Abstract
For some bacteria and algae, it has been proposed that inorganic polyphosphates and transport of metal-phosphate complexes could participate in heavy metal tolerance. To test for this possibility in Acidithiobacillus ferrooxidans, a microorganism with a high level of resistance to heavy metals, the polyphosphate levels were determined when the bacterium was grown in or shifted to the presence of a high copper concentration (100 mM). Under these conditions, cells showed a rapid decrease in polyphosphate levels with a concomitant increase in exopolyphosphatase activity and a stimulation of phosphate efflux. Copper in the range of 1 to 2 μM greatly stimulated exopolyphosphatase activity in cell extracts from A. ferrooxidans. The same was seen to a lesser extent with cadmium and zinc. Bioinformatic analysis of the available A. ferrooxidans ATCC 23270 genomic sequence did not show a putative pit gene for phosphate efflux but rather an open reading frame similar in primary and secondary structure to that of the Saccharomyces cerevisiae phosphate transporter that is functional at acidic pH (Pho84). Our results support a model for metal detoxification in which heavy metals stimulate polyphosphate hydrolysis and the metal-phosphate complexes formed are transported out of the cell as part of a possibly functional heavy metal tolerance mechanism in A. ferrooxidans.
Acidithiobacillus ferrooxidans (formerly Thiobacillus ferrooxidans) is a chemolithoautotrophic bacterium that obtains its energy from the oxidation of ferrous iron, elemental sulfur, or partially oxidized sulfur compounds (19, 24). This ability makes it of great industrial importance due to its application in biomining to recover metals such as copper, gold, and uranium (19, 23). These microorganisms are normally subjected to stress in their environment, such as temperature and pH changes and the presence of toxic heavy metals and nutrient starvation, which affect their physiological state (30).
Unlike most heterotrophic bacteria, A. ferrooxidans is capable of resisting high concentrations of heavy metals such as copper, zinc, arsenic, and uranium (9). The genetic basis for mercury and arsenic resistance has been studied in detail in this acidophile (6, 26). Copper is an essential trace element for all cells. However, it can cause serious cell damage through radical formation (10). Information regarding copper resistance in A. ferrooxidans is scarce. Although copper-tolerant strains have been obtained by growth and adaptation to increasingly higher concentrations of this metal (5, 8, 18), only a few genes were recently identified by RNA arbitrarily primed PCR as being induced or repressed in A. ferrooxidans subjected to copper (21). Nevertheless, the role of these genes in the mechanism of copper resistance is still unclear, and their expression may be related to indirect metabolic responses to stress (21).
Many heavy metal resistance systems involve either active efflux or detoxification of metal ions by different transformations (27). For copper, these include intracellular complexation, reduced accumulation, extracellular complexation, and sequestration in the periplasm (13, 25). One of the proposed mechanisms for metal tolerance is the sequestration of metal cations with long polymers of inorganic polyphosphate (17). Polyphosphate is a linear polymer of hundreds of orthophosphate residues linked by phosphoanhydride bonds. Several physiological functions have been attributed to polyphosphate in addition to being a reservoir of phosphate, such as a substitute for ATP, chelator of metals, and adaptation to stress conditions in the cell (17). The main enzyme involved in the biosynthesis of polyphosphate is the polyphosphate kinase, which catalyzes the reversible conversion of the terminal phosphate of ATP into polyphosphate (17). An exopolyphosphatase (PPX), on the other hand, is known to hydrolyze polyphosphate, liberating inorganic phosphate (Pi) (17). These enzymes have been purified from Escherichia coli, and their genes have been identified in several bacteria, including A. ferrooxidans (30). These genes show a relatively high degree of sequence conservation (7, 28).
