Abstract
A ccpA gene that encodes global catabolite control protein A (CcpA) in Streptococcus bovis was identified and characterized, and the involvement of CcpA in transcriptional control of a gene (ldh) encoding lactate dehydrogenase (LDH) and a gene (pfl) encoding pyruvate formate-lyase (PFL) was examined. The ccpA gene was shown to be transcribed as a monocistronic operon. A catabolite-responsive element (cre) was found in the promoter region of ccpA, suggesting that ccpA transcription in S. bovis is autogenously regulated. CcpA required HPr that was phosphorylated at the serine residue at position 46 (HPr-[Ser-P]) for binding to the cre site, but glucose 6-phosphate, fructose 1,6-bisphosphate, and NADP had no effect on binding. Diauxic growth was observed when S. bovis was grown in a medium containing glucose and lactose, but it disappeared when ccpA was disrupted, which indicates that CcpA is involved in catabolite repression in S. bovis. The level of ccpA mRNA was higher when cells were grown on glucose than when they were grown on lactose, which was in line with the level of ldh mRNA. When cells were grown on glucose, the ldh mRNA level was lower but the pfl mRNA level was higher in a ccpA-disrupted mutant than in the parent strain, which suggests that ldh transcription is enhanced and pfl transcription is suppressed by CcpA. The ccpA-disrupted mutant produced less lactate and more formate than the parent, probably because the mutant had reduced LDH activity and elevated PFL activity. In the upper region of both ldh and pfl, a cre-like sequence was found, suggesting that the complex consisting of CcpA and HPr-[Ser-P] binds to the possible cre sites. Thus, CcpA appears to be involved in the global regulation of sugar utilization in S. bovis.
Streptococcus bovis, an important amylolytic and lactate-producing bacterium, is one of the predominant bacteria in the rumen when ruminants are fed high-concentrate diets (18, 27). Rapid fermentation of starch often leads to an increase in ruminal lactate production, which causes a drop in the ruminal pH. S. bovis is relatively acid tolerant among ruminal bacteria (36) and produces higher percentages of lactate when the culture pH is low (37). Thus, S. bovis is likely to contribute to the progress of lactic rumen acidosis (37). Therefore, it is desirable to suppress the overproduction of lactate by S. bovis when high-concentrate diets are used.
On the other hand, lactate usually undergoes secondary fermentation in the rumen, and propionate is generally a major product (38). Propionate is important for ruminant nutrition as a glycogenic substance. Propionate is formed by accepting electrons, which generally leads to a reduction in ruminal methanogenesis. This is particularly important, because methanogenesis represents an energy loss for ruminants and, in addition, methane contributes to the greenhouse effect. Furthermore, lactate serves as an electron donor for sequential nitrate reduction to ammonia (20, 21), which also leads to a decrease in ruminal methanogenesis (19, 20). In addition, stimulation of nitrite reduction by lactate may alleviate toxic effects of nitrite (20, 21). Hence, it may be beneficial to modulate lactate production so that lactate is always present at appropriate levels in the rumen.
Lactate production by S. bovis is regulated by the ratio of lactate dehydrogenase (LDH) activity to pyruvate formate-lyase (PFL) activity (2, 7). The activities of LDH and PFL are regulated by allosteric effectors, such as fructose 1,6-bisphosphate (FBP), phosphoenolpyruvate, and triose phosphates, and also by the amounts of enzyme proteins (1, 2, 3). The synthesis of both enzymes is regulated at the transcriptional level (6, 7). S. bovis increases lactate production by increasing LDH synthesis and simultaneously decreases formate production by decreasing PFL synthesis in response to a drop in the pH and an excess energy supply (2, 6, 7). In addition, PFL activity is posttranslationally regulated by PFL activase and PFL deactivase (4). In S. bovis, PFL activase is usually present at a level that is high enough to activate PFL (4). However, since the amount of LDH is much larger than the amount of PFL, the ratio of formate to lactate produced is usually low (4). Therefore, control of LDH synthesis is important for the control of lactate production by S. bovis.
In Streptococcus thermophilus (41) and Lactococcus lactis (25), transcription of the gene encoding LDH (ldh) is regulated by catabolite control protein A (CcpA), which is a member of the LacI/GalR family of transcriptional repressors/activators (15). HPr (heat-stable protein), a component of the phosphoenolpyruvate-dependent phosphotransferase system, is phosphorylated at the Ser-46 residue by ATP-dependent HPr kinase, which forms seryl-phosphorylated HPr (HPr-[Ser-P]) (9, 13, 17). CcpA forms a complex with HPr-[Ser-P], and then the complex binds to the catabolite-responsive elements (cre) (11, 13) that are located upstream of, or within, the 5′ region of many operons (17, 33). As a consequence, gene transcription is activated or repressed (9, 28).
In low-G+C-content gram-positive bacteria, such as bacilli, streptococci, and lactococci, the phosphotransferase system catalyzes the transport and phosphorylation of mono- and disaccharides and also plays a key role in the control of sugar metabolism by regulating the expression of catabolic genes, modulating the activity of key metabolic enzymes, and controlling the activity of sugar transport systems (14, 29, 35).
It has been reported previously that genes encoding HPr (ptsH) and HPr kinase (hprK) were identified in S. bovis (5). Expression of HPr was found to be regulated at the transcriptional level in response to the sugar supply (5). HPr kinase had both HPr-phosphorylating and -dephosphorylating activities, and these activities were affected by the concentration of inorganic phosphate (Pi) (5). Thus, HPr-[Ser-P] formation appears to be regulated by the bifunctional activity of HPr kinase in response to the intracellular Pi concentration in S. bovis. However, how HPr-[Ser-P] is involved in the transcriptional regulation through CcpA is unknown at present.
