Abstract
Over the 13-month period from October 2000 to November 2001 (inclusive), the Food Safety Authority of Ireland (FSAI) carried out surveillance of Irish bulk raw (n = 389) and commercially pasteurized (n = 357) liquid-milk supplies to determine the incidence of Mycobacterium paratuberculosis. The pasteurization time-temperature conditions were recorded for all pasteurized samples. Overall, 56% of whole-milk pasteurized samples had been heat treated at or above a time-temperature combination of 75°C for 25 s. All analyses were undertaken at the Department of Food Science (Food Microbiology) laboratory at Queen's University Belfast. Each milk sample was subjected to two tests for M. paratuberculosis: immunomagnetic separation-PCR (IMS-PCR; to detect the presence of M. paratuberculosis cells, live or dead) and chemical decontamination and culture (to confirm the presence of viable M. paratuberculosis). Overall, M. paratuberculosis DNA was detected by IMS-PCR in 50 (12.9%; 95% confidence interval, 9.9 to 16.5%) raw-milk samples and 35 (9.8%; 95% confidence interval, 7.1 to 13.3%) pasteurized-milk samples. Confirmed M. paratuberculosis was cultured from one raw-milk sample and no pasteurized-milk samples. It is concluded that M. paratuberculosis DNA is occasionally present at low levels in both raw and commercially pasteurized cows' milk. However, since no viable M. paratuberculosis was isolated from commercially pasteurized cows' milk on retail sale in the Republic of Ireland, current pasteurization procedures are considered to be effective.
Mycobacterium paratuberculosis is an organism which can cause chronic inflammation of the intestine in cattle, known as Johne's disease. Clinically infected animals may shed large numbers of M. paratuberculosis organisms in their feces and smaller numbers in their milk (6). Subclinically infected animals also shed the organism, though usually in smaller amounts. Generally, cattle are infected early in life by ingestion of M. paratuberculosis through colostrum, milk, fecally contaminated teats, water, feeds, or surfaces (6, 28, 38). The problems associated with Johne's disease include failure to thrive, reduced milk production, reduced reproductive performance, premature culling, reduced culling value, increased replacement costs, and more recently, potential concerns about the safety of the herd's milk (6, 38). It has been estimated that the economic cost of Johne's disease in the United States may exceed $1.5 billion per year (21, 25).
In the Republic of Ireland, it has been compulsory to notify the Department of Agriculture and Food (DAF) of any incidence of Johne's disease since 1955 (39). All confirmed infected animals and their immediate progeny are removed from herds and slaughtered. Historically, the prevalence of Johne's disease in the Republic of Ireland has been comparatively low, with a total of just 92 cases reported in the 50-year period from 1932 to 1982 (35). However, since 1992, when the European Union single market was introduced and free movement of animals within the European Union was permitted, Johne's disease has increased in Ireland through the importation of asymptomatic carrier animals from other European Union member states where the incidence is higher (33). A study of 16 herds with imported animals in the Republic of Ireland, published in 2002, showed that 3.7% (8 of 225) tested positive for Johne's disease by the enzyme-linked immunosorbent assay and 4.1% (9 of 221) were positive by fecal culture testing (33).
Crohn's disease is characterized by a relapsing inflammatory process in the digestive tracts of humans. Crohn's disease in humans has features that resemble Johne's disease in animals, and the question has been raised of whether the causal organism of Johne's disease, M. paratuberculosis, could have the same role in the etiology of Crohn's disease. A recent review reports that Koch's postulates, the generally accepted standard for establishing a specific infectious agent as the cause of a human disease (27), may have been fulfilled for M. paratuberculosis and Crohn's disease (20). However, scientific opinion differs on the existence of any causal association between this bacterium and Crohn's disease, and worldwide, there is no consensus. Proposed etiologies for Crohn's disease include bacterial or viral infection, diet or smoking, genetic susceptibility, and immune dysfunction (36). It is, of course, possible that the disease has a multifactorial causation. Crohn's disease occurs in Western European countries at an estimated incidence of 5.6 per 100,000 individuals per year (37).
The European Union (EU) Scientific Committee on Animal Health and Animal Welfare (10), the Microbiology Sub-Committee of the Food Safety Authority of Ireland's (FSAI) Scientific Committee (11), the Advisory Committee on the Microbiological Safety of Food of the Food Standards Agency of the United Kingdom (FSA UK) (1), the U.S. National Academy of Sciences, Board on Agriculture and Natural Resources (6), and other expert groups (36) in the field have concluded that there is insufficient evidence at present to establish a link between Crohn's disease and M. paratuberculosis. These groups have reported that there are not sufficient data available at present on the incidence and prevalence of both Johne's disease and Crohn's disease and on the precise etiologies of Crohn's disease to substantiate evidence of a link between the two diseases.
As a consequence of research findings on the thermal tolerance of M. paratuberculosis in milk (3, 14, 15, 16, 32) and the debate surrounding the organism suggesting a possible association between M. paratuberculosis and Crohn's disease (4, 22, 23, 24, 30), in 1999, the FSAI undertook to review the public health implications of M. paratuberculosis (11). The FSAI also put in place a strategy to reduce the likelihood of consumers being exposed to the intact M. paratuberculosis organism when consuming pasteurized cows' milk. This included having Irish manufacturers raise milk pasteurization times and temperatures to ≥72°C for 25 s, the time-temperature combination recommended by the EU Scientific Committee on Animal Health and Animal Welfare, as a precautionary measure (10).
