Abstract
Glycerol dialkyl glycerol tetraethers (GDGTs) are core membrane lipids of the Crenarchaeota. The structurally unusual GDGT crenarchaeol has been proposed as a taxonomically specific biomarker for the marine planktonic group I archaea. It is found ubiquitously in the marine water column and in sediments. In this work, samples of microbial community biomass were obtained from several alkaline and neutral-pH hot springs in Nevada, United States. Lipid extracts of these samples were analyzed by high-performance liquid chromatography-mass spectrometry and by gas chromatography-mass spectrometry. Each sample contained GDGTs, and among these compounds was crenarchaeol. The distribution of archaeal lipids in Nevada hot springs did not appear to correlate with temperature, as has been observed in the marine environment. Instead, a significant correlation with the concentration of bicarbonate was observed. Archaeal DNA was analyzed by denaturing gradient gel electrophoresis. All samples contained 16S rRNA gene sequences which were more strongly related to thermophilic crenarchaeota than to Cenarchaeum symbiosum, a marine nonthermophilic crenarchaeon. The occurrence of crenarchaeol in environments containing sequences affiliated with thermophilic crenarchaeota suggests a wide phenotypic distribution of this compound. The results also indicate that crenarchaeol can no longer be considered an exclusive biomarker for marine species.
The western United States has a long geological history of volcanic activity caused by the collision of the Pacific and North American tectonic plates. Associated with this volcanism are convective hydrothermal systems, which are manifested as surficial hot springs (19). Meteoric water enters the subsurface hydrothermal system along boundary faults and reaches about 200 to 280°C (6, 17) before coming up again along vertical fractures. Temperatures in these springs range from 20°C to >100°C at depth; the majority, however, have temperatures below 100°C (14). Typical features of the hot springs include dense communities of floating microbial mats; biodiversity within the mats is high, and in particular, the archaeal communities appear to be dominated by members of the crenarchaeota.
Cultivated and uncharacterized crenarchaeota synthesize a wide variety of glycerol dialkyl glycerol tetraether (GDGT) core membrane lipids (11, 12, 39). In addition to the stability afforded by the ether linkages and by the tetraether (monolayer) structure, the presence of cyclopentane rings in the C40 isoprenoid backbone has been shown to increase the thermal tolerance of membranes (16). Indeed, both in culture studies and in the marine system, the average number of rings correlates positively with temperature (40, 42, 50).
The molecular structure of crenarchaeol represents an unusual exception to this relationship because crenarchaeol contains five rings. However, unique among archaeal lipids, it has one C40 isoprenoid containing two cyclopentane rings (C40:2) and one C40 isoprenoid containing two cyclopentane rings plus a cyclohexane ring (C40:3) (43). Molecular modeling suggests that the presence of cyclohexane prevents dense packing of the lipid membranes and thus could be an adaptation to cold temperatures (43).
Until now, crenarchaeol has been found only in the marine system between 2 and 30°C (9, 20, 40). Relatives of the marine Crenarchaeota have also been detected in fresh water lakes or reservoirs (22, 28, 44, 45), soils (3, 5, 23, 36), and in the terrestrial subsurface (49). However, concomitant analyses of archaeal lipids from these environments have not been performed. The presence of diverse Crenarchaeota in low-temperature environments could support the hypothesis that crenarchaeol evolved during the physical adaptation to colder temperatures. However, recent work suggests that ancient oceans were as warm as 36°C and still contained crenarchaeol (41), and crenarchaeol has also been found in hydrothermal marine sediments (42). Here we demonstrate that crenarchaeol can be found in springs with temperatures ranging from 40 to 84°C, that it is not an exclusive product of marine planktonic crenarchaeota, and that the TEX86 index of GDGTs in terrestrial hot springs is correlated with HCO3− concentration but not with temperature.
We suggest that water chemistry, in addition to temperature, is an important determinant of the archaeal population and therefore of the distribution of GDGTs. The marine planktonic crenarchaeota are believed to be autotrophic organisms dependent on fixation of HCO3− into biomass (35, 52). The data from Nevada hot springs show that the lipid distribution of the integrated archaeal community correlates more strongly with HCO3− than it does with temperature. Individual species may change the proportions of GDGTs within their membranes in response not only to physiological (temperature) conditions but also to biochemical (substrate) requirements.
MATERIALS AND METHODS
Sampling.
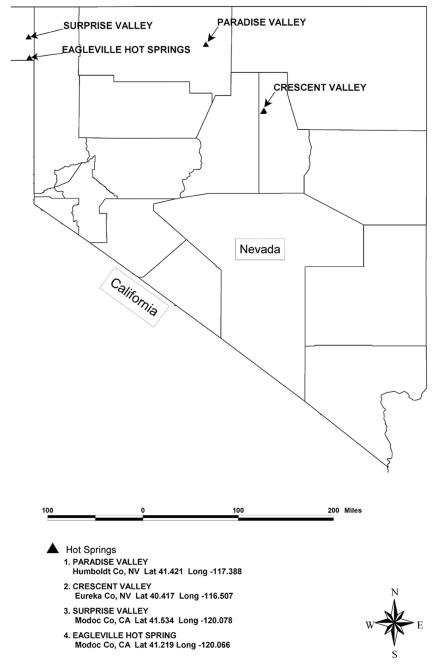
Samples were collected from five locations in Nevada and northeast California (Fig. 1). The hot springs were selected to represent a range of temperatures and chemistries. At each spring, temperature and pH were measured in situ prior to collection of bacterial mats. A standard pH electrode with temperature compensation was used; standards at pH 4, 7, and 10 were used for calibration. Alkalinity was measured with a Hach alkalinity kit by colorimetric titration. Floating mat material was picked with sterilized spatulas and stored in autoclaved, 100-ml wide-mouthed glass jars. The jars were filled with the corresponding spring water to the rim and sealed with sterile black butyl rubber stoppers and screw caps. Samples were transported and stored anaerobically at 4°C before freeze-drying and subsequent lipid extraction.
FIG. 1.
