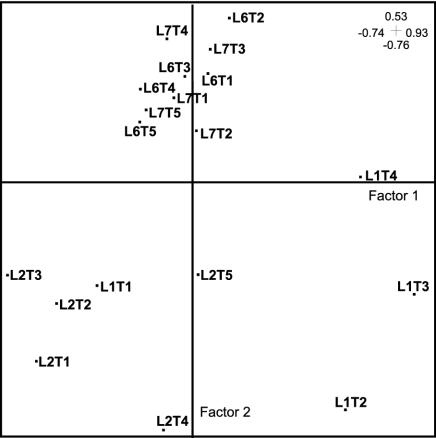
Results from CA ordination of the sampling sites and dates based on aquatic hyphomycete assemblages on decomposing alder leaves are shown in Fig. 3. Factor 2 explained 17.7% of the total variance and separated upstream (L1 and L2) from downstream (L6 and L7) sites, while factor 1 explained 19.9% of the total variance and mainly separated L1 from L2. In addition, there was considerable overlap between L6 and L7, suggesting great similarity in the structures of their aquatic hyphomycete assemblages on leaves.
FIG. 3.
CA of sampling sites and dates based on aquatic hyphomycete assemblages associated with decomposing alder leaves in the Ave River. L1, L2, L6, and L7, sampling sites; T1 = 9 days, T2 = 14 days, T3 = 22 days, T4 = 34 days, and T5 = 42 days. Factors 1 and 2 accounted for 19.9 and 17.7% of the total variance, respectively.
Bacterial production on decomposing leaves.
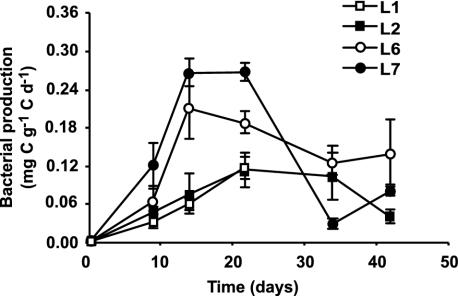
Addition of eukaryotic inhibitors did not significantly change the bacterial production estimated from incorporation rates of l-[4,5-3H]leucine into protein (data not shown). Bacterial production on decomposing leaves reached maximum values between 14 and 22 days, corresponding to 0.22 and 0.28 mg of C g of C−1 day−1 at L6 and L7, respectively (Fig. 4). Differences in bacterial production were significant between upstream (L1 and L2) and downstream (L6 and L7) sites, for both peak values (one-way ANOVA, P = 0.0001, Tukey's test, P < 0.01 for those comparisons) and complete data sets (randomized-block ANOVA, P = 0.001; Tukey's test, P < 0.05 for those comparisons). Bacterial production was significantly correlated with nitrate concentration (r = 0.62, P = 0.04), COD (r = 0.74, P = 0.03), and fecal coliforms (r = 0.73, P = 0.03).
FIG. 4.
Bacterial production, determined from the rates of l-[4,5-3H]leucine incorporation into protein (milligram of bacterial carbon per gram of leaf detrital carbon per day), on alder leaves during decomposition at four sites (L1, L2, L6, and L7) in the Ave River. Values are means and SEM (n = 3).
At downstream sites, the total bacterial net production was 3.0 and 3.5 mg of C g of initial leaf C−1, which accounted for 0.4 and 0.8% of the leaf carbon loss at L7 and L6, respectively (Table 3). The percentage of the initial leaf carbon assimilated by bacteria reached a maximum at L6 (6.0%) and corresponded to 13.9% of the overall leaf carbon loss. In addition, the contribution of bacterial assimilation to overall leaf carbon loss was at least twice as great at L6 as at the other sites.
DISCUSSION
Leaf decomposition in a polluted river.
The decomposition rate of alder leaves in the Ave River varied from 0.013 to 0.042 day−1 and was within the range of values reported for this leaf species by other authors (8, 14, 18, 36, 40). Leaf decomposition was generally faster in spring (this study) than in the previous autumn (37), which may be due to the stimulation of microbial decomposing activity by higher temperatures.
