Abstract
The acute acetoclastic methanogenic inhibition of several inorganic and organic arsenicals was assayed. Trivalent species, i.e., methylarsonous acid and arsenite, were highly inhibitory, with 50% inhibitory concentrations of 9.1 and 15.0 μM, respectively, whereas pentavalent species were generally nontoxic. The nitrophenylarsonate derivate, roxarsone, displayed moderate toxicity.
Arsenic is a high-priority pollutant that is widely distributed in the environment. Arsenic is a naturally occurring element in rocks and can be released into soils and aquatic environments upon weathering or natural dissolution. The predominant form of inorganic arsenic in oxic, aqueous environments is arsenate [As(V)]. In reducing environments, arsenic occurs primarily as arsenite [As(III)]. Methylated organoarsenicals, chiefly monomethylarsonic acid [MMA(V)] and dimethylarsinic acid [DMA(V)], are introduced into the environment as defoliants and herbicides in agriculture (2) or as biotransformation products of inorganic arsenicals (3, 13). MMA(V) is among the top pesticides utilized in the United States, with approximately 2.6 million kg applied annually to 3.8 million acres (2). Roxarsone (4-hydroxy-3-nitrophenylarsonic acid) and p-arsanilic acid (4-aminophenylarsonic acid) are used as growth-promoting and antibiotic agents in poultry. Roxarsone is recovered at a high rate from poultry litter that is subjected to composting and land application. The annual emission of roxarsone from poultry operations in the United States has been estimated at 900,000 kg per year (11).
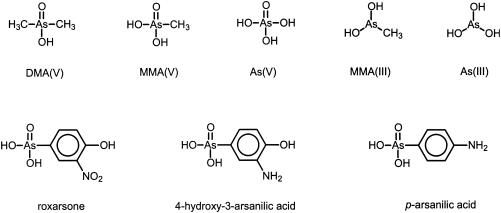
Microorganisms catalyze important transformations of the arsenic cycle, including oxidation, reduction, methylation, and demethylation reactions (3, 20). Microbial conversion of arsenic compounds and other environmental contaminants can be affected by the inhibitory effects displayed by arsenical compounds. In spite of the environmental significance of arsenical compounds, few studies have addressed microbial inhibition by arsenicals in anaerobic environments. The aim of the present study was to assess the inhibitory effects of important inorganic and organic arsenical compounds (Fig. 1) on acetoclastic methanogens in a mixed anaerobic consortium. Methanogenic-toxicity data are important, since methanogenesis is the final step in the degradation of organic matter in many anaerobic environments, including sediments, wastewater treatment systems, and gastrointestinal tracts.
FIG. 1.
Chemical structures of the arsenical compounds investigated in this study.
Two methanogenic consortia used in our study were granular anaerobic sludges obtained from upflow industrial anaerobic treatment plants treating recycled paper wastewater (Industriewater Eerbeek, Eerbeek, The Netherlands) and distillery wastewater (Royal Nedalco BV, Bergen op Zoom, The Netherlands). A third methanogenic consortium was anaerobically digested sewage sludge (ADSS) obtained from the Ina Road wastewater treatment plant (Tucson, Ariz.). The organic contents of the inocula (expressed as percentages of volatile suspended solids [VSS]) were 12.9% for Eerbeek sludge, 10.0% for Nedalco sludge, and 1.1% for ADSS. The maximum specific acetoclastic methanogenic activities of Eerbeek sludge, Nedalco sludge, and ADSS were 240, 928 and 28 mg of CH4 chemical oxygen demand g of VSS−1 day−1, respectively. The inhibitory effect of arsenic compounds on acetoclastic methanogens was evaluated at 30 ± 2°C in shaken-batch bioassay mixtures supplied with 33 mM acetate as outlined previously (7). Lactate (10 mM) was used as an electron-donating substrate in one assay. When hydrogen was the substrate, the headspace of the vials was supplied with H2-CO2 (80:20, vol/vol). On the day following electron donor addition, the desired amount of toxicant was added to triplicate flasks by using neutralized concentrated stock solutions. Stock solutions of 4-hydroxy-3-arsanilic acid, an aminophenol which is prone to autoxidation, were prepared fresh with 200 mg of ascorbic acid liter−1 added to prevent oxidative coupling. Triplicate substrate controls were based on assay mixtures where no toxicant was added. After toxicant additions, the assay mixtures were incubated for 1 h prior to the