It has been proposed that polyphosphate sequesters the heavy metals, thereby reducing their intracellular concentration and, on the other hand, that the hydrolysis of polyphosphate detoxifies the metals (1, 14). Van Veen (29) has shown that the inorganic phosphate transport system (Pit) in E. coli and Acinetobacter johnsonii can reversibly transport metal phosphates. Later, Keasling and Hupf (15), using genetically engineered strains of E. coli, obtained results indicating that not only a large quantity of intracellular polyphosphate but also the ability to synthesize and degrade polyphosphate is important for tolerance to heavy metals. Based on these results and those mentioned above, Keasling (14) proposed a model in which the intracellular cation concentration in bacteria would regulate the activity of PPX, which would in turn degrade polyphosphate and the Pi generated accompanied by cation transport out of the cell through the Pit system.
In the present work, it was found that A. ferrooxidans normally accumulates high amounts of polyphosphate granules and that the levels of intracellular polyphosphate are greatly reduced when the bacterium is grown in or shifted to 100 mM Cu2+ ions. In the presence of this metal, PPX activity and Pi efflux increased greatly. Our results support a model for metal tolerance mediated through polyphosphate in A. ferrooxidans.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. ferrooxidans ATCC 19859 was grown in spherical prills, 0.15 to 0.20 cm in diameter, of elementary sulfur (2) in the presence of 1.75 mM Pi (phosphate-sufficient conditions), unless otherwise indicated. Growth was monitored by measuring cell numbers or the optical density at 600 nm (OD600). Experiments were conducted when cells reached the late exponential phase (OD600 = 0.2). Cultures were adapted to grow in the presence of CuSO4 by continuous subculturing. During the first culture in the presence of Cu2+ (10 mM), a 4- to 5-day lag period was observed. Bacteria were completely adapted to grow in the presence of Cu2+ (10 to 100 mM) in the second subculture. It is a known phenomenon that A. ferrooxidans cells adapted to grow under these conditions lose this tolerance when they are cultured again in medium without cupric ions (18).
Electron microscopy and X-ray microanalyses.
Unstained and unfixed cells were examined for the presence of electron-dense granules by transmission electron microscopy (12). A suspension (10 μl) of A. ferrooxidans cells (OD600 = 0.25) was placed on a Formvar-coated grid and left for 2 min to allow the cells to sediment. Excess liquid was removed with a piece of filter paper, and the grids were air dried. For analysis, a transmission electron microscope (Philips Tecnai 12) operating at 80 kV was used. Energy-dispersive X-ray microanalysis (EDAX) was performed with an EDAX-PV 9800 energy-dispersive microanalyzer at an accelerating voltage of 120 kV (11).
Polyphosphate quantification.
Polyphosphate was quantified with a two-step conversion of polyphosphate into ATP by polyphosphate kinase and quantification of the ATP formed by using luciferase to generate light (3). First, polyphosphate was extracted from cell extracts with Glassmilk and then assayed by the reverse reaction of E. coli polyphosphate kinase in ADP excess. Finally, the ATP content was assayed with the firefly luciferase ATP assay, and luminescence was measured with a luminometer (BioScan Lumi/96). The concentration of polyphosphate is given in terms of Pi residues.
Preparation of crude cell extracts from A. ferrooxidans.
Cultures (200 ml) grown to the late exponential phase were harvested by centrifugation (10,000 × g for 20 min), and the cell pellets were resuspended in 500 μl of a buffer containing 50 mM Tris-HCl (pH 7) and 10% sucrose and lysed by four cycles of freezing (−80°C) and thawing by sonication. The lysate was centrifuged (5,000 × g for 5 min) to eliminate cellular debris, and the supernatant (crude cell extract) was used to measure PPX activity. These cell extracts were also obtained from nonadapted cells shifted to copper.
Assay for PPX activity.
PPX activity was determined as previously reported (16). The 20-μl reaction mixture contained 50 mM Tris-HCl (pH 6.5), 1 mM MgCl2, 100 mM KCl, 250 μM [33P]polyP750 (polyphosphate with an average of 750 Pi residues). After incubation of the mixture at 30°C for 60 min, the reaction was stopped by loading the mixture in polyethyleneimine-cellulose plates for thin-layer chromatography (Aldrich) and development in 0.75 M KH2PO4, pH 3.5. Radioactive spots corresponding to the Pi liberated by the hydrolysis of polyphosphate were visualized and quantified with a phosphor imager (Molecular Imager FX; Bio-Rad). [33P]polyP750 was synthesized in vitro by the method of Ault-Riché et al. (3) as described in Cardona et al. (7). One unit of enzyme was defined as the amount releasing 1 pmol of Pi from polyphosphate per min.