The concentration of inorganic and organic nutrients in the stream water was high and was significantly increased at downstream sites. Previous studies conducted at six sites in the same reach of the Ave River, which did not include L6, showed a longitudinal gradient of pollution that was positively correlated with rates of leaf breakdown (37). In the present study, the fastest leaf decomposition (k = 0.042 day−1) was also found at the farthest downstream polluted site (L7). Accelerated leaf decomposition due to nutrient enrichment has been found in laboratory (45), stream (23, 36, 46), and mixed (41) studies. Despite the high organic and inorganic load at L6, the lowest leaf decomposition rate (k = 0.013 day−1) was found at this site. The most striking differences between L6 and L7 were the current velocity and dissolved oxygen in the stream water, which were much lower at L6. In addition, bags taken at later sampling dates from this site were filled with mud and leaves had a dark color, suggesting the presence of hypoxic conditions, which could have inhibited leaf decomposition. These findings are in agreement with the expected pattern in a sewage-impacted stream, which involves slower leaf breakdown just below the sewage effluent as a result of low levels of dissolved oxygen and accelerated breakdown further downstream as a result of nutrient enrichment (49).
In this work, the initial nitrogen concentration in alder leaves was higher than generally reported for this leaf species (see, e.g., reference 40), and it further increased during the first 14 days of leaf decomposition at all sites. The magnitude of the increase was greater at the most nutrient-enriched sites, as reported by other authors (23, 44), supporting earlier evidence that the major source of nitrogen for fungi growing in leaves is the water (23, 46). Immobilization of nitrogen in leaves has been at least partially attributed to the accumulation of microbial biomass (49). At L7, the initial increase in nitrogen concentration in leaves was associated with high microbial biomass and production and the subsequent decrease in nitrogen concentration was probably due to release of large numbers of conidia (see 44). In contrast, the high nitrogen concentration in leaves at L6 could not be fully explained by microbial immobilization, since low fungal biomass and production were found at this site. However, nitrogen immobilization may also result from the formation of complexes between nitrogen and other compounds (e.g., lignin) in leaves (35).
Fungal diversity and activity on decomposing leaves.
Significant correlations between leaf decomposition rate and fungal parameters, namely, maximum sporulation rate and biomass, were found in this study, in agreement with the results obtained by other authors (18, 46). In the Ave River, peaks of fungal biomass on alder leaves (32 to 120 mg of C g of C−1) were within the range found in other streams (13, 14, 26) while the peak of sporulation at the most downstream nutrient-enriched site was extremely high (24 conidia μg of C−1 day−1), surpassing the highest values reported for alder leaves (ca. 14 conidia μg of C−1 day−1 [18]). In the Ave River, peaks of fungal production (3.6 to 7.2 mg of C g of C−1 day−1) were higher than those measured in a large river (2) but lower than the maximum reported in the literature (13, 44). In the present study, a great part of fungal production was allocated to reproduction, and at the farthest-downstream site, total conidial net production (106.9 mg of C g of initial leaf C−1) even exceeded total fungal net production estimated from the acetate incorporation method (Fig. 2; Table 3). This finding was not expected, since the acetate method takes into account the amount of biomass being lost as conidia. Whether that disparity was caused by (i) an overproduction of conidia under laboratory conditions, (ii) the use of an inappropriate conversion factor, relating acetate incorporation rate and biomass production, or (iii) a greater peak of fungal production before the first sampling date, as suggested by the decline in fungal biomass and production after that time, remains an open question. Nevertheless, maximum fungal production was correlated with maximum sporulation rate, suggesting that both measures were suitable indicators of fungal activity on decomposing leaves in the Ave River.
Higher fungal biomass, production, and sporulation rates of aquatic hyphomycetes were found at the most downstream nutrient-enriched site (L7), which could have contributed to the fastest leaf decomposition. In spite of the high nutrient concentration, fungal activity was inhibited at L6, which was consistent with the observed slower leaf decomposition. These results suggested that the stimulation of fungal activity on leaves by increased nutrient concentrations in the stream water might be offset by other factors. For the Ave River, low current velocity and decreased dissolved oxygen seemed to be important factors affecting fungal activity and leaf decomposition.
The species richness of aquatic hyphomycetes associated with alder leaves at studied sites (20 to 25 species) was similar to that found in a nonpolluted stream from central Portugal (5) and slightly higher than that observed during the previous autumn in the Ave River (37). Although aquatic hyphomycetes have been associated with clean and well-aerated waters (4) and low diversity has been reported in an organically polluted stream (3 or 4 species [38]), they appear to be rather well represented in streams with either heavy-metal (13 or 14 species [42]) or organic and inorganic (18 to 23 species [37]) pollution. CA ordination of sampling sites and dates on the basis of aquatic hyphomycete assemblages on leaves separated reference (L1 and L2) from polluted (L6 and L7) sites, suggesting that shifts in the structure of fungal communities were associated with changes in the water chemistry. Since similar dominant fungal species were found at L6 and L7, the species identity per se did not seem to account for the differences in fungal decomposing activity at polluted sites.