The specific aims of the surveillance were (i) to determine the incidence of M. paratuberculosis in the Irish raw-milk supply, (ii) to determine the incidence of M. paratuberculosis in pasteurized liquid milk on the Irish market, and (iii) to establish the effectiveness of the milk pasteurization time-temperature combinations in use in the Republic of Ireland at inactivating any M. paratuberculosis organisms which may have been present in the milk.
MATERIALS AND METHODS
Scope of M. paratuberculosis milk testing.
Over a 13-month period from October 2000 to November 2001 (inclusive), the FSAI carried out surveillance of Irish liquid-milk supplies for the presence of M. paratuberculosis. The time period of the study was designed to eliminate any variations due to seasonal effects on the milk supply. Initially a 12-month study was planned; however, the study had to be extended by 1 month due to unforeseen foot-and-mouth disease restrictions on milk sampling in March 2001.
Bulk raw milk, commercially pasteurized milk, and cream samples were collected from all approved milk pasteurizing plants (n = 27) throughout the Republic of Ireland. Limited sampling of cartons of pasteurized milk produced in Northern Ireland that were on retail sale in the Republic of Ireland was also carried out. In total, 746 bulk raw milk samples and commercially pasteurized cows' milk and cream samples (comprising 389 bulk raw and 357 heat-treated samples) were examined for the presence of M. paratuberculosis. The 357 heat-treated samples included 277 whole-milk, 31 semiskim-milk, 8 skim-milk, and 41 cream samples. Nineteen (5%) of the heat-treated milk samples tested were pasteurized milk produced in Northern Ireland and sampled from cartons at the retail level in the Republic of Ireland.
Sample collection and transport.
Samples were taken by an authorized officer of the FSAI following agreed national protocols for official sampling. A total of 253 sampling visits to milk pasteurization plants throughout the Republic of Ireland were carried out over the course of the survey. The number of plant visits, and consequently the number of milk samples taken at each milk processing plant, was dependent on the annual plant throughput. The larger milk pasteurization plants were visited up to 16 times, and the smaller plants were visited a minimum of 4 times, over the 13-month period. At each sampling visit, raw and heat-treated milk samples were collected as available. In collecting samples at each liquid-milk processing plant, the aim was to obtain as many “matched” samples as possible, i.e., to obtain raw and heat-treated samples originating from the same batch of milk. This aim was achieved for the majority (66%) of the samples taken. Each milk sample was assigned a numerical code, which was recorded on the sample bottle and on the laboratory sample submission form. Information on the origin and type of each milk sample, its sampling point, the corresponding sample code, and time-temperature processing details for pasteurized-milk samples (collected at the time of sampling) was held centrally at the FSAI. No information regarding the origin of the milk samples accompanied the samples to the laboratory. The temperature of the milk samples was maintained at or below 4°C at all times between collection and the commencement of laboratory testing by means of a controlled temperature cool box.
Milk testing.
All analyses of the milk samples were undertaken at the Department of Food Microbiology, Queen's University Belfast, and all milk testing commenced within 48 h of sampling.
(i) Tests for M. paratuberculosis.
Each milk sample was subjected to two tests for M. paratuberculosis: immunomagnetic separation-PCR (IMS-PCR), to detect the presence of M. paratuberculosis cells, live or dead, and chemical decontamination and culture (to confirm the presence of viable M. paratuberculosis), as follows.
For IMS-PCR, a 50-ml portion of each milk sample was centrifuged (15 min at 2,500 × g), and the pellet was resuspended in 1 ml of phosphate buffered saline (pH 7.4) containing 0.05% Tween 20 (PBS-T; Sigma Chemical Co. Ltd., Poole, Dorset, United Kingdom) prior to IMS, which was carried out as described by Grant et al. (15). To maximize test sensitivity, samples were subjected to a modified DNA extraction and purification protocol after IMS and before PCR. This protocol involved overnight incubation of the resuspended beads after IMS at 37°C in 700 μl of lysis buffer (2 mM EDTA, 400 mM NaCl, 10 mM Tris [pH 8.0], 0.6% sodium dodecyl sulfate) containing 20 μg of proteinase K (Sigma), mechanical disruption of the sample in “blue-capped tubes” in a Hybaid Ribolyser (both from Hybaid Ltd., Middlesex, United Kingdom), and subsequent extraction, purification, and precipitation of the DNA using phenol, chloroform-isoamyl alcohol (24:1), and isopropanol (all from Sigma), respectively. Precipitated DNA was washed once in 70% ethanol and resuspended in 50 μl of Tris-EDTA buffer. PCR was performed as described previously (19). IMS-PCR results were reported as positive if a band of the correct size (400 bp) was observed on the gel. The minimum detection limit of this modified IMS-PCR protocol is estimated to be in the region of 1 CFU of M. paratuberculosis/50 ml of milk. Negative (water-only) and positive (M. paratuberculosis DNA) PCR controls, and negative (PBS-T used to resuspend milk pellets) and positive (M. paratuberculosis broth culture) IMS controls, were run in parallel with each batch of milk samples tested.
