Abstract
Vibrio vulnificus is a marine bacterium that causes human wound infections and septicemia with a high mortality rate. V. vulnificus strains from different clinical and environmental sources or geographic regions have been successfully characterized by ribotyping and several other methods. Pulsed-field gel electrophoresis (PFGE) is a highly discriminative method, but previous studies suggested that it was not suitable for examining the correlation of V. vulnificus strains from different origins. We employed PFGE to determine its efficacy for characterizing V. vulnificus strains from different geographic regions, characterizing a total of 153 strains from clinical and environmental origins from the United States and Taiwan after SfiI or NotI digestion. V. vulnificus strains showed a high intraspecific diversity by PFGE after SfiI or NotI digestion, and about 12% of the strains could not be typed by the use of either of these enzymes. For PFGE with SfiI digestion, most of the clinical and environmental strains from the United States were grouped into cluster A, while the strains from Taiwan were grouped into other clusters. Clinical strains from the United States showed a higher level of genetic homogeneity than clinical strains from Taiwan, and environmental strains from both regions showed a similarly high level of heterogeneity. PFGE with NotI digestion was useful for studying the correlation of clinical strains from the United States and Taiwan, but it was not suitable for analyzing environmental strains. The results showed that PFGE with SfiI digestion may be used to characterize V. vulnificus strains from distant geographic regions, with NotI being a recommended alternative enzyme.
Vibrio vulnificus is an autochthonous marine and estuarine bacterium that is commonly associated with shellfish and the intestinal contents of fish (8, 13). This bacterium may cause septicemia and severe wound infections in susceptible persons (19). V. vulnificus strains are generally subgrouped into biotype 1 and biotype 2. Biotype 1 strains are ubiquitous in the marine environment and some cause human infections, while biotype 2 strains, comprised of serovars O3 and O4 and some untypeable strains, are largely restricted to diseased eels (12). New biotype 3 strains were isolated from an outbreak of wound infections and bacteremia in Israel, and they differ from biotypes 1 and 2 in several biochemical characteristics and by molecular typing (4).
Several molecular methods have been developed to analyze the intraspecific diversity of V. vulnificus, including amplified fragment length polymorphism (2), randomly amplified polymorphic DNA (RAPD) (3), pulsed-field gel electrophoresis (PFGE), and ribotyping (11). These methods have been applied to differentiate between V. vulnificus and other Vibrio species (29) or to analyze stressed cells of V. vulnificus (28), but they are most frequently applied to characterize strains from different sources and to determine their genetic relatedness (1, 12, 27). For instance, Tamplin et al. (27) indicated that 50 to 83% of clinical strains of V. vulnificus were associated with the specific ribotype clusters A, B, and D. Arias et al. (1) showed that close genetic relatedness exists among isolates from Spanish fish farms, Spanish eel farms, and some Mediterranean isolates.
These methods have been compared for their efficacy for subspecific grouping of V. vulnificus. Among these molecular methods, PFGE has been found to produce a high degree of variation in the patterns obtained from clinical and environmental strains of V. vulnificus, while ribotyping usually produces fewer patterns and clusters than PFGE, with the ribotypes showing a good relationship between geography and genotype (27). However, PFGE is known to be a reliable tool for differentiating strains of many other pathogenic bacteria, including several Vibrio species, such as V. cholerae (20), V. anguillarum (25), and V. parahaemolyticus (36). PFGE is the method of choice among ribotyping and different PCR typing methods for the molecular typing of V. parahaemolyticus (31-33, 36). The application of PFGE for typing of V. vulnificus needs more evaluation. In addition, a large quantity of seafood is imported from foreign countries to Taiwan, with Chile, the United States, Thailand, Japan, and Australia topping the list (24), and it is thus likely that vibrios are imported from different continents (35). For this study, we evaluated the effectiveness of applying PFGE to characterize V. vulnificus strains from different sources and from different continents. To this end, we collected a large number of clinical and environmental strains of V. vulnificus from the United States and the southern and northern parts of Taiwan and then analyzed them by PFGE.
MATERIALS AND METHODS
Bacterial strains and cultivation.