Relative contribution of fungi and bacteria to leaf decomposition in a polluted river.
In the Ave River, peaks of bacterial production (0.12 to 2.80 mg of C g of leaf C−1 day−1), determined from incorporation rates of [3H]leucine into protein, were within the range of values estimated by others using either this method (28, 50) or [3H]thymidine incorporation into DNA (2). Bacterial production on decomposing leaves in the Ave River was significantly higher downstream and was positively correlated with COD and nitrate. This finding agrees with the stimulation of bacterial activity on leaves by increased concentrations of nutrients (23). Although total bacterial net production tended to be higher at polluted sites (3.0 and 3.5 mg of C g of initial leaf C−1 at L7 and L6, respectively), fungal production was always much greater, accounting for 94.4 to 98.2% of the total microbial production. Similarly, a more extensive role of fungi than of bacteria in microbial assemblages associated with leaf litter decomposing in freshwaters has been reported, regardless of whether biomass (16, 23) or production (2, 50) is considered.
The fraction of leaf carbon converted into fungal production on decomposing leaves differed among streams (11 to 15%, [44] and 1.5 to 7.5% [50]). In this study, fungal yield coefficients varied from 10.2 to 13.6% whereas those for bacteria were less than 1%. Data in the literature also point to low bacterial yield coefficients, e.g., 0.09 to 1.84%, with greater values at higher nutrient concentrations and in the absence of fungi (24, 25), indicating that only a small portion of the leaf is channeled to bacterial production. However, the contribution of bacteria to leaf decomposition might not be neglected if there is intense bacterial respiration. Unfortunately, little is known about the magnitude of bacterial respiration on decomposing leaves in freshwater environments and about the factors that regulate bacterial growth efficiency, i.e., the relationship between bacterial production and respiration. Recently, Gulis and Suberkropp (25) found bacterial growth efficiencies on decomposing leaves of 1.6 and 5.8%, with the greater value occurring at the highest nutrient concentration. If one assumes a bacterial growth efficiency of 5.8%, the contribution of bacterial assimilation to leaf decomposition in the Ave River would vary from 4.2 to 13.9%, with the highest value at L6, representing 26.4% of the total microbial contribution. This estimate suggests that bacteria may make a greater contribution to leaf litter decomposition in polluted rivers, particularly when fungal activity is depressed. However, the most common values for bacterial growth efficiency in aquatic environments range from 10 to 30% (11). Thus, if bacterial growth efficiency in the Ave River was higher than that assumed in this work, the contribution of this group of microorganisms to leaf decomposition would be overestimated. Bacteria explained 9 to 13% of overall leaf mass loss in a third-order stream, where shredders and fungi accounted for 51 to 64% and 15 to 18%, respectively (26). In the Ave River, the contribution of fungi to overall leaf carbon loss was higher (29.0 to 38.8%), but it was lower than that found in a large river (41.9 to 65.5% [3]). Fungi accounted for 73.6 to 89.7% of the total microbial contribution to overall leaf carbon loss, suggesting that fungi were actually the main agents of leaf decomposition in this polluted river. Since invertebrates were excluded from the present experimental design, a major contribution of microorganisms to leaf carbon loss might be expected. The presence of microbial assemblages on decomposing alder leaves explained 40.6 to 52.7% of leaf carbon loss. The remaining 47.3 to 59.4% was probably lost as dissolved organic carbon and fine particulate organic carbon.
Acknowledgments
This work was supported by Portuguese project grant POCTI/34024/BSE/2000.
We are grateful to M. A. S. Graça, V. Gulis, and the anonymous reviewers for helpful comments on the manuscript.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 2.Baldy, V., E. Chauvet, J.-Y. Charcosset, and M. O. Gessner. 2002. Microbial dynamics associated with leaves decomposing in the mainstem and floodplain pond of a large river. Aquat. Microb. Ecol. 28:25-36. [Google Scholar]
- 3.Baldy, V., M. O. Gessner, and E. Chauvet. 1995. Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos 74:93-102. [Google Scholar]
- 4.Bärlocher, F. 1992. The ecology of aquatic hyphomycetes. Springer-Verlag KG, Berlin, Germany.
- 5.Bärlocher, F., C. Canhoto, and M. A. S. Graça. 1995. Fungal colonization of alder and eucalypt leaves in two streams in central Portugal. Arch. Hydrobiol. 133:457-470. [Google Scholar]
- 6.Bärlocher, F., and M. Schweizer. 1983. Effects of leaf size and decay rate on colonization by aquatic hyphomycetes. Oikos 41:205-210. [Google Scholar]
- 7.Benfield, E. F. 1996. Leaf breakdown in stream ecosystems, p. 579-589. In F. R. Hauer and G. A. Lamberti (ed.), Methods in stream ecology. Academic Press, Ltd., London, United Kingdom.
