Abstract
Phylogenetic analysis of 16S rRNA gene sequences from deep marine sediments identified a deeply branching clade, designated candidate division JS1. Primers for PCR amplification of partial 16S rRNA genes that target the JS1 division were developed and used to detect JS1 sequences in DNA extracted from various sedimentary environments, including, for the first time, coastal marine and brackish sediments.
A significant microbial biomass exists buried deep within the sediments of the world's oceans (20, 35). Cultivation of organisms from this deep marine biosphere is difficult (2, 10), but it has been the focus of several molecular microbial ecology studies to investigate prokaryotic diversity (8, 11, 14, 17, 25, 26, 34). Studies have revealed a previously unknown and diverse prokaryotic microbial community within this deep biosphere. Many 16S rRNA gene sequences retrieved from these subseafloor sediments belong to previously unidentified and uncultured groups of organisms, some of which have no clear phylogenetic affiliation (9, 11, 14, 25, 26).
Recent observations report novel sequences representing similar taxa in near-surface, subsurface, and gas hydrate-bearing sediments from deep marine sites such as the Guaymas Basin (4, 31), the Japan Trench (14), the Nankai Trough and forearc basin (11, 19, 25), and the Gulf of Mexico (13). Some of these sequences fall into a single group which has been variously named, as follows: JAP504 cluster (26), OP9 associated (9, 31), methane- and hydrocarbon-rich sediment group (4), hydrocarbon associated (11), and the deep sediment group (19). However, this group has not yet been firmly placed phylogenetically at the division level within the domain Bacteria (3, 19). Sequences from the group were originally found in sediment from the Japan Sea (26) and dominated 16S rRNA gene libraries from sediment of the Nankai Trough (19, 34) and the Sea of Okhotsk (9), suggesting that the organisms from which these sequences derive play an important role in biogeochemical processes of deep marine sediments.
In this paper we have investigated the phylogenetic assignment of these 16S rRNA gene sequences from our own studies and from others reported elsewhere. We present evidence to suggest that they constitute a novel, deeply branching monophyletic clade within the domain Bacteria, which we propose to be called the candidate division JS1 in recognition of the Japan Sea as the first reported source of these sequences (26). Furthermore, we report the design and use of 16S rRNA gene PCR primers for amplification of JS1 candidate division sequences from deep subseafloor sediments and other environments.
Deep sediment samples were collected as whole round cores from locations in the Pacific Ocean by the Ocean Drilling Program (ODP) Legs 190 and 201 (Nankai Trough and Peru Margin) (27, 28) and the Chile Continental Margin (ChCM), Cruise SO156. In addition, soil, peat, river water, seawater, and marine sediment were collected from various locations around the United Kingdom and elsewhere (see Table 1). DNA was extracted directly from samples with use of the FastDNA spin kit for soil (Bio 101, Vista, Calif.) with the modifications described by Webster et al. (34).
TABLE 1.
Location of environmental samples used in this study and the presence or absence of JS1 candidate division 16S rRNA gene sequences determined by nested PCR (63F-665R and 357FGC-518R)a
| Sample (location) | Environment | PCR product of expected size observed from primersb
|
Identification of JS1 sequencec | |
|---|---|---|---|---|
| 63F-665R | 357F-518Rd | |||
| Nankai Trough site 1173, Japan (32° 14.6′N, 135°1.5′E) | Marine sediment, 4.15 mbsf, 4,791 mbsl | + | + | + |
| Peru Margin site 1228 (11°3.8S, 78°4.7′W) | Marine sediment, 25.75 mbsf, 262 mbsl | − | + | + |
| Peru Margin site 1229 (10°58.5′S, 77°57.4′W) | Marine sediment, 86.67 mbsf, 152 mbsl | +/− | + | + |
| ChCM core GeoB 7112-3 (24°2.0′S, 70°49.4′W) | Marine sediment, 2.2 mbsf, 2,507 mbsl | +/− | + | + |
| ChCM core GeoB 7132-5 (29°28.0′S, 71°53.4′W) | Marine sediment, 1.0 mbsf, 3,248 mbsl | +/− | + | + |
| ChCM core GeoB 7190-3 (44°16.9′S, 75°51.9′W) | Marine sediment, 3.1 mbsf, 3,285 mbsl | +/− | + | + |
| Penarth, Bristol Channel, UK (51°26.3′N, 3°9.4′W) | Marine channel surface sediment, 0- to 30-cm depth | + | + | + |
| Beaulieu, UK (50°49.1′N, 1°27.2′W) | Brackish sediment, 35-cm depth | + | + | + |
| Arne Peninsula, UK (50°41.6′N, 2°1.6′W) | Salt marsh, 30-cm depth | + | + | + |
| Arabian Sea (23°46.0′N, 59°52.0′E) | Seawater, 10 mbsl | − | + | − |
| River Taff, UK (51°31.4′N, 3°15.1′W) | River water with freshwater sediment | + | + | + |
| Chartley Moss, UK (52°51.0′N, 1°57.5′W) | Peatland, 35-cm depth | − | + | − |
| Cardiff University, UK (51°29.1′N, 3°10.5′W) | Garden soil, 0- to 20-cm depth | + | + | − |
Abbreviations: UK, United Kingdom; mbsf, meters below seafloor; mbsl, meters below sea level.