In vivo labeling of A. ferrooxidans with 32Pi.
Cells were grown in sulfur medium to the late exponential phase in Pi-sufficient conditions (1.75 mM). Cells were collected by centrifugation and resuspended at a higher cell density (1010 cells/ml) in medium with reduced Pi (0.18 mM Pi). To label the cells, H332PO4 (100 μCi/ml) was added, and the microorganisms were further incubated for 17 h, after which the radioactively labeled cells were harvested by centrifugation.
Pi efflux measurements.
The 32Pi-labeled cells were exhaustively washed by resuspension and centrifugation with fresh medium containing sufficient Pi (1.75 mM) to eliminate the nonincorporated radioactive label and finally resuspended in the same medium to an OD600 of 0.26 (109 cells/ml) in the presence or absence of CuSO4. To determine the amount of 32Pi released into the medium, samples (1.5 ml) were taken periodically, and the radioactivity in the supernatants obtained by centrifugation at 12,000 × g for 10 min (1.0 ml) was determined by scintillation counting.
Genome sequence analysis.
Preliminary sequence data for A. ferrooxidans strain 23270 was obtained from the Institute for Genomic Research website at http://www.tigr.org. Identity and similarity searches in the databases were done with the tBlastn program from NCBI (http://www.ncbi.nlm.nih.gov). The finished available A. ferrooxidans ATCC 23270 genomic sequence (http://www.tigr.org), which is not yet annotated, was used. The amino acid sequences of the PitA and PitB transporters from E. coli and the Pho84 transporter from Saccharomyces cerevisiae were used as probes. The possible presence of transmembrane domains in the open reading frames analyzed was studied with the Top-Pred program (http://bioweb.pasteur.fr/seqanal/interfaces/toppred.html).
RESULTS AND DISCUSSION
Accumulation of polyphosphate in A. ferrooxidans.
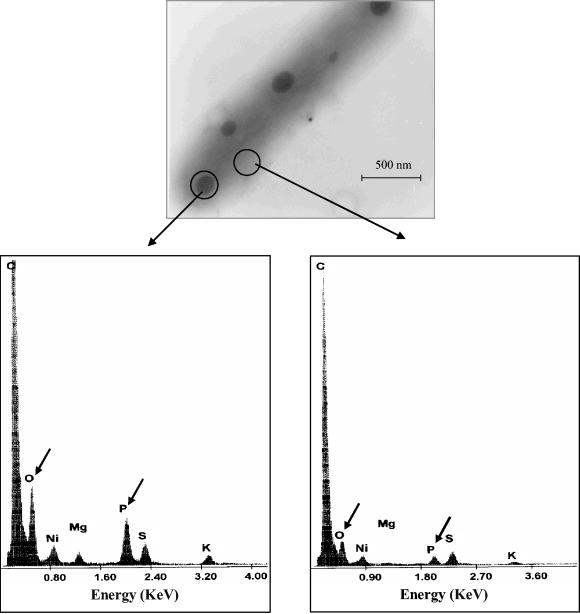
Previously, it was demonstrated that A. ferrooxidans possesses a polyphosphate kinase activity and the corresponding gene, suggesting the existence of polyphosphate in this bacterium (30). Typically, accumulation of polyphosphates in the form of electron-dense granules has been reported in many bacteria (12). To detect the presence of such granules in A. ferrooxidans, cells grown in sulfur medium in a Pi-sufficient condition were analyzed by transmission electron microscopy. As Fig. 1 shows, abundant spherical electron-dense granules were observed. All cells grown under these conditions presented at least two granules, and over 90% of the cells presented three to four granules. These granules disappeared when the cells were subjected to phosphate starvation (results not shown).