determination of the methane production-versus-time curve. The methane content in the headspace of each assay bottle was determined hourly during the subsequent 8- to 24-h incubation period, unless otherwise indicated below. The specific methanogenic activities were calculated from the linear increases in the amounts of methane in the vials and the biomass concentrations at the end of the assay as the means of the values for triplicate culture flasks. The IC20, IC50, and IC80 are the inhibitory concentrations of a given compound causing, respectively, 20, 50, and 80% decreases in methanogenic activity compared to the activity in an uninhibited control. Methane and acetic acid contents were determined by gas chromatography as described previously (21). Arsenic speciation in liquid samples was analyzed by ion chromatography-inductively coupled plasma-mass spectrometry with a method adapted from the work of Gong et al. (12). The evolution of arsines as a result of microbial activity was determined by flushing the headspace of incubated culture flasks with a gentle stream of N2-CO2 gas for 22 to 24 h and subsequently trapping the arsenic species in a 2 M HNO3 solution. The samples were analyzed by ion chromatography-inductively coupled plasma-mass spectrometry for total arsenic. Other parameters (e.g., pH and levels of VSS) were determined according to Standard Methods for the Examination of Water and Wastewater (5). Sodium arsenate (Chemical Abstracts Registry number [CAS] 15584-04-0), sodium arsenite (CAS 15502-74-6), MMA(V) (CAS 124-58-3), roxarsone (CAS 121-19-7), and p-arsanilic acid (CAS 98-50-0) were obtained from Sigma-Aldrich Corp. (St. Louis, Mo.). DMA(V) (CAS 75-60-5) was purchased from Chem Service, Inc. (West Chester, Pa.), and 4-hydroxy-3-arsanilic acid (3-amino-4-hydroxyphenylarsonic acid; CAS 2163-77-1) was purchased from Platz and Bauer, Inc. (Waterbury, Conn.). Monomethylarsonous acid [MMA(III); CAS 25400-23-1] was kindly provided by H. V. Aposhian, Department of Molecular and Cellular Biology, University of Arizona.
Table 1 lists the IC20, IC50, and IC80 determined for the various inorganic and organic arsenicals tested in this study in short-term acetoclastic methanogenic bioassays with Eerbeek sludge. MMA(III) and As(III) displayed the highest inhibitory effect towards acetate-utilizing methanogens, with IC50 of only 9.1 and 15.5 μM, respectively. The inhibitory effect of As(III) was also evaluated in anaerobic assay mixtures supplied with H2 or lactate as the electron-donating substrate (Table 1). The highest toxicity was determined in the assays with lactate, indicating that syntrophic acetogens are particularly sensitive to As(III) inhibition. Hydrogen-utilizing methanogens, on the other hand, appear to be less inhibited by As(III) than acetoclastic methanogens. In contrast to the trivalent arsenicals, the pentavalent species, As(V), MMA(V), and DMA(V), did not cause noteworthy methanogenic inhibition at the highest concentrations tested. The anaerobic sludge consortium used in this study has been shown to result in the dissimilatory reduction of As(V) to the more toxic As(III) (9). Nonetheless, As(III) was not detected in the medium at the completion of the As(V) toxicity assay with Eerbeek sludge, due to the short duration of the assay incubations (8 to 24 h).
TABLE 1.
IC20, IC50, and IC80 of arsenical compounds against the acetoclastic methanogenic activity of Eerbeek sludge with acetate as the substrate
| Compound (alternate substrate) | Mol wt | IC20 (μM) | IC50 (μM) | IC80 (μM) |
|---|---|---|---|---|
| Inorganic species | ||||
| As(III) | 122.9 | 9.1 | 15.5 | 23.5 |
| As(III) (H2a) | 122.9 | 10.2 | 27.1 | 79.2 |
| Arsenite (lactatea) | 122.9 | 3.4 | 4.4 | 6.1 |
| As(V) | 138.9 | >500 | >500 | >500 |
| As(V) (H2a) | 138.9 | >500 | >500 | >500 |
| Methylated organoarsenic compounds | ||||
| MMA(V) | 140.0 | >5,000 | >5,000 | >5,000 |
| DMA(V) | 138.0 | >5,000 | >5,000 | >5,000 |
| MMA(III) | 123.9 | 2.2 | 9.1 | 17.9 |
| Phenylarsonic compounds | ||||
| p-Arsanilic acid | 217.1 | >2,300 | >2,300 | >2,300 |
| Roxarsone | 263.0 | 251 | 425 | 780 |
| 4-Hydroxy-3-arsanilic acid | 233.1 | >600 | >600 | >600 |
H2 or lactate (as indicated) instead of acetate was the substrate supplied in this assay.
