Abstract
1,2-sn-Diacylglycerols (DAGs) are activators of protein kinase C (PKC), which is involved in the regulation of colonic mucosal proliferation. Extracellular DAG has been shown to stimulate the growth of cancer cell lines in vitro and may therefore play an important role in tumor promotion. DAG has been detected in human fecal extracts and is thought to be of microbial origin. Hitherto, no attempts have been made to identify the predominant fecal bacterial species involved in its production. We therefore used anaerobic batch culture systems to determine whether fecal bacteria could utilize phosphatidylcholine (0.5% [wt/vol]) to produce DAG. Production was found to be dependent upon the presence of the substrate and was enhanced in the presence of high concentrations of deoxycholate (5 and 10 mM) in the growth medium. Moreover, its production increased with the pH, and large inter- and intraindividual variations were observed between cultures seeded with inocula from different individuals. Clostridia and Escherichia coli multiplied in the fermentation systems, indicating their involvement in phosphatidylcholine metabolism. On the other hand, there was a significant decrease in the number of Bifidobacterium spp. in the presence of phosphatidylcholine. Pure-culture experiments showed that 10 of the 12 strains yielding the highest DAG levels (>50 nmol/ml) were isolated from batch culture enrichments run at pH 8.5. We found that the strains capable of producing large amounts of DAG were predominantly Clostridium bifermentans (8 of 12), followed by Escherichia coli (2 of 12). Interestingly, one DAG-producing strain was Bifidobacterium infantis, which is often considered a beneficial gut microorganism. Our results have provided further evidence that fecal bacteria can produce DAG and that specific bacterial groups are involved in this process. Future strategies to reduce DAG formation in the gut should target these species.
Colon cancer is one of the most common causes of mortality in Western civilization (25). Studies have shown that certain environmental factors, especially a diet high in fat and meat, may play a significant role in the risk of the development of colon cancer (36). However, the mechanisms by which such diets affect its incidence have not been determined, although it is considered that a high fat intake results in an increased generation of secondary bile acids through activities of the gut bacteria. Hill et al. (19) showed increased levels of secondary bile acids in the feces of patients with colon cancer. Many subsequent studies have attempted to explain this relationship, and increased levels of secondary bile acids have been associated with enhanced cell proliferation and the activity of ornithine decarboxylase, a rate-limiting enzyme in polyamine synthesis (8). They have also been shown to stimulate protein kinase C (PKC), an enzyme that plays a key role in growth control and signal transduction (22).
PKC is normally activated by endogenous 1,2-sn-diacylglycerol (DAG) released from membrane phospholipids (27). However, exogenous DAG can stimulate intracellular signaling pathways, inducing mitosis in adenoma, and some carcinoma, cells in vitro (14). Thus, increased levels of exogenous DAG can also be considered a tumor-promoting risk factor. Morotomi et al. (31) showed that human fecal bacteria generated DAG when they were incubated with phosphatidylcholine and secondary bile salts. They proposed that bacteria expressing phospholipase C would be capable of such a conversion and that this was dependent upon the presence of secondary bile acids. Several studies have shown that a high-fat diet results in increased fecal concentrations of DAG (7, 35, 39). The interaction of DAG with PKC is thought to be stereospecific and stimulated only by DAG with a 1,2-sn configuration (33). DAG produced from the action of digestive or hepatic lipases upon dietary triacylglycerides does not share such stereospecificity (30).
The large intestine is by far the most heavily colonized part of the human body, with several hundred culturable species of bacteria, whose numbers can exceed 1012 per g of dry matter (10). These bacteria are able to produce a wide range of compounds that have been found to exert both positive and negative effects on gut physiology and host health (16). Given the diversity and number of bacterial species found in the human large intestine, it is plausible that DAG found in the feces is a result of microbial activity. The aims of this study were to quantify 1,2-sn-DAG production in phosphatidylcholine fermentations inoculated with fecal material obtained from healthy individuals and to assess the effects of enhanced DAG levels on the major bacterial groups found in the colon.
MATERIALS AND METHODS
Chemicals.
Unless otherwise stated, all chemicals and reagents were obtained from Sigma-Aldrich Co. Ltd. (Poole, United Kingdom) and bacteriological growth medium supplements were obtained from Oxoid Ltd. (Basingstoke, United Kingdom).
Preparation and collection of fecal samples.
Fecal samples were obtained from six healthy human volunteers (two males and four females; age range, 25 to 43; mean age, 30) on two separate occasions. None of the volunteers had been prescribed antibiotics for at least 6 months prior to the study or had any history of gastrointestinal disease. The samples were collected on site and used immediately after collection. A 1/10 (wt/vol) dilution in anaerobic phosphate buffer (0.1 M, pH 7.4) was prepared, and the samples were homogenized in a stomacher for 2 min.
Batch fermentations.
Sterile stirred batch culture fermentation vessels (300 ml) were filled with 135 ml of sterilized basal nutrient medium (peptone water, 2 g/liter; yeast extract, 2 g/liter; NaCl, 0.1 g/liter; K2HPO4, 0.04 g/liter; KH2PO4, 0.04 g/liter; MgSO4 · 7H2O, 0.01 g/liter; CaCl2 · 6H2O, 0.01 g/liter; NaHCO3, 2 g/liter; Tween 80, 2 ml/liter; hemin, 0.02 g/liter; vitamin K1, 10 μl/liter; cysteine-HCl, 0.5 g/liter; and bile salts [sodium glycocholate and sodium taurocholate], 0.5 g/liter [pH 7.0]) with or without 0.5% (wt/vol) phosphatidylcholine and were gassed overnight with O2-free N2. Prior to the addition of the fecal slurry, the temperature was controlled at 37°C by means of a circulating water bath, and the culture pH was maintained at 6.8, 7.5, and 8.5 in separate vessels by use of an Electrolab pH controller. The vessels were inoculated with 15 ml of fresh fecal slurry and continuously sparged with O2-free N2 at a rate of 15 ml/min. Samples (5 ml) from each vessel were obtained for culture on selective agars, for fluorescence in situ hybridization, for analyses of short-chain fatty acids (SCFA) by high-performance liquid chromatography, and for DAG measurements. The batch cultures were run for 48 h, and samples were taken at the start and end of the incubations (T0 and T48).