For chemical decontamination and culture, a second 50-ml aliquot of each milk sample was centrifuged (15 min at 2,500 × g), and the pellet was resuspended in 10 ml of freshly prepared 0.75% (wt/vol) hexadecylpyridinium chloride (HPC; Sigma). Following incubation at room temperature (21°C) for 5 h and further centrifugation, as above, the pellet was resuspended in 1 ml of PBS-T. Two slopes of Herrold's egg yolk medium containing 2 μg of mycobactin J/ml were each inoculated with 250 μl of the resuspended pellet. One vial of BACTEC 12B radiometric medium (Becton Dickinson, Cowley, Oxford, United Kingdom) supplemented with 0.5 ml of Difco egg yolk emulsion (Becton Dickinson), 2 μg of mycobactin J (Synbiotics Europe SAS, Lyon, France)/ml, and PANTA antibiotic supplement (Becton Dickinson) reconstituted according to the manufacturer's instructions (100 μl for raw-milk cultures and 50 μl for pasteurized-milk cultures) was inoculated with 500 μl of the resuspended pellet. Both media were incubated at 37°C for as long as 18 weeks. Slopes were examined periodically for the presence of colonies. BACTEC vials were read regularly on a BACTEC 460TB instrument (Becton Dickinson). When growth was observed in either medium, an acid-fast stain was performed by the Ziehl-Neelsen method to confirm the presence of acid-fast organisms. Further confirmatory tests to confirm slow growth rate, typical colony morphology, and mycobactin J dependence, and IS900 PCR for a colony, were carried out on each suspect acid-fast isolate to determine whether it was M. paratuberculosis or some other Mycobacterium sp. (19).
(ii) Phosphatase testing.
In order to verify whether milk samples had been adequately pasteurized at the standard milk pasteurization time-temperature combination, all pasteurized-milk samples were subjected to phosphatase testing by the method of Aschaffenburg and Mullen (2).
Statistical analysis.
The numbers of IMS-PCR-positive results and confirmed M. paratuberculosis isolates obtained for each type of milk were expressed as percentages of the total number of samples of that type of milk tested. The standard error of this value was calculated, and 95% confidence limits are presented. The chi-square test was used to test for significant differences. Statistically significant effects were defined at the 95% significance level (P < 0.05).
RESULTS
Detection of M. paratuberculosis by IMS-PCR.
Since the IMS-PCR method essentially detects M. paratuberculosis DNA, it cannot differentiate between viable and dead cells. IMS-PCR-positive results were found in milk samples from 23 of the 27 (85.2%) milk-pasteurizing plants that participated in the survey. IMS-PCR results for raw- and pasteurized-milk samples tested during the survey are summarized in Table 1. Overall, M. paratuberculosis DNA was detected by IMS-PCR in 50 (12.9%; 95% confidence interval, 9.9 to 16.5%) raw-milk samples and 35 (9.8%; 95% confidence interval, 7.1 to 13.3%) pasteurized-milk samples. Two of the 35 (5.7%) pasteurized-milk samples found to be positive by IMS-PCR were from milk that was produced in Northern Ireland and sampled from cartons at the retail level in the Republic of Ireland. There was no significant difference in the probability of finding a positive IMS-PCR result between the raw- and pasteurized-milk samples (P > 0.05). This result is to be expected, since IMS-PCR is unable to distinguish between viable and dead cells.
TABLE 1.
IMS-PCR and culture results for 389 bulk raw and 357 commercially pasteurized cows' milk samplesa
| Type of milk | No. (%) of samples with the indicated resultb by:
|
|||
|---|---|---|---|---|
| IMS-PCR
|
Culture
|
|||
| + | − | + | − | |
| Raw | 50 (12.9) | 339 (87.1) | 1 (0.3) | 388 (99.7) |
| Pasteurized | 35 (9.8) | 322 (90.2) | 0 (0) | 357 (100) |
Samples came from the 27 approved liquid-milk processing plants throughout the Republic of Ireland and from limited sampling of cartons of pasteurized milk produced in Northern Ireland that were on retail sale in the Republic of Ireland.
+, positive; −, negative.
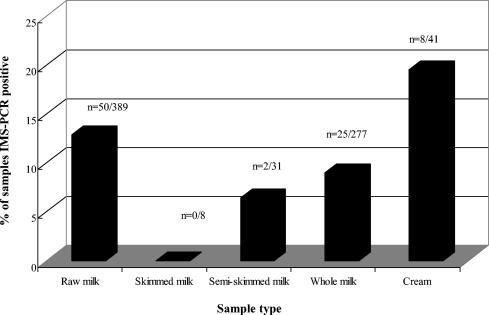
Figure 1 outlines the breakdown of the IMS-PCR results for each of the pasteurized sample types. No skim-milk samples were found to be positive by IMS-PCR; however, 6.5, 9.0, and 19.5% of semiskim-milk, whole-milk, and cream samples, respectively, were found to be positive by IMS-PCR. There was a significantly higher probability (P < 0.05) of finding an IMS-PCR-positive result in cream samples than in pasteurized-milk samples. Cream samples were pasteurized by using a combination of temperatures and times within the range of 79 to 88°C and 5 to 22 s, respectively. IMS-PCR positive results were obtained across the range of time temperature combinations.