In order to elucidate the mechanism for transcriptional regulation by CcpA in S. bovis, we initially analyzed the gene encoding CcpA (ccpA) and then examined the transcriptional regulation of the gene. We also examined whether HPr-[Ser-P] affects the binding of CcpA to the cre site. In addition, we examined whether CcpA is involved in the transcriptional regulation of ldh and pfl (a gene encoding PFL) in S. bovis. To do these things, we constructed a ccpA-disrupted mutant. No report is available at present on the involvement of CcpA in pfl transcription even in other bacteria.
MATERIALS AND METHODS
Sources of bacterial strains and plasmids.
The sources of S. bovis JB1 and 12U1 were described previously (4). Unless indicated otherwise, the JB1 strain was used. Escherichia coli DH5α was purchased from a commercial source (Toyobo, Tokyo, Japan). Plasmid pUC18 was purchased from the same commercial source in order to clone S. bovis ccpA and to construct a ccpA-disrupted mutant. Plasmid pGEX-6P-3 was purchased from Amersham Bioscience in order to express a glutathione S-transferase (GST) fusion protein, and plasmid pQE30 was purchased from QIAGEN in order to express a His-tagged fusion protein. The source of plasmid pSBE11, a shuttle vector between E. coli and S. bovis (32), was described previously (4).
Growth conditions.
S. bovis was grown anaerobically in batch culture as described previously (1). The medium contained (per liter) 0.45 g of K2HPO4, 0.45 g of KH2PO4, 0.9 g of (NH4)2SO4, 0.9 g of NaCl, 0.12 g of CaCl2 · 2H2O, 0.19 g of MgSO4 · 7H2O, 0.1 g of Fe(NH4)2(SO4)2, 1.0 g of Trypticase (BBL, Becton Dickinson), 1.0 g of yeast extract (Difco Laboratories), and 0.6 g of cysteine HCl. As an energy source, either glucose or lactose (3 g/liter) was provided. Culture incubation was performed in triplicate, and the pH was maintained between 6.8 and 7.0 (1). Cell growth was estimated by measuring the optical density at 600 nm. Unless indicated otherwise, S. bovis was grown until the late log phase. To prepare the fusion protein, E. coli was grown aerobically in Luria-Bertani medium.
Determination of sugars, fermentation products, and cellular nitrogen.
Glucose and lactose (16), as well as organic acids (16), were analyzed by high-performance liquid chromatography as described previously. The cellular nitrogen (cell-N) content was determined by the Kjeldahl method as described previously (3).
Sequencing of the ccpA gene.
Unless indicated otherwise, we used the standard methods for general cloning procedures (39). Genomic DNA was extracted from S. bovis, and nucleotide sequences were determined on both strands as described previously (6, 7). The sequence data were evaluated as previously described (7).
Based on the sequences of the ccpA gene in Streptococcus mutans (GenBank accession number AF014460), L. lactis (AF106673), Lactococcus delbrueckii (Z54205), and Bacillus subtilis (M85182), two oligonucleotide primers for PCR were designed and prepared commercially (Hokkaido System Science, Sapporo, Japan), and they were designated ccpA-p1 (5′-GTN TCN ATG GCA ACN GT-3′; from position 89 to position 105) and ccpA-p2 (5′-ACN GCW CCN ANR TCA TA-3′; from position 939 to position 923) (N is any base, W is A or T, and R is A or G). The PCR product obtained from the genomic DNA of S. bovis JB1 was a 851-bp fragment, which was homologous with a high degree of identity to part of the ccpA gene reported for the bacteria described above (BLAST search). Subsequently, the 851-bp fragment was labeled with a digoxigenin DNA-labeling and detection kit (Roche) and used as a hybridization probe for Southern blotting.
S. bovis genomic DNA was digested with EcoRI or Sau3AI, and the fragments were ligated to plasmid pUC18. Restriction and modification enzymes were used according to the recommendations of the manufacturer (TaKaRa Shuzo, Kyoto, Japan). The recombinant plasmids were then introduced into E. coli DH5α by electroporation. A fragment containing the upstream and downstream regions of the 851-bp fragment (part of a possible ccpA gene) was selected with the probe described above.
Primer extension analysis.
Primer extension analysis was carried out with an IRD800-labeled primer, ccpA-PEX (5′-TCT TTA TCG TCG TCT TCA TCA CTT G-3′; from position 363 to position 339), and Moloney murine leukemia virus reverse transcriptase RNase H Minus (Toyobo). Products obtained from the primer extension analysis were separated by using a Li-cor DNA sequencer (Aloka, Tokyo, Japan) as previously described (7).
Northern blot analysis.
Cultures were promptly frozen by immersing them in liquid nitrogen and were stored at −80°C to stop the degradation of mRNA after sampling (7). Northern blot analysis was performed as previously described (6). A probe specific for ccpA for Northern blotting was designed to cover the open reading frame (ORF) and was labeled with a digoxigenin DNA-labeling and detection kit (Roche). Probes specific for S. bovis ldh (accession number U60997) and pfl (AB014686) were prepared as described previously (6, 7). The amounts of ccpA, ldh, and pfl mRNA in 10 μg of total RNA were estimated from the peak areas and intensities of the bands on a 1.0% (wt/vol) agarose-0.6% (wt/vol) formaldehyde gel by using a Fluor-S Multi Imager (Bio-Rad) as described previously (6). Northern blotting was carried out three times.
Estimation of the rate of degradation of ccpA mRNA.
Rifampin (100 μg/ml) was added to cultures at the mid-log growth phase, and cells were harvested every 2.5 min after the addition of rifampin. The rifampin concentration added was confirmed to be high enough to inhibit RNA synthesis (3). The rate of degradation of ccpA mRNA was estimated from the decay curve.
Preparation of recombinant proteins.