+, +/−, and −, high yield, low yield, and no PCR product, respectively.
JS1 sequence type was determined by sequencing excised DGGE bands.
Nested PCR amplification with use of primers 63F-665R and 357FGC-518R.
16S rRNA gene sequences thought to belong to the candidate division JS1, including those previously reported to be associated with the OP9 candidate division (3, 31) and the deep sediment group (19), were retrieved from the National Center for Biotechnology Information database along with other related nucleotide sequences and analyzed for possible priming sequences with use of the computer software programs PRIMROSE (1) and OligoCheck version 1.0 (www.cf.ac.uk/biosi/research/biosoft/). The reverse primer sequence 665R (5′-ACC GGG AAT TCC ACY TYC CT-3′), corresponding to Escherichia coli 16S rRNA nucleotide positions 665 to 684, was identified as having the best potential for specific detection of JS1 sequences. When used in combination with the forward primer 63F (16) a 640-bp PCR product was obtained with the deep sediment bacterial group clone sequences NankB-5, NankB-7, and NankB-14, previously obtained from the Nankai Trough (19). PCR amplification conditions were optimized further by using DNA from the above clones and from DNA preparations of Pseudomonas putida strain PP3, Halobacterium sp., and Desulfovibrio desulfuricans subsp. desulfuricans (DSM 1924) as negative controls, in a DNA Engine Dyad Thermal Cycler gradient block (MJ Research, Boston, Mass.). The reaction mixture consisted of 0.2 pmol of primers μl−1, 1 μl of DNA template (≤30 pg [deep sediment] to 1 to 2 ng [coastal sediment]), 1× reaction buffer (Bioline, London, United Kingdom), 1.5 mM MgCl2, 1.5 U of Biotaq DNA polymerase (Bioline), 0.125 mM (each) deoxynucleoside triphosphates, and 10 μg of bovine serum albumin in a 50-μl PCR mixture with molecular-grade water (Severn Biotech Ltd., Kidderminster, United Kingdom). The optimized thermal cycling protocol used was an initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and elongation at 72°C for 45 s, with a final elongation step of 5 min at 72°C. PCR operations were carried out under sterile conditions, and all disposable plasticware was autoclaved and UV treated prior to use.
PCR products amplified from DNA extracted from the ChCM, sediment core GeoB 7132-5, were cloned using the plasmid vector pGEM-T Easy (Promega, Madison, Wis.). Recombinant plasmids were purified from selected clones and sequenced in both directions with the vector-specific M13F and 665R primers in an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, Calif.) according to the manufacturer's protocol. PCR products obtained from DNA extracted from other samples were analyzed by denaturing gradient gel electrophoresis (DGGE). Nested PCR amplification with bacterial 16S rRNA gene primers 357FGC and 518R (18) was carried out on the 640-bp amplimers as template in a 50-μl reaction mix as described above without bovine serum albumin. Thermal cycling conditions and DGGE analysis were as described previously (33, 34). Dominant DGGE bands were excised and sequenced with the 518R primer, and partial bacterial 16S rRNA gene sequences were analyzed to identify sequences with highest identity (33, 34).
