Abstract
Wolbachia strains are endosymbiotic bacteria typically found in the reproductive tracts of arthropods. These bacteria manipulate host reproduction to ensure maternal transmission. They are usually transmitted vertically, so it has been predicted that they have evolved a mechanism to target the host's germ cells during development. Through cytological analysis we found that Wolbachia strains display various affinities for the germ line of Drosophila. Different Wolbachia strains show posterior, anterior, or cortical localization in Drosophila embryos, and this localization is congruent with the classification of the organisms based on the wsp (Wolbachia surface protein) gene sequence. This embryonic distribution pattern is established during early oogenesis and does not change until late stages of embryogenesis. The posterior and anterior localization of Wolbachia resembles that of oskar and bicoid mRNAs, respectively, which define the anterior-posterior axis in the Drosophila oocyte. By comparing the properties of a single Wolbachia strain in different host backgrounds and the properties of different Wolbachia strains in the same host background, we concluded that bacterial factors determine distribution, while bacterial density seems to be limited by the host. Possible implications concerning cytoplasmic incompatibility and evolution of strains are discussed.
Wolbachia strains are obligate intracellular bacteria found in arthropods and nematodes, and they use several strategies to manipulate the reproduction of their arthropod hosts, thus ensuring maternal transmission (39). The most widespread Wolbachia-induced phenotype in Drosophila is cytoplasmic incompatibility (CI), a form of embryonic lethality in crosses between infected males and uninfected females. Crosses of infected males with infected females are not affected, which has led to a model proposing that sperm of infected males carries an imprint which is erased in infected oocytes (44).
The induction of various reproductive alterations and the maternal transmission have led to the suggestion that this microbe evolved mechanisms that specifically target the host's germ cells during development. Concentration of Wolbachia in the germ plasm of embryos has been reported previously for the Drosophila melanogaster Canton-S strain (16), the wasp Nasonia (4), some Trichogramma species (32, 40), and Aphytis (47). On the other hand, Wolbachia cells were found to be equally distributed in the cortex of Drosophila simulans Riverside embryos (3, 31). Interestingly, the presence of bacteria in the anterior part of the embryo, next to the micropyle, was observed for the first time in the mosquito Aedes polynesiensis (45).
Recent work has shown that there is extreme variation in the bacterial load and distribution in Drosophila testes in different host-symbiont combinations (10, 12, 43), as well as in different stages of development in an individual male (11). This variation correlates with different CI levels and is due to both bacterial and host factors. A comparison of Wolbachia growth during spermatogenesis in D. simulans, which can have nearly complete CI, to the bacterial growth observed in D. melanogaster, which rarely expresses high levels of CI, revealed a crucial difference. Within infected D. simulans testes, abundant Wolbachia cells were seen in cysts at different stages of development at or before the premeiotic growth phase through spermatid elongation. In D. melanogaster, high levels of Wolbachia were observed only in elongated spermatids (11). These differences in Wolbachia growth and proliferation in different host-symbiont combinations during spermatogenesis could result from differences in Wolbachia distribution earlier in development (e.g., pole cell formation), from active host suppression of bacterial entrance into the testes, from differences in bacterial replication in larval testes, or from a combination of these factors.
Unlike Wolbachia's behavior during spermatogenesis, the behavior of this organism during oogenesis has been poorly described, although this is the site of the rescue activity for the imprint of infected sperm (5). Moreover, bacterial incorporation into the oocytes forms the basis for efficient maternal transmission. Following this line of reasoning, we monitored Wolbachia from early oogenesis to late embryogenesis in Drosophila. Specifically, below we describe the density and distribution of a variety of bacterial strains infecting six Drosophila species, and our results revealed several important aspects of Wolbachia-host interactions.
MATERIALS AND METHODS
Drosophila lines.
The Drosophila lines and Wolbachia strains used in the present study are listed in Table 1. Flies were routinely grown at 25°C on standard medium in uncrowded conditions.
TABLE 1.