FIG. 1.
Transmission electron microscopy and energy dispersive X-ray analysis of A. ferrooxidans. Unstained and unfixed cells taken from sulfur-containing medium were examined directly for the presence of electron-dense granules. The elemental composition of a granule (left spectrum) and a cytoplasmic area (right spectrum) was analyzed by energy dispersive X-ray analysis. Arrows indicate the signals corresponding to oxygen and phosphorus.
To confirm the chemical nature of the electron-dense granules, an elemental analysis with EDAX in the transmission electron microscopy mode was carried out. As can be seen in Fig. 1, the EDAX spectra showed that the electron-dense bodies present in A. ferrooxidans were mainly composed of phosphorus and oxygen (Fig. 1, left spectrum), in contrast to the elemental composition of a cytoplasmic area (Fig. 1, right spectrum), indicating that A. ferrooxidans synthesizes and accumulates electron-dense granules containing phosphate, which is most likely polyphosphate. Cells grown under control conditions (Pi sufficient) showed a very high level of polyphosphate, determined enzymatically, of approximately 400 nmol of Pi residues/mg of protein (Fig. 2). This high level of the polymer was in agreement with the presence of abundant electron-dense granules in the cells (Fig. 1). A. ferrooxidans can therefore be considered a polyphosphate-accumulating microorganism, like Acinetobacter johnsonnii (29).
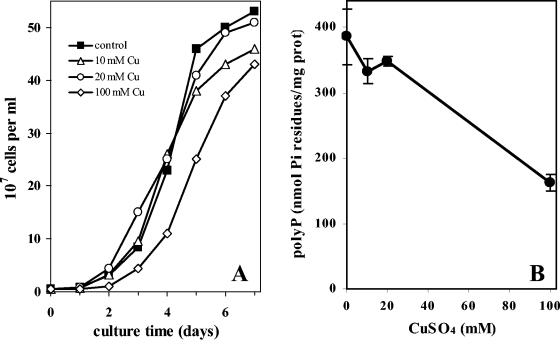
FIG. 2.
Growth and polyphosphate levels of A. ferrooxidans in the presence of copper ions. A. ferrooxidans cultures were inoculated in sulfur medium with 1.75 mM Pi in the presence of the indicated concentration of CuSO4, and cells were counted daily (A). To determine polyphosphate levels (B), the cells in A were harvested in the early stationary phase, and polyphosphate was extracted and quantified by the nonradioactive enzymatic method. Two independent determinations were performed. The error bars represent the standard deviations.
Effect of CuSO4 on the growth of A. ferrooxidans and its polyphosphate levels.
To evaluate the effect of Cu2+ on polyphosphate levels, cells were grown in the presence of different concentrations of Cu2+ and the cellular polyphosphate content was determined. The bacterial cells used in these experiments were previously adapted to grow at different concentrations of copper. There was a small decrease in the bacterial growth rates, and the curves reached the plateaus at slightly lower cell densities when cells were grown in the presence of increasing Cu2+ concentrations compared with the control culture in the absence of the metal (Fig. 2A). On the other hand, polyphosphate levels showed a clear drop (more than 50%) only when the Cu2+ concentration was raised to 100 mM (Fig. 2B). These results indicate a possible relationship between the polyphosphate level and the adaptation of A. ferrooxidans to growth in the presence of Cu2+.
Polyphosphate levels in cells shifted to medium containing copper ions.