Phylogenetic analysis to resolve the division-level relationship of clade JS1 was undertaken on 16S rRNA sequences (>1,400 nucleotides) available in the public databases under the accession numbers listed in Fig. 1. Sequences were aligned using ClustalX (32) and edited manually using GDE running within the ARB software package (www.arb-home.de). Regions of ambiguous alignment were removed, leaving 1,180 sites. The LogDet/Paralinear distances (12) was used as the primary tool for estimating phylogenetic relationships, but other methods including maximum parsimony gave similar tree topologies. LogDet/Paralinear distances of variable sites (15) was used and implemented in PAUP*4.0b 10 (30). The maximum-likelihood method was used to estimate the proportion of invariable sites (6). All LogDet/Paralinear distances trees were constructed by using the minimum evolution criterion, and the data were bootstrapped 1,000 times to assess support for nodes.
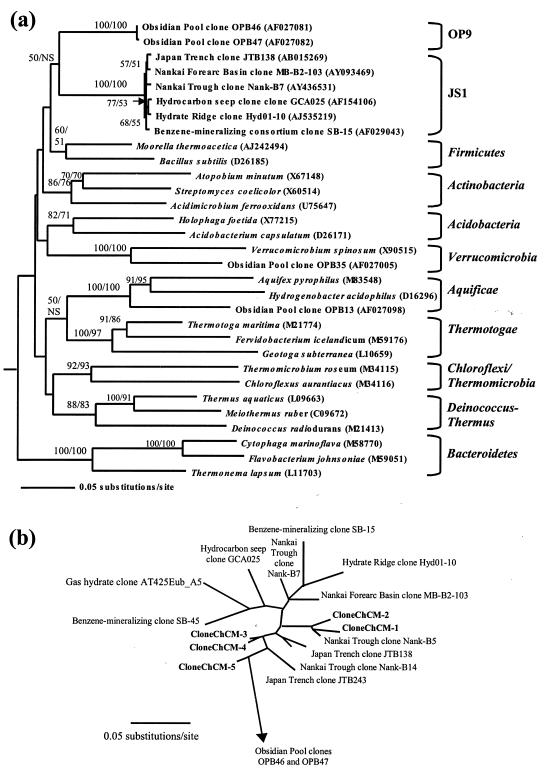
FIG. 1.
Phylogenetic trees showing the candidate division JS1. (a) Phylogenetic relationship between the candidate division JS1 and selected bacterial divisions. The minimum evolution tree is derived from LogDet/Paralinear distances of variable sites (estimated value of proportion of invariable sites = 0.4441). The tree is based on 1,428 bases of aligned 16S rRNA gene sequences. Bootstrap support values over 50% (1,000 replicates) are shown; NS denotes support below 50%. First value, bootstrap derived by LogDet/Paralinear distances of variable sites; second value, derived by maximum parsimony. Representative proteobacterial sequences were used as outgroups: Sinorhizobium fredii (D14516), Agrobacterium tumefaciens (M11223), Pseudomonas aeruginosa (AY268175), and Nitrosomonas europaea (AF353160). (b) An unrooted tree showing the relationship of sequences derived from the ChCM core GeoB 7132-5 with representative 16S rRNA gene sequences of candidate divisions JS1 and OP9. The minimum evolution tree is derived from LogDet/Paralinear distances of variable sites (estimated value of proportion of invariable sites = 0.6115). The tree is based on 606 bases of aligned 16S rRNA gene sequences. Accession numbers of sequences are as listed in panel a. Additional sequences are SB-45 (AF029050), Nank-B5 (AY436530), Nank-B14 (AY436527), JTB243 (AB015271), and AT425Eub_A5 (AY053496).
A bacterial division has been defined as a lineage consisting of two or more 16S rRNA gene sequences that is reproducibly monophyletic and unaffiliated with other known division-level groups of the bacterial domain (7). A multiple-outgroup approach was implemented to resolve the division-level phylogenetic relationship of the JS1 group (3). This approach repeatedly resulted in monophyly of the JS1 ingroup when different sets of division-level outgroups were used; an example is shown in Fig. 1a. Bootstrap analysis supported the inferred tree topologies and confirmed that the evolutionary distances are sufficient to consider the JS1 clade a novel bacterial division (Fig. 1a). Poor support was consistently found for the branch node between the candidate divisions OP9 and JS1 (<50%), demonstrating that these two divisions are unaffiliated as previously suggested by Dalevi et al. (3), and their association may be caused artificially by long-branch attraction (21).