The effect of deoxycholic acid (DCA) was assessed in fermentors under the same conditions described above, with 0.5% (wt/vol) phosphatidylcholine added to the medium. The only difference was that the bile salts in the basal medium were replaced with 1.2, 2.5, 5, or 10 mM DCA, which was filter sterilized at the appropriate concentration and added after autoclaving. Batch cultures were run in duplicate. The production of DAG was assessed for 48 h, with samples taken after 4, 8, 10, 24, and 48 h.
Selective agar culture.
Samples (1 ml) were serially diluted in half-strength peptone water in an anaerobic cabinet (10% H2, 10% CO2, 80% N2). Triplicate plates, with media that were selective for populations of total anaerobes, total aerobes, bacteroides, bifidobacteria, lactobacilli, clostridia, or coliforms, were inoculated from each dilution tube. The agars used were those described by Wang and Gibson (46). They were incubated at 37°C aerobically or anaerobically, as appropriate. After incubation, the bacterial populations were enumerated and individual colonies were randomly removed for confirmation of culture identities. These were subcultured onto fresh agar, checked for purity by Gram staining, and stored at −70°C.
Bacterial enumeration.
Differences in bacterial populations were assessed by fluorescence in situ hybridization with oligonucleotide probes designed to target diagnostic regions of 16S rRNA. These were commercially synthesized and labeled with the fluorescent dye Cy3 (Eurogentec UK Ltd.). The probes used were Bif164 (26), Bac303 (29), Lab158 (18), Erec482 (13), His150 (13), Ec1531 (37), and Srb687 (11), specific for bifidobacteria, bacteroides, Lactobacillus and Enterococcus spp., the Clostridium coccoides-Eubacterium rectale group, the Clostridium histolyticum group, Escherichia coli, and Desulfovibrio spp., respectively. For total bacterial counts, the nucleic acid stain 4,6-diamidino-2-phenylindole (DAPI) was used. Samples obtained from fermentation vessels were diluted in 4% (wt/vol) paraformaldehyde and fixed overnight at 4°C. The cells were then centrifuged at 1,500 × g for 5 min, washed twice with phosphate-buffered saline (0.1 M, pH 7.0), resuspended in a mixture of phosphate-buffered saline and 99% ethanol (1:1), and stored at −20°C for at least 1 h. The cell suspension was then added to the hybridization mixture and left to hybridize at the appropriate temperature for each probe (11, 13, 18, 26, 29, 37). The resultant mixture was washed and vacuum filtered through a 0.2-μm-pore-size Isopore membrane filter (Millipore Corporation, Watford, Herts, United Kingdom). The filter was removed, placed onto a glass slide with SlowFade (Molecular Probes, Eugene, Oreg.), and examined by fluorescence microscopy (Nikon Eclipse E400 microscope). The DAPI-stained cells were examined under UV light, and hybridized cells were viewed by use of a DM510 filter. For each slide, at least 15 different fields of view were counted. The following equation was used to calculate the number of bacteria: number of bacteria = log10[15.56 × A1 × 14,873.74 × (1,000/S1)], where 15.56 is the dilution factor, A1 is the average count derived from 15 fields of view, 14,873.74 is the area of the field of view, and S1 is the sample size expressed in microliters. The number of cells obtained is expressed per milliliter of culture.
Analysis of SCFA.
The production of lactic, acetic, propionic, and butyric acids during fermentations was quantified. Samples were centrifuged at 1,500 × g for 15 min, and the resultant supernatants were used for analysis. A model 1801 UV high-pressure liquid chromatograph (Bio-Rad) with an integrated oven compartment (50°C) and data system was used in combination with a differential refractometer (Knauer). The column was a prepacked Aminex HPX-87-H strong-cation-exchange resin column (150 by 7.8 mm [internal diameter]) fitted with an ion exclusion microguard refill cartridge (Bio-Rad). Samples (25 μl) were injected via a sample loop valve. The eluent used was 0.005 M sulfuric acid.
Assay of 1,2-sn-DAG production.
In order to quantify DAG, we first extracted the lipid fraction of the culture supernatants by a modification of the Bligh-Dyer technique, using chloroform-methanol-water (5). Briefly, chloroform (1.25 ml) and methanol (2.5 ml) were added to 1 ml of sample and vortexed thoroughly. A further 1.25 ml of chloroform and 1.25 ml of water were added, and the liquid was thoroughly mixed. The lower chloroform phase was removed and the aqueous phase was reextracted twice. Pooled lower phases were evaporated to dryness under oxygen-free nitrogen, and lipids were redissolved in 500 μl of chloroform prior to storage at −20°C. These extracts were then assayed for physiologically relevant (1,2-sn-configured) DAG by use of a commercially available kit (Amersham International, Little Chalfont, United Kingdom) by which DAG was converted to [32P]phosphatidic acid by a bacterial DAG kinase in the presence of [γ-32P]ATP. [32P]phosphatidic acid was then measured by liquid scintillation spectrometry.
Identification of DAG-producing bacteria.
Isolates were subcultured on Wilkins-Chalgren plates and checked for purity before they were screened. Screw-top bottles (20 ml) were filled with the basal nutrient medium described above supplemented with 1% (wt/vol) phosphatidylcholine as a substrate (pH 8.5), autoclaved at 121°C for 15 min, left overnight in an anaerobic cabinet, and seeded the following day with a 1% inoculum of individual bacterial strains. In total, 198 bacterial isolates obtained from the fermentation work described above were screened for DAG production. The cultures were incubated (anaerobically) for 48 h, after which samples were removed for measurements of the final culture pH and the DAG concentration (as described above). Strains that were positive for DAG production were then identified by 16S rRNA gene sequencing.
PCR and sequencing of 16S rRNA-encoding genes of strains.
DNAs were extracted with InstaGene matrix (Bio-Rad). The 16S rRNA genes were amplified by PCRs performed according to the method of Hoyles et al. (21), using ∼10 ng of DNA, and partial sequencing was performed with an automated DNA sequencer (model 373A; Applied Biosystems) and a Taq Dye-Deoxy Terminator cycle sequencing kit (Applied Biosystems) using primer γ (MWG Biotech) (21). The partial sequences were subjected to BLAST searches via the EMBL web site to confirm the identities of the strains.
Statistical analysis.
Differences in all of the results were analyzed with Student's paired t test.
RESULTS
The ability of gut bacteria to produce DAG was assessed by batch culture fermentations of 0.5% (wt/vol) phosphatidylcholine. Replicate fermentors were run at three different pH values to simulate different physiological conditions of the gut during health and disease. Namely, pH 6.8 was used to approximate healthy gut contents, and pH 7.5 and pH 8.5 were used because they are indicative of many diseases, including bowel cancer (4, 40).