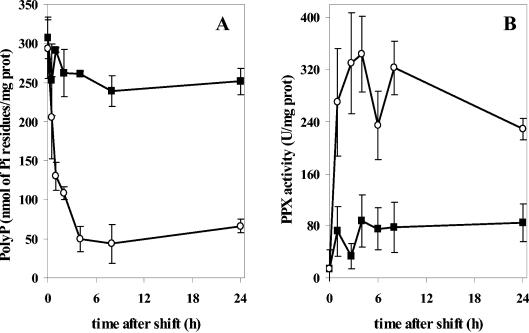
To determine the effect of Cu2+ in unadapted A. ferrooxidans, cells grown under Pi-sufficient conditions to accumulate a large amount of polyphosphate (Fig. 2B) were shifted to the presence of 20 mM Cu2+, and the polyphosphate levels were determined at different times postshift (Fig. 3A). A great decrease in polyphosphate levels was seen after 1 h, reaching the lowest level by 4 h, with polyphosphate rapidly dropping to about 20% of the level at time zero, and this level was maintained for the next 24 h. These results indicate that the presence of Cu2+ ions affects the polyphosphate content in A. ferrooxidans, probably by stimulating the degradation of this polymer, as has been suggested in other systems (1, 14).
FIG. 3.
(A) Reduction in polyphosphate content during exposure to copper ions. A. ferrooxidans cells grown in sulfur medium in the absence of copper to the early stationary phase were divided into two portions. CuSO4 (20 mM final concentration) was added to one sample (○), and an equal volume of fresh medium was added to the control sample (▪). Both were then incubated at 30°C. Aliquots were taken at the indicated times, and polyphosphate was quantified. (B) PPX activity in cells of A. ferrooxidans shifted to copper. A. ferrooxidans was cultured and transferred to medium with copper as in A. Cell extracts were then prepared at each of the indicated postshift times from control cells (▪) and cells exposed to 20 mM copper (○), and the PPX activity was determined. The error bars represent the standard deviations.
Effect of copper ions on PPX activity.
A possible mechanism to explain the observed decrease in polyphosphate levels when cells were exposed to copper ions is an increase in PPX activity. Previously, a putative ppx gene was found in A. ferrooxidans which appears to be part of a Pho regulon in this microorganism (30). To investigate the effect of copper ions on PPX activity, nonadapted cells were shifted to the presence of 20 mM CuSO4. Cells were collected at different time intervals, and PPX activity was measured in the cell extracts. A rapid increase in PPX activity was seen when cells were exposed to copper (Fig. 3B). This increase corresponded in time to the decrease in polyphosphate levels seen in Fig. 3A, strongly suggesting that the decrease in polyphosphate levels observed when cells were shifted to the presence of copper could be due to an increase in this exopolyphosphatase activity.
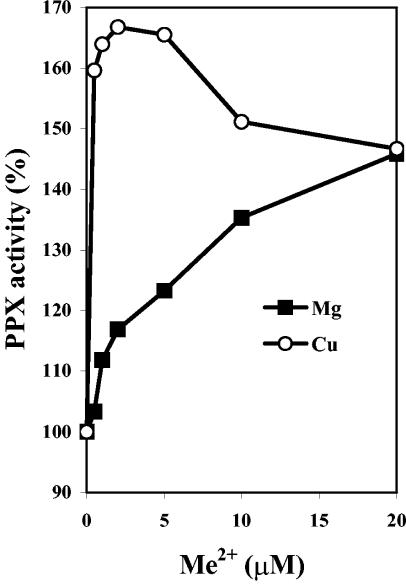
To further analyze the previous phenomenon, the in vitro effect of copper and other metals on the PPX activity present in cell extracts of nonadapted A. ferrooxidans was determined, as shown in Fig. 4. It is clear that copper greatly stimulated PPX activity at very low concentrations. The same maximal PPX activity was reached with both copper and magnesium. However, the metal concentration required for this maximal activity was 1 to 2 μM for copper (Fig. 4) and 1,000 μM for magnesium (results not shown). At concentrations of copper higher than 5 μM, there was an inhibition of the activity, whereas magnesium continued to stimulate PPX. Cadmium and zinc also stimulated PPX activity at concentrations of 1 to 2 μM. However, this effect was only half that seen with copper (not shown). These results suggest an effect of the heavy metals on PPX activity and polyphosphate hydrolysis.
FIG. 4.