We used the new JS1 primers to retrieve five nearly identical 16S rRNA gene sequences (98.6 to 99.3% identity) from the ChCM core GeoB 7132-5 described in Table 1. These sequences also had a high percent identity (93.7 to 99.5%) to JS1 cloned sequences previously retrieved from the Nankai Trough (11, 19) and other deep marine sediments (14, 25, 31). For example, the five ChCM clone sequences retrieved in this study were 98.4 to 99.1% similar to clone JTB138 from the Japan Trench (14) and 98.2 to 98.9% similar to clone MB-B2-103 from the forearc basin of the Nankai Trough (25). All methods used for phylogenetic analysis consistently placed all five of the novel 16S rRNA sequences within the monophyletic JS1 group (Fig. 1b), which was distantly related to all other bacterial divisions (Fig. 1a).
The depth of phylogenetic divergence (sequence difference) within JS1, which indicates the extent of diversity represented by members of the group, seems to be markedly less than that of other known divisions of Bacteria. For example, the JS1 group as represented in Fig. 1b showed only 6% sequence difference, compared to 26 and 33% in the candidate divisions WS6 and OP11, respectively, and 13 and 23% in Cyanobacteria and Proteobacteria, respectively (5). The observed small sequence divergence for the JS1 division may reflect a closely related group of slow-growing bacterial taxa (35) that have evolved under strong selection pressures imposed by the subsurface habitat. However, it is quite possible that this low observed diversity in sequence difference may increase as new sequences from this division are retrieved from the environment.
PCR amplifications with the JS1-specific primers resulted in both visible and undetectable PCR products, presumably due to differing target copy numbers within the different environmental samples used (Table 1). A second amplification step using general bacterial primers was therefore necessary to consistently obtain a DNA yield that could be analyzed by DGGE (Fig. 2) to ascertain the presence or absence of the group within environmental samples. This approach was used very successfully to screen for JS1 candidate division organisms in deep marine sediments (Fig. 2a), and it was observed that JS1 16S rRNA gene sequences were present in all deep marine sediments analyzed in this study (Fig. 2a; Table 1). All of the JS1 sequences retrieved from DGGE bands were very similar (98.7 to 100%), suggesting that they were derived from very closely related organisms present in sediment at different geographical locations in the Pacific Ocean. This was consistent with the suggestion that the JS1 candidate division has a low phylogenetic diversity and demonstrated that they are widespread throughout anoxic deep marine sediment environments.
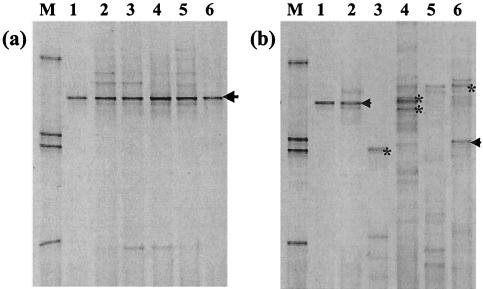
FIG. 2.
DGGE analysis of 16S rRNA gene sequences from various environmental samples amplified by nested PCR with primers 63F-665R and 357FGC-518R. (a) Deep marine sediment samples (Table 1). Lanes: M, DGGE marker (34); 1, clone Nank-B7, positive control; 2, ChCM core GeoB 7112-3; 3, ChCM core GeoB 7132-5; 4, ChCM core GeoB 7190-3; 5, Nankai Trough site 1173; 6, Peru Margin site 1229. (b) Other environmental samples (Table 1). Lanes: M, DGGE marker; 1, clone Nank-B7, positive control; 2, River Taff; 3, Chartley Moss; 4, Arabian Sea; 5, Cardiff University; 6, Arne Peninsula. Labeled DGGE bands represent bands that were excised and sequenced. JS1-like sequences are represented by arrows, and γ-proteobacteria are represented by asterisks.