Production of 1,2-sn-DAG by fecal bacteria.
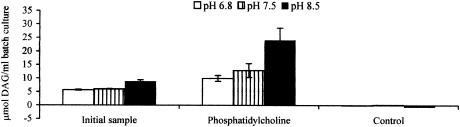
The in vitro production of DAG at pHs 6.8, 7.5, and 8.5 is shown in Fig. 1. The amounts of DAG in the initial samples (T0; immediately after inoculation) were similar across all of the pHs. However, there was a reproducible trend toward more DAG production at pH 8.5 than at pH 6.8 or 7.5. DAG concentrations rose in all vessels containing phosphatidylcholine after a 48-h fermentation. No DAG was identified in the absence of phosphatidylcholine (control) after a 48-h fermentation. This finding was consistent at all pH values examined and indicated either that fecal bacteria utilize residual DAG levels in vitro or that DAG was unstable under the conditions used.
FIG. 1.
Production of 1,2-sn-DAG by fecal bacteria in batch culture fermentations run at pHs 6.8, 7.5, and 8.5. Fecal samples were collected from six individuals on two separate occasions and were incubated with 0.5% (wt/vol) phosphatidylcholine and 135 ml of basal nutrient medium in batch culture systems at 37°C. Control fermentors contained no phosphatidylcholine. Samples were obtained at time zero and after 48 h of incubation, and lipids were extracted prior to the measurement of DAG by use of a commercially available kit. The data presented are mean values of initial DAG levels (T0) and levels after 48 h of fermentation with and without phosphatidylcholine.
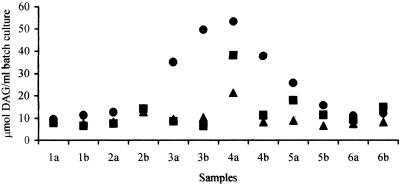
The extent of DAG production is illustrated in more detail in Fig. 2, which shows the levels after 48 h of fermentation for each run. These data clearly demonstrate both interindividual and intraindividual variation in DAG production in vitro, suggesting that the composition of the microbiota may play as important a role in DAG production as the pH. Data for subjects 1, 2, and 6 were consistent between runs and across the three pH levels examined, with only slight increases in DAG levels during fermentation (compared to the T0 levels) (data not shown), which were generally independent of the pH. In contrast, inocula from subjects 3, 4, and 5 produced significantly higher DAG levels (P < 0.01, 0.01, and 0.05, respectively) after 48 h of fermentation at pH 8.5, and fecal bacteria from one individual (subject 4) showed an increased ability to produce DAG at pH 7.5 (P < 0.05).
FIG. 2.
1,2-sn-DAG production by fecal bacteria in batch culture fermentations, measured at the end of the phosphatidylcholine run. Fecal samples were collected from six individuals (numbers 1 to 6) on two separate occasions (a and b) and were incubated with 0.5% (wt/vol) phosphatidylcholine and 135 ml of basal nutrient medium in batch culture systems at 37°C. DAG production was measured after 48 h of fermentation. •, pH 8.5; ▪, pH 7.5; ▴, pH 6.8.
Effect of phosphatidylcholine on bacterial populations and production of lactic, acetic, propionic, and butyric acids.
Overall, the total bacterial counts and levels of lactobacilli-enterococci decreased significantly (P < 0.01) in all fermentations, irrespective of the culture pH and the presence or absence of lipids (Table 1). Concurrently, E. coli numbers increased in all systems and the numbers of Desulfovibrio spp. increased (P < 0.01) in all vessels except for the phosphatidylcholine fermentations run at pH 8.5, compared to initial levels. Bifidobacterial numbers decreased (P < 0.01) in the presence of phosphatidylcholine compared to the initial levels and controls at all pH levels tested. Bacteroides levels were broadly similar under all conditions. C. histolyticum levels increased (P < 0.01) during batch cultures with phosphatidylcholine. A similar increase was seen for C. histolyticum in the control fermentation at pH 6.8 (P < 0.05). C. coccoides-E. rectale levels remained relatively stable during fermentation, with the only notable difference between T0 and T48 occurring at pH 6.8 in the absence of lipids (lower at T48; P < 0.05).
TABLE 1.
Mean bacterial populations in all samples over the experimental period for pHs 6.8, 7.5, and 8.5, as determined by fluorescence in situ hybridization
| Bacterial group | Bacterial population (log10 cells/ml of batch culture)a
|
||||||
|---|---|---|---|---|---|---|---|
| Initial sample | pH 6.8 (T48)
|
pH 7.5 (T48)
|
pH 8.5 (T48)
|
||||
| Lipids | Control | Lipids | Control | Lipids | Control | ||
| Total bacteria | 9.8 ± 0.1 | 9.5 ± 0.1c,d | 9.3 ± 0.2c | 9.4 ± 0.1c | 9.3 ± 0.3c | 9.4 ± 0.1c,d | 9.3 ± 0.2c |
| Bifidobacterium spp. | 8.4 ± 0.1 | 8.1 ± 0.2c,e | 8.4 ± 0.1 | 8.1 ± 0.2c,e | 8.3 ± 0.1 | 8.0 ± 0.2c,e | 8.3 ± 0.0 |
| Bacteroides spp. | 8.7 ± 0.2 | 8.5 ± 0.2d | 8.6 ± 0.1 | 8.5 ± 0.1 | 8.6 ± 0.1 | 8.5 ± 0.1b | 8.5 ± 0.1 |
| Lactobacilli-enterococci | 7.9 ± 0.1 | 7.3 ± 0.3c | 7.4 ± 0.1c | 7.3 ± 0.2c | 7.5 ± 0.3c | 7.2 ± 0.2c,d | 7.5 ± 0.2c |
| C. coccoides-E. rectale | 8.8 ± 0.2 | 8.6 ± 0.1h | 8.4 ± 0.3b | 8.6 ± 0.1h | 8.4 ± 0.3 | 8.7 ± 0.1e | 8.4 ± 0.3 |
| C. histolyticum | 7.5 ± 0.2 | 7.9 ± 0.2c,g | 7.9 ± 0.2c,f,h | 8.0 ± 0.2c,d | 7.6 ± 0.2 | 8.0 ± 0.2c,e | 7.7 ± 0.4 |
| E. coli | 7.3 ± 0.1 | 7.5 ± 0.3b | 7.5 ± 0.2b | 7.6 ± 0.2c,g | 7.5 ± 0.1c | 7.4 ± 0.1b | 7.5 ± 0.2b |
| Desulfovibrio spp. | 6.9 ± 0.2 | 7.2 ± 0.1c | 7.2 ± 0.1c | 7.2 ± 0.2c | 7.2 ± 0.2c | 7.6 ± 0.4 | 7.2 ± 0.1c |
Bacterial numbers are means ± standard deviations (n = 12). The inoculum was human feces (1% [wt/vol]), and the incubations were carried out in batch culture for 48 h with phosphatidylcholine as the main substrate at a concentration of 0.5% (wt/vol). Control fermentors did not contain phosphatidylcholine.