PPX response to divalent cations in vitro. PPX activity was determined in the standard assay with cell extracts from A. ferrooxidans grown in the absence of copper. The indicated amounts of MgSO4 or CuSO4 were added. The enzyme activity in the absence of added metal was set at 100%.
Effect of copper ions on efflux of Pi from A. ferrooxidans cells.
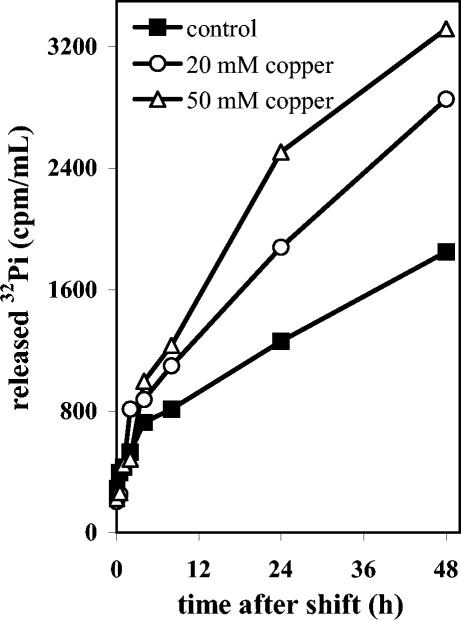
The decrease in polyphosphate levels due to increased PPX activity in A. ferrooxidans cells subjected to Cu2+ should generate free Pi. To evaluate if this Pi was transported out of the cells, A. ferrooxidans was grown to the late exponential phase and labeled in vivo with 32Pi as indicated in Materials and Methods. After exhaustive washing of the radioactively labeled cells to eliminate nonincorporated label, they were resuspended in sulfur medium with or without Cu2+ ions, and Pi efflux was determined, as shown in Fig. 5. There was a continuous basal release of label from the control cells. However, an increase in the Pi efflux over this basal level was observed when cells were exposed to Cu2+ ions. The amount of 32Pi released into the medium was higher in cells exposed to 50 mM Cu2+. By analyzing the radioactivity released into the medium by thin-layer chromatography on polyethyleneimine-cellulose, it was found that it consisted mainly of Pi (not shown). No other radioactively labeled cellular metabolites appeared on the thin-layer chromatograph, indicating that the label released into the medium was not the product of cellular lysis during treatment with copper. These results strongly suggest that at least part of the Pi generated from polyphosphate hydrolysis in the presence of Cu2+ ions was transported out of the cells.
FIG. 5.
Effect of copper ions on the efflux of Pi from A. ferrooxidans cells. A. ferrooxidans was grown in sulfur medium with 1.75 mM Pi to the exponential phase. These cells were then labeled in vivo with H332PO4 (100 μCi/ml) for 17 h in the presence of 0.18 mM Pi, as indicated in Materials and Methods. After the cells were exhaustively washed with unlabeled standard medium, they were shifted to the same fresh medium containing the indicated concentrations of CuSO4. At the times indicated, the cells were removed by centrifugation, and the radioactive Pi released into the supernatants was determined.
In silico search for Pi transporters in A. ferrooxidans.
The proposed model for metal ion detoxification based on the hydrolysis of polyphosphate involves the transport of metal-phosphate complexes out of the cell. It has been proposed that the inorganic phosphate transport (Pit) system is a candidate for this purpose because it can reversibly transport metal-phosphate complexes (14, 29). Pi transporters have not been described in A. ferrooxidans, although the bacterium possesses a putative Pho regulon (30). A Pit-like phosphate transport system was searched for in the available genome of A. ferrooxidans ATCC 23270. A Pit-like transporter was not found in this bacterium, but instead we found an open reading frame coding for a protein similar to the Pho84 Pi transporter from S. cerevisiae. An alignment of the predicted amino acid sequences of the putative Pho84-like protein of A. ferrooxidans with Pho84 from S. cerevisiae showed 26% identity and 43% similarity, with the same number of highly conserved transmembrane segments (results not shown). On the other hand, experimental evidence indicates that yeast Pho84, like Pit, transports metal-phosphate complexes (22).