Further application of the JS1-specific primers detected the presence of JS1-like sequences for the first time in samples from a number of different environments other than the deep marine biosphere (Fig. 2b; Table 1). For example, sequences that were very closely related (97.4 to 100%) to those retrieved from the ChCM were also present in aquatic samples taken from an English coastal salt marsh, a brackish sediment, a Welsh river, and a marine channel sediment. The presence of these deep marine sequences in the anaerobic depths of a coastal salt marsh and marine and brackish sediments may be easily understood. However, their presence in river water is interesting and may be explained by their association with sediment in the water sample. The absence of JS1 sequences in aerobic soil, peatland, and seawater environments sampled in this study suggested that they are derived from bacteria of sedimentary origin and have an anaerobic metabolism.
Extensive sequencing of dominant DGGE bands revealed that the JS1-specific nested PCR amplified some sequences from other bacteria when nonmarine sediment samples with a more diverse community structure were used. Sequences were retrieved with these primers that belonged to γ-Proteobacteria (Fig. 2b). As these sequences were sufficiently different from the novel JS1 group, they were easily identified and eliminated by sequencing. Analysis of the JS1 primer 665R with use of PRIMROSE and OligoCheck version 1.0 and allowing for one base mismatch between primer and target revealed that 665R hit 2.9% of relevant records from nontarget species (RDP database release 8.1; Ribosomal Database Project II [http://rdp.cme.msu.edu/]) and could potentially amplify other bacterial phyla, including γ-Proteobacteria.
It is curious that JS1 sequences have not been reported previously in microbial diversity studies of coastal marine, brackish, or freshwater sediments, and this may be due to the bacteria represented by these sequences being present in low numbers. However, population size is not necessarily an indication of importance within an ecosystem, as it is well documented that other important functional groups of prokaryotes are present in relatively low numbers. For example, ammonia-oxidizing bacteria may be only 0.01% of the total soil bacterial community (22). Knowledge of the habitats in which JS1 clones are found and their geographical distribution may provide important clues to the true physiological role of the organisms represented within this division. Representative sequences of the novel group described here have previously been found in methane-rich subsurface sediments where gas hydrates are present and in near-surface sediment with very deep overlying waters. This suggests that some members of the division may be associated with methanogenic consortia and that others are adapted to or can tolerate high pressures.
Currently, the only clue to the metabolism of JS1 bacteria is that they are probably anaerobic. We also do not know how abundant they are in their environment, but studies to assess their abundance and role within natural communities using a combination of molecular techniques like quantitative real-time PCR (29) and stable-isotope probing (23, 24) are under way. In turn, these methods may also provide important information on how to obtain pure laboratory cultures (36), which will be essential to resolve the true role of these microorganisms in global biogeochemical processes.
Nucleotide sequence accession numbers.
The nucleotide sequence accession numbers determined in this study have been deposited in the EMBL database under accession numbers AJ605559 to AJ605563.
Acknowledgments
We are very grateful to Martin Embley (Natural History Museum, London) for helpful discussions regarding phylogenetic analyses. We acknowledge Johannes Scholten (University of Warwick) and Graeme Nicol (University of Aberdeen) for providing the cultures of D. desulfuricans subsp. desulfuricans and Halobacterium sp., respectively, and Natasha Banning, Fiona Brock, Carole Newberry, Catrin Thomas, and Kevin Ashelford (Cardiff University) for kindly donating environmental DNA samples for use in this study. We thank the ODP for Leg 190 and 201 samples and the many scientists (especially Barry Cragg, Cardiff University) who obtained these samples for us. We also acknowledge Tim Ferdelman, Jens Kallmeyer, and Bo Barker Jørgenson, Max Planck Institute for Marine Microbiology, Bremen, for supplying samples from ChCM Cruise SO156; English Nature for access to Chartley Moss NNR; Beaulieu Estate (Alex Glanville) for access to Beaulieu; and The Royal Society for the Protection of Birds for access to the Arne Peninsula.
This work was funded by the NERC Marine and Freshwater Microbial Biodiversity thematic program (grant number NER/T/S/2000/636) and by the EU DeepBUG project (contract number EVK3-CT-1999-00017). ODP is sponsored by the U.S. National Science Foundation (NSF) and participating countries under the management of Joint Oceanographic Institutions (JOI), Inc.