Density of Wolbachia in Drosophila embryos
| Host
|
Wolbachia straina | No. of Wolbachia cells (104) in embryosb
|
% of infected sperm cystsc | CI leveld (%) | Reference(s) | |||
|---|---|---|---|---|---|---|---|---|
| Species | Strain | Whole embryos | Posterior | Anterior | ||||
| D. melanogaster | yw67C23 | wMel | 1.65 ± 0.45 | 0.13 ± 0.07 | 0.05 ± 0.02 | 11.5 ± 10.4 | 25.1 | 1 |
| D. melanogaster | Canton-S | wMelCS | 2.35 ± 1.25 | 0.24 ± 0.09 | 0.09 ± 0.06 | 10.0 ± 9.2 | 0 | 20, 34 |
| D. melanogaster | popcorn | wMelPop | 3.75 ± 2.06 | 0.44 ± 0.25 | 0.18 ± 0.16 | 4.0 ± 6.0 | 0 | 29 |
| D. simulans | NhaTCe | wMel | 4.84 ± 2.38 | 0.84 ± 0.38 | 0.20 ± 0.26 | 72.9 ± 10.3 | 97.3 | 33 |
| D. simulans | Coffs Harbor | wCof | 5.84 ± 3.12 | 0.47 ± 0.25 | 0.36 ± 0.30 | 78.3 ± 16.2 | 0 | 19 |
| D. yakuba | SA3 (Africa) | wSty | 1.16 ± 1.20 | 0.40 ± 0.37 | 0.09 ± 0.11 | 4.2 ± 6.2 | 0 | 24 |
| D. teissieri | Bloomington #1015 | wSty | 1.21 ± 0.61 | 0.57 ± 0.39 | 0.08 ± 0.08 | 8.3 ± 9.3 | 0 | 24 |
| D. santomea | STO9 (Africa) | wSty | 1.19 ± 0.57 | 0.40 ± 0.17 | 0.09 ± 0.06 | 9.5 ± 8.3 | 0 | 24 |
| D. simulans | Riverside | wRi | 9.74 ± 4.34 | 0.82 ± 0.38 | 0.69 ± 0.41 | 85.0 ± 18.3 | 97.6 | 18 |
| D. yakuba | SA3Te | wRi | 10.36 ± 3.34 | 0.73 ± 0.36 | 0.64 ± 0.25 | 60.4 ± 28.9 | 92.4 | 46 |
| D. teissieri | Bloomington #1015Te | wRi | 11.06 ± 4.01 | 0.80 ± 0.40 | 0.75 ± 0.42 | 41.5 ± 32.7 | 86.0 | 46 |
| D. santomea | STO9Te | wRi | 10.60 ± 6.45 | 0.90 ± 0.51 | 0.85 ± 0.69 | 70.5 ± 16.7 | 94.3 | 46 |
| D. simulans | Noumea | wNo | 4.13 ± 2.20 | 0.31 ± 0.25 | 0.92 ± 0.57 | 27.9 ± 14.3 | 48.7 | 27 |
| D. simulans | Watsonvillee | wMa | 6.12 ± 2.32 | 0.42 ± 0.27 | 1.03 ± 0.62 | 23.2 ± 15.5 | 0 | 15 |
| D. mauritiana | Bloomington #31 | wMa | 5.15 ± 3.69 | 0.32 ± 0.24 | 1.14 ± 0.84 | 76.0 ± 22.1 | 0 | 43 |
| D. simulans | Kilimanjaro | wKi | 3.03 ± 1.13 | 0.38 ± 0.30 | 0.69 ± 0.41 | 19.8 ± 17.3 | 0 | 28 |
Based on wsp gene sequences.
Bacterial density in 15 early embryos of each strain (mean ± standard deviation).
Percentage of infected sperm cysts (mean ± standard deviation), adapted from the study of Veneti et al. (43).
Average CI levels expressed as percentage of embryo mortality, adapted from the study of Veneti et al. (43).
Transinfected strain.
Cytological study. (i) Embryos.