Map of hot spring locations in Nevada and California.
The material from Santa Monica Basin and Santa Barbara Basin was described in reference 35.
Lipid extractions.
Lipids were extracted from the freeze-dried Nevada samples according to published methods (51). The neutral lipid, glycolipid, and polar lipid fractions were dissolved in 1.3% isopropanol in hexane for screening by high-performance liquid chromatography-mass spectrometry (HPLC-MS); GDGTs were detected only in the glycolipid fraction. For comparison to the marine system, a sample from Santa Monica Basin was selected. The sample was freeze-dried and Soxhlet extracted with 93:7 CH2Cl2-CH3OH (Fisher GC Resolv or Burdick & Jackson GC2) for 72 h. The total lipid extract was transesterified, and lipid classes were separated on SiO2 gel (23). GDGTs eluted with fraction 9, 100% ethyl acetate; an aliquot was dissolved in 1.3% isopropanol in hexane for screening by HPLC-MS.
HPLC-MS.
Intact GDGTs were identified with an Agilent 1100 series high performance liquid chromatograph with atmospheric pressure chemical ionization-MS. Following earlier methods (21), the column was a Zorbax NH2 (4.6 by 250 mm, 5 μm) at 30°C. GDGTs were eluted isocratically in 1.3% isopropanol in hexane. Conditions for atmospheric pressure chemical ionization-MS were nebulizer pressure of 60 lb/in2, drying gas flow of 6.0 liters/min and 350°C, vaporizer temperature of 375°C, voltage of 3 kV, and corona of 5 μA. Spectra were scanned over the m/z range from 1,000 to 1,350.
HI digestion.
Aliquots of each glycolipid fraction and of Santa Monica Basin fraction 9 were saved for acid digestion. The ether linkages of GDGTs were cleaved by reflux in 55% HI, followed by reduction under LiAlH4 following the procedure described before (20). Samples were redissolved in hexane for analysis by gas chromatography (GC) and GC-MS.
GC and GC-MS.
GC analyses were performed on an Agilent 6890 GC with flame ionization detector, equipped with a 60-m DB-5MS column (5% phenyl) and programmable temperature vaporizing inlet. The temperature program for samples in hexane was 60°C for 0 min, 10°C/min to 180°C, and 4°C/min to 320°C (20 min). GC-MS analyses were performed on an Agilent HP6890 GC coupled to a 5873 mass selective detector equipped with a 60-m, 100% methylpolysiloxane column (CP-Sil5) and programmable temperature vaporizing inlet.
GC-isotope ratio-monitoring MS.
Compound-specific δ13C values were obtained by gas chromatography-isotope ratio-monitoring mass spectrometry as described before (31). The column stationary phase was 100% methylpolysiloxane (DB-1), and the temperature program was 65°C (2 min), 20°C/min to130°C, and 4°C/min to 320°C (40 min).
Bulk carbon isotopic measurements.
Carbon isotopic compositions of total biomass were determined by combustion in an elemental analyzer followed by measurement of the 13C/12C ratio with a DeltaPlus isotope ratio mass spectrometer. Precision was ±0.2 ‰. Bulk samples were first treated in 10% HCl for 4 to 5 h to remove carbonate. The residual material was rinsed with distilled H2O and dried at 50°C prior to analysis.
DNA amplification.
Genomic DNA was extracted from approximately 0.5 g of mat material with a commercial extraction kit (MoBio Lab Inc., Solana Beach, Calif.). DNA was quantified by UV spectrophotometry at 260 to 280 nm. The DNA concentration used for PCR was 0.3 μg/μl.
A nested PCR approach was used to amplify partial 16S ribosomal DNA sequences representative of Crenarchaeota. The first round used primers Arch 21F and Arch 958R (8, 15); the second round used internal denaturing gradient gel electrophoresis primer 344F(GC-clamp) and primer 519R (8, 33). One unit of HotStarTaq (Qiagen Inc., Valencia, Calif.) was used in 20-μl reactions for the first round, and 1 μl of product was used as template for the second round of PCR.
Reaction conditions for the first round of PCR were 20 cycles of denaturation (50 s) at 95°C, annealing at 50°C (45 s), and elongation at 72°C (2 min). Reaction conditions for the second round were denaturation at 94°C (30 s), annealing at 65°C for 10 cycles (40 s), 60°C for 10 cycles (40 s), and 55°C for 10 cycles (40 s), and extension at 72°C (1 min). All PCRs were performed with an MJ thermal cycler (MJ Research Co.).
Denaturing gradient gel electrophoresis.
PCR products were analyzed with a modified double-gradient procedure (18, 33). Denaturing gradient gel electrophoresis was performed with the DCode Universal Mutation Detection system (Bio-Rad Lab, Hercules, Calif.) following the manufacturer's instruction. PCR products (20 μl) were electrophoresed for 5 h at 200 V and 60°C on 1-mm-thick, 6 to 12% (wt/vol) denaturing gradient polyacrylamide gels with 30 to 55% denaturant (100% denaturant is 7 M urea with 40% formamide) in 0.5× TAE running buffer (20 mM Tris-HCl, pH 8.3, 10 mM acetic acid, 0.5 mM EDTA). Gels were stained with SYBR Green I (Molecular Probes, Eugene, Oreg.) for 30 min and photographed under UV illumination with an AlphaImager 3400 gel imaging system (Alpha Innotech Co., San Leandro, Calif.).
Sequencing and phylogenetic analysis.
Bidirectional sequencing reactions were carried out with the BigDye kit version 3.1 (Applied Biosystems) with primers 344F and 517R; products were analyzed on an ABI 377 DNA sequencer (Perkin Elmer Inc., Wellesley, Mass.). Sequences were edited by hand, and consensus sequences were assembled in Sequencher version 4.0 (Gene Codes Corp., Ann Arbor, Mich.). The consensus sequences were compared with those from GenBank with the BLAST algorithm. Phylogenetic inference and evolutionary distance calculations were performed with ClustalX and Phylip (http://evolution.genetics.washington.edu/phylip.html). Maximum likelihood phylogenies were reconstructed with the Kimura two-parameter model (27).