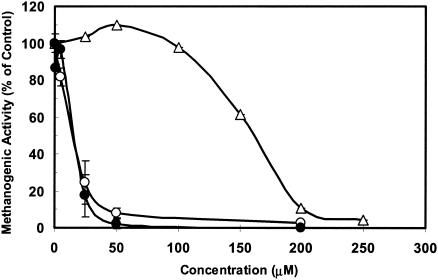
The short-term toxicity response determined for As(III) and As(V) in assays with Eerbeek granular sludge were compared with those from tests utilizing two other microbial consortia, i.e., Nedalco sludge and ADSS. Figure 2 shows that exposure to As(III) led to comparable levels of acetoclastic methanogenic inhibition in the assays of the other anaerobic granular sludge (Nedalco). Acetoclastic methanogens in the ADSS were also highly inhibited by As(III); however, the sharp response started at a threshold of about 100 μM. The IC50 was 10-fold higher than those of the granular sludge inocula. The threshold may have been due to the sequestration of As(III) by the high surface area of fine ADSS solids. Likewise, As(V) was tested in ADSS, and concentrations up to 500 μM were found to be nontoxic, as was observed for the Eerbeek sludge. The results therefore indicate a relatively high toxicity for As(III) and a low toxicity for As(V) for methanogens from diverse environments.
FIG. 2.
Inhibitory effects of As(III) on the methanogenic activity of an anaerobic consortium in bioassays inoculated with various anaerobic consortia: Eerbeek sludge (•), Nedalco sludge (○), and ADSS (▵). The maximum concentration of 4-hydroxy-3-arsanilic acid tested was limited by the aqueous solubility of the compound. Data are means of results from three replicated culture flasks. Error bars indicate standard errors of the means.
Additional experiments were conducted to test the toxicity of As(III) and As(V) in long-term assays. As(III) supplied to acetate-fed Eerbeek sludge assays at 15.5 and 23.5 μM initially resulted in 61.7 and 82.6% inhibition, respectively, in the first 10 h of exposure. However, the inhibition decreased to 29.9 and 52.6% inhibition, respectively, after 3 days, and to 17.7 and 36.9% inhibition, respectively, after 7 days. The decrease in inhibition was clearly due to the sequestration of As(III), which decreased in concentration by 88.4% ± 2.0% and 84.2% ± 3.8%, after 10 days, respectively, in the assays initially receiving 15.5 and 23.5 μM As(III). The formation of As2S3 precipitates (15, 19, 22) is plausible since the background sulfide in Eerbeek sludge was 0.053 mM. This sulfide concentration together with the neutral pH value in the toxicity assays is well within the range considered suitable for precipitation of amorphous As2S3 (15). Volatile arsenicals were also measured and found to account for only 0.04 to 0.40% of added As(III). These low yields are consistent with arsine production data from methanogenic cultures (17, 18).
Arsenate (500 μM) was initially nontoxic to acetoclastic methanogens in ADSS; however, prolonged exposure of 4 days or more resulted in complete methanogenic inhibition sustained for the remainder of a 10-day experiment. The sudden change in toxicity can be attributed to the facile reduction of As(V) to As(III) in anaerobic sludge (9). The experiment also demonstrates that no immediate physiological adaptation to As(III) occurs in methanogens when they receive sustained exposure to As(III) via As(V) reduction.
Few studies have evaluated methanogenic inhibition by arsenicals. As(III) was found to be completely inhibitory to methanogenesis in marsh sediments when it was tested at a single concentration of 13.3 mmol kg−1 (dry weight) (4). As(III) was also shown to inhibit fermentative activities of rumen microorganisms (10). The general trends observed in two studies with aerobic mixed cultures (activated sludge and microflora from agriculture drainage) indicated a considerably higher toxicity for As(III) than for As(V), MMA(V), and DMA(V) (14, 23), as was observed here for the methanogenic consortium.