Embryos were collected from apple juice plates and dechorionated in 50% commercial bleach for 5 min. After a quick rinse with washing buffer (0.7% NaCl, 0.3% Triton X-100), they were transferred to a 1:1 heptane-methanol solution and shaken vigorously for a couple of minutes. Fixed and devitellinized embryos were allowed to settle to the bottom of the methanol layer. The embryos were briefly washed three times with methanol, and this was followed by three washes with TBST (50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween, 0.05% NaN3; pH 7.5) for 15 min each time. They were then blocked in 1% bovine serum albumin in TBST and incubated with the WSP (Wolbachia surface protein) antibody (14) at a 1:500 dilution overnight at 4°C. After three washes with TBST, the embryos were incubated for 1 h at room temperature with a 1:500 dilution of Alexa Fluor 488 goat anti-rabbit immunoglobulin G-labeled antibody (Molecular Probes) and 2 mg of RNase A (Sigma) per ml in TBST. After several washes in TBST, the embryos were stained with 5 μg of propidium iodide (Molecular Probes) per ml for 20 min, rinsed, and mounted with a ProLong antifade kit (Molecular Probes).
(ii) Ovaries.
Ovaries were removed from 2- to 3-day-old females in TBST and dissected on glass slides. Tissue samples were flattened under a coverslip and frozen in liquid nitrogen. The coverslips were removed with a razor blade, and the slides were placed in ice-cold ethanol for 3 min and fixed in 4% paraformaldehyde for 12 min. The slides were rehydrated in TBST, blocked, and incubated with antibodies and propidium iodide as previously described.
(iii) Image analysis.
Optical sections were obtained with a confocal laser scanning microscope (Leica TCS-NT), and they were projected onto single images. The images were processed further by using Photoshop 6.0 (Adobe).
Wolbachia load in embryos.
Fifteen early embryos resulting from 1 to 13 mitotic cycles and stained with the WSP antibody were analyzed for each strain. For each embryo, 20 1-μm-thick sections were obtained. Optical sections were projected onto a single image and analyzed by using the Scion Image program (Scion Corporation). The numbers of pixels from clear stained regions were determined for the whole embryos and the posterior (10% of the total volume) and anterior (10% of the total volume) parts of the embryos. Taking in consideration that on average every embryo was 20 μm thick, each Wolbachia cell was 0.5 to 1 μm in diameter, and the pixel size was 0.5 by 0.5 μm, we assumed that the number of pixels roughly correlated with the true number of bacteria present in every embryo. Data were statistically analyzed by using SPSS (version 11).
RESULTS AND DISCUSSION
Wolbachia during embryogenesis.
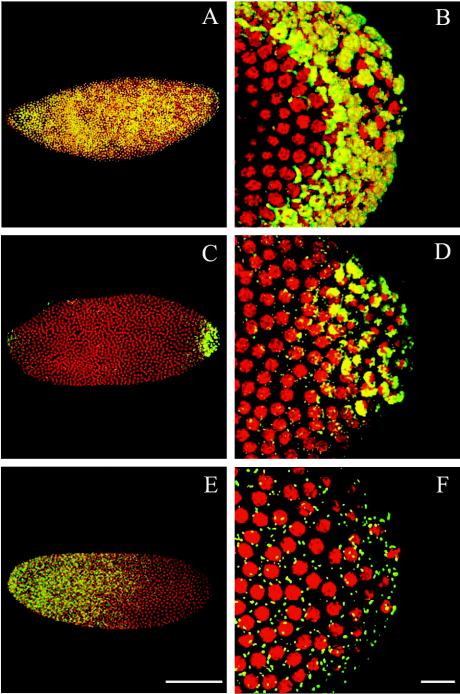
A confocal analysis was performed with embryos of several Wolbachia-infected strains by using an anti-WSP antiserum (14). This analysis revealed remarkable differences in bacterial distribution between strains. Specifically, the distributions of nine bacterial strains infecting six Drosophila species were studied, which revealed three distinct categories of Wolbachia-host associations. A representative embryo from each category is shown in Fig. 1. While strain wRi bacteria were evenly distributed throughout the cortex of the embryo (Fig. 1A and B), strain wMel, wCof, and wSty bacteria were concentrated more in the