Nucleotide sequence accession numbers.
Sequence data have been deposited with GenBank and accession numbers have been assigned: AY597003 to AY597005 for sequences EV1 to EV3; AY597006 for EV5; AY597007 for PV1; AY597008 for EV4; AY597009 for PV2; and AY597010 for CV1.
RESULTS
The temperature, pH, and alkalinity data for the five hot springs are summarized in Table 1. The Eagleville site had the highest pH (9.2) and lowest temperature (40 to 41°C) among the analyzed samples. The highest alkalinity was found in the samples from Crescent Valley, and the highest temperature was recorded in a sample from Surprise Valley (84 to 85°C). This spring was sampled in two locations, at the southern, hot end (Surprise Valley 5), and in the cooler middle (Surprise Valley 6). The carbonate chemistry of the water (as well as dissolved cations, unpublished data) was uniform in both locations.
TABLE 1.
Chemistry and lipid data for Nevada hot springs and Santa Monica Basin
| Location | Temp (°C) | pH | Alkalinity (ppm) | Log[HCO3−]a (mol/kg) | GDGT I/ GDGT IIb | Ring indexc | TEX86d | Temp TEX86 (°C) |
|---|---|---|---|---|---|---|---|---|
| Eagleville | 40-41 | 9.2 | 55 | −4.91 | 22.8 | 4.6 | 0.93 | 44 |
| Paradise Valley | 54-56 | 6.4-6.5 | 850 | −3.07 | 4.5 | 3.6 | 0.53 | 16 |
| Crescent Valley | 58 | 5.9-6.1 | 900 | −3.06 | 1.3 | 2.6 | 0.72 | 29 |
| Surprise Valley (5) | 84-85 | 7.9-8.3 | 68 | −4.39 | 2.3 | 2.9 | 0.79 | 34 |
| Surprise Valley (6) | 55-56 | 8.2-8.3 | 68 | −4.31 | 1.2 | 2.4 | 0.68 | 27 |
| Santa Monicae | 6.4f | 7.7 | 2,300 | −2.66 | 0.59 | 2.0 | 0.54 | 17 |
Dissolved inorganic carbon concentrations for Nevada were calculated from temperature, pH, and carbonate alkalinity with the equations and pK values given before (37).
Weighted average number of rings, calculated as [(% peak III) + 2(% peak IV) + 3(% peak V) + 5(% peak I + VI)]/100.
TEX86 = [IV + V + VI]/[III + IV + V + VI] (40).
From reference 35.
16°C surface water temperature (4).
To illustrate the diverse chemical environments in which crenarchaeol can be found, temperature, pH, and dissolved inorganic carbon data are also presented for a sample from Santa Monica Basin (Table 1). Radiocarbon data obtained for archaeal lipids from Santa Monica Basin surface sediments place the approximate depth of biomass production in the mid- to deep water column, around 400 to 500 m (35). The Santa Monica Basin water column data in Table 1 represent an average of the carbonate system that was reported for GEOSECS stations 201 and 347 at 400 to 500 m water depth, both of which are off the coast of southern California (7). The temperature in Santa Monica Basin at 500 m water depth is 6.4°C, while annual average sea surface temperature is ≈16°C (4).
Identification of crenarchaeol.
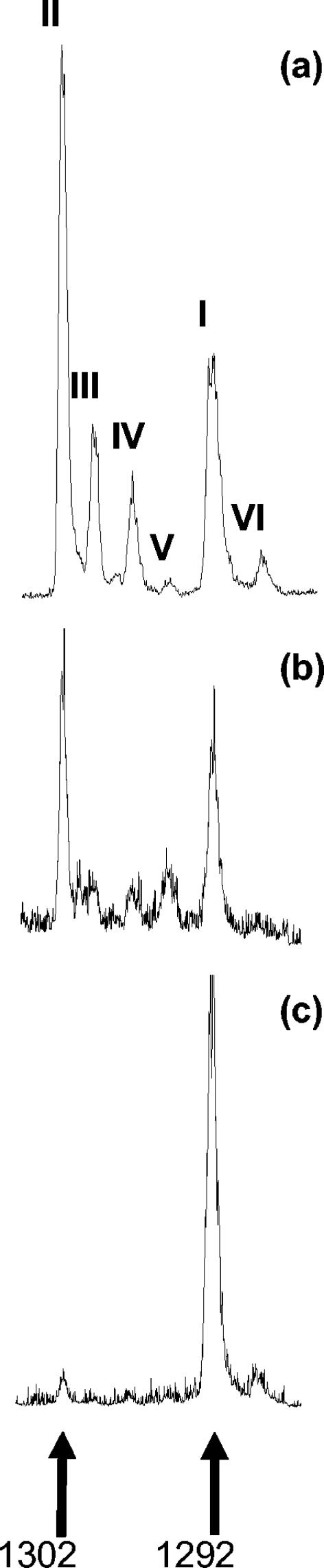
Analysis of the lipid extracts from each hot spring and from Santa Monica Basin sediment revealed the presence of GDGTs with masses ranging from 1,302 (no rings) through 1,292 (five rings). No GDGTs with six or more total rings were detected (mass < 1,292), nor was there detection of any GDGTs having four rings (mass 1,294; equivalent to GDGT-4 [42]). Figure 2 shows HPLC-MS total ion chromatograms of the GDGT region as measured for Santa Monica Basin surface sediments, Crescent Valley, and Eagleville. Each chromatogram shows a prominent peak with a mass of 1292, corresponding to crenarchaeol (I). In addition, a second peak with mass 1,292 (VI) was also detected. This compound has been tentatively identified as an isomer of crenarchaeol (40). Other GDGTs having 0, 1, 2, and 3 rings were also detected (peaks II, III, IV, and V, respectively).
FIG. 2.