A total of 153 biotype 1 strains of V. vulnificus were collected and examined for this study, including 30 clinical and 17 environmental strains from the United States, 17 clinical and 32 environmental strains from southern Taiwan (Tainan and adjacent regions), and 38 environmental strains from northern Taiwan (Taipei and adjacent regions). The clinical strains were isolated from patients with wound or septicemia infections, while environmental strains were isolated from seawater, sediments, or oysters (14). Stock cultures were maintained in tryptic soy broth (Difco Laboratories, Detroit, Mich.) with 10% glycerol at −85°C. V. vulnificus was cultured at 37°C on tryptic soy agar (Difco) with a supplement of 3% NaCl.
PFGE.
DNA extraction, DNA digestion, and PFGE were performed according to procedures that are described elsewhere (36). Bacteria grown on tryptic soy agar-3% NaCl were transferred to 5 ml of trytpic soy broth-3% NaCl and then incubated at 37°C for 16 h with shaking at 160 rpm. The cells were harvested by centrifugation and resuspended in 2 ml of buffer containing 10 mM Tris, 100 mM EDTA, and 1 mM NaCl, pH 8.0. Agarose plugs were prepared by mixing equal volumes of bacterial suspensions with 1.5% low-melting-point agarose (FMC Corp., Rockland, Maine). Cells in the agarose plugs were lysed by treatment with a lysis solution containing 1 mg of lysozyme/ml and 0.1% N-sodium lauroyl sarcosine at 37°C for 24 h. The cells were then treated with proteinase K (0.5 mg/ml in 0.5 M EDTA and 1% N-sodium lauroyl sarcosine) at 45°C for 48 h and washed three times (30 min each time) with TE buffer (10 mM Tris-HCl, 1 mM EDTA). One section of the plug (4 by 9 by 1.2 mm) was equilibrated with an enzyme buffer, placed in 100 μl of fresh buffer containing 10 U of SfiI or NotI (New England Biolabs, Beverly, Mass.), and incubated at 37°C for 4 h.
High-molecular-weight restriction fragments were resolved in 1% agarose gels in 0.5% Tris-borate-EDTA buffer by use of a contour-clamped homogeneous electric field apparatus (CHEF-DR II; Bio-Rad Laboratories, Richmond, Calif.). The running conditions were 190 V for 22.4 h at 14°C, with a 3- to 80-s pulse time. A lambda ladder PFGE marker (New England Biolabs) was used as a molecular size marker. After electrophoresis, the gels were stained with ethidium bromide (Sigma Co., St. Louis, Mo.), destained in distilled water, and photographed over a UV transilluminator (V028490; Vilber Lourmat, Torey, France). The PFGE assay for each strain was repeated twice, and reproducible patterns were obtained.
Statistical analysis.
Digitized images were converted, normalized, and analyzed with the software package Gel Compar, version 4.0 (Applied Maths, Inc., Kortrijk, Belgium). The similarity between two tracks was calculated by use of the coefficient of Jaccard (Sj) and the band positions. Hierarchical cluster analysis was performed by the unweighted pair group method by arithmetic averages, and a dendrogram was produced with the software. The Pearson correlation coefficient and factor analysis with principal component extraction methods were used to correlate clusters to the clinical or environmental source or geographic location by the use of SPSS software for Windows, release 11.01, network version (SPSS Inc., Chicago, Ill.).
RESULTS
Of the 153 strains of V. vulnificus examined in this study, 134 were successfully characterized by PFGE after genomic DNA digestion with SfiI or NotI. Of the 153 strains, 20 or 21% of them failed to yield discernible PFGE patterns by SfiI or NotI digestion, respectively. Some strains could be typed by one enzyme but not the other. An average of about 12% of the strains could not be typed by either of these enzymes, with about 21, 7, and 6% of the strains from southern Taiwan, northern Taiwan, and the United States, respectively, being untypeable.