FIG. 1.
Breakdown of IMS-PCR-positive results for raw-milk and pasteurized skim-milk, semiskim-milk, whole-milk, and cream samples.
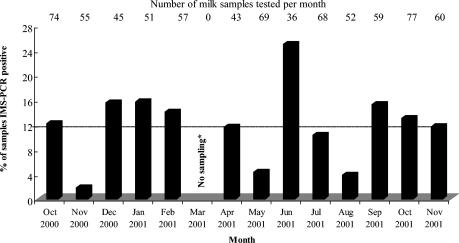
The distribution of IMS-PCR positive results by month of survey is shown in Fig. 2. In all months of the survey in which samples were collected, IMS-PCR-positive results were obtained. The percentage of IMS-PCR-positive samples ranged from 1.8 to 25.0% of samples tested per month (mean, 12%). The largest peak for IMS-PCR-positive results occurred in June, when 25.0% of the total number of milk samples tested were found to be positive. Less significant peaks occurred in December (15.6%), January (15.7%), and September (15.3%).
FIG. 2.
Distribution of IMS-PCR-positive results for raw and commercially pasteurized cows' milk by month of survey. Dashed line indicates the overall mean percentage of samples that were IMS-PCR positive. “No sampling*,” no sampling due to foot-and-mouth disease sampling restrictions.
The number of IMS-PCR-positive results in raw and commercially pasteurized cows' milk as a percentage of the total number of milk samples examined for M. paratuberculosis for each plant is shown in Table 2. The number of IMS-PCR-positive results as a percentage of the total number of milk samples per plant ranged from 0 to 26.3%. Liquid-milk processing plants were visited a minimum of 4 times and a maximum of 16 times depending on plant throughput. For 17 of the 27 liquid-milk processing plants (63.0%), IMS-PCR-positive results were found again on subsequent sampling visits to the plant (Table 2).
TABLE 2.
Number of milk sampling visits and distribution of IMS-PCR-positive results for raw and commercially pasteurized cows' milk per liquid-milk processing plant
| Plant | No. of visits to plant | Total no. of milk samples testeda | No. (%) of samples testing IMS-PCR positive | M. paratuberculosis detected on more than one sampling visit |
|---|---|---|---|---|
| A | 4 | 6 | 1 (16.7) | No |
| B | 11 | 33 | 4 (12.1) | Yes |
| C | 11 | 30 | 3 (10.0) | Yes |
| D | 5 | 8 | 1 (12.5) | No |
| E | 11 | 29 | 3 (10.3) | Yes |
| F | 9 | 31 | 4 (12.9) | Yes |
| G | 10 | 32 | 3 (9.4) | Yes |
| H | 9 | 23 | 4 (17.4) | Yes |
| I | 8 | 15 | 3 (20.0) | Yes |
| J | 8 | 14 | 0 (0) | No |
| K | 10 | 30 | 5 (16.7) | Yes |
| L | 16 | 76 | 10 (13.2) | Yes |
| M | 16 | 67 | 8 (11.9) | Yes |
| N | 10 | 40 | 3 (7.5) | Yes |
| O | 8 | 22 | 0 (0) | No |
| P | 13 | 28 | 2 (7.1) | Yes |
| Q | 8 | 24 | 2 (8.3) | Yes |
| R | 5 | 9 | 1 (11.1) | No |
| S | 6 | 11 | 0 (0) | No |
| T | 10 | 19 | 5 (26.3) | Yes |
| U | 11 | 23 | 1 (4.3) | No |
| V | 10 | 18 | 1 (5.6) | No |
| W | 9 | 21 | 4 (19.0) | Yes |
| X | 10 | 37 | 8 (21.6) | Yes |
| Y | 9 | 21 | 2 (9.5) | No |
| Z | 10 | 48 | 5 (10.4) | Yes |
| AA | 6 | 12 | 0 (0) | No |
This does not include the 19 heat-treated milk samples produced in Northern Ireland and sampled from cartons at the retail level in the Republic of Ireland.
When the IMS-PCR results of the FSAI study are expressed similarly to the FSA UK results, by plant (i.e., number of IMS-PCR-positive dairies on a single initial visit per total number of dairies tested), similar trends can be seen. When the data from the first individual visit to each dairy in Ireland are analyzed, 6 (22.2%) of the 27 plants were found to have IMS-PCR positives in raw or pasteurized milk; in comparison, in the United Kingdom, 56 (23.5%) of 241 plants were found to be positive on a single visit. However, we contend that the results presented in this fashion for a single visit are misleading. The full set of results of the present study are more representative and take account of the seasonal factors associated with the milk supplied to each plant, as repeat visits to each plant took place at intervals over the course of 1 year. This may have given rise to the higher overall results, where 23 of the 27 plants were found to have IMS-PCR positives. Had the previous United Kingdom study involved more than a single sampling visit, the results may have been comparable.
Isolation of viable M. paratuberculosis by culture.
The culture results for raw- and pasteurized-milk samples tested during the survey are also summarized in Table 1. Confirmed M. paratuberculosis was cultured from one raw-milk sample (0.3%; 95% confidence interval, 0.06 to 1.4%) and no (0%; 95% confidence interval, 0.007 to 1.0%) pasteurized-milk samples following chemical decontamination.