The ORFs of S. bovis ccpA, ptsH (accession number AB027569), and hprK (AB027460) were amplified with primers having BamHI and SalI restriction sites at the 5′ and 3′ termini, respectively. Then a ccpA-containing fragment and a ptsH-containing fragment were ligated into plasmid pGEX-6P-3. A fragment containing hprK was ligated into plasmid pQE30. The recombinant plasmids were introduced into E. coli DH5α, as described previously (2). The GST fusion protein produced by E. coli was purified by affinity column chromatography with a glutathione-Sepharose 4B column (Amersham Bioscience) and then by chromatography with a Superdex 200 HR 10/30 column (1.0 by 30 cm; AKTA System; Amersham Bioscience). The GST part of the fusion protein was cut off with prescission protease (Amersham Bioscience). The His-tagged HPr kinase produced by E. coli was purified by affinity column chromatography with HiTrap Chelating HP (Amersham Bioscience) and by Superdex 200 HR 10/30 column chromatography. The purified proteins were confirmed to include the target proteins, as judged from the molecular weights estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (24).
Phosphorylation of HPr.
The recombinant HPr was phosphorylated with recombinant HPr kinase as previously described (5). The reaction mixture contained 100 mM Tris-HCl (pH 7.0), 5 mM MgCl2, 10 mM NaCl, 5 mM ATP, 10 μg of HPr, and an appropriate amount of His-tagged HPr kinase. The reaction was initiated by adding HPr kinase, and then incubation was carried out at 37°C for 10 min. The reaction was terminated by heating the mixture at 70°C for 5 min. After it was confirmed that HPr was completely converted to HPr-[Ser-P] by nondenaturing 15% PAGE, the reaction mixture was applied to a HiTrap Chelating HP column to separate His-tagged HPr kinase. The eluate containing HPr-[Ser-P] was further purified by Superdex 200 HR 10/30 column chromatography.
Electrophoretic mobility shift assay.
A DNA fragment (48 bp; from position −83 to position −36) containing a cre sequence in ccpA was synthesized by Hokkaido System Science. Binding of CcpA to the DNA fragment containing a cre sequence was examined as follows. The reaction mixture contained 100 mM Tris-HCl (pH 7.5), 1 mM EDTA, 10 mM NaCl, a 48-bp cre-containing DNA fragment at a concentration of 5 μM, 5 μM CcpA (dimer), and 10 μM HPr-[Ser-P]. To distinguish specific binding from nonspecific binding, 50 μg of poly(dI-dC) per μl (an amount much larger than the amount of the cre-containing DNA fragment) was also added. Then the reaction mixture was incubated at 37°C for 15 min and subjected to nondenaturing 10% PAGE. The gel was stained with ethidium bromide and then with Coomassie brilliant blue.
Construction of ccpA-disrupted mutant of S. bovis.
The upper and lower regions of ccpA were amplified by PCR with oligonucleotide primer pairs. One pair was ccpA-pi1 (5′-AGA GTT GAA GCC GAA AAA-3′; from position −249 to position −232) and ccpA-pi2 (5′-CAT TGA GAC GCC TGC TTC-3′; from position 97 to position 80), and the other pair was ccpA-pi3 (5′-TTT GTT TCA GGA CCA CTT-3′; from position 590 to position 607) and ccpA-pi4 (5′-TAG GTT TGG ACG AGT GTA-3′; from position 901 to position 884). The ccpA-pi1 and ccpA-pi4 primers were designed to introduce BamHI and SalI restriction sites, respectively, at the 5′ end of each primer. Both of the PCR products were blunt ended by using T4 DNA polymerase (TaKaRa Shuzo). An erythromycin resistance gene, ermB (8), was inserted between the ccpA-pi1-ccpA-pi2 fragment and the ccpA-pi3-ccpA-pi4 fragment with T4 DNA ligase (TaKaRa Shuzo). The ligated product was digested with BamHI and SalI and then introduced into plasmid pUC18. The recombinant plasmid was electroporated into a competent strain of S. bovis, strain 12U1, with a Genepulser (Bio-Rad) operated at 12.5 kV/cm and 200 Ω. Transformants were selected with 10 μg of erythromycin per ml.
Reintroduction of ccpA into the ccpA-disrupted mutant of S. bovis.
The gene including the ORF of ccpA was amplified by PCR as described above. The PCR product was blunted with T4 DNA polymerase and phosphorylated with T4 polynucleotide kinase (TaKaRa Shuzo). The resulting fragment was introduced into SmaI-digested pSBE11. The recombinant plasmid was electroporated into the ccpA-disrupted mutant described above. Transformants harboring the recombinant plasmid were selected by Southern blotting with a ccpA-specific probe.
Assay for the activities of LDH and PFL.
S. bovis cells collected by centrifugation (20,000 × g, 10 min, 4°C) were repeatedly disrupted by ultrasonication until approximately 95% of the cells were broken (1). After recentrifugation, the supernatant was immediately subjected to analysis. LDH activity was assayed as described previously (1, 2), and PFL activity was measured by using coupled reactions with malate dehydrogenase and citrate synthase (2, 23). Enzyme activity was expressed as specific activity (in micromoles of NADH changed per minute per microgram of cell-N). Since cell-N was proportional to cell mass or cell dry weight, the specific activity represented the enzyme content in cells.
Evaluation of data.
Data were analyzed by the Tukey test or the Student t test by using the SigmaStat statistical analysis system (Jandel Scientific).
Nucleotide sequence accession number.
The nucleotide sequence of S. bovis ccpA reported in this paper has been deposited in the GenBank database under accession number AB028599.
RESULTS AND DISCUSSION
Characterization of S. bovis ccpA.
S. bovis ccpA consisted of 1,005 nucleotides, which included a putative ATG start codon (from position 38 to position 40) and a TAA termination codon (from position 1,040 to position 1,042) (Fig. 1A). The protein encoded by the ccpA ORF was deduced to consist of 333 amino acids with a molecular mass of 36,568 Da. The isoelectric point of S. bovis CcpA was 5.33. The amino acid sequence of CcpA in S. bovis showed high levels of identity and similarity to the sequences of CcpA of other bacteria. The levels of identity and similarity to streptococcal proteins were especially high (more than 70%) (BLAST search), and levels of identity and similarity to B. subtilis CcpA (accession number M85182) were 50 and 65%, respectively.