Several restriction enzymes that are commonly used for PFGE, including ApaI, BglI, CpoI, NotI, SmaI, SfiI, and SpeI, were tried in a preliminary study. NotI and SfiI were the only enzymes that yielded suitable banding patterns. With SfiI digestion, 1 to 20 bands were discernible. With NotI digestion, 3 to 18 bands could be detected. All of the NotI and SfiI restriction bands ranged from 48.5 to 582.0 kb. All strains showed a high degree of genetic diversity, with unique PFGE patterns resulting from either NotI or SfiI digestion.
The strains were grouped into different categories according to their isolation location and source (clinical or environmental), and the similarity indices of strains in each category were calculated and compared (27). The U.S. strains showed a higher degree of homology than the Taiwanese strains, mainly because the clinical strains from the United States showed a comparatively higher degree of homology than the Taiwanese clinical strains. The environmental strains from both geographic locations were notable for their low level of homology (Table 1).
TABLE 1.
Percentages of V. vulnificus strains at selected similarity indices as analyzed by SfiI-PFGE
| Type and origin of isolates | % of isolates at similarity level of:
|
|||
|---|---|---|---|---|
| 30% | 45% | 60% | 75% | |
| Total | ||||
| United States | 100 | 94 | 40 | 18 |
| Taiwan | 86 | 50 | 20 | 15 |
| Clinical | ||||
| United States | 100 | 79 | 46 | 24 |
| Taiwan | 55 | 11 | 0 | 0 |
| Environmental | ||||
| United States | 90 | 86 | 19 | 10 |
| Taiwan | 86 | 64 | 22 | 17 |
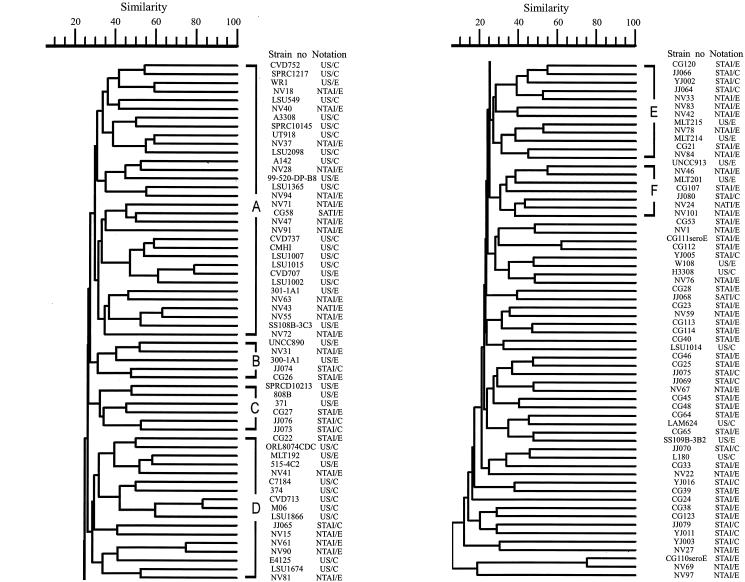
The SfiI restriction patterns were clustered, and a dendrogram for the strains examined is presented in Fig. 1. Strains with similarity indices of 30% or more were arbitrarily grouped into five clusters (A to E). Cluster A comprised 39.7% of the strains, while clusters B to E comprised 4.1 to 9.1% of the strains. About 35.5% of the strains belonged to other, unnamed clusters. Cluster A comprised 79.2, 4.2, and 16.7% of the strains from the United States and from southern and northern Taiwan, respectively. A high percentage (88.4%) of the clinical and environmental strains from the United States were grouped into cluster A, while 60% of the southern Taiwan and 50% of the northern Taiwan strains were grouped into other, unnamed clusters. Factor analysis using a principal component extraction method showed that the clusters were related to the geographic locations of the strains but not to their clinical or environmental origins. The correlation coefficients for clinical or environmental strains from the United States and for those from Taiwan were significantly different at the 0.01 level. The clustering of domestic strains (Taiwanese strains) was not significantly related to their isolation locations (southern or northern Taiwan), as indicated by the Pearson correlation coefficient.
FIG. 1.