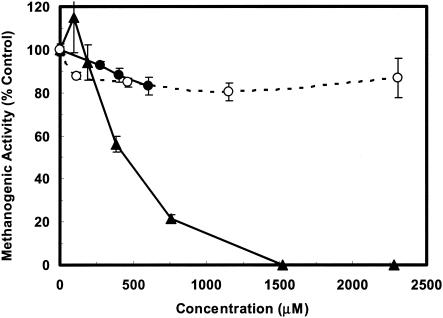
The microbial toxicity of As(III) and As(V) has been attributed to different mechanisms. As(III) binds to protein sulfydryl groups (13) and has been reported to inhibit over 200 different enzymes (1). The sensitivity of methanogens to As(III) might be related to the reaction of coenzyme M, which is a thiol-containing compound central to the biochemistry of methanogenesis (6, 8). As(V) is a phosphate analog that can cause uncoupling of substrate-linked and respiratory chain phosphoprylation (13, 24). Figure 3 shows the acetoclastic methanogenic inhibition displayed by various phenylarsonic compounds as a function of their concentration in the culture medium. No major inhibition was observed for the aminophenyl arsenic compounds 4-hydroxy-3-arsanilic acid and p-arsanilic acid at concentrations as high as 600 and 2,300 μM, respectively. On the other hand, roxarsone caused 50% inhibition at a concentration of 425 μM. No previous studies evaluating the anaerobic microbial toxicity of phenylarsonic compounds have been conducted. However, the IC50 of roxarsone was determined by a Microtox assay to be 3,160 μM for the aerobic luminescent marine bacterium Photobacterium phosphoreum (16). Roxarsone is much less toxic to methanogens than other structurally related nitroaromatic compounds (7). The lower toxicity is probably related to the arsonate substituent, which is dissociated at the culture pH. This observation is in keeping with the fact that nitroaromatics bearing dissociated carboxylic groups are less inhibitory than homologous compounds lacking ionized groups (7). In anaerobic environments, As(V) is readily reduced to As(III) (9, 20). Based on results presented here, it can be concluded that accumulation of the highly toxic trivalent arsenic species, resulting from the reductive biotransformation of pentavalent arsenicals, could potentially have a serious impact on methanogenesis.
FIG. 3.
Inhibitory effects of several orgarsenic compounds, p-arsanilic acid (○), roxarsone (▴), and 4-hydroxy-3-arsanilic acid (•), on the acetoclastic methanogenic activity of an anaerobic microbial consortium. Data are means of results from three replicates. Error bars indicate standard errors of the means.
Acknowledgments
This research was funded in part by a grant from the National Science Foundation (NSF-0137368 to R.S.-A.), by a USGS-National Institute for Water Resources 104B grant, and by a seed grant from the NIEHS-supported Superfund Basic Research Program (NIH ES-04940).
Arsenic speciation analyses were performed by the Analytical Section of the Hazard Identification Core (Superfund Basic Research Program grant NIH ES-04940).
REFERENCES
- 1.Abernathy, C. O., D. L. Y. P. Liu, H. V. Aposhian, B. Beck, B. Fowler, R. Goyer, B. Menzer, T. Rossman, C. Thompson, and M. Waalkes. 1999. Arsenic: health effects, mechanisms of actions, and research issues. Environ. Health Perspect. 107:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bednar, A. J., J. R. Garbarino, J. F. Ranville, and T. R. Wildeman. 2002. Presence of organoarsenicals used in cotton production in agricultural water and soil of the southern United States. J. Agric. Food Chem. 50:7340-7344. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, R., and T. G. Chasteen. 2002. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev. 66:250-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capone, D. G., D. D. Reese, and R. P. Kiene. 1983. Effects of metals on methanogenesis, sulfate reduction, carbon dioxide evolution, and microbial biomass in anoxic salt marsh sediments. Appl. Environ. Microbiol. 45:1586-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, D.C.