Significantly different from the initial sample (P < 0.05).
Significantly different from the initial sample (P < 0.01).
Significantly different from T48 control (P < 0.05).
Significantly different from T48 control (P < 0.01).
Significantly different from T48 sample at pH 7.5 (P < 0.01).
Significantly different from T48 sample at pH 8.5 (P < 0.05).
Significantly different from T48 sample at pH 8.5 (P < 0.01).
In general, the reduction in total bacterial counts was significantly larger in control systems than in those containing phosphatidylcholine (P < 0.05). Levels of lactobacilli-enterococci were significantly higher (P < 0.05) in fermentations run at pH 8.5 without phosphatidylcholine than in those with lipids. The culture pH had a lesser effect on bacterial populations than did the presence of lipids. Batch cultures containing phosphatidylcholine and run at pH 8.5 contained higher numbers of the C. coccoides/E. rectale group after 48 h of fermentation than the cultures run at pHs 6.8 and 7.5 (P < 0.01). Similarly, the numbers of the C. histolyticum group were significantly higher at T48 in lipid vessels operated at pH 8.5 than in those operated at pH 6.8 (P < 0.05). However, control vessels run at pH 6.8 contained unusually high levels of C. histolyticum which were significantly higher (P < 0.01) than the control levels for both pHs 7.5 and 8.5. The only other significant difference with respect to pH was seen in the E. coli populations in the presence of phosphatidylcholine between pHs 7.5 and 8.5 (P < 0.05).
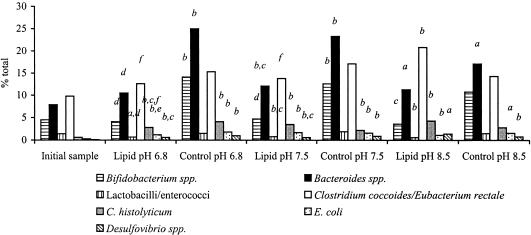
An examination of the relative proportions of each bacterial group, taking into account the decrease in total bacterial numbers during all fermentations, revealed a slightly different trend in the data (Fig. 3). In the case of the bifidobacterial population, for example, the presence of lipids resulted in an overall reduction in numbers while the control systems maintained initial bifidobacterial numbers. However, the relative proportion of bifidobacteria remained stable during the 48-h fermentation in the presence of lipids and increased significantly in the controls (P < 0.01 for pHs 6.8 and 7.5). Lactobacillus-Enterococcus proportions decreased in the presence of phosphatidylcholine (P < 0.05 for pH 6.8 and P < 0.01 for pHs 7.5 and 8.5) while remaining constant in the controls. In the cases of all of the other bacterial populations examined, the proportions increased during the 48-h fermentation period. The only effects of pH on bacterial distribution were seen in the presence of lipids. The C. coccoides/E. rectale group comprised a larger proportion of the microbiota at pH 8.5 than at pHs 6.8 and 7.5 (P < 0.05), C. histolyticum made up a larger part at pH 8.5 than at pH 6.8 (P < 0.05), and the E. coli proportion was higher at pH 7.5 than at pH 6.8 (P < 0.05).
FIG. 3.
Mean bacterial proportions in all samples over the experimental period for pHs 6.8, 7.5, and 8.5, as determined by fluorescence in situ hybridization. Population proportions are expressed as percentages of total bacteria. The inocula were human feces (1% [wt/vol]), and the incubations were carried out in batch cultures for 48 h with phosphatidylcholine as the main substrate at a concentration of 0.5% (wt/vol). Control fermentors did not contain phosphatidylcholine. a, significantly different from the initial sample (P < 0.05); b, significantly different from the initial sample (P < 0.01); c, significantly different from the T48 control (P < 0.05); d, significantly different from the T48 control (P < 0.01); e, significantly different from the T48 sample at pH 7.5 (P < 0.05); f, significantly different from the T48 sample at pH 8.5 (P < 0.05).
The levels of metabolic end products such as SCFA increased during fermentations, regardless of the presence of lipids or the pH, with the only exception being a significant reduction in butyrate in control cultures at pHs 7.5 and 8.5 (P < 0.05) (Table 2). Similarly, a reduction in lactic acid levels was observed in all fermentations, regardless of the pH or the presence of lipids.
TABLE 2.
Average SCFA levels over the experimental period for pHs 6.8, 7.5, and 8.5, as determined by high-performance liquid chromatography
| Fatty acid | Amt of SCFA (mM)f
|
||||||
|---|---|---|---|---|---|---|---|
| Initial sample | pH 6.8 (T48)
|
pH 7.5 (T48)
|
pH 8.5 (T48)
|
||||
| Lipids | Control | Lipids | Control | Lipids | Control | ||
| Lactic acid | 8.3 ± 3.1 | 3.2 ± 3.0b,d,e | 3.5 ± 0.6b,d,e | 2.7 ± 2.5b,e | 1.3 ± 0.7b | 2.1 ± 2.1b | ND |
| Acetic acid | 9.7 ± 4.2 | 17.7 ± 6.6a,d | 13.7 ± 0.2e | 19.4 ± 6.4b | 13.9 ± 0.4 | 18.1 ± 4.9b | 14.5 ± 0.2 |
| Propionic acid | 5.1 ± 4.2 | 13.2 ± 7.7b | 12.9 ± 3.7b | 13.4 ± 8.2b | 12.5 ± 1.7 | 13.0 ± 5.8b | 12.8 ± 0.8 |
| Butyric acid | 2.2 ± 2.1 | 10.8 ± 5.3b,c | 1.6 ± 2.3 | 12.7 ± 8.2b,c | 1.9 ± 0.1a,e | 15.6 ± 11.4b,c | ND |
Significantly different from the initial sample (P < 0.05).