REFERENCES
- 1.Ashelford, K. E., A. J. Weightman, and J. C. Fry. 2002. PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res. 30:3481-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale, S. J., K. Goodman, P. A. Rochelle, J. R. Marchesi, J. C. Fry, A. J. Weightman, and R. J. Parkes. 1997. Desulfovibrio profundis sp. nov., a novel barophilic sulfate-reducing bacterium from the deep sediment layers in the Japan Sea. Int. J. Syst. Bacteriol. 47:515-521. [DOI] [PubMed] [Google Scholar]
- 3.Dalevi, D., P. Hugenholtz, and L. L. Blackall. 2001. A multiple-outgroup approach to resolving division-level phylogenetic relationships using 16S rDNA data. Int. J. Syst. Evol. Microbiol. 51:385-391. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon, A., A. Teske, J. Dillon, D. A. Stahl, and M. L. Sogin. 2003. Molecular characterization of sulfate-reducing bacteria in the Guaymas Basin. Appl. Environ. Microbiol. 69:2765-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dojka, M. A., J. K. Harris, and N. R. Pace. 2000. Expanding the known diversity and environmental distribution of an uncultured phylogenetic division of bacteria. Appl. Environ. Microbiol. 66:1617-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirt, R. P., J. M. Logsdon, B. Healy, M. W. Dorey, W. F. Doolittle, and T. M. Embley. 1999. Microsporidia are related to fungi: evidence from the largest subunit of RNA polymerase II and other proteins. Proc. Natl. Acad. Sci. USA 96:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki, F., K. Takai, T. Komatsu, T. Kanamatsu, K. Fujioka, and K. Horikoshi. 2001. Archaeology of Archaea: geomicrobiological record of Pleistocene thermal events concealed in a deep-sea subseafloor environment. Extremophiles 5:385-392. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki, F., M. Suzuki, K. Takai, H. Oida, T. Sakamoto, K. Aoki, K. H. Nealson, and K. Horikoshi. 2003. Microbial communities associated with geological horizons in coastal subseafloor sediments from the sea of Okhotsk. Appl. Environ. Microbiol. 69:7224-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato, C., L. Li, Y. Nogi, Y. Nakamura, J. Tamaoka, and K. Horikoshi. 1998. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl. Environ. Microbiol. 64:1510-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kormas, K. A., D. C. Smith, V. Edgcomb, and A. Teske. 2003. Molecular analysis of deep subsurface microbial communities in Nankai Trough sediments (ODP Leg 190, site 1176). FEMS Microbiol. Ecol. 45:115-125. [DOI] [PubMed] [Google Scholar]
- 12.Lake, J. A. 1994. Reconstructing evolutionary trees from DNA and protein sequences: paralinear distances. Proc. Natl. Acad. Sci. USA 91:1455-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanoil, B. D., R. Sassen, M. T. La Duc, S. T. Sweet, and K. H. Nealson. 2001. Bacteria and Archaea physically associated with Gulf of Mexico gas hydrates. Appl. Environ. Microbiol. 67:5143-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, L., C. Kato, and K. Horikoshi. 1999. Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Mar. Biotechnol. 1:391-400. [DOI] [PubMed] [Google Scholar]
- 15.Lockhart, P. J., A. W. D. Larkum, M. A. Steel, P. J. Waddel, and D. Penny. 1996. Evolution of chlorophyll and bacteriochlorophyll: the problem of invariant sites in sequence analysis. Proc. Natl. Acad. Sci. USA 93:1930-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchesi, J. R., T. Sato, A. J. Weightman, T. A. Martin, J. C. Fry, S. J. Hiom, and W. G. Wade. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchesi, J. R., A. J. Weightman, B. A. Cragg, R. J. Parkes, and J. C. Fry. 2001. Methanogen and bacterial diversity and distribution in deep gas hydrate sediments from the Cascadia Margin as revealed by 16S rRNA molecular analysis. FEMS Microbiol. Ecol. 34:221-228. [DOI] [PubMed] [Google Scholar]