Dendrogram and banding patterns of V. vulnificus strains analyzed by SfiI-PFGE. Several clusters were designated as clusters A to E, at similarity indices of 30%. Clinical and environmental strains from the United States, southern Taiwan, and northern Taiwan are designated as US/C (clinical U.S. strains), US/E (environmental U.S. strains), STAI/C (clinical southern Taiwanese strains), STAI/E (environmental southern Taiwanese strains), or NTAI/E (environmental northern Taiwanese strains).
Strains analyzed by PFGE after NotI digestion also showed unique banding patterns (Fig. 2), although the similarity indices for the strains were lower than those for the SfiI restriction patterns (Fig. 1). Strains with similarity indices of 30% or more were also grouped into six clusters (A to F). Clusters A and D were the major ones and comprised 26.2 and 13.9% of the strains, respectively, while clusters B, C, E, and F comprised 4.1 to 9.8% of the strains. About 35.2% of the strains were in other, unnamed clusters. The clinical strains from the United States were grouped into cluster A (55.6% of the strains), cluster D (29.6%), and others (14.8%), while the environmental strains from the United States were evenly distributed in these named clusters. Clinical strains from Taiwan were distributed widely in the named or other clusters, while the environmental strains from Taiwan were grouped mainly in cluster A (21.3%) and other clusters (45.9%). A factor analysis showed that the clusters were related to the geographic origins of these strains. The correlation coefficients for the clinical strains from the United States and Taiwan were significantly different at the 0.01 level, while the environmental strains from these two geographically distant regions were not significantly different. The clustering of domestic environmental strains was significant at the 0.01 level with regards to their geography (southern or northern Taiwan), as indicated by the Pearson correlation coefficient.
FIG. 2.
Dendrogram and banding patterns of V. vulnificus strains analyzed by NotI-PFGE. Several clusters were designated as clusters A to F, at similarity indices of 30%. Clinical and environmental strains from the United States, southern Taiwan, and northern Taiwan are designated as US/C (clinical U.S. strains), US/E (environmental U.S. strains), STAI/C (clinical southern Taiwanese strains), STAI/E (environmental southern Taiwanese strains), or NTAI/E (environmental northern Taiwanese strains).
DISCUSSION
The application of molecular methods for the characterization of microorganisms depends on the advantages of the selected method, the nature of the target organism, and the purpose of the study. Amplified fragment length polymorphism fingerprinting (AFLP) has been successfully applied to determine the genetic diversity of clinical and environmental strains of V. cholerae. In AFLP, the enzyme-restricted fragments of genomic DNAs are amplified to generate highly discriminative patterns by which the environmental and pandemic O1 and O139 strains can be differentiated and their phylogenetic relationships can be clarified (16). The clustering of AFLP patterns also reveals the temporal pattern of change of the pandemic strains of V. cholerae (18). The PFGE method is also a highly discriminative and reproducible method, and it has been applied to examine the pandemic strains of V. cholerae (20, 30) and the new O3:K6 strains of V. parahaemolyticus (34), although it is more time-consuming and costly than AFLP. Since the pandemic strains of V. cholerae and V. parahaemolyticus are mostly derived from a single clone or from closely related clones and since environmental strains are usually heterogenous (15, 35), these clinical strains can be clearly separated from their environmental strains by clustering analyses of their AFLP or PFGE patterns (16, 34). In contrast, the high intraspecific diversity of genomes from environmental and clinical V. vulnificus strains has been demonstrated by both PFGE (5, 27) and several other methods (2, 11), and it has been confirmed in this study by the examination of a large number of strains isolated from distant geographic regions by the use of PFGE with NotI or SfiI digestion (Fig. 1 and 2). The high level of genetic diversity in clinical strains of V. vulnificus is probably due to the high proportion of genetically heterogenous environmental strains (57%) which are virulent (14), which may be involved in human infections, and which would otherwise appear to be clinical isolates. Also, V. vulnificus infections are usually sporadic, and the spread of specific clinical strains in both the human population and the environment is scarce (26). Therefore, it is less likely for PFGE to be able to separate the clinical and the environmental strains of V. vulnificus into distinct clusters and to trace the ancestry of these clinical strains than it is for other pandemic vibrios.