FIG. 1.
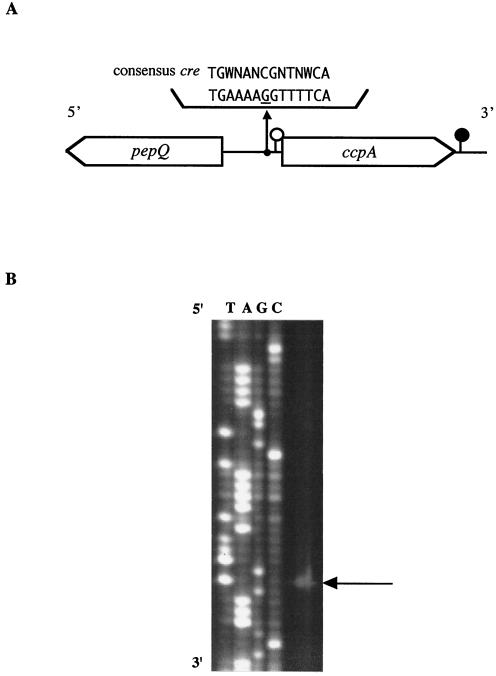
(A) Schematic representation of the ccpA and pepQ genes in S. bovis. A putative transcriptional start site and a termination site for ccpA are indicated by open and solid circles, respectively. A possible cre sequence is shown along with the cre consensus sequence. (B) Primer extension analysis of S. bovis ccpA. Sequence ladders were electrophoresed with the same primer, and the transcriptional start site is indicated by an arrow.
A part of the presumptive pepQ gene (from position −304 to position −1209) encoding a proline peptidase (PepQ; 301 amino acids) was found to be located 341 bp upstream of the ccpA ORF in S. bovis, which was present in the opposite strand. The levels of amino acid identity of part of S. bovis PepQ to the proteins reported for S. mutans (accession number AF014460) and Lactobacillus plantarum (AJ310777) were 68 and 43%, respectively. The arrangement of the pepQ and ccpA genes in S. bovis was the same as that in S. mutans (40). In contrast to streptococcal pepQ, the pepQ gene in L. plantarum has been reported to be located in an area downstream of ccpA on the same strand (31). In L. plantarum, however, ccpA and pepQ are transcribed as distinct transcriptional units.
Transcription of ccpA in S. bovis.
A putative ribosome-binding site, the Shine-Dalgarno sequence (GAAAG, from position 22 to position 26), was detected 12 bp upstream from the ATG initiation codon. Primer extension analysis with a ccpA-PEX primer demonstrated that there was a single transcriptional start site 37 bp upstream of the ccpA start codon (Fig. 1B). Putative −35 (TTAAAA, from position −34 to position −29) and −10 (TAAAAT, from position −11 to position −6) promoter regions were also present. An inverted repeat sequence that is one of the characteristics of a transcriptional terminator was found to be located 40 bp downstream from the ccpA termination codon (TGA TTT TTT TTA GTC TTA GTG AAA AAA ATT A, from position 1,082 to position 1,112). Calculation of the free energy change for this region of the corresponding mRNA (−10.0 kcal/mol) suggested the presence of a stem-loop structure. Northern blot analysis gave a single band that hybridized with a ccpA-specific probe (Fig. 2A). The ccpA mRNA was estimated to be approximately 1.1 kb long, which is consistent with the length of the transcriptional unit of ccpA deduced from nucleotide sequence analysis (ccpA operon; 1,112 bp). These results indicate that the ccpA gene is transcribed as a monocistronic mRNA.
FIG. 2.
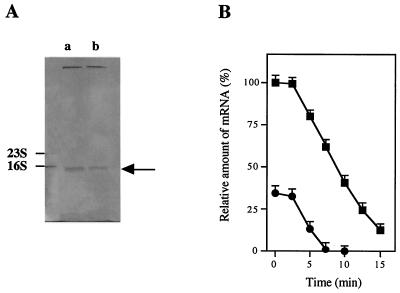
(A) Level of ccpA mRNA in S. bovis grown on glucose (lane a) or lactose (lane b), as estimated by Northern blotting. The arrow indicates the position of 1.1-kb ccpA mRNA. The ratio of mRNA levels (level of ccpA mRNA in S. bovis grown on glucose/level of ccpA mRNA in S. bovis grown on lactose) was 3/1. (B) Decay of ccpA mRNA in S. bovis cells grown on glucose (▪) or lactose (•).
The Shine-Dalgarno sequence (GAAGG in the opposite strand, from position −293 to position −297) was located 7 bp upstream from the ATG initiation codon of the pepQ gene. Potential −35 (TTGATT in the opposite strand, from position −249 to position −254) and −10 (AAAAAT in the opposite strand, from position −272 to position −277) promoter regions for the pepQ gene were also present. As deduced from the gene arrangement, pepQ and ccpA may be transcribed separately in S. bovis. However, whether S. bovis pepQ is monocistronic or polycistronic is unknown at present.
A 14-bp palindromic sequence (TGAAAAGGTTTTCA, from position −68 to position −51) was found in the intergenic region between pepQ and ccpA in S. bovis (Fig. 1A). This sequence was 88 and 236 bp upstream of the ORFs of ccpA and pepQ, respectively. The sequence differed from the consensus cre sequence (TGWNANCGNTNWCA) defined by Weickert and Chambliss (42) by one nucleotide (indicated by underlining in the palindromic sequence). In S. mutans, a cre site has been reported to be present in the promoter region between pepQ and ccpA (40). Therefore, the palindromic sequence in S. bovis is probably a cre site. Autogenous regulation of ccpA has been described for L. plantarum (31), Lactobacillus pentosus (26), and Staphylococcus xylosus (12).
Binding of CcpA to the cre site.