- 8.Canhoto, C., and M. A. S. Graça. 1996. Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333:79-85. [Google Scholar]
- 9.Carpenter, R. S., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley, and V. H. Smith. 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 8:559-568. [Google Scholar]
- 10.Chauvet, E., and K. Suberkropp. 1998. Temperature and sporulation of aquatic hyphomycetes. Appl. Environ. Microbiol. 64:1522-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, J. J. 1999. Aquatic microbiology for ecosystem scientists: new and recycled paradigms in ecological microbiology. Ecosystems 2:215-225. [Google Scholar]
- 12.Dangles, O., and E. Chauvet. 2003. Effects of stream acidification on fungal biomass in decaying beech leaves and leaf palatability. Water Res. 37:533-538. [DOI] [PubMed] [Google Scholar]
- 13.Duarte, S., C. Pascoal, and F. Cássio. Effects of zinc on leaf decomposition by fungi in streams: studies in microcosms. Microb. Ecol., in press. [DOI] [PubMed]
- 14.Fabre, E., and E. Chauvet. 1998. Leaf breakdown along an altitudinal stream gradient. Arch. Hydrobiol. 141:167-179. [Google Scholar]
- 15.Findlay, S. E. G., and R. D. Arsuffi. 1989. Microbial growth and detritus transformations during decomposition of leaf litter in a stream. Freshwater Biol. 21:261-269. [Google Scholar]
- 16.Findlay, S., J. Tank, S. Dye, H. M. Valett, P. J. Mulholland, W. H. McDowell, S. L. Johnson, S. K. Hamilton, J. Edmonds, W. K. Dodds, and W. B. Bowden. 2002. A cross-system comparison of bacterial and fungal biomass in detritus pools of headwater streams. Microb. Ecol. 43:55-66. [DOI] [PubMed] [Google Scholar]
- 17.Gessner, M. O., and E. Chauvet. 1993. Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl. Environ. Microbiol. 59:502-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gessner, M. O., and E. Chauvet. 1994. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807-1817. [Google Scholar]
- 19.Gessner, M. O., E. Chauvet, and M. Dobson. 1999. A perspective on leaf litter breakdown in streams. Oikos 85:377-384. [Google Scholar]
- 20.Gessner, M. O., and S. Y. Newell. 2002. Biomass, growth rate, and production of filamentous fungi in plant litter, p. 390-408. In C. J. Hurst, R. L. Crawford, C. J. Knudsen, M. J. McInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 21.Gessner, M. O., and A. L. Schmitt. 1996. Use of solid-phase extraction to determine ergosterol concentrations in plant tissue colonized by fungi. Appl. Environ. Microbiol. 62:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grattan, R. M., and K. Suberkropp. 2001. Effects of nutrient enrichment on yellow poplar leaf decomposition and fungal activity in streams. J. North Am. Benthol. Soc. 20:33-43. [Google Scholar]
- 23.Gulis, V., and K. Suberkropp. 2003. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshwater Biol. 48:123-134. [Google Scholar]
- 24.Gulis, V., and K. Suberkropp. 2003. Effect of inorganic nutrients on relative contributions of fungi and bacteria to carbon flow from submerged decomposing leaf litter. Microb. Ecol. 45:11-19. [DOI] [PubMed] [Google Scholar]
- 25.Gulis, V., and K. Suberkropp. 2003. Interactions between stream fungi and bacteria associated with decomposing leaf litter at different levels of nutrient availability. Aquat. Microb. Ecol. 30:149-157. [Google Scholar]
- 26.Hieber, M., and M. O. Gessner. 2002. Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83:1026-1038. [Google Scholar]
- 27.Kirchman, D. L. 1993. Leucine incorporation as a measure of biomass production by heterotrophic bacteria, p. 509-512. In P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 28.Kuehn, K. A., M. J. Lemke, K. Suberkropp, and R. G. Wetzel. 2000. Microbial biomass and production associated with decaying leaf litter of the emergent macrophyte Juncus effusus. Linmol. Oceanogr. 45:862-870. [Google Scholar]
- 29.Legendre, P., and L. Legendre. 1998. Numerical ecology, p. 387-476. In Developments in environmental modelling, vol. 20, 2nd ed. Elsevier Science BV, Amsterdam, The Netherlands.