Pho84 and Pho89 are the major Pi transporters in S. cerevisiae. Pho84, like Pit, belongs to the family of Pi:H+ symporters and is a member of the major facilitator superfamily (20). The Pho84 transporter is functional only in acidic environmental conditions (22). Although currently there is no experimental evidence for a Pho84-like transporter in A. ferrooxidans, it is remarkable that this microorganism, an acidophilic bacterium, possesses a putative Pi transporter of this kind. In this regard, genes encoding proteins similar to Pho84 but not to Pit (or Pho89) were also found in the genomes of other acidophilic microorganisms, such as Sulfolobus tokodaii, Sulfolobus solfataricus, Thermoplasma acidophilum, Thermoplasma volcanicum, and Ferroplasma acidarmanus (results not shown).
Finally, the A. ferrooxidans ATCC 23270 genome sequence also shows the presence of a putative CopA uptake Cu+ P-type ATPase and the CopB efflux Cu+ P-type ATPase present in other bacteria (4). This suggests that A. ferrooxidans might have a copper homeostasis mechanism similar to that of other microorganisms, but no experimental evidence supporting this proposal is available for this bacterium. Irrespective of the existence of such metal cation uptake and efflux mechanisms, it is plausible that a polyphosphate-mediated metal tolerance mechanism such as the one described here is also of great functional survival value for this extremophilic microorganism.
Acknowledgments
This work was supported in part by grants from ICGEB (project CRP/CHI00-04, contract 01/001, and ICM-P99-031-F).
We acknowledge Arthur Kornberg for supplying E. coli strain NR 100.
REFERENCES
- 1.Aiking, H., A. Stijnman, C. van Garderen, H. van Heerikhuizen, and J. van't Riet. 1984. Inorganic phosphate accumulation and cadmium detoxification in Klebsiella aerogenes CTC 418 growing in continuous culture. Appl. Environ. Microbiol. 47:374-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arredondo, R., A. García, and C. A. Jerez. 1994. The partial removal of lipopolysaccharide from Thiobacillus ferrooxidans affects its attachment to solids. Appl. Environ. Microbiol. 60:2846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ault-Riché, D., C. D. Fraley, C. M. Tzeng, and A. Kornberg. 1998. A novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 180:1841-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreto, M., R. Quatrini, S. Bueno, C. Arriagada, J. Valdes, S. Silver, E. Jedlicki, and D. S. Holmes. 2003. Aspects of the predicted physiology of Acidithiobacillus ferrooxidans deduced from analysis of its partial genome sequence. Hydrometallurgy 71:97-105. [Google Scholar]
- 5.Boyer, A., J-P. Magnin, and P. Ozil. 1998. Copper ion removal by Thiobacillus ferrooxidans biomass. Biotechnol. Lett. 20:187-190. [Google Scholar]
- 6.Butcher, B. G., S. M. Deane, and D. E. Rawlings. 2000. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 66:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardona, S. T., F. P. Chávez, and C. A. Jerez. 2002. The exopolyphosphatase gene from Sulfolobus solfataricus: characterization of the first gene found to be involved in polyphosphate metabolism in Archaea. Appl. Environ. Microbiol. 68:4812-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das, A., J. M. Modak, and K. A. Natarajan. 1998. Surface chemical studies of Thiobacillus ferrooxidans with reference to copper tolerance. Antonie van Leeuwenhoek 73:215-222. [DOI] [PubMed] [Google Scholar]
- 9.Dopson, M., C. Baker-Austin, P. R. Koppineedi, and P. L. Bond. 2003. Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic microorganisms. Microbiology 149:1959-1970. [DOI] [PubMed] [Google Scholar]