Significantly different from the initial sample (P < 0.01).
Significantly different from T48 control (P < 0.05).
Significantly different from T48 sample at pH 7.5 (P < 0.05).
Significantly different from T48 sample at pH 8.5 (P < 0.05).
Amounts of SCFA are means ± standard deviations (n = 12). The inoculum was human feces (1% [wt/vol]), and the incubations were carried out in batch culture for 48 h with phosphatidylcholine as the main substrate at a concentration of 0.5% (wt/vol). Control fermentors did not contain phosphatidylcholine. ND, not detected.
Identification of DAG-producing bacteria.
Thirty-four of the 198 strains screened for DAG production failed to grow in medium containing 1% (wt/vol) phosphatidylcholine and were discarded. In contrast, 12 organisms produced concentrations in excess of 50 nmol/ml in pure culture and were subsequently characterized by partial 16S rRNA gene sequencing. Of the remaining isolates, 5 produced only trace amounts of DAG and 147 yielded between 8.1 and 42.9 nmol/ml (average, 26.2 ± 7.9 nmol/ml). Clostridium bifermentans was the predominant species identified, comprising 8 of the 12 high DAG producers (Table 3). Other bacterial isolates that were shown to be high DAG producers were E. coli, Bifidobacterium infantis, and an isolate that was most closely related to an uncultured rumen bacterium. Ten of the 12 high DAG producers originated from phosphatidylcholine fermentations at pH 8.5, with the other two coming from pH 7.5 systems. Interestingly, those strains which were isolated from the pH 7.5 batch culture systems produced notably less DAG than those from pH 8.5 fermentors. Four of the high DAG producers were isolated from batch cultures inoculated with a fecal slurry from subject 5, three each were isolated from subjects 3 and 4, and two were isolated from subject 2.
TABLE 3.
Identities of strains that produced the highest amounts of DAG in static batch cultures, as determined by sequencing
| Isolate | Subject no. | Fermentation pH | Bacterial species (% similarity)a | Amt of DAG (nmol/ml) |
|---|---|---|---|---|
| WCC6a | 3 | 8.5 | C. bifermentans (99%) | >135 |
| CF4 | 5 | 8.5 | C. bifermentans (99%) | >135 |
| WCC6b | 3 | 8.5 | C. bifermentans (99%) | >135 |
| WCS2a | 4 | 8.5 | C. bifermentans (99%) | >135 |
| BAC5 | 3 | 8.5 | C. bifermentans (99%) | >135 |
| BAF6b | 5 | 8.5 | C. bifermentans (99%) | >135 |
| WCB9a | 2 | 8.5 | C. bifermentans (99%) | >135 |
| WCF4a | 5 | 7.5 | C. bifermentans (99%) | 70.7 |
| WC2D11 | 4 | 8.5 | E. coli (98%) | >135 |
| BAD7 | 4 | 7.5 | E. coli (98%) | 99.9 |
| BAF6a | 5 | 8.5 | Uncultured rumen bacterium (99%) | 130.9 |
| BE1B9 | 2 | 8.5 | B. infantis (99%) | >135 |
The sequences were submitted as BLAST searches via the EMBL website (http://www.ebi.ac.uk), and anything over 98% recognition was accepted as identity.
All cultures of C. bifermentans strains had a final culture pH of 6.9, except the one isolated from the pH 7.5 fermentor, which produced less DAG and reduced the pH of the culture medium to 7.2. Similarly, an E. coli strain that was shown to produce more DAG reduced the medium pH to 6.8, whereas the E. coli strain that produced less DAG had a final culture pH of 7.2. The uncultured rumen bacterium and the B. infantis strain yielded final culture pHs of 7.0 and 7.8, respectively. These results suggest that 11 of the strains (all but the B. infantis strain) were able to metabolize lipids or to ferment other components in the medium. More DAG was produced by those strains that were able to reduce the pH of the medium to neutral, suggesting that the ability to produce DAG in pure culture could be closely related to substrate solubility and utilization. However, this was not the case for B. infantis, which produced high levels of DAG without any marked reduction in the pH of the culture medium. It is not clear whether the B. infantis strain was able to metabolize lipids (producing DAG) or whether phosphatidylcholine stimulated DAG yields by another route.
Effect of DCA on DAG production.
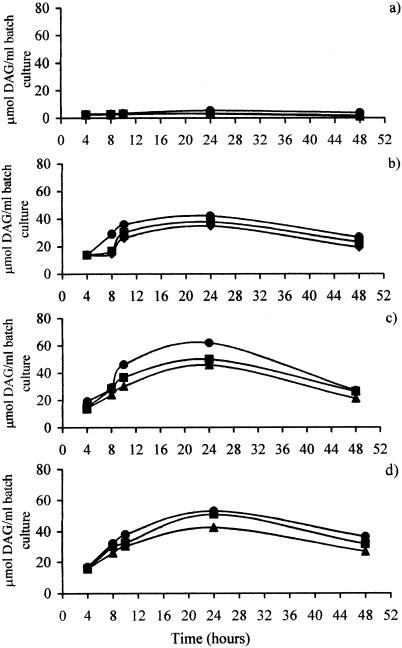
No DAG was detected at any of the pH values tested when the bile salts in the basal medium were replaced with DCA at the same concentration (i.e., 1.2 mM DCA) (Fig. 4a). It is not clear why this amount of DCA had an inhibitory effect upon DAG production. However, at higher concentrations, the production of DAG was markedly increased, although a significantly higher (P < 0.01) DAG concentration after 48 h compared to that shown in Fig. 1 was only seen with 10 mM DCA. Maximum DAG production was achieved by 24 h, after which the DAG levels declined (P < 0.01). This was consistent for all pH levels used and all DCA concentrations examined, suggesting that either fecal bacteria reduce DAG over time or DAG is unstable. The observation that pH appears to be an important factor affecting the rate of DAG production (Fig. 1) was corroborated. DAG production again increased with a higher culture pH (P < 0.01 for 5 mM DCA and P < 0.05 for 2 and 10 mM DCA). Furthermore, DAG production was also affected by the DCA concentration. A significantly smaller (P < 0.01) amount of DAG was generated with 2 mM DCA, at all pH levels tested, than was generated with 5 or 10 mM DCA. Overall, DCA appeared to enhance DAG production at higher concentrations, with the optimum being about 5 mM under these conditions.