- 6.Deppenmeier, U. 2002. The unique biochemistry of methanogenesis. Prog. Nucleic Acid Res. Mol. Biol. 71:223-283. [DOI] [PubMed] [Google Scholar]
- 7.Donlon, B. A., E. Razo-Flores, J. A. Field, and G. Lettinga. 1995. Toxicity of N-substituted aromatics to acetoclastic methanogenic activity in granular sludge. Appl. Environ. Microbiol. 61:3889-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahey, R. C. 2001. Novel thiols of prokaryotes. Annu. Rev. Microbiol. 55:333-356. [DOI] [PubMed] [Google Scholar]
- 9.Field, J. A., R. Sierra-Alvarez, I. Cortinas, G. Feijoo, M. T. Moreira, M. Kopplin, and A. J. Gandolfi. 2004. Facile reduction of arsenate in methanogenic sludge. Biodegradation 15:185-196. [DOI] [PubMed] [Google Scholar]
- 10.Foresberg, W. 1978. Some effects of arsenic on rumen microflora—an in vitro study. Can. J. Microbiol. 24:36-44. [DOI] [PubMed] [Google Scholar]
- 11.Garbarino, J. R., A. J. Bednar, D. W. Rutherford, R. S. Beyer, and R. L. Wershaw. 2003. Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environ. Sci. Technol. 37:1509-1514. [DOI] [PubMed] [Google Scholar]
- 12.Gong, Z. L., X. F. Lu, W. R. Cullen, and X. C. Le. 2001. Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J. Anal. At. Spectrom. 16:1409-1413. [Google Scholar]
- 13.Hughes, M. F. 2002. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 133:1-16. [DOI] [PubMed] [Google Scholar]
- 14.Huysmans, K. D., and W. Frankenberger. 1990. Arsenic resistant microorganisms isolated from agricultural drainage water and evaporation pond sediments. Water Air Soil Pollut. 53:159-168. [Google Scholar]
- 15.Inskeep, W. P., T. R. McDermott, and S. Fendorf. 2002. Arsenic(V)/(III) cycling in soils and natural waters: chemical and microbiological processes, p. 183-215. In W. T. Frankenberger (ed.), Environmental chemistry of arsenic. Marcel Dekker, Inc., New York, N.Y.
- 16.Kaiser, K. L. E., M. B. McKinnon, and F. L. Fort. 1994. Interspecies toxicity correlations of rat, mouse and Photobacterium phosphoreum. Environ. Toxicol. Chem. 13:1559-1606. [Google Scholar]
- 17.McBride, B. C., and R. S. Wolfe. 1971. Biosynthesis of dimethylarsine by a methanobacterium. Biochemistry 10:4312-4317. [DOI] [PubMed] [Google Scholar]
- 18.Michalke, K., E. B. Wickenheiser, M. Mehring, A. V. Hirner, and R. Hensel. 2000. Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 66:2791-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman, D. K., T. J. Beveridge, and F. M. M. Morel. 1997. Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl. Environ. Microbiol. 63:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oremland, R. S., and J. F. Stolz. 2003. The ecology of arsenic. Science 300:939-944. [DOI] [PubMed] [Google Scholar]
- 21.Paulo, P. L., A. J. M. Stams, J. A. Field, C. Dijkema, J. B. van Lier, and G. Lettinga. 2003. Pathways of methanol conversion in a thermophilic anaerobic (55°C) sludge consortium. Appl. Microbiol. Biotechnol. 63:307-314. [DOI] [PubMed] [Google Scholar]
- 22.Rochette, E. A., B. C. Bostick, G. C. Li, and S. Fendorf. 2000. Kinetics of arsenate reduction by dissolved sulfide. Environ. Sci. Technol. 34:4714-4720. [Google Scholar]
- 23.Stasinakis, A. S., N. S. Thomaidis, A. S. Giannes, and T. D. Lekkas. 2003. Effect of arsenic and mercury speciation on inhibition of respiration rate in activated sludge systems. Environ. Sci. Pollut. Res. Int. 10:177-182. [DOI] [PubMed] [Google Scholar]
- 24.ter Welle, H. F., and E. C. Slater. 1967. Uncoupling of respiratory-chain phosphorylation by arsenate. Biochim. Biophys. Acta. 143:1-17. [DOI] [PubMed] [Google Scholar]