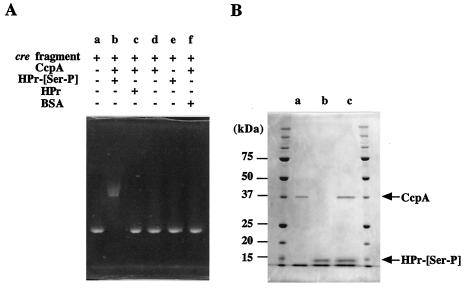
Binding of CcpA to the deduced cre site in the upper region of ccpA was examined by electrophoretic mobility shift analysis. The migration during nondenaturing PAGE was slower when a cre-containing fragment had been incubated with both CcpA and HPr-[Ser-P] previously than when the cre-containing fragment alone was loaded (Fig. 3A). However, when HPr-[Ser-P] was replaced by HPr or bovine serum albumin (used to distinguish specific binding from nonspecific binding) and then the preparation was incubated, the migration was similar to that when the cre-containing fragment alone was applied. Incubation of the cre-containing fragment with either CcpA or HPr-[Ser-P] did not alter the migration. These results indicate that the complex consisting of CcpA and HPr-[Ser-P] binds to the cre site. In other words, HPr-[Ser-P] is needed for the binding of CcpA to the cre site. In addition, binding of the complex consisting of CcpA and HPr-[Ser-P] strongly supported the hypothesis that the deduced cre site is actually a cre site.
FIG. 3.
(A) Gel shift assay showing the binding of the complex consisting of CcpA and HPr-[Ser-P] to the cre site. A cre-containing fragment was incubated in the presence (plus sign) or in the absence (minus sign) of CcpA, HPr, and/or HPr-[Ser-P]. Bovine serum albumin (BSA) was used as a nonspecific protein. (B) SDS-PAGE of the complex consisting of CcpA and HPr-[Ser-P] after the gel shift assay. Lanes a and b contained CcpA and HPr-[Ser-P], respectively. Lane c contained the complex consisting of CcpA, HPr-[Ser-P], and the cre-containing fragment.
The ternary complex consisting of HPr-[Ser-P], CcpA, and the cre-containing fragment eluted from the gel after a nondenaturing PAGE gel was subjected to SDS-PAGE. The results showed that the ternary complex was dissociated into two proteins (Fig. 3B). The behavior on the gel indicated that the two proteins corresponded to CcpA and HPr-[Ser-P], confirming that the ternary complex contained HPr-[Ser-P], CcpA, and the cre-containing fragment.
Binding of the complex consisting of CcpA and HPr-[Ser-P] to the cre-containing fragment was not affected by 30 mM glucose 6-phosphate, FBP, or NADP (data not shown), which was different from the finding that these phosphates affected the binding of CcpA to the cre site at a concentration of 30 mM or less in B. subtilis (10, 22, 30). Because the concentration of glucose 6-phosphate, FBP, or NADP never exceeded 30 mM in S. bovis cells (3), it is unlikely that these compounds affect the binding in vivo.
Regulation of the transcription of ccpA.
As shown in Fig. 2A, the level of intracellular ccpA mRNA was approximately threefold higher when S. bovis was grown on glucose than when it was grown on lactose. This result suggests that ccpA expression was greater when glucose was an energy source, which is in contrast to the previously reported observation that the amount of CcpA protein was at least twofold larger when S. thermophilus was grown on lactose than when it was grown on glucose (41). S. bovis ferments glucose more rapidly than it ferments lactose (4), whereas S. thermophilus utilizes lactose more rapidly than it utilizes glucose (34, 41). Therefore, it is conceivable that ccpA expression is higher when a more rapidly utilizable energy source is supplied.
The rate of degradation of ccpA mRNA when S. bovis was grown on glucose did not differ significantly from the rate when the organism was grown on lactose (Fig. 2B), suggesting that the level of ccpA mRNA reflects the rate of transcription. Therefore, CcpA synthesis in S. bovis appears to be regulated at the transcriptional level. However, why ccpA transcription is greater when a more rapidly utilizable energy source is supplied remains to be clarified.
Involvement of ccpA in catabolite repression.
A clone of S. bovis 12U1 carrying a null mutation in the ccpA gene was constructed by a two-step homologous recombination process. Disruption of ccpA was confirmed by Southern blotting and PCR analysis (data not shown). The growth rate of the ccpA-disrupted mutant (12U1-ccpA−) was similar to that of the parent strain (12U1), indicating that growth was not affected by the disruption of ccpA (Table 1).
TABLE 1.
Effect of disruption of ccpA on the ratio of formate to lactate produced in S. bovisa
| Strain | Energy substrate | Growth rateb | Relative amt of mRNAc
|
Sp act
|
Ratio of formate to lactatee | |||
|---|---|---|---|---|---|---|---|---|
| ldh | pfl | LDHd | PFLd | PFL/LDH | ||||
| 12U1 | Glucose | 0.98 A | 1.0 A | 1.0 C | 61 A | 1.8 C | 0.03 | 0.07 D |
| Lactose | 0.78 B | 0.5 B | 3.6 B | 29 B | 5.5 B | 0.19 | 0.48 B | |
| 12U1-ccpA− | Glucose | 0.94 A | 0.5 B | 9.4 A | 27 B | 14.2 A | 0.53 | 0.39 C |
| Lactose | 0.82 B | 0.5 B | 9.2 A | 31 B | 14.5 A | 0.47 | 0.59 A | |
| 12U1-ccpA+ | Glucose | 0.97 A | 1.1 A | 1.2 C | 59 A | 1.9 C | 0.03 | 0.09 D |
| Lactose | 0.80 B | 0.5 B | 3.4 B | 31 B | 5.1 B | 0.20 | 0.49 B | |
Different letters within a column indicate a significant difference (P < 0.05; n = 3).
The growth rate is expressed as an increase in the amount of cell-N (in milligrams) per hour during the log stage.
Relative amount of mRNA as estimated by Northern blotting.