On a number of occasions, suspect M. paratuberculosis colonies (based on colony morphology) were detected on Herrold's egg yolk medium. A number of tests are necessary to confirm that a suspect colony is indeed M. paratuberculosis and not some other Mycobacterium sp. However, because it is impossible to carry out several confirmatory tests (acid-fast staining, PCR, and subculture to confirm mycobactin J dependency and slow growth) on a single colony, subculturing was attempted prior to the confirmatory tests if only a single suspect colony was available. Subculturing was unsuccessful on a number of occasions, and thus, no further confirmatory tests could be carried out. In such instances, the sample was reported as culture negative. This highlights the difficulties associated with the culture detection methods for M. paratuberculosis, as a result of which the level of culture detection may be underestimated. The problems associated with purifying M. paratuberculosis from mixed cultures have been acknowledged by other groups trying to confirm the presence of viable M. paratuberculosis in pasteurized milk (13, 18, 32).
Viable M. paratuberculosis was isolated from one raw-milk sample that tested negative by IMS-PCR. The corresponding pasteurized-milk sample, heat treated at 78°C for 27 s, also tested negative by IMS-PCR, and M. paratuberculosis was not isolated by culture.
Processing details for pasteurized-milk samples.
Current Irish regulations require that milk must have been pasteurized by a treatment involving a high temperature for a short time (at least 71.7°C for 15 s or any equivalent combination) or a pasteurization process using different time and temperature combinations to obtain an equivalent effect (40). All milk pasteurization time and temperature conditions recorded during this survey complied with the legal minimum for the high-temperature, short-time (HTST) process. All pasteurized-milk samples tested were found to be phosphatase negative. A negative phosphatase result indicates that the milk has been subjected to a pasteurization process in compliance with the regulatory 71.7°C for 15 s, since the enzyme phosphatase is destroyed during HTST pasteurization. The pasteurization records demonstrated that 90% of the whole-milk samples collected were treated at temperatures of ≥75°C and that 62% of samples were treated at holding times of ≥25 s. Overall, 56% of whole-milk samples were treated at or above a time-temperature combination of 75°C for 25 s (i.e., ≥75°C and ≥25 s).
DISCUSSION
The present survey carried out by the FSAI was similar in certain respects to a survey carried out in the United Kingdom by the FSA UK. The FSAI survey was similar to the FSA UK survey in terms of the number of samples tested, the detection methods employed, and the laboratory used for analyses. However, the FSAI survey did differ from the United Kingdom study in many respects, and these differences may explain why the findings of the present study are different from those reported previously. The differences included the following. (i) The FSAI survey used a different experimental design; all of the approved liquid-milk processing plants (n = 27) in the Republic of Ireland were visited on multiple occasions (4 to 16 times). In contrast, during the FSA UK survey, around 32% (241 of 754) of approved liquid-milk processing plants in the United Kingdom were visited on a single occasion only (12, 17). In order to ensure that the protocol used was representative, this study involved matching the number of milk samples sampled and analyzed with the throughput of each of the individual liquid-milk processing establishments. (ii) The geographical location is different from that of previous studies, and thus, the Johne's disease status in the national herd, national control and intervention policies to eradicate Johne's disease on the farm, and industrial milk-processing parameters are different and may influence the findings. (iii) Preliminary unpublished research suggests that the incidence of clinical Johne's disease in the Republic of Ireland is low but that there may be a far greater problem of subclinical disease in dairy herds in the Republic of Ireland. (iv) The nationally adopted pasteurization time-temperature combinations for milk heat treatment in Ireland are substantially more stringent than those recorded in previous studies examining the incidence of M. paratuberculosis in cows' milk in the United Kingdom. In the United Kingdom survey (12), 85% of milk samples had been pasteurized at 72 to 74.9°C and 49% had been pasteurized for 15 s (the legal minimum holding time), whereas in the Irish study 90% of milk samples were pasteurized at ≥75°C and 62% were pasteurized for holding times of 25 s or more. The results of the present study suggest that commercial pasteurization as carried out currently in Irish liquid-milk processing plants is effective at killing M. paratuberculosis, if it is present.
The FSA UK M. paratuberculosis survey was part of a wider national study on the microbiological quality and heat processing of cows' milk that took place between March 1999 and August 2000. In that study, M. paratuberculosis DNA was detected by IMS-PCR in 19 (7.8%) of the raw-milk samples and 67 (11.8%) of the pasteurized-milk samples (12, 17). Similar levels of M. paratuberculosis DNA were detected in the raw-milk (12.9%) and pasteurized-milk (9.8%) samples in the FSAI survey of Irish liquid milk on the retail market (Table 1).
The FSA UK survey found that 4 (1.6%) raw-milk samples and 10 (1.8%) pasteurized-milk samples were culture positive for M. paratuberculosis (12, 17). However, M. paratuberculosis was cultured from only one raw-milk sample (0.3%) in the present study. In addition, culture-positive M. paratuberculosis was not detected in any pasteurized-milk product, suggesting that the pasteurization regimens employed were efficient. The lower culture-positive rates in the FSAI survey (0.3% in raw milk and 0% in pasteurized milk), despite an IMS-PCR-positive rate similar to that in the FSA UK survey, may be due in part to the repeated testing of culture-negative plants or to culturing difficulties.