- 30.Maltby, L. 1992. Heterotrophic microbes, p. 165-187. In P. Calow and G. E. Petts (ed.), The rivers handbook: hydrological and ecological principles, vol. 1. Blackwell Scientific Publications Ltd., Oxford, United Kingdom.
- 31.Mills, H. A., and J. B. Jones, Jr. 1996. Plant analysis handbook, vol. II. MicroMacro Publishing Inc., Athens, Ga.
- 32.Newell, S. Y., and R. D. Fallon. 1991. Toward a method for measuring instantaneous fungal growth rates in field samples. Ecology 72:1547-1559. [Google Scholar]
- 33.Niyogi, D. K., W. M. Lewis, Jr., and D. M. McKnight. 2001. Litter breakdown in mountain streams affected by mine drainage: biotic mediation of abiotic controls. Ecol. Appl. 11:506-516. [Google Scholar]
- 34.Niyogi, D. K., W. M. Lewis, Jr., and D. M. McKnight. 2002. Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 5:554-567. [Google Scholar]
- 35.Odum, W. E., P. W. Kirk, and J. C. Zieman. 1978. Nonprotein nitrogen compounds associated with particles of vascular plant detritus. Oikos 32:363-367. [Google Scholar]
- 36.Pascoal, C., F. Cássio, and P. Gomes. 2001. Leaf breakdown rates: a measure of water quality? Int. Rev. Hydrobiol. 86:407-416. [Google Scholar]
- 37.Pascoal, C., M. Pinho, F. Cássio, and P. Gomes. 2003. Assessing structural and functional ecosystem condition using leaf breakdown: studies on a polluted river. Freshwater Biol. 48:2033-2044. [Google Scholar]
- 38.Raviraja, N. S., K. R. Sridhar, and F. Bärlocher. 1998. Breakdown of Ficus and Eucalyptus leaves in an organically polluted river in India: fungal diversity and ecological functions. Freshwater Biol. 39:537-545. [Google Scholar]
- 39.Royer, T. V., and G. W. Minshall. 2001. Effects of nutrient enrichment and leaf quality on the breakdown of leaves in a hardwater stream. Freshwater Biol. 46:603-610. [Google Scholar]
- 40.Sampaio, A., R. Cortes, and C. Leão. 2001. Invertebrate and microbial colonisation in native and exotic leaf litter species in a mountain stream. Int. Rev. Hydrobiol. 86:527-540. [Google Scholar]
- 41.Sridhar, K. R., and F. Bärlocher. 2000. Initial colonization, nutrient supply, and fungal activity on leaves decaying in streams. Appl. Environ. Microbiol. 66:1114-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sridhar, K. R., G. Krauss, F. Bärlocher, N. S. Raviraja, R. Wennrich, R. Baumbach, and G.-J. Krauss. 2001. Decomposition of alder leaves in two heavy-metal-polluted streams in central Germany. Aquat. Microb. Ecol. 26:73-80. [Google Scholar]
- 43.Suberkropp, K. 1991. Relationships between growth and sporulation of aquatic hyphomycetes on decomposing leaf litter. Mycol. Res. 95:843-850. [Google Scholar]
- 44.Suberkropp, K. 1995. The influence of nutrients on fungal growth, productivity, and sporulation during leaf breakdown in streams. Can. J. Bot. 73:1361-1369. [Google Scholar]
- 45.Suberkropp, K. 1998. Effect of dissolved nutrients on two aquatic hyphomycetes growing on leaf litter. Mycol. Res. 102:998-1002. [Google Scholar]
- 46.Suberkropp, K., and E. Chauvet. 1995. Regulation of leaf breakdown by fungi in streams: influences of water chemistry. Ecology 76:1433-1445. [Google Scholar]
- 47.Suberkropp, K., and H. S. Weyers. 1996. Application of fungal and bacterial production methodologies to decomposing leaves in streams. Appl. Environ. Microbiol. 62:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thioulouse, J., D. Chessel, S. Dolédec, and J.-M. Olivier. 1997. ADE-4: a multivariate analysis and graphical display software. Stat. Comput. 7:75-83. [Google Scholar]
- 49.Webster, J. R., and E. F. Benfield. 1986. Vascular plant breakdown in freshwater ecosystems. Annu. Rev. Ecol. Syst. 17:567-594. [Google Scholar]
- 50.Weyers, H. S., and K. Suberkropp. 1996. Fungal and bacterial production during the breakdown of yellow poplar leaves in 2 streams. J. North Am. Benthol. Soc. 15:408-420. [Google Scholar]
- 51.Zar, J. H. 1996. Biostatistical analysis, 3rd ed. Prentice-Hall, Englewood Cliffs, N.J.