- 18.Muyzer, G., E. C. De Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newberry, C. J., G. Webster, A. J. Weightman, and J. C. Fry. 2004. Diversity of prokaryotes and methanogenesis in deep subsurface sediments from the Nankai Trough, Ocean Drilling Program Leg 190. Environ. Microbiol. 6:274-287. [DOI] [PubMed] [Google Scholar]
- 20.Parkes, R. J., B. A. Cragg, S. J. Bale, J. M. Getliff, K. Goodman, P. A. Rochelle, J. C. Fry, A. J. Weightman, and S. M. Harvey. 1994. Deep bacterial biosphere in Pacific Ocean sediments. Nature 371:410-413. [Google Scholar]
- 21.Philippe, H., and J. Laurent. 1998. How good are deep phylogenetic trees? Curr. Opin. Genet. Dev. 8:616-623. [DOI] [PubMed] [Google Scholar]
- 22.Phillips, C. J., D. Harris, S. L. Dollhopf, K. L. Gross, J. I. Prosser, and E. A. Paul. 2000. Effects of agronomic treatments on structure and function of ammonia-oxidizing communities. Appl. Environ. Microbiol. 66:5410-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radajewski, S., P. Ineson, N. R. Parekh, and J. C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646-649. [DOI] [PubMed] [Google Scholar]
- 24.Radajewski, S., G. Webster, D. S. Reay, S. A. Morris, P. Ineson, D. B. Nedwell, J. I. Prosser, and J. C. Murrell. 2002. Identification of active methylotroph populations in an acidic forest soil by stable-isotope probing. Microbiology 148:2331-2342. [DOI] [PubMed] [Google Scholar]
- 25.Reed, D. W., Y. Fujita, M. E. Delwiche, D. B. Blackwelder, P. P. Sheridan, T. Uchida, and F. S. Colwell. 2002. Microbial communities from methane hydrate-bearing deep marine sediments in a forearc basin. Appl. Environ. Microbiol. 68:3759-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochelle, P. A., B. A. Cragg, J. C. Fry, R. J. Parkes, and A. J. Weightman. 1994. Effect of sample handling on estimation of bacterial diversity in marine sediments by 16S rRNA gene sequence diversity. FEMS Microbiol. Ecol. 15:215-226. [Google Scholar]
- 27.Shipboard Scientific Party. 2001. Leg 190 summary, p. 1-87. In G. F. Moore, A. Taira, A. Klaus, et al. (ed.), Proceedings of the Ocean Drilling Program, initial reports. Ocean Drilling Program, College Station, Tex.
- 28.Shipboard Scientific Party. 2003. Leg 201 summary, p. 1-81. In S. L. D'Hondt, B. B. Jørgenson, D. J. Miller, et al. (ed.), Proceedings of the Ocean Drilling Program, initial reports. Ocean Drilling Program, College Station, Tex.
- 29.Stubner, S. 2002. Enumeration of 16S rDNA of Desulfotomaculum lineage 1 in rice field soil by real-time PCR with SybrGreen detection. J. Microbiol. Methods 50:155-164. [DOI] [PubMed] [Google Scholar]
- 30.Swofford, D. L. 1998. PAUP*: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Mass.
- 31.Teske, A., K. U. Hinrichs, V. Edgcomb, A. de Vera Gomez, D. Kysela, S. P. Sylva, M. L. Sogin, and H. W. Jannasch. 2002. Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl. Environ. Microbiol. 68:1994-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX-Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster, G., T. M. Embley, and J. I. Prosser. 2002. Grassland management regimens reduce small-scale heterogeneity and species diversity of β-proteobacterial ammonia oxidizer populations. Appl. Environ. Microbiol. 68:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster, G., C. J. Newberry, J. C. Fry, and A. J. Weightman. 2003. Assessment of bacterial community structure in the deep sub-seafloor biosphere by 16S rDNA-based techniques: a cautionary tale. J. Microbiol. Methods 55:155-164. [DOI] [PubMed] [Google Scholar]
- 35.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. USA 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zengler, K., G. Toledo, M. Rappé, J. Elkins, E. J. Mathur, J. M. Short, and M. Keller. 2002. Cultivating the uncultured. Proc. Natl. Acad. Sci. USA 99:15681-15686. [DOI] [PMC free article] [PubMed] [Google Scholar]