HPLC-MS chromatograms of intact GDGTs from Santa Monica Basin surface sediment (a), Crescent Valley (b), and Eagleville (c). GDGTs are enumerated I to VI as in reference 29. Peak II is acyclic GDGT, while peak I is crenarchaeol.
To confirm the identities of the GDGTs from the Nevada samples, aliquots of the lipid extracts were hydrolyzed in 55% HI and reduced with LiAlH4 to yield a series of C40 isoprenoid hydrocarbons. The hydrocarbon samples were analyzed by GC and GC-MS. Four C40 isoprenoids containing between 0 and 3 rings were found in each sample: C40:0, C40:1, C40:2, and C40:3. The proportion of each C40 compound corresponded to the expected amount based on analysis by HPLC-MS; for example, the relative yield of C40:0 was very small for the sample from Eagleville.
Mass spectra were obtained for each of the HI-hydrolyzed C40 compounds identified in the Nevada hot springs. The fragmentograms were indistinguishable from the equivalent peaks found in the Santa Monica Basin sample, and the data also agreed with published spectra (9, 38). In particular, there was no difference between the C40:3 isomers found in the hot spring and marine samples, and each included the diagnostic fragment at mass 263.
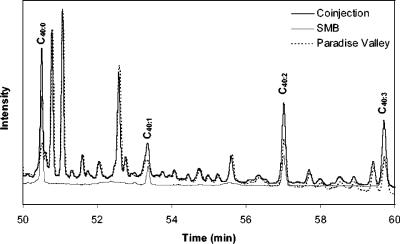
The unique feature of crenarchaeol is the presence of a cyclohexane ring. In the absence of enough material to perform 13C nuclear magnetic resonance, we cannot be absolutely certain that the cyclohexane ring is present and therefore that the GDGT found in Nevada hot springs is crenarchaeol. However, analysis of the terrestrial and marine samples by coinjection showed that the C40:3 isomer was the same in all samples. Figure 3 shows the GC chromatograms for Santa Monica Basin sediment and Paradise Valley samples. All four C40 isoprenoids overlapped when an equal mixture of the two samples was analyzed by coinjection. Back-calculation based on the peak areas confirmed that the C40:2 and C40:3 peaks in the coinjected sample contained a 47:53 mixture of Paradise Valley and Santa Monica Basin components.
FIG. 3.
Coinjection of C40 isoprenoid hydrocarbons from Santa Monica Basin (SMB) and from Paradise Valley.
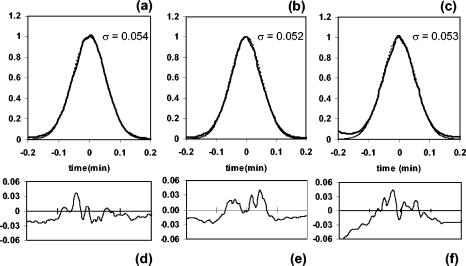
If there were multiple isomers of the C40:3 isoprenoid present in the samples, or if the hot spring compound did not contain the diagnostic cyclohexane ring, the GC peak shape could be expected to show asymmetry or a visible shoulder. In Fig. 4, the peaks for C40:3 were compared to an ideal Gaussian model. For each peak, the data were centered about an artificial time zero and normalized to a peak height of 1.0. Perfectly symmetrical peaks were calculated according to the equation y = e−(Δt2)/2σ2. The raw data for each sample were slightly irregular in shape. However, examination of the residuals (calculated values minus data; Fig. 4d to f) showed that the coinjections were no more irregular than the pure Santa Monica Basin sample. Additionally, the one-sigma (1σ) peak widths are the same for Santa Monica Basin and for the coinjections. All of the above data support the interpretation that the C40:3 isoprenoids found in Nevada hot springs and in Santa Monica Basin surface marine sediment are identical and derived from crenarchaeol.
FIG. 4.
Coinjections of C40:3 isoprenoid hydrocarbons: pure Santa Monica Basin surface sediment (a), Eagleville (25%) plus Santa Monica Basin (75%) (b), and Paradise Valley (47%) plus Santa Monica Basin (53%) (c). Solid lines represent raw data; calculated Gaussian curves are shown as dashed lines. Residuals for each model are shown in panels d, e, and f. One-sigma peak widths are in minutes.
Relationship of GDGTs to water chemistry and temperature.
In the marine system, changes in the relative proportions of GDGTs are known to correlate with the temperature of the upper water column (40). Specifically, the parameter TEX86, calculated as TEX86 = [IV + V + VI]/[III + IV + V + VI] has been used as a marine paleoproxy for sea surface temperature (40, 41). The ratio of the sums of these minor components scales linearly with temperature according to the equation TEX86 = 0.015 × sea surface temperature (°C) + 0.28 (40); crenarchaeol (peak I) and GDGT II are excluded from TEX86 due to the variability in both their absolute and relative concentrations.
Similar to these observations for marine samples, in Nevada the relative abundance of crenarchaeol (peak I) and GDGT II was different at each location studied. The ratio of GDGT I to GDGT II ranged from 0.59 in Santa Monica Basin to 22.8 in Eagleville (Table 1), and it did not correlate significantly with any of the physical or chemical parameters.
Interestingly, the other GDGTs did not scale with temperature either. This contrasts with expectations derived from marine samples and with TEX86 calculations. Marine samples display a ring index, or weighted average number of rings, between 1.4 and 3.3; a higher index correlates with higher surface water temperatures. Our Santa Monica Basin sample had a weighted average value of 2.0 rings and a TEX86 value of 0.54; as expected, these numbers predict an annual mean sea surface temperature of around 14 and 17°C, respectively. Both means of estimation are consistent with the observational data from Santa Monica Basin, which yields a sea surface temperature of ≈16°C (4).