The results show a clear correlation between the fat content of the pasteurized samples and M. paratuberculosis DNA positivity, as shown in Fig. 1. As the fat content of the pasteurized samples increased (from skim milk to semiskim milk to whole milk to cream), the number of IMS-PCR positives also increased accordingly. This may relate to the hydrophobic nature of the lipid-rich M. paratuberculosis cell wall, making it more likely to associate with the fat fraction rather than the skim fraction of the milk. However, the centrifugation of samples for M. paratuberculosis testing may have an effect; thus, the above observation would require further investigation. We acknowledge that some M. paratuberculosis will go undetected if the cream fraction is not tested after centrifugation (as in this milk survey and the earlier United Kingdom milk survey, where the resuspended pellet was analyzed), and therefore the incidence of M. paratuberculosis may be underestimated. However, in our experience, the majority of M. paratuberculosis cells (in terms of CFU) segregate to the pellet upon centrifugation at 2,500 × g for 15 min and not to the cream fraction, and therefore, by testing the pellet fraction, the chances of detecting an M. paratuberculosis-positive sample are maximized.
M. paratuberculosis is an important animal pathogen and is the causative agent of Johne's disease in cattle (29). The IMS-PCR results from the present study and other Irish studies (31) suggest that there is a significant level of Johne's disease in the national herd. Successful culture of M. paratuberculosis from milk is dependent on many factors, including the number of M. paratuberculosis organisms present in the original milk sample, strain differences, the impact of HPC decontamination treatment on viability (9), antagonistic interference from non-acid-fast microorganisms during incubation, and stringent confirmatory tests requiring subculture of suspect colonies. Therefore, there is a high risk of false-negative culture results. As expected, suspect culture positives were obtained during this study. Some of the suspect colonies obtained were not subcultured successfully, which meant that confirmatory tests could not be performed, leading to the reporting of these milk samples as culture negative when in fact viable M. paratuberculosis may have been present. Because the success of methods used to culture M. paratuberculosis is dependent on a number of factors (as outlined above), studies inevitably underestimate the true prevalence of these difficult-to-culture organisms (34). There will also be disagreement between incidence estimates obtained by using molecular and cultural methods, as the detection sensitivity of the IMS-PCR method used (1 CFU/50 ml) is at least 10 times greater than the detection sensitivity of culture when HPC decontamination has been performed (ca. 10 CFU/50 ml [9]).
It has been reported that cows are most likely to excrete M. paratuberculosis during periods of stress, which may coincide with calving or feeding of a lower-quality diet (34). In all months of the present survey when samples were collected, IMS-PCR-positive results were obtained. The percentage of IMS-PCR-positive samples ranged from 1.8 to 25.0% of samples tested per month (mean, 12%). The largest peak for IMS-PCR-positive results occurred in June, and less significant peaks occurred in December, January, and September. A study carried out in the United Kingdom by Millar et al. (32) on the detection of M. paratuberculosis by IS900 PCR in retail supplies of whole pasteurized cows' milk in England and Wales also reported PCR-positive peaks at various times of the year. Millar et al. (32) identified peaks during January to March and September to November. Also, the study of the incidence of M. paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy-processing establishments in the United Kingdom (17) reported that, in all 17 months of the FSA UK survey except the final 2 months, IMS-PCR-positive results were obtained. The percentage of IMS-PCR-positive samples in the United Kingdom study ranged from 2 to 27.3% of samples tested per month. Peaks in incidence of IMS-PCR-positive samples were identified in the United Kingdom survey in June, July, and November (17).
It is widely recognized that the most effective way of reducing any potential human health risk of exposure to M. paratuberculosis through the consumption of cows' milk is to control and prevent Johne's disease in the national dairy herd (5, 7, 26, 33, 34). The farm is the critical control point at which to prevent the possibility of M. paratuberculosis entering the food chain. In April 2003 the Irish DAF announced a new voluntary strategy for addressing the problem of Johne's disease in the national dairy herd (8). Tackling the disease on the farm is central to the DAF strategy. The new strategy acknowledges that success in tackling the problem of Johne's disease is achievable only on the basis of a sustained commitment by all of the key players, including farmers and their representative organizations, dairy co-ops, cattle breed societies, veterinary surgeons, Teagasc (the Irish Agriculture and Food Development Authority), and DAF, with each playing a defined role within an integrated policy. Also, DAF has produced and circulated practical information on the control and prevention of Johne's disease for farmers. It has also circulated guidelines for on-farm management in relation to Johne's disease-infected cattle (7, 8).
In 2000, the Microbiology Sub-Committee of the FSAI's Scientific Committee published its review of the evidence of a link between M. paratuberculosis and Crohn's disease. The review concluded that there was insufficient evidence to establish such a link (11). In March 2003, the Microbiology Sub-Committee of the FSAI revisited its opinion on M. paratuberculosis in light of ongoing scientific publications on the issue. Again, the Committee was of the opinion that there is no proven link between M. paratuberculosis and Crohn's disease and that the report published in January 2000 (11) is still relevant.