- 10.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931-936. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg, J., H. Gonzalez, T. E. Jensen, and W. A. Corpe. 2001. Quantitative analysis of the elemental composition and the mass of bacterial polyphosphate bodies using STEM EDX. Microbios 106:177-188. [PubMed] [Google Scholar]
- 12.Gonzalez, H., and T. E. Jensen. 1998. Nickel sequestering by polyphosphate bodies in Staphylococcus aureus. Microbios 93:179-185. [PubMed] [Google Scholar]
- 13.Harwood, V. J., and A. S. Gordon. 1994. Regulation of extracellular copper-binding proteins in copper-resistant and copper-sensitive mutants of Vibrio alginolyticus. Appl. Environ. Microbiol. 60:1749-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keasling, J. D. 1997. Regulation of intracellular toxic metals and other cations by hydrolysis of polyphosphate. Ann. N. Y. Acad. Sci. 829:242-249. [DOI] [PubMed] [Google Scholar]
- 15.Keasling, J. D., and G. A. Hupf. 1996. Genetic manipulation of polyphosphate metabolism affects cadmium tolerance in Escherichia coli. Appl. Environ. Microbiol. 62:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keasling, J. D., L. Bertsch, and A. Kornberg. 1993. Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long-chain exopolyphosphatase. Proc. Natl. Acad. Sci. USA 90:7029-7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornberg, A., N. N. Rao, and D. Ault-Riché. 1999. Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 68:89-125. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan, K. A., K. Sudeesha, and G. R. Rao. 1994. Stability of copper tolerance in Thiobacillus ferrooxidans. Antonie van Leeuwenhoek 66:303-306. [DOI] [PubMed] [Google Scholar]
- 19.Olson, G. J., J. A. Brierley, and C. L. Brierley. 2003. Bioleaching review, part B. Progress in bioleaching: applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 63:249-257. [DOI] [PubMed] [Google Scholar]
- 20.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulino, L. C., M. P. de Mello, and L. M. M. Ottoboni. 2002. Differential gene expression in response to copper in Acidithiobacillus ferrooxidans analyzed by RNA arbitrarily primed polymerase chain reaction. Electrophoresis 23:520-527. [DOI] [PubMed] [Google Scholar]
- 22.Persson, B. L., J. O. Lagerstedt, J. R. Pratt, J. Pattison-Granberg, K. Lundh, S. Shokrollahzadeh, and F. Lundh. 2003. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 43:225-244. [DOI] [PubMed] [Google Scholar]
- 23.Rawlings, D. E. 2002. Heavy metal mining using microbes. Annu. Rev. Microbiol. 56:65-91. [DOI] [PubMed] [Google Scholar]
- 24.Rohwerder, T., T. Gehrke, K. Kinzler, and W. Sand. 2003. Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 63:239-248. [DOI] [PubMed] [Google Scholar]
- 25.Rouch, D. R., B. T. Lee, and J. Camakaris. 1989. Genetics and molecular basis of copper resistance in Escherichia coli, p. 439-446. In D. H. Hamer and D. R. Winge (ed.), Metal homeostasis. Alan Liss Inc., New York, N.Y.
- 26.Shiratori, T., C. Inoue, K. Sugawara, T. Kusano, and Y. Kitagawa. 1989. Cloning and expression of Thiobacillus ferrooxidans mercury ion resistance genes in Escherichia coli. J. Bacteriol. 171:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver, S., and L. T. Phung. 1996. Bacterial heavy metal resistance: new surprises. Annu. Rev. Microbiol. 50:753-789. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng, C. M., and A. Kornberg. 1998. Polyphosphate kinase is highly conserved in many bacterial pathogens. Mol. Microbiol. 29:381-382. [DOI] [PubMed] [Google Scholar]
- 29.van Veen, H. W. 1997. Phosphate transport in prokaryotes: molecules, mediators and mechanisms. Antonie van Leeuwenhoek. 72:299-315. [DOI] [PubMed] [Google Scholar]
- 30.Vera, M., N. Guiliani, and C. A. Jerez. 2003. Proteomic and genomic analysis of the phosphate starvation response of Acidithiobacillus ferrooxidans. Hydrometallurgy 71:125-132. [Google Scholar]