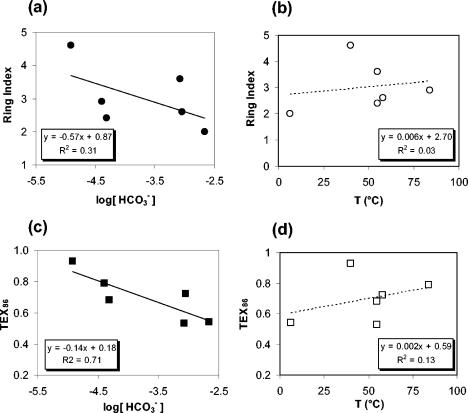
The Nevada samples had ring index values between 2.6 and 4.6; these values did not correlate with the temperatures of the hot springs (Fig. 5b), nor did the ring index correlate significantly with the concentration of bicarbonate (Fig. 5a). The TEX86 values for Nevada samples ranged between 0.53 and 0.93. This is equivalent to 16 to 44°C on the TEX86 scale, and it did not correspond to the measured growth temperatures for these samples, which fell between 40 and 84°C (Fig. 5d; Table 1). While there may be spatial and physical heterogeneity within the floating bacterial mats which could permit the growth of stratified communities, the difference between some of the measured temperatures and the calculated TEX86 values is so large that it is unlikely that a temperature gradient could produce such discrepancy. Additionally, the differences between temperature and TEX86 are not offset consistently in magnitude or direction. However, a significant relationship was found between TEX86 and the calculated concentration of HCO3− in the water. Linear regression of TEX86 versus log[HCO3−] yielded an R2 of 0.71 (Fig. 5c).
FIG. 5.
Ratios of the peak areas of GDGTs in Nevada hot springs and Santa Monica Basin as a function of HCO3− concentration and water temperature. Ring index is given versus log[HCO3−] (a) and versus temperature (b). TEX86 is given versus log[HCO3−] (c) and versus temperature (d).
16S rRNA gene sequences.
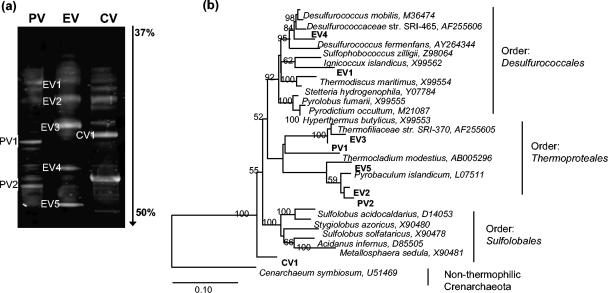
Bands exhibiting the greatest degree of amplification were excised from denaturing gradient gel electrophoresis gels for sequencing (Fig. 6a). These included two prominent bands (PV1 and PV2) for the Paradise Valley sample, five (EV1 to EV5) for the Eagleville sample, and two (CV1 and CV2) for the Crescent Valley sample. Numerous bands of minor intensity were also observed in the gel but were not investigated further in this preliminary sequencing study. Because amplification by PCR is a nonquantitative method, it is possible that some of these minor bands also represent significant members of the hot-spring archaeal community.
FIG. 6.
Denaturing gradient gel electrophoresis gel (a) and neighbor-joining tree (b) for environmental sequences from Nevada hot springs. PV, Paradise Valley; EV, Eagleville Valley; CV, Crescent Valley. The scale bar in panel b indicates 10 substitutions per 100 nucleotide positions.
All of the 16S rRNA genes that were sequenced belong to two of the three orders of Crenarchaeota (Fig. 6b). Sequences EV1 and EV4 from Eagleville align with the Desulfurococcales. Sequences EV2, EV3, and EV5 from Eagleville and sequences PV1 and PV2 from Paradise Valley align with the Thermoproteales. Sequence CV1 from Crescent Valley is distinct from the above sequences and may be related to the Sulfolobales or Thermoproteales (Fig. 6b). The sequence obtained for CV2 was too short for a meaningful interpretation, and thus this sequence is not reported. All sequences (excluding CV2) have 91.2 to 99.9% similarities to known species of Desulfurococcales and Thermoproteales and are only distantly related to nonthermophilic crenarchaeota such as Crenarchaeum symbiosum (Fig. 6b). Crenarchaeol in Eagleville Spring was >20 times more concentrated than any other GDGT. The sequences identified here suggest, therefore, that a member(s) of the Thermoprotales or Desulfurococcales may synthesize crenarchaeol.
DISCUSSION
Biology and chemistry of crenarchaeota.
The ecological and physiological diversity of archaea is now known to far surpass the original classification of archaea as extremophiles. Culture-independent surveys of archaeal populations in general (1) and of crenarchaeota in particular (24, 29, 32) revealed the ubiquity of archaea. The cold-adapted marine pelagic group I crenarchaeota are found throughout the world's oceans (13) and could account for 20% of the total prokaryotic population in the marine environment (24). Here, the lipid data as well as sequence information demonstrate the presence of crenarchaeota in the thermal environments of these nonacidic hot springs. Most of the cultured thermophilic Crenarchaeota grow in acidic conditions (47, 48). However, based on 16S rRNA gene sequences, Barns et al. (1, 2) reported diverse Crenarchaeota in a hot spring from Yellowstone National Park (United States) at neutral pH and temperatures from 74 to 93°C. These novel crenarchaeotal species have not been isolated, and their lipid biomarkers remain unknown. Our sequencing results indicate that the nonacidic Nevada hot springs may harbor similar alkaliphilic Crenarchaeota with diverse ecology and physiology.
Further attempts to culture these species may permit greater understanding of the diversity of archaeal metabolism. The cultured neutraliphilic Crenarchaeota include the anaerobe genera Thermoproteus and Sulfophobococcus. Aeropyrum pernix, however, is an aerobe, and the genus Pyrobaculum contains facultative species. Therefore, there may be culturable analogues for the aerobic neutraliphiles in the Nevada springs.
It remains unknown how the marine pelagic crenarchaeota obtain their energy, but recent evidence, based on measurements of natural-level radiocarbon concentrations (35) and on 13C-labeled bicarbonate uptake experiments (52), suggests that group I marine archaea are chemoautotrophs. Cultured thermophilic relatives, including Sulfolobus metallicus and Acidianus infernus, use the 3-hydroxypropionate cycle for carbon fixation (30). The enzymes of the 3-hydroxypropionate pathway are specific for HCO3− as a substrate rather than CO2; this affinity for HCO3− apparently occurs despite an optimal growth pH of 2.0 among the Sulfolobales. It is unknown yet whether the 3-hydroxypropionate pathway operates among the crenarchaeota from Nevada hot springs.