On the basis of the results of FSAI surveillance of Irish liquid milk reported here, it can be concluded that M. paratuberculosis DNA is occasionally present in both raw and commercially pasteurized cows' milk. However, no viable M. paratuberculosis was isolated from commercially pasteurized cows' milk on retail sale in the Republic of Ireland. If the prevalence of subclinical Johne's disease in Ireland continues to increase, then the number of M. paratuberculosis organisms present in milk being processed at Irish milk pasteurizing plants could increase and the consumer protection afforded by pasteurization could be compromised at some point in the future. It has been suggested that the survival or inactivation of M. paratuberculosis by HTST pasteurization may be governed to some extent by the numbers initially present in the raw milk (14), although the propensity of M. paratuberculosis to clump confuses this issue in terms of conventional methods for viable counting. While the results of the FSAI survey of Irish liquid milk to detect M. paratuberculosis are encouraging, there is no room for complacency. The FSAI will remain watchful watching brief in this area and take appropriate precautionary measures that are proportionate to the perceived risk and the current state of knowledge on the issue.
Acknowledgments
The FSAI thanks the 27 approved milk pasteurization plants for their cooperation in the collection of samples and provision of processing information. Thanks are also due to John Early, Robin Kirk, and Natalie Totton for technical assistance during milk testing.
REFERENCES
- 1.Advisory Committee on the Microbiological Safety of Food. December 2001. Minutes of the 42nd meeting of the Advisory Committee on the Microbiological Safety of Food, Tower Hill, London. Advisory Committee on the Microbiological Safety of Food, London, United Kingdom.
- 2.Aschaffenburg, R., and J. E. C. Mullen. 1949. A rapid and simple phosphatase test for milk. J. Dairy Res. 16:108-115. [Google Scholar]
- 3.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurisation. J. Vet. Diagn. Investig. 5:90-117. [DOI] [PubMed] [Google Scholar]
- 4.Chiodini, R. J., and C. A. Rossiter. 1996. Paratuberculosis: a potential zoonosis? Vet. Clin. N. Am. 12:457-467. [DOI] [PubMed] [Google Scholar]
- 5.Collins, M. T. 2001. Prevention of paratuberculosis. On-farm control and diagnosis of paratuberculosis. Bull. Int. Dairy Fed. 364:46-47. [Google Scholar]
- 6.Committee on Diagnosis and Control of Johne's Disease, National Research Council. 2003. Diagnosis and control of Johne's disease. The U.S. National Academy of Sciences Board on Agriculture and Natural Resources Report. National Academy Press, Washington, D.C.
- 7.Department of Agriculture and Food. 2002. Johne's disease. Principal guidelines in relation to Johne's disease infected cattle. Department of Agriculture and Food, Dublin, Ireland.
- 8.Department of Agriculture and Food. 15 April 2003. Aylward announces review of approach to dealing with Johne's disease. Press release. Department of Agriculture and Food, Dublin, Ireland.
- 9.Dundee, L., I. R. Grant, H. J. Ball, and M. T. Rowe. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subspecies paratuberculosis from milk. Lett. Appl. Microbiol. 33:173-177. [DOI] [PubMed] [Google Scholar]
- 10.European Union Scientific Committee on Animal Health and Animal Welfare. 2000. Possible links between Crohn's disease and paratuberculosis. Report of the EU Scientific Committee on Animal Health and Animal Welfare (adopted 21 March 2000). European Commission, Brussels, Belgium. http://www.europa.eu.int/comm/food/fs/sc/scah/out38_en.pdf.
- 11.Food Safety Authority of Ireland. 2000. Mycobacterium paratuberculosis: does it contribute to Crohn's disease? Food Safety Authority of Ireland, Dublin, Ireland. http://www.fsai.ie/publications/reports/myco.pdf
- 12.Food Standards Agency UK. 2003. Report of the national study on the microbiological quality and heat processing of cows' milk 2003. Food Standards Agency UK, London, United Kingdom. http://www.foodstandards.gov.uk/multimedia/pdfs/milksurvey.pdf
- 13.Gao, A., L. Mutharia, S. Chen, K. Rahn, and J. Odumeru. 2002. Effect of pasteurization on survival of Mycobacterium paratuberculosis in milk. J. Dairy Sci. 85:3198-3205. [DOI] [PubMed] [Google Scholar]
- 14.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Effect of high-temperature, short-time (HTST) pasteurization on milk containing low numbers of Mycobacterium paratuberculosis. Lett. Appl. Microbiol. 26:166-170. [DOI] [PubMed] [Google Scholar]
- 15.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Isolation of Mycobacterium paratuberculosis from milk by immunomagnetic separation. Appl. Environ. Microbiol. 64:3153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant, I. R., H. J. Ball, and M. T. Rowe. 1999. Effect of higher pasteurisation temperatures, and longer holding times at 72°C, on the inactivation of Mycobacterium paratuberculosis in milk. Lett. Appl. Microbiol. 28:461-465. [DOI] [PubMed] [Google Scholar]
- 17.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant, I. R., E. I. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial-scale high-temperature, short-time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant, I. R., C. M. Pope, L. M. O'Riordan, H. J. Ball, and M. T. Rowe. 2000. Improved detection of Mycobacterium avium subspecies paratuberculosis in milk by immunomagnetic PCR. Vet. Microbiol. 77:369-378. [DOI] [PubMed] [Google Scholar]
- 20.Greenstein, R. J. 2003. Is Crohn's disease caused by a mycobacterium? Comparisons with leprosy, tuberculosis, and Johne's disease. Lancet Infect. Dis. 3:507-514. [DOI] [PubMed] [Google Scholar]
- 21.Gutknecht, K. 1997. Dire warnings about Johne's disease. A wake up call to the dairy industry? Wisc. Agric. 1997:8-15. [Google Scholar]
- 22.Hermon-Taylor, J. 1998. The causation of Crohn's disease and treatment with antimicrobial drugs. Ital. J. Gastroenterol. Hepatol. 30:607-610. [PubMed] [Google Scholar]
- 23.Hermon-Taylor, J. 2000. Mycobacterium avium subspecies paratuberculosis in the causation of Crohn's disease. World J. Gastroenterol. 6:630-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermon-Taylor, J., J. M. Sheridan, J. Cheng, M. L. Stellakis, and N. Sumar. 2000. Causation of Crohn's disease by Mycobacterium avium subspecies paratuberculosis. Can. J. Gastroenterol. 14:521-539. [DOI] [PubMed] [Google Scholar]
- 25.Jones, R. L. 1989. Review of the economic impact of Johne's disease in the United States, p. 46-50. In A. R. Milner and P. R. Wood (ed.), Johne's disease. Current trends in research, diagnosis and management. Commonwealth Scientific Industry Research Organisation, Melbourne, Australia.