Increase or decrease in the amount of NADH (in micromoles per minute per 10 μg of cell-N).
Ratio of the amount of formate to the amount of lactate produced in 1 h.
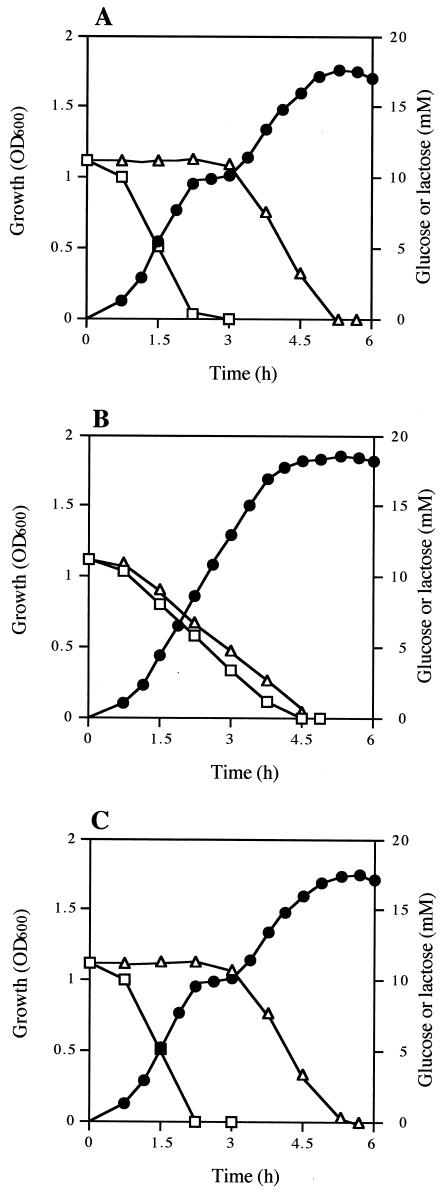
Diauxic growth was observed when 12U1 was grown in a medium containing both glucose and lactose (Fig. 4A). Lactose was utilized after the glucose was exhausted, indicating that 12U1 utilizes glucose in preference to lactose; i.e., 12U1 has a system of catabolite repression for lactose utilization. However, when 12U1-ccpA− was grown in the same medium, glucose and lactose were utilized simultaneously, and diauxic growth was not observed (Fig. 4B). When a 12U1-ccpA− clone into which a ccpA-containing plasmid was introduced (12U1-ccpA+) was grown, diauxic growth was observed again (Fig. 4C). These results indicate that CcpA is involved in catabolite repression in S. bovis.
FIG. 4.
Growth (•) and glucose (□) and lactose (▵) concentrations in the medium when S. bovis 12U1 (A), 12U1-ccpA− (B), and 12U1-ccpA+ (C) were grown in a medium containing both glucose and lactose. OD600, optical density at 600 nm.
Involvement of CcpA in the expression of LDH and PFL.
The level of intracellular ldh mRNA was approximately twofold higher when 12U1 was grown on glucose than when it was grown on lactose (Table 1 and Fig. 5A). A similar trend was observed for the specific activity of LDH (amount of activity per unit of cell-N). These results are essentially the same as previous observations (4). However, when 12U1-ccpA− was used, there was no significant difference in the ldh mRNA level or the LDH specific activity between glucose-grown cells and lactose-grown cells. When 12U1-ccpA+ was grown on glucose, the ldh mRNA level and the LDH specific activity were similar to the corresponding values for 12U1 (Table 1), suggesting that ccpA transcription was similar irrespective of whether ccpA was included in plasmid or chromosomal DNA.
FIG. 5.
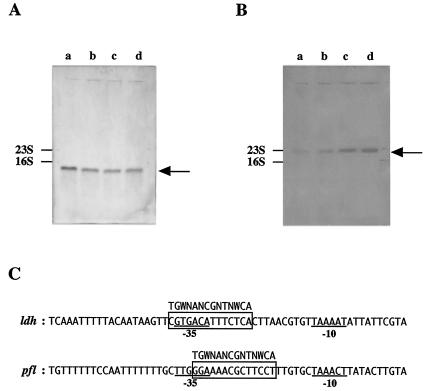
(A) ldh mRNA level in S. bovis 12U1 grown on glucose (lane a) or lactose (lane b) and ldh mRNA level in S. bovis 12U1-ccpA− grown on glucose (lane c) or lactose (lane d), as estimated by Northern blotting. The arrow indicates the position of 1.0-kb ldh mRNA. (B) pfl mRNA level in 12U1 grown on glucose (lane a) or lactose (lane b) and pfl mRNA level in 12U1-ccpA− grown on glucose (lane c) or lactose (lane d). The arrow indicates the position of 2.3-kb pfl mRNA. (C) Alignment of the ldh (accession number U60997) and pfl (AB014686) promoter regions of S. bovis. The −35 and −10 sequences are underlined. Possible cre sites are enclosed in boxes, and the consensus cre sequence is indicated.
The ldh mRNA level and LDH specific activity in glucose-grown 12U1-ccpA− were one-half of the corresponding values for 12U1 (Table 1). These results support the idea that CcpA positively regulates ldh transcription in S. bovis. However, there was no significant difference in the ldh mRNA level or LDH specific activity between 12U1 and 12U1-ccpA− when lactose was a substrate (Table 1), suggesting that ldh transcription is not enhanced by CcpA when cells are grown on lactose. This is consistent with the finding described above that ccpA expression was much lower when lactose was an energy source.
In contrast to the ldh mRNA level, the pfl mRNA level was 3.6-fold higher when 12U1 was grown on lactose than when it was grown on glucose (Table 1 and Fig. 5B). The difference in PFL specific activity between lactose-grown 12U1 cells and glucose-grown 12U1 cells (3.1-fold) was similar to the difference in the pfl mRNA level. When glucose was an energy substrate, the pfl mRNA level and PFL specific activity of 12U1-ccpA− were 9.4- and 7.9-fold, respectively, higher than the values for 12U1. Even when lactose was a substrate, the pfl mRNA level and PFL specific activity in 12U1-ccpA− were 2.6-fold higher than the values for 12U1. These results strongly suggest that pfl transcription is suppressed by CcpA in S. bovis.