FIG. 4.
Production of 1,2-sn-DAG in the presence of DCA by fecal bacteria in batch culture fermentations run at pHs 6.8 (▴), 7.5 (▪), and 8.5 (•). Fecal samples were incubated with DCA, 0.5% phosphatidylcholine, and 135 ml of basal nutrient medium at 37°C. Samples were obtained at 4, 8, 10, 24, and 48 h, and lipids were extracted prior to the measurement of DAG by use of a commercially available kit. The data presented are mean values from duplicate runs. DCA was used at a concentration of 1.2 (a), 2 (b), 5 (c), or 10 (d) mM.
DISCUSSION
The principal aim of these studies was to determine whether human fecal bacteria could produce DAG in vitro. The results demonstrate that this is possible when phosphatidylcholine is used as a substrate. However, the data also indicate the potential of the gut flora to elicit deleterious fermentations under certain conditions.
Bacterial fermentation is often considered one of the most important factors in determining the colonic pH, and this work demonstrates that pH has a direct effect upon DAG levels, with an increase in pH resulting in a significant elevation of the DAG level. One reason for this may be that phosphatidylcholine is a neutral phospholipid and, as such, requires a neutral or slightly alkaline pH for its solubility. It has been demonstrated that the degradation of phosphatidylcholine is negligible below pH 7 (31). However, the increased levels of DAG were not only determined by the culture pH. The addition of DCA to the medium also increased DAG production, with an optimum concentration of 5 mM DCA, in line with similar observations from previous studies (34). The effect of DCA and other bile acids on DAG production is, however, not fully understood. One hypothesis is that bile acids act simply as detergents, increasing the solubility of phospholipids. However, studies that examined this relationship found that DAG production was effective only in the presence of bile acids and that other detergents that are known to solubilize phosphatidylcholine in the reaction medium, such as Triton X-100, had no such stimulatory effect (32). Morotomi et al. (31) suggested that the steric feature of a hydrophobic domain containing two hydroxyl groups in DCA and the amphiphilic character of these groups were responsible for the enhancing effect. No studies have examined this relationship in detail, and as such, the precise mechanism for the involvement of bile acids, and especially DCA, remains unknown. The biological significance of this finding, however, is that secondary bile acids are found in the lumen of the large intestine at a concentration of 2.4 mM in healthy individuals (20). In individuals who have a tendency to develop cancer, such as those consuming a high-fat diet, the concentration of DCA can be even higher (17). Therefore, the potential to create within the large intestine an environment that would enhance the production of DAG exists.
The results presented in this article demonstrate both inter- and intraindividual variations in DAG production in vitro. It is essential not only to have the appropriate conditions for DAG production but also for bacteria capable of this transformation to be present. This finding strongly supports the suggestion that the composition of the microbiota plays an important role in human health. Inter- and intraindividual variations in bacterial composition, enzyme activities, moisture, and pH in healthy individuals have been previously demonstrated, regardless of DAG production (23). Moreover, variations in fecal DAG content have been demonstrated in vivo, and it has been suggested that this is due to changes in the proportion of bacterial species possessing phospholipase C, an enzyme involved in DAG conversion (31).
In this study, bacteria that were capable of producing considerable amounts of DAG were identified as predominantly C. bifermentans and E. coli. The activity of phospholipase C was not assessed, but it is well known that C. bifermentans possesses phospholipase C activity (45). Moreover, C. bifermentans has been associated with colonic and hematological malignancies (24, 41) and is also known to produce a 7α-dehydroxylase enzyme (2, 12). Bacterial 7α-dehydroxylase catalyzes the removal of the 7α-hydroxyl group from primary bile acids to form secondary bile acids (28). Thus, C. bifermentans has the ability to create a favorable environment for enhanced DAG production. Indeed, one of the reasons that primary bile acids were used in the medium for the majority of the experiments in this study was the fact that the conversion to secondary bile acids occurs naturally under appropriate conditions (i.e., the presence of bacteria and an optimal pH of 7 to 8) (28).
There are several potential mechanisms for the production of DAG from phosphatidylcholine during fermentations by the fecal microbiota. Increased DAG levels may reflect an exchange of exogenous phospholipids with those of the bacterial cell membrane. For example, E. coli does not contain phosphatidylcholine in its membrane but has other phospholipids, such as phosphatidylethanolamine (PE), phosphatidylglycerol, and diphosphatidylglycerol. The outer membrane exclusively contains PE, which is a neutral lipid, as is phosphatidylcholine (9). Studies have shown that the incubation of E. coli with PE results in the production of DAG (38). Although no studies have shown that phosphatidylcholine exerts a similar effect, the exchange of phospholipids between E. coli and the environment has been established (15). Additionally, membrane incorporation of phosphatidylcholine has been demonstrated for bacteria that are unable to produce this phospholipid naturally (3). Cell lysis may also occur, releasing enzymes (such as phospholipase A) (9) which are capable of hydrolyzing substrates such as phosphatidylcholine (44) to yield DAG. Furthermore, DAG may be formed during the synthesis of membrane-derived oligosaccharides (42). The present study did not attempt to elucidate the mechanisms of DAG production.
The observation that B. infantis has the potential to generate DAG was unexpected, as this bacterium is thought to be among the beneficial bacteria present in the colon (16) and because higher fecal DAG levels are associated with an increased risk of colon cancer. Indeed, the ability of the majority of isolates from the phosphatidylcholine fermentations to produce DAG was somewhat of a surprise (80.3% of the bacteria produced ≥8.1 nmol/ml). One explanation may be that the experimental procedure was selective for isolation of the predominant species. Alternatively, enrichment may have occurred, making these strains better adapted to a lipid environment and thus able to produce DAG. We propose that many bacterial species are involved in DAG production to varying degrees (beyond those identified herein) and that together the consortia contribute to overall DAG production. One such species would most certainly be Clostridium perfringens, which has been shown to possess phospholipase C activity (43) and which has the ability to produce DAG in pure culture (34). Of course, it is not unexpected that we did not isolate C. perfringens, as it generally comprises <1% of the colonic microbiota of healthy individuals (13). C. perfringens may, however, play an important role in DAG production in subjects with a compromised gut ecology (including colon cancer patients or subjects at risk for colon cancer) (6).