Crenarchaeol has been proposed as a unique biomarker for the marine group I archaea. Although crenarchaeol has not been found in cultured species of crenarchaeota such as Sulfolobus solfataricus and Thermoplasma acidophilum (10, 12, 26), these species are acidophiles that grow optimally at pHs of less than 6. Our data show that crenarchaeol can be found in a wide variety of ecologically divergent Crenarchaeota. This unusual membrane lipid appears to have a wide environmental distribution, and the taxonomic diversity of crenarchaeol may therefore be equally diverse. Regardless, the current hypothesis that crenarchaeol, or specifically its unique cyclohexane ring (43), provided for the adaptation of thermophilic crenarchaeota to the cold marine environment should be reevaluated. The archaea in the springs thrive at high temperatures and would not appear to require a cold-adapted membrane lipid. In contrast, the similar chemistry of the springs and the ocean suggests that crenarchaeol could correlate with metabolic function. The increased porosity of a membrane containing a cyclohexane ring may facilitate transport of solutes or provide some other biochemical advantage associated with permeability.
The measured values of δ13C for the C40 isoprenoids are consistent with the biochemical similarity between marine and hot spring crenarchaeota. In Table 2, data for the total biomass and for C40 lipids are shown for the Nevada samples and for the Santa Monica Basin and Santa Barbara Basin (34). The carbon isotopic values for C40 isoprenoids are similar between the marine and terrestrial samples, although the δ13C values for total organic carbon are substantially different. This suggests that the metabolic pathway used by the Nevada groups is the same as that used by the marine group I archaea despite being found within a different ecological consortium. Carbonate speciation, both in the marine system and in these Nevada hot springs, strongly favors HCO3− and CO32−, while the concentration of dissolved CO2 is relatively low. This chemistry would favor import of HCO3− into autotrophic cells rather than passive uptake of CO2. The 3-hydroxypropionate pathway exists in archaea living at a wide range of pHs, from the Sulfolobales at pH 2 (30) to the marine group I archaea at pH 8 (35, 52). We suggest, therefore, that crenarchaeol may be a biomarker for the presence of the 3-hydroxypropionate pathway when crenarchaeota are found in mid- to high-pH environments. Crenarchaeol may participate in the control of membrane permeability.
TABLE 2.
Isotopic data for C40 isoprenoids from Nevada hot springs and Santa Monica and Santa Barbara basins
| Location | δ13C (‰)
|
||||
|---|---|---|---|---|---|
| TOCa | C40:0 | C40:1 | C40:2 | C40:3 | |
| Eagleville | −18.3 ± 0.2 | NAb | NA | −19.2 ± 0.2 | −20.6 ± 0.6 |
| Paradise Valley | −15.6 ± 1.1 | NA | NA | −23.3 ± 1.1 | NA |
| Crescent Valley | −10.1 ± 0.4 | −26.1 ± 0.9 | −22.8 ± 0.4 | −19.4 ± 0.1 | −22.5c |
| Santa Monica Basind | −22.8 ± 0.3 | −20.8 ± 0.4 | NA | −20.3 ± 0.4 | −20.5 ± 0.4 |
| Santa Barbara Basind | −22.0 ± 0.3 | NA | NA | −22.1 ± 0.4 | −22.5 ± 0.4 |
TOC, total organic carbon, average and standard deviation for three replicates (two for Crescent Valley).
NA, not analyzed.
No replicates.
From reference 34.
This interpretation also may explain why the TEX86 and ring index temperature correlations work well for marine samples (40, 41) but do not work for the Nevada samples. The chemistry of the marine environment displays little variation relative to the range of alkalinity and pH recorded for the Nevada hot springs. If the relative concentrations of GDGTs I, II, III, IV, V, and VI are controlled by variations in archaeal populations that are sensitive both to temperature and to the chemistry of the water in which they grow, then the relative effect of temperature may be expressed more strongly in the marine system. Further examination of the TEX86 proxy over a wider range of aqueous chemistries is warranted.
Conclusions.
Five samples representing four hot springs from Nevada each contained a complex assemblage of archaeal GDGT membrane lipids. Crenarchaeol was detected in every sample; previously this compound was only found in marine locations. The presence of crenarchaeol in Nevada hot springs having pHs between 6.0 and 9.2 and temperatures between 40 and 84°C suggests that the archaea that produce this compound occupy a vast physiological niche. The 16S ribosomal DNA sequences show the presence of organisms affiliated with the Desulfurococcales and Thermoproteales. This work expands the environmental and potentially the taxonomic realm in which crenarchaeol can be found. The neutral to alkaline pHs of these thermal springs are similar to aspects of oceanic chemistry, while the thermal environment does not resemble that of the ocean. Thus, it is possible that the GDGT lipid composition of archaeal communities responds in a complex manner both to temperature and to chemical environment. Much more remains to be learned about the physiology, ecology, and diversity of archaeal communities, both on land and in the ocean.
Acknowledgments
We thank J. Hutchings at the Eureka County Natural Resources of Nevada for help with sampling hot springs in Beoware and Crescent and the owners of the Surprise Valley for their hospitality. We thank J. Cantu for helping with lipid extraction from the Nevada samples and J. Sachs for use of the GC-MS. Comments from Christopher Bagwell and two anonymous reviewers improved an early version of the manuscript.
This work was supported by NSF grants EAR-0311937 and OCE-0241363 to A.P. and by NSF grant MCB-0348180 to C.L.Z. C.L.Z. and C.S.R. are also supported by the Environmental Remediation Sciences Division of the Office of Biological and Environmental Research, U.S. Department of Energy, financial assistance award DE-FC09-96SR18546 to the University of Georgia Research Foundation.