Thus, the impact of disruption of ccpA on the pfl mRNA level and PFL specific activity was opposite the impact on the ldh mRNA level and LDH specific activity, suggesting that CcpA negatively regulates pfl transcription in S. bovis. The pfl mRNA level and PFL specific activity in glucose-grown 12U1-ccpA− were comparable to the values for lactose-grown 12U1-ccpA−. This result is in line with the finding described above that the ldh mRNA level and LDH specific activity in 12U1-ccpA− were not affected by the substrate supplied.
In the upper region of ldh and pfl, a cre-like sequence was found (Fig. 5C), suggesting that the complex consisting of CcpA and HPr-[Ser-P] binds to the possible cre sites. The levels of HPr[Ser-P] and CcpA are higher in glucose-grown cells than in lactose-grown cells. It is presumed that an increase in HPr[Ser-P] and CcpA causes an increase in the binding of the complex consisting of CcpA and HPr-[Ser-P] to the cre sites. Binding to the cre site in the upper region of ldh enhances ldh transcription directly or indirectly, whereas binding to the cre site in the upper region of pfl triggers suppression of pfl transcription. Experiments to confirm this presumption are in progress.
Based on these results, it is likely that CcpA is involved in the catabolite control of sugar utilization; in addition, the transcription of ldh and pfl is regulated through CcpA in opposite directions. How CcpA is involved in the regulation of ldh and pfl transcription remains to be clarified.
Impact of ccpA disruption on the fermentation pattern.
The ratio of formate to lactate produced by 12U1 was much greater when cells were grown on lactose (0.48) than when cells were grown on glucose (0.07) (Table 1), which agreed with the previous results obtained with the JB1 and 12U1 strains (3, 4). When cells were grown on glucose, the formate-to-lactate ratio in 12U1-ccpA− (0.39) was much higher than that in 12U1 (0.07). The formate-to-lactate ratio appears to have been altered by a decrease in LDH specific activity and a simultaneous increase in PFL specific activity.
However, the formate-to-lactate ratio in 12U1-ccpA− grown on glucose (0.39) was lower than that in 12U1 grown on lactose (0.48), although the ratio of PFL specific activity to LDH specific activity was higher in the latter organism. This discrepancy may be explained as follows. Specific activity values reflect the amounts of enzymes, but they do not necessarily represent reaction rates. It is possible that the LDH reaction was actually faster than the PFL reaction (4), because the concentration of intracellular FBP, an activator of LDH, is much higher when cells are grown on glucose than when cells are grown on lactose and, in addition, the levels of dihydroxyacetone phosphate and d-glyceraldehyde-3-phosphate, which are inhibitors of PFL, are lower when cells are grown on lactose than when cells are grown on glucose (3). In 12U1-ccpA−, the formate-to-lactate ratio was higher when cells were grown on lactose (0.59) than when cells were grown on glucose (0.39), although there was no difference in LDH or PFL activity. This difference is also likely to have been caused by the allosteric effects of glycolytic intermediates.
In conclusion, S. bovis has a ccpA gene, which is transcribed as a single operon. Transcription of ccpA may be autogenously regulated through a cre sequence that is present in the promoter region of ccpA. S. bovis CcpA requires HPr-[Ser-P] for binding to the cre site. Transcription of ccpA appears to be greater when a more rapidly utilizable energy source is supplied. S. bovis utilizes glucose in preference to lactose, indicating that it possesses a system of catabolite repression for lactose utilization. CcpA is probably involved in catabolite repression. Transcription of ldh and pfl is regulated reciprocally through CcpA, which alters the formate-to-lactate ratio. There is a cre-like sequence in the upper region of both ldh and pfl, suggesting that the complex consisting of CcpA and HPr-[Ser-P] binds to the cre sites. However, how CcpA acts in the global catabolite control system in S. bovis remains to be clarified. If it is possible to control CcpA synthesis, lactate production by S. bovis could be modulated.
Acknowledgments
This study was supported in part by grants-in-aid for scientific research 16780190 and 15580240 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Asanuma, N., and T. Hino. 1997. Tolerance to low pH and lactate production in rumen bacteria. Anim. Sci. Technol. 68:367-376. [Google Scholar]
- 2.Asanuma, N., and T. Hino. 2000. Effect of pH and energy supply on the activity and amount of pyruvate formate-lyase in Streptococcus bovis. Appl. Environ. Microbiol. 66:3773-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma, N., and T. Hino. 2002. Fructose bisphosphate aldolase activity and glycolytic intermediate concentrations in relation to lactate production in Streptococcus bovis. Anaerobe 8:1-8. [Google Scholar]
- 4.Asanuma, N., and T. Hino. 2002. Molecular characterization and expression of pyruvate formate-lyase-activating enzyme in a ruminal bacterium, Streptococcus bovis. Appl. Environ. Microbiol. 68:3352-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asanuma, N., and T. Hino. 2003. Molecular characterization of HPr and related enzymes, and regulation of HPr phosphorylation in the ruminal bacterium Streptococcus bovis. Arch. Microbiol. 179:205-213. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma, N., M. Iwamoto, and T. Hino. 1997. Regulation of lactate dehydrogenase synthesis in a ruminal bacterium, Streptococcus bovis. J. Gen. Appl. Microbiol. 43:325-331. [DOI] [PubMed] [Google Scholar]
- 7.Asanuma, N., M. Iwamoto, and T. Hino. 1999. Structure and transcriptional regulation of the gene encoding pyruvate formate-lyase of a ruminal bacterium, Streptococcus bovis. Microbiology 145:151-157. [DOI] [PubMed] [Google Scholar]
- 8.Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164:123-128. [DOI] [PubMed] [Google Scholar]
- 9.Deutscher, J., and M. H. Saier, Jr. 1983. ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 80:6790-6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutscher, J., J. Reizer, C. Fischer, A. Galinier, M. H. Saier, Jr., and M. J. Steinmetz. 1994. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J. Bacteriol. 176:3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deutscher, J., E. Kuster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol. Microbiol. 15:1049-1053. [DOI] [PubMed] [Google Scholar]
- 12.Egeter, O., and R. Bruckner. 1996. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol. Microbiol. 21:739-749. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, Y., Y. Miwa, A. Galinier, and J. Deutscher. 1995. Specific recognition of the Bacillus subtilis gut cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol. Microbiol. 17:953-960. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton, I. R. 1987. Effect of changing environment on sugar transport and metabolism by oral bacteria, p. 94-133. In J. Reizer and A. Peterkofsky (ed.), Sugar transport and metabolism by Gram-positive bacteria. Ellis Horwood, Chichester, England.