Some molecular methods appear able to correlate genotypes with the phenotypic traits or geographic origins of V. vulnificus isolates. RAPD-PCR and ribotyping have been used to reveal the genetic proximity existing among Spanish fish farm isolates and a close relationship between Spanish eel farm isolates and some Mediterranean isolates of V. vulnificus (1), whereas these eel-pathogenic strains are usually highly homogenous (1). Ribotyping of Hawaiian isolates has also demonstrated a relationship between geography and genotype (27). Compared to RAPD-PCR and ribotyping, PFGE, with its higher degree of discrimination, is often unsuccessful in demonstrating a correlation between genotype and phenotype, and thus it is used relatively less often for environmental research (27). When the purpose of a study is to examine the spatial and temporal genetic diversity of V. vulnificus, PFGE may be applicable, as was the application of AFLP for studying the dynamic nature of V. cholerae in Chesapeake Bay (15).
In this study, PFGE was able to effectively differentiate strains isolated from the United States and Taiwan. The clinical strains from the United States showed a comparatively higher degree of homology than the Taiwanese clinical strains (Fig. 1). It was recently demonstrated that all clinical strains from patients with V. vulnificus septicemia after the ingestion of oysters have a type B 16S rRNA sequence, while those strains from oysters are mostly type A (22). This suggests that in vivo selection may occur and thus increase the intraspecific homology of the clinical strains. In this study, among the strains with known clinical sources, about 70% of the strains from the United States were isolated from patients with septicemia. Although in Taiwan most V. vulnificus infections are also caused by the ingestion of seafood or the exposure of abraded skin to salt water (6), raw oysters are not consumed as frequently as in the United States. Differences in the origins and characteristics of V. vulnificus in these two regions may account for the phenomenon of high homology among the U.S. strains. More domestic (Taiwanese) clinical strains must be examined before we make a conclusion about the difference in the genetic diversities of strains from these highly separated geographic regions.
PFGE employing SfiI digestion was preferable to that employing NotI digestion. PFGE with SfiI digestion produced patterns with higher similarity indices than those of NotI, and clinical or environmental strains obtained from distant geographic regions could be grouped into different clusters (Fig. 1). PFGE with NotI digestion was able to differentiate the clinical strains isolated from the United States and Taiwan, but it was not of value for differentiating the environmental strains (Fig. 2). Some strains that failed to be typed by PFGE with SfiI digestion could be successfully typed with NotI digestion, for example, strains CG64, CG65, YJ011, JJ064, JJ065, JJ066, JJ068, JJ075, and JJ079. Considering the large untypeable portion of strains, PFGE with NotI digestion is recommended as an alternative choice.
A major concern as a result of this study was the presence of a high level of untypeable V. vulnificus strains (about 20%) by the present PFGE method. Strains that are untypeable by PFGE are probably a result of the degradation of genomic DNA during the process. A high ratio of untypeable strains by PFGE has also been demonstrated in other studies for V. vulnificus (23) and V. parahaemolyticus (21), as well as for other DNA-degradation-sensitive species, such as Clostridium difficile (7). The ratio of untypeable strains varied for the different geographic regions in our study, which may be attributed to the presence of a high level of DNA-degrading activity in some of these strains. The degradation of genomic DNA can be lessened by changing the electrophoresis buffer to HEPES (17), by adding thiourea to the gel and buffer (9), or by inactivation of the degrading enzymes by formaldehyde before lysis of the bacterial cells (10).
In conclusion, PFGE with SfiI digestion showed that clinical strains of V. vulnificus from Taiwan have a lower level of genetic homogeneity than U.S. strains and that they also grouped into different clusters. This method may be used for subspecies typing of V. vulnificus, for strains isolated from distant geographic regions, and for studying spatial and temporal shifts of genotypes.
Acknowledgments
We thank the Department of Health of the Republic of China for financially supporting this research under contract no. DOH92-TD-1081 and DOH91-DC-1006.