REFERENCES
- 1.Barns, S. M., R. E. Fundyga, M. W. Jeffries, and N. R. Pace. 1994. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc. Natl. Acad. Sci. USA 91:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barns, S. M., C. F. Delwiche, J. D. Palmer, and N. R. Pace. 1996. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc. Natl. Acad. Sci. USA 93:9188-9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bintrim, S. B., T. J. Donohue, J. Handelsman, G. P. Robert, and R. M. Goodman. 1997. Molecular phylogeny of archaea from soil. Proc. Natl. Acad. Sci. USA 94:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CalCOFI Database. 1996. CalCOFI cruise 9610, SIO reference series, University of California. Scripps Institution of Oceanography, San Diego, Calif.
- 5.Chelius, M. K., and E. W. Triplett. 2001. The diversity of Archaea and Bacteria in association with the roots of Zea mays L. Microbiol. Ecol. 41:252-263. [DOI] [PubMed] [Google Scholar]
- 6.Cole, D. R., and L. I. Ravinsky. 1984. Hydrothermal alteration zoning in the Beoware geothermal system, Eureka and Lander counties, Nevada. Econ. Geol. 79:759-767. [Google Scholar]
- 7.Craig, H., D. Spencer, A. E. Bainbridge, and W. Broecker. 1980. GEOSECS Pacific Expedition, vol. 3. Hydrographic data. National Science Foundation, Washington, D.C.
- 8.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLong, E. F., L. L. King, R. Massana, H. Cittone, A. Murray, C. Schleper, and S. G. Wakeham. 1998. Dibiphytanyl ether lipids in nonthermophilic crenarchaeotes. Appl. Environ. Microbiol. 64:1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRosa, M., S. DeRosa, and A. Gambacorta. 1977. 13C-NMR assignments and biosynthetic data for the ether lipids of Caldariella. Phytochemistry 16:1909-1912. [Google Scholar]
- 11.DeRosa, M., A. Gambacorta, and A. Gliozzi. 1986. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 50:70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeRosa, M., and A. Gambacorta. 1988. The lipids of Archaebacteria. Prog. Lipid Res. 27:153-175. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrman, J. A., and A. A. Davis. 1997. Widespread Archaea and novel Bacteria from the deep sea as shown by 16S rRNA gene sequences. Mar. Ecol. Prog. Ser. 150:275-285. [Google Scholar]
- 14.Garside L. J., and J. H. Schilling. 1979. Thermal waters of Nevada, p. 1-163. Mackay School of Mines, University of Nevada, Las Vegas.
- 15.Giovannoni, S. J., E. F. DeLong, G. J. Olsen, and N. R. Pace. 1988. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 170:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gliozzi, A., G. Paoli, M. DeRosa, and A. Gambacorta. 1983. Effect of isoprenoid cyclization on the transition temperature of lipids in thermophilic archaebacteria. Biochim. Biophys. Acta 735: 234-242. [Google Scholar]
- 17.Goff, F., H. A. Wollenberg, D. C. Brookings, and R. W. Kistler. 1991. A Sr-isotopic comparison between thermal waters, rocks, and hydrothermal calcites, Long Valley caldera, California. J. Volcanol. Geotherm. Res. 48:265-281. [Google Scholar]
- 18.Haruta, S., M. Kondo, K. Nakamura, H. Aiba, S. Ueno, M. Ishii, and Y. Igarashi. 2002. Microbial community changes during organic solid waste treatment analyzed by double gradient-denaturing gradient gel electrophoresis and fluorescence in situ hybridization. Appl. Microbiol. Biotechnol. 60:224-231. [DOI] [PubMed] [Google Scholar]
- 19.Hermance, J. F. 1983. The Long Valley/Mono Basin volcanic complex in eastern California: status of present knowledge and future research needs. J. Geophys. Res. 21:1545-1565. [Google Scholar]
- 20.Hoefs, M. J. L., S. Schouten, J. W. deLeeuw, L. L. King, S. G. Wakeham, and J. S. Sinninghe Damsté. 1997. Ether lipids of planktonic Archaea in the marine water column. Appl. Environ. Microbiol. 63:3090-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopmans, E. C., S. Schouten, R. D. Pancost, M. T. J. van der Meer, and J. S. Sinninghe Damsté. 2000. Analysis of intact tetraether lipids in archaeal cell material and sediments by high performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 14:585-589. [DOI] [PubMed] [Google Scholar]
- 22.Jurgens, G., F.-O. Glockner, R. Amann, A. Saano, L. Montonen, M. Likolammi, and U. Münster. 2000. Identification of novel archaea in bacterioplankton of a boreal forest lake by phylogenetic analysis and fluorescent in situ hybridization. FEMS Microbiol. Ecol. 34:45-56. [DOI] [PubMed] [Google Scholar]
- 23.Jurgens, G., K. Lindstrom, and A. Saano. 1997. Novel group within the kingdom Crenarchaeota from boreal forest soil. Appl. Environ. Microbiol. 63:803-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 25.Kevbrin V. V., C. S. Romanek, and J. Wiegel. Alkalithermophiles: a double challenge from extreme environments. In J. Seckbach (ed.), Cellular origins: life in extreme habitats and astrobiology, vol. 6A, in press. Kluwer Academic Publishers. Dordrecht, The Netherlands.