One interesting observation from the present work was that DAG levels declined over time. DAG can be hydrolyzed to yield fatty acids and monoacylglycerols in a reaction catalyzed by DAG kinase (44). Therefore, it is feasible that certain bacteria may be involved in this degradation (31). Moreover, it was observed that bacteria classified as high DAG producers can be isolated from certain individuals (subject 2 in the present study) from an environment in which overall DAG production is not significant. This perhaps suggests that certain individuals may possess DAG-degrading bacteria as well as DAG producers. This may explain the inability of 34 isolates (17.5%) to produce DAG in pure culture.
In addition, the present studies have shown that the presence of phosphatidylcholine, as well as the pH, had more of an effect upon the population levels of beneficial groups of bacteria, namely bifidobacteria and lactobacilli, than on the other groups assessed. Counts for both of these groups significantly decreased, and not surprisingly, levels of lactic acid were low in the fermentations. Clostridial levels generally increased during phosphatidylcholine fermentation, and since neither of the probes used to enumerate levels of clostridial subgroups in this study included C. bifermentans (the most commonly isolated high DAG producer), it is quite possible that the total clostridium count was an underestimate.
High fat intake is one of the major dietary links with the risk of developing colon cancer. In healthy adults, approximately 95% of the fat consumed is digested and absorbed, leaving only 5% to enter the colon and potentially be utilized by gut bacteria. A high-fat diet increases the fecal excretion of phospholipids such as phosphatidylcholine to levels as high as 400 mg/day (1), elevating fecal levels of DAG, >90% of which is in the 1,2-sn configuration (35). Therefore, it is not unreasonable to assume that, given the diversity of potential substrates, sufficient DAG will be produced by fecal bacteria to activate PKC and to inappropriately stimulate cell proliferation. The evidence presented here suggests that more DAG is produced at higher colonic pHs, a condition found in individuals who have a tendency to develop tumors. Therefore, DAG production by fecal bacteria and its subsequent modulation of PKC form a credible hypothesis for the mechanism whereby diets high in fat can be detrimental to the distal gut.
The evidence presented here suggests that phospholipid metabolism by gut bacteria is detrimental to beneficial bacteria such as lactobacilli and bifidobacteria. It is well known that more acidic environments are favorable to these bacteria. Thus, increasing numbers of such organisms (and/or their activity) would potentially benefit the health of individuals who are predisposed to a higher-than-normal colonic pH. This not only would have the effect of decreasing the amount of DAG produced but would also exert several other benefits that such bacteria are known to possess, including anticarcinogenic properties and stimulation of both the host immune response and host resistance to pathogens.
Acknowledgments
This research was sponsored by the World Cancer Research Fund, London, United Kingdom.
REFERENCES
- 1.Ali, S. S., and A. Kuksis. 1967. Excretion of phospholipids by men on high fat diets. Can. J. Biochem. 45:703-714. [DOI] [PubMed] [Google Scholar]
- 2.Archer, R. H., I. S. Maddox, and R. Chong. 1982. Transformation of cholic acid by Clostridium bifermentans. J. Appl. Bacteriol. 52:49-56. [Google Scholar]
- 3.Barsukov, L. I., V. I. Kulikov, I. M. Simakova, G. V. Tikhonova, D. N. Ostrovskii, and L. D. Bergelson. 1978. Manipulation of phospholipid composition of membranes with the aid of lipid exchange proteins. Eur. J. Biochem. 90:331-336. [DOI] [PubMed] [Google Scholar]
- 4.Bingham, S. A. 1996. Epidemiology and mechanisms relating diet to risk of colorectal cancer. Nutr. Res. Rev. 9:197-239. [DOI] [PubMed] [Google Scholar]
- 5.Bligh, E. G., and W. J. A. Dyer. 1964. A rapid method of total lipid extraction. Can. J. Biochem. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 6.Bodey, G. P., S. Rodriguez, V. Fainstein, and L. S. Elting. 1991. Clostridial bacteremia in cancer patients. Cancer 67:1928-1942. [DOI] [PubMed] [Google Scholar]
- 7.Choe, M., E. S. Kris, L. Rajesh, J. C. Pelling, T. E. Donnelly, and D. F. Birt. 1992. Protein kinase C is activated and diacylglycerol is elevated in epidermal cells from Sencer mice fed high fat diets. J. Nutr. 122:2322-2329. [DOI] [PubMed] [Google Scholar]
- 8.Craven, P. A., J. Pfanstiel, R. Saito, and F. R. DeRubertis. 1986. Relationship between loss of rat colonic surface epithelium induced by deoxycholate and initiation of the subsequent proliferative response. Cancer Res. 46:5754-5759. [PubMed] [Google Scholar]
- 9.Cronan, J. E., and C. O. Rock. 1996. Biosynthesis of membrane lipids, p. 612-636. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. ASM Press, Washington, D.C.