REFERENCES
- 1.Arias, C. R., M. J. Pujalte, E. Garay, and R. Aznar. 1998. Genetic relatedness among environmental, clinical, and diseased-eel Vibrio vulnificus isolates from different geographic regions by ribotyping and randomly amplified polymorphic DNA PCR. Appl. Environ. Microbiol. 64:3403-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias, C. R., L. Verdonck, J. Swings, E. Garay, and R. Aznar. 1997. Intraspecific differentiation of Vibrio vulnificus biotypes by amplified fragment length polymorphism and ribotyping. Appl. Environ. Microbiol. 63:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aznar, R., W. Ludwig, and K.-H. Schleifer. 1993. Ribotyping and randomly amplified polymorphic DNA analysis of Vibrio vulnificus biotypes. Syst. Appl. Microbiol. 16:303-309. [Google Scholar]
- 4.Bisharat, N., V. Agmon, R. Finkelstein, R. Raz, G. Ben Dror, L. Lerner, S. Soboh, R. Colodner, D. N. Cameron, D. L. Wykstra, D. L. Swerdlow, and J. J. Farmer III. 1999. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 354:1421-1424. [DOI] [PubMed] [Google Scholar]
- 5.Buchrieser, C., V. V. Gangar, R. L. Murphree, M. L. Tamplin, and C. W. Kaspar. 1995. Multiple Vibrio vulnificus strains in oysters as demonstrated by clamped homogeneous electric field gel electrophoresis. Appl. Environ. Microbiol. 61:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang, Y. C., C. Y. Yuan, C. Y. Liu, C. K. Lan, and A. H. Huang. 1992. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin. Infect. Dis. 15:271-276. [DOI] [PubMed] [Google Scholar]
- 7.Corkill, J. E., R. Graham, C. A. Hart, and S. Stubbs. 2000. Pulsed-field gel electrophoresis of degradation-sensitive DNAs from Clostridium difficile PCR ribotype 1 strains. J. Clin. Microbiol. 38:2791-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePaola, A., G. M. Capers, and D. Alexander. 1994. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast. Appl. Environ. Microbiol. 60:984-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawley, W. N., and M. H. Wilcox. 2002. Pulsed-field gel electrophoresis can yield DNA fingerprints of degradation-susceptible Clostridium difficile strains. J. Clin. Microbiol. 40:3546-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hielm, S., J. Bjorkroth, E. Hyytia, and H. Korkeala. 1998. Genomic analysis of Clostridium botulinum group II by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 64:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Høi, L., A. Dalsgaard, J. L. Larsen, J. M. Warner, and J. D. Oliver. 1997. Comparison of ribotyping and randomly amplified polymorphic DNA PCR for characterization of Vibrio vulnificus. Appl. Environ. Microbiol. 63:1674-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Høi, L., I. Dalsgaard, A. DePaola, R. J. Siebeling, and A. Dalsgaard. 1998. Heterogeneity among isolates of Vibrio vulnificus recovered from eels (Anguilla angulla) in Denmark. Appl. Environ. Microbiol. 64:4676-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Høi, L., J. L. Larsen, I. Dalsgaard, and A. Dalsgaard. 1998. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Appl. Environ. Microbiol. 64:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hor, L. I., C. T. Gao, and L. Wan. 1995. Isolation and characterization of Vibrio vulnificus inhabiting the marine environment of the Southwestern area of Taiwan. J. Biomed. Sci. 2:384-389. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, S. C., V. Louis, N. Choopun, A. Sharma, A. Huq, and R. R. Colwell. 2000. Genetic diversity of Vibrio cholerae in Chesapeake Bay determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, S. C., M. Matte, G. Matte, A. Huq, and R. R. Colwell. 2000. Genetic diversity of clinical and environmental isolates of Vibrio cholerae determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koort, J. M., S. Lukinmaa, M. Rantala, E. Unkila, and A. Siitonen. 2002. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:3497-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan, R., and P. R. Reeves. 