- 26.Langworthy, T. A. 1977. Long-chain diglycerol tetraether from Thermoplasma acidophilum. Biochim. Biophys. Acta 487:37-50. [DOI] [PubMed] [Google Scholar]
- 27.Lanoil, B. D., R. Sassen, M. T. La Duc, S. T. Sweet, and K. H. Nealson. 2001. Bacteria and archaea physically associated with Gulf of Mexico gas hydrates. Appl. Environ. Microbiol. 67:5143-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacGregor, B. J., D. P. Moser, E. Wheeler Alm, K. H. Nealson, and D. A. Stahl. 1997. Crenarchaeota in Lake Michigan sediment. Appl. Environ. Microbiol. 63:1178-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massana, R., A. E. Murray, C. M. Preston, and E. F. DeLong. 1997. Vertical distribution and phylogenetic characterization of marine planktonic archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 63:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menendez, C., Z. Bauer, H. Huber, N. Gad'on, K.-O. Stetter, and G. Fuchs. 1999. Presence of acetyl coenzyme A (CoA) carboxylase and propionyl-CoA carboxylase in autotrophic Crenarchaeota and indication for operation of a 3-hydroxypropionate cycle in autotrophic carbon fixation. J. Bacteriol. 181:1088-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt, D. A., K. H. Freeman, M. P. Ricci, S. A. Studley, and J. M. Hayes. 1995. Performance and optimization of a combustion interface for isotope ratio monitoring gas chromatography/mass spectrometry. Anal. Chem. 67:2461-2473. [DOI] [PubMed] [Google Scholar]
- 32.Murray, A. E., C. M. Preston, R. Massana, L. T. Taylor, A. Blakis, K. Wu, and E. F. DeLong. 1998. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters near Anvers Island, Antarctica. Appl. Environ. Microbiol. 64:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muyzer, G., E. C. de Waal, and A. G. Utterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson, A. 2000. Ph.D. thesis. Massachusetts Institute of Technology/Woods Hole Oceanographic Institution, Cambridge, Mass.
- 35.Pearson, A., A. P. McNichol, B.-C. Benitez-Nelson, J. M. Hayes, and T. I. Eglington. 2001. Origins of lipid biomarkers in Santa Monica Basin surface sediment: a case study using compound-specific 14C analysis. Geochem. Cosmochim. Acta 65:3123-3137. [Google Scholar]
- 36.Pesaro, M., and F. Widmer. 2002. Identification of novel Crenarchaeota and Euryarchaeota clusters associated with different depth layers of a forest soil. FEMS Microbiol. Ecol. 42:89-98. [DOI] [PubMed] [Google Scholar]
- 37.Roy, R. N., L. N. Roy, K. M. Vogel, C. Porter-Moore, T. Pearson, C. E. Good, F. J. Millero, and D. M. Campbell. 1993. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45°C. Mar. Chem. 44:249-267. [Google Scholar]
- 38.Schouten, S., M. J. L. Hoefs, M. P. Koopmans, H. J. Bosch, and J. S. Sinninghe Damsté. 1998. Structural characterization, occurrence and fate of archaeal etherbound acyclic and cyclic biphytanes and corresponding diols in sediments. Org. Geochem. 29:1305-1319. [Google Scholar]
- 39.Schouten, S., E. C. Hopmans, R. D. Pancost, and J. S. Sinninghe Damsté. 2000. Widespread occurrence of structurally diverse tetraether membrane lipids: evidence for the ubiquitous presence of low-temperature relatives of hyperthermophiles. Proc. Natl. Acad. Sci. USA 26:14421-14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schouten, S., E. C. Hopmans, E. Schefuss, and J. S. Sinninghe Damsté. 2002. Distributional variations in marine crenarchaeotal membrane lipids: a new tool for reconstructing ancient sea water temperatures? Earth Planet. Sci. Lett. 204:265-274. [Google Scholar]
- 41.Schouten, S., E. C. Hopmans, A. Forster, Y. van Breugel, M. M. M. Kuypers, and J. S. Sinninghe Damsté. 2003. Extremely high sea-surface temperatures at low latitudes during the middle Cretaceous as revealed by archaeal membrane lipids. Geology 31:1069-1072. [Google Scholar]
- 42.Schouten, S., S. G. Wakeham, E. C. Hopmans, and J. S. Sinninghe Damsté. 2003. Biogeochemical evidence that thermophilic archaea mediate the anaerobic oxidation of methane. Appl. Environ. Microbiol. 69:1680-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinninghe Damsté, J. S., S. Schouten, E. C. Hopmans, A. C. T. van Duin, and J. A. J. Geenevasen. 2002. Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota. J. Lipid Res. 43:1641-1651. [DOI] [PubMed] [Google Scholar]
- 44.Stein, L. Y., G. Jones, B. Alexander, K. Elmund, C. Wright-Jones, and K. H. Nealson. 2002. Intriguing microbial diversity associated with metal-rich particles from a freshwater reservoir. FEMS Microbiol. Ecol. 42:431-440. [DOI] [PubMed] [Google Scholar]
- 45.Stein, L. Y., M. T. La Duc, T. J. Grundl, and K. H. Nealson. 2001. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 3:10-18. [DOI] [PubMed] [Google Scholar]
- 46.Stetter, K. O. 1989. Extremely thermophilic chemolithoautotrophic archaebacteria, p. 167-176. In H. G. Schlegel and B. Bowien (ed.), Autotrophic bacteria. Science Tech Publishers, Madison, Wis.
- 47.Stetter, K. O. 1995.Microbial life in hyperthermal environments. ASM News 61:285-290. [Google Scholar]
- 48.Stetter, K. O. 1996. Hyperthermophilic procaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 49.Takai, K., D. P. Moser, M. DeFlaun, T. C. Onstott, and J. K. Fredrickson. 2001. Archaeal diversity in waters from deep South African gold mines. Appl. Environ. Microbiol. 67:5750-5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uda, I., A. Sugai, Y. H. Itoh, and T. Itoh. 2001. Variation in molecular species of polar lipids from Thermoplasma acidophilum depends on growth temperature. Lipids 36:103-105. [DOI] [PubMed] [Google Scholar]
- 51.White, D. C., W. M. Davis, and J. S. Nickels. 1979. Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51-62. [DOI] [PubMed] [Google Scholar]
- 52.Wuchter, C., S. Schouten, H. T. S. Boschker, and J. S. Sinninghe Damsté. 2003. Bicarbonate uptake by marine Crenarchaeota. FEMS Microbiol. Lett. 219:203-207. [DOI] [PubMed] [Google Scholar]
- 53.Zeebe, R. E., and D. Wolf-Gladrow. 2001. CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier Science B.V., Amsterdam, The Netherlands.