- 10.Cummings, J. H., and G. T. Macfarlane. 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70:443-459. [DOI] [PubMed] [Google Scholar]
- 11.Devereux, R., M. D. Kane, J. Winfrey, and D. A. Stahl. 1992. Genus- and group-specific hybridisation probes for determinative and environmental studies of sulphate-reducing bacteria. Syst. Appl. Microbiol. 15:601-609. [Google Scholar]
- 12.Ferrari, A., C. Scolastico, and L. Berreta. 1977. On the mechanism of cholic acid 7α-dehydroxylation by Clostridium bifermentans cell-free extracts. FEBS Lett. 75:166-168. [DOI] [PubMed] [Google Scholar]
- 13.Franks, A. H., H. J. M. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman, E., P. Isaksson, J. Rafter, B. Marian, S. Winawer, and H. Newmark. 1989. Fecal diglycerides as selective endogenous mitogens for premalignant and malignant human colonic epithelial cells. Cancer Res. 49:544-548. [PubMed] [Google Scholar]
- 15.Galdiero, F., L. Sommese, C. Capasso, M. Galdiero, M. Cappello, and M. A. Tufano. 1993. Exchange of phospholipids between Escherichia coli cells and environment. Microb. Pathog. 14:85-94. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 17.Goldin, B. R. 1986. The metabolism of the intestinal microflora and its relationship to dietary fat, colon and breast cancer. Prog. Clin. Biol. Res. 222:655-685. [PubMed] [Google Scholar]
- 18.Harmsen, H. J. M., P. Elfferich, F. Schut, and G. W. Welling. 1999. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridisation. Microb. Ecol. Health Dis. 11:3-12. [Google Scholar]
- 19.Hill, M. J., J. S. Crowther, B. S. Drasar, G. Hawksworth, V. Aries, and R. E. O. Williams. 1971. Bacteria and aetiology of cancer of large bowel. Lancet i:95-100. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann, A. F., and J. R. Poley. 1972. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. Gastroenterology 62:918-934. [PubMed] [Google Scholar]
- 21.Hoyles, L., M. D. Collins, E. Falsen, N. Nikolaitchouk, and A. L. McCartney. 2004. Transfer of members of the genus Falcivibrio to the genus Mobiluncus, and emended description of the genus Mobiluncus. Syst. Appl. Microbiol. 27:72-83. [DOI] [PubMed] [Google Scholar]
- 22.Huang, X. P., X. T. Fan, J. F. Desjeux, and M. Castagna. 1992. Bile acids, non-phorbol-ester-type tumor promoters, stimulate the phosphorylation of protein kinase C substrates in human platelets and colon cell line HT29. Int. J. Cancer 52:444-450. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda, N., Y. Saito, J. Shimizu, A. Ochi, J. Mitzutani, and J. Watabe. 1994. Variation in concentrations of bacterial metabolites, enzyme activities, moisture, pH and bacterial composition between and within individuals in faeces of seven healthy adults. J. Appl. Bacteriol. 77:185-194. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis, W., O. Nunez-Montiel, M. Towns, F. Thompson, J. Morris, V. Dowell, E. Hill, W. Vogler, E. Winton, and J. Hughes. 1983. Clostridium bifermentans in a cohort of patients with hematologic malignancies. Clin. Res. 31:366A. [Google Scholar]
- 25.Key, T. J., N. E. Allen, E. A. Spencer, and R. C. Travis. 2002. The effect of diet on risk of cancer. Lancet 360:861-868. [DOI] [PubMed] [Google Scholar]
- 26.Langendijk, P. S., F. Schut, G. J. Jansen, G. W. Raangs, G. R. Kamphuis, M. H. F. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liscovitch, M., and L. Cantley. 1994. Lipid second messengers. Cell 77:329-334. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald, I. A., G. Singh, D. E. Mahony, and C. E. Meier. 1978. Effect of pH on bile salt degradation by mixed faecal cultures. Steroids 32:245-256. [DOI] [PubMed] [Google Scholar]
- 29.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 30.Morley, N., A. Kuskis, and D. Buchnea. 1974. Hydrolysis of synthetic triacylglycerols by pancreatic and lipoprotein lipase. Lipids 9:481-488. [DOI] [PubMed] [Google Scholar]
- 31.Morotomi, M., J. G. Guillem, P. LoGerfo, and I. B. Weinstein. 1990. Production of diacylglycerol, an activator of protein kinase C, by human intestinal microflora. Cancer Res. 50:3595-3599. [PubMed] [Google Scholar]
- 32.Nomoto, K., M. Morotomi, M. Miyake, D. B. Xu, P. P. LoGerfo, and I. B. Weinstein. 1994. The effects of bile acids on phospholipase C activity in extracts of normal human colon mucosa and primary colon tumors. Mol. Carcinog. 9:87-94. [DOI] [PubMed] [Google Scholar]
- 33.Nomura, H., H. Nakanishi, K. Ase, U. Kikkawa, and Y. Nishizuka. 1986. Inositol phospholipid turnover in stimulus-response coupling. Prog. Hemost. Thromb. 8:143-158. [PubMed] [Google Scholar]
- 34.Ochi, S., T. Miyawaki, H. Matsuda, M. Oda, M. Nagahama, and J. Sakurai. 2002. Clostridium perfringens α-toxin induces rabbit neutrophil adhesion. Microbiology 148:237-245. [DOI] [PubMed] [Google Scholar]
- 35.Pickering, J. S., J. R. Lupton, and R. S. Chapkin. 1995. Dietary fat, fiber and carcinogen alter fecal diacylglycerol composition and mass. Cancer Res. 55:2293-2298. [PubMed] [Google Scholar]
- 36.Potter, J. D. 1995. Risk-factors for colon neoplasia—epidemiology and biology. Eur. J. Cancer 31A:1033-1038. [DOI] [PubMed] [Google Scholar]
- 37.Poulsen, L. K., F. Lan, C. S. Kristensen, P. Hobolth, S. Molin, and K. A. Krogfelt. 1994. Spatial distribution of Escherichia coli in the mouse large intestine inferred from rRNA in situ hybridization. Infect. Immun. 62:5191-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proulx, P., and H. Aubry. 1989. Diacylglycerol-phosphatidylethanolamine exchange in Escherichia coli. Biochim. Biophys. Acta 1003:217-220. [DOI] [PubMed] [Google Scholar]
- 39.Reddy, B. S., B. Simi, N. Patel, C. Aliaga, and C. V. Rao. 1996. Effect of amount and types of dietary fat on intestinal bacterial 7α-dehydroxylase and phosphatidylinositol-specific phospholipase C and colonic mucosal diacylglycerol kinase and PKC activities during different stages of colon tumor promotion. Cancer Res. 56:2314-2320. [PubMed] [Google Scholar]
- 40.Salminen, S., C. Bouley, M. C. Boutron-Ruault, J. H. Cummings, A. Franck, G. R. Gibson, E. Isolauri, M. C. Moreau, M. Roberfroid, and I. Rowland. 1998. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 80:S147-S171. [DOI] [PubMed] [Google Scholar]
- 41.Scanlan, D. R., M. A. Smith, H. D. Isenberg, S. Engrassia, and E. Hilton. 1994. Clostridium bifermentans bacteremia with metastatic osteomyelitis. J. Clin. Microbiol. 32:2867-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulman, H., and E. P. Kennedy. 1977. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J. Biol. Chem. 252:4250-4255. [PubMed] [Google Scholar]
- 43.Titball, R. W. 1993. Bacterial phospholipase C. Microbiol. Rev. 57:347-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tronchere, H., M. Record, F. Terce, and H. Chap. 1994. Phosphatidylcholine cycle and regulation of phosphatidylcholine biosynthesis by enzyme translocation. Biochim. Biophys. Acta 1212:137-151. [DOI] [PubMed] [Google Scholar]
- 45.Tso, J. Y., and C. Siebel. 1989. Cloning and expression of the phospholipase C gene from Clostridium perfringens and Clostridium bifermentans. Infect. Immun. 57:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, X., and G. R. Gibson. 1993. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 75:373-380. [DOI] [PubMed] [Google Scholar]