- 15.Henkin, T. M., F. J. Grundy, W. L. Nicholson, and G. H. Chambliss. 1991. Catabolite repression of α-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacI and galR repressors. Mol. Microbiol. 5:575-584. [DOI] [PubMed] [Google Scholar]
- 16.Hino, T., K. Shimada, and T. Maruyama. 1994. Substrate preference in a strain of Megasphaera elsdenii, a ruminal bacterium, and its implications in propionate production and growth competition. Appl. Environ. Microbiol. 60:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueck, C. J., W. Hillen, and M. H. Saier, Jr. 1994. Analysis of a cis-active sequence mediating catabolite repression in Gram-positive bacteria. Res. Microbiol. 145:503-518. [DOI] [PubMed] [Google Scholar]
- 18.Hungate, R. E., R. W. Dougherty, M. P. Bryant, and R. M. Cello. 1952. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 42:423-449. [PubMed] [Google Scholar]
- 19.Iwamoto, M., N. Asanuma, and T. Hino. 1999. Effect of nitrate combined with fumarate on methanogenesis, fermentation, and cellulose digestion by mixed ruminal microbes in vitro. Anim. Sci. J. 70:471-478. [Google Scholar]
- 20.Iwamoto, M., N. Asanuma, and T. Hino. 2001. Effects of pH and electron donors on nitrate and nitrite reduction in ruminal microbiota. Anim. Sci. J. 72:117-125. [Google Scholar]
- 21.Iwamoto, M., N. Asanuma, and T. Hino. 2001. Effects of protozoa on nitrate and nitrite reduction in ruminal microbiota. Kanto J. Anim. Sci. 51:9-15. [Google Scholar]
- 22.Kim, J. H., M. I. Voskuil, and G. H. Chambliss. 1998. NADP, corepressor for the Bacillus catabolite control CcpA. Proc. Natl. Acad. Sci. USA 95:9590-9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knappe, J., and H. P. Blaschkowski. 1975. Pyruvate formate-lyase from Escherichia coli and its activation system. Methods Enzymol. 41:508-518. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Luesink, E. J., R. E. M. A. van Herpen, B. P. Grossiord, O. P. Kuipers, and W. M. de Vos. 1998. Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol. Microbiol. 30:789-798. [DOI] [PubMed] [Google Scholar]
- 26.Mahr, K., W. Hillen, and F. Titgemeyer. 2000. Carbon catabolite repression in Lactobacillus pentosus: analysis of the ccpA region. Appl. Environ. Microbiol. 66:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marounek, M., and S. Bartos. 1987. Interactions between rumen amylolytic and lactate-utilizing bacteria in growth on starch. J. Appl. Bacteriol. 63:233-238. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Verstraete, I., J. Stulke, A. Klier, and G. Rapoport. 1995. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177:6919-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meadow, N. D., D. K. Fox, and S. Roseman. 1990. The bacterial phosphoenolpyruvate:glucose phosphotransferase system. Annu. Rev. Biochem. 59:497-542. [DOI] [PubMed] [Google Scholar]
- 30.Miwa, Y., K. Nagura, S. Eguchi, H. Fukuda, J. Deutscher, and Y. Fujita. 1997. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol. Microbiol. 23:1203-1213. [DOI] [PubMed] [Google Scholar]
- 31.Muscariello, L., R. Marasco, M. de Felice, and M. Sacco. 2001. The functional ccpA gene is required for carbon catabolite repression in Lactobacillus plantarum. Appl. Environ. Microbiol. 67:2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura, M., K. Ogata, T. Nagamine, K. Tajima, H. Matsui, and Y. Benno. 2001. The replicon of the cryptic plasmid pSBO1 isolated from Streptococcus bovis JB1. Curr. Microbiol. 43:11-16. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson, W. L., and G. H. Chambliss. 1985. Isolation and characterization of a cis-acting mutation conferring catabolite repression resistance to α-amylase synthesis in Bacillus subtilis. J. Bacteriol. 161:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poolman, B. 1993. Energy transduction in lactic acid bacteria. FEMS Microbiol. Rev. 12:125-148. [DOI] [PubMed] [Google Scholar]
- 35.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carboxyhydrate phosphotransferase system of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell, J. B., and D. B. Dombrowski. 1980. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl. Environ. Microbiol. 39:604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell, J. B., and T. Hino. 1985. Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis. J. Dairy Sci. 68:1712-1721. [DOI] [PubMed] [Google Scholar]
- 38.Russell, J. B., and R. J. Wallace. 1997. Energy-yielding and energy-consuming reactions, p. 246-282. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem, 2nd ed. Blackie Academic and Professional, London, England.
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Simpson, C. L., and R. R. B. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 66:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Bogaard, P. T. C., M. Kleerebezem, O. P. Kuipers, and W. M. de Vos. 2000. Control of lactose transport, β-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar. J. Bacteriol. 182:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repressor operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:6238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]