2002. Pandemic spread of cholera: genetic diversity and relationships within the seventh pandemic clone of Vibrio cholerae determined by amplified fragment length polymorphism. J. Clin. Microbiol. 40:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 20.Mahalingam, S., Y.-M. Cheong, S. Kan, R. M. Yassin, J. Vadivelu, and T. Pang. 1994. Molecular epidemiologic analysis of Vibrio cholerae O1 isolates by pulsed-field gel electrophoresis. J. Clin. Microbiol. 32:2975-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall, S., C. G. Clark, G. Wang, M. Mulvey, M. T. Kelly, and W. M. Johnson. 1999. Comparison of molecular methods for typing Vibrio parahaemolyticus. J. Clin. Microbiol. 37:2473-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson, W. B., R. N. Paranjype, A. DePaola, and M. S. Strom. 2003. Sequence polymorphism of the 16S rRNA gene of Vibrio vulnificus is a possible indicator of strain virulence. J. Clin. Microbiol. 41:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryang, D. W., S. B. Koo, M. G. Shin, J. H. Shin, and S. P. Suh. 1999. Molecular typing of Vibrio vulnificus isolated from clinical specimens by pulsed-field gel electrophoresis and random amplified polymorphic DNA analysis. Jpn. J. Infect. Dis. 52:38-41. [PubMed] [Google Scholar]
- 24.Shiao, C. Y. 1997. Trend of seafood export and import in Taiwan. Fishery Promotion 126:13-18. [Google Scholar]
- 25.Skov, M. N., K. Pedersen, and J. L. Larsen. 1995. Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar O1. Appl. Environ. Microbiol. 61:1540-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 27.Tamplin, M. L., J. K. Jackson, C. Buchrieser, R. L. Murphhree, K. M. Portier, V. Gangar, L. G. Miller, and C. W. Kaspar. 1996. Pulsed-field gel electrophoresis and ribotype profiles of clinical and environmental Vibrio vulnificus isolates. Appl. Environ. Microbiol. 62:3572-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner, J. M., and J. D. Oliver. 1998. Randomly amplified polymorphic DNA analysis of starved and viable but nonculturable Vibrio vulnificus cells. Appl. Environ. Microbiol. 64:3025-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warner, J. M., and J. D. Oliver. 1999. Randomly amplified polymorphic DNA analysis of clinical and environmental isolates of Vibrio vulnificus and other Vibrio species. Appl. Environ. Microbiol. 65:1141-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong, H. C., D. P. Liu, S. H. Liu, Y. C. Chung, and T. Shimada. 2002. Characterization of Vibrio cholerae O139 isolated in Taiwan. Food Microbiol. 19:653-661. [Google Scholar]
- 31.Wong, H. C., C. Y. Ho, L. P. Kuo, T. K. Wang, C. L. Lee, and D. Y. Shih. 1999. Ribotyping of Vibrio parahaemolyticus isolates obtained from food poisoning outbreaks in Taiwan. Microbiol. Immunol. 43:631-636. [DOI] [PubMed] [Google Scholar]
- 32.Wong, H. C., and C. H. Lin. 2001. Evaluation of typing of Vibrio parahaemolyticus by three PCR methods using specific primers. J. Clin. Microbiol. 39:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong, H. C., C. C. Liu, T. M. Pan, T. K. Wang, C. L. Lee, and D. Y. C. Shih. 1999. Molecular typing of Vibrio parahaemolyticus isolates, obtained from patients involved in food poisoning outbreaks in Taiwan, by random amplified polymorphic DNA analysis. J. Clin. Microbiol. 37:1809-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong, H. C., S. H. Liu, T. K. Wang, C. L. Lee, C. S. Chiou, D. P. Liu, M. Nishibuchi, and B. K. Lee. 2000. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 66:3981-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong, H. C., M. C. Chen, S. H. Liu, and D. P. Liu. 1999. Incidence of highly genetically diversified Vibrio parahaemolyticus in seafood imported from Asian countries. Int. J. Food Microbiol. 52:181-188. [DOI] [PubMed] [Google Scholar]
- 36.Wong, H. C., K.-T. Lu, T.-M. Pan, C.-L. Lee, and D. Y. C. Shih. 1996. Subspecies typing of Vibrio parahaemolyticus by pulsed-field gel electrophoresis. J. Clin. Microbiol. 34:1535-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]