- 26.Kennedy, D. 2001. Control of paratuberculosis. On-farm control and diagnosis of paratuberculosis. Bull. Int. Dairy Fed. 364:29-34. [Google Scholar]
- 27.Koch, R. 1884. Die Aetiologie der Tuber-kulose. Mitt. Kaiserl. Ges. 2:1-88. [Google Scholar]
- 28.Larsen, A. B., R. S. Merkal, and R. C. Cutlip. 1975. Age of cattle as related to infection with Mycobacterium paratuberculosis. Am. J. Vet. Res. 36:255-257. [PubMed] [Google Scholar]
- 29.Linnabary, R. D., G. L. Meerdink, M. T. Collins, J. R. Stabel, R. W. Sweeney, M. K. Washington, and S. J. Wells. 2001. Johne's disease in cattle. CAST issue paper no. 17. Council for Agricultural Science and Technology, Ames, Iowa.
- 30.Mason, O., M. T. Rowe, and H. J. Ball. 1997. Is Mycobacterium paratuberculosis a possible agent in Crohn's disease? Implications for the dairy industry. Milchwissenschaft 52:311-316. [Google Scholar]
- 31.McKee, R., I. R. Grant, M. T. Rowe, H. G. Buckley, J. F. Buckley, and S. Fanning. 2002. Examination of in-line milk filters to detect Mycobacterium avium subsp. paratuberculosis infection at farm level, p. 258-260. In R. A. Juste, M. V. Geijo, and J. M. Garrido (ed.), Proceedings of the 7th International Colloquium on Paratuberculosis. International Association for Paratuberculosis, Madison, Wisc.
- 32.Millar, D., J. Ford, J. Sanderson, S. Withey, M. Tizard, T. Doran, and J. Hermon-Taylor. 1996. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows' milk in England and Wales. Appl. Environ. Microbiol. 62:3446-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O' Doherty, A., D. O'Grady, K. O'Farrell, T. Smith, and J. Egan. 2002. Survey of Johne's disease in imported animals in the Republic of Ireland. Vet. Rec. 18:634-636. [DOI] [PubMed] [Google Scholar]
- 34.O' Doherty, A., D. O'Grady, T. Smith, and J. Egan. 2002. Mycobacterium avium subsp. paratuberculosis in pasteurised and unpasteurised milk in the Republic of Ireland. Irish J. Agric. Food Res. 41:117-121. [Google Scholar]
- 35.O'Reilly, L. M., and B. N. MacClancy. 1983. Paratuberculosis in Ireland, p. 99-102.. In J. Berg Jorgensen and O. Aalund (ed.), Paratuberculosis: diagnostic methods, practical application and experience with vaccination. Report EUR 9000EN. Commission of the European Communities, Agriculture, Brussels, Belgium.
- 36.Rubery, E. 2002. A review of the evidence for a link between exposure to Mycobacterium paratuberculosis (MAP) and Crohn's disease (CD) in humans. A report for the UK Food Standards Agency. Food Standards Agency UK, London, United Kingdom. http://www.foodstandards.gov.uk/multimedia/pdfs/mapcrohnreport.pdf
- 37.Shivananda, S., J. Lennard-Jones, R. Logan, N. Fear, A. Price, L. Carpenter, and M. van Blankenstein. 1996. Incidence of inflammatory bowel disease across Europe: is there a difference between North and South? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 39:690-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stabel, J. R. 1998. Johne's disease: a hidden threat. J. Dairy Sci. 81:283-288. [DOI] [PubMed] [Google Scholar]
- 39.Statutory Instrument no. 86 of 1955. 1955. Johne's Disease Order. Irish Statute Book. Government Publications Office, Dublin, Ireland.
- 40.Statutory Instrument no. 9 of 1996. 1996. European Communities (hygienic production and placing on the market of raw milk, heat-treated milk and milk-based products) Regulations, 1996. Irish Statute Book. Government Publications Office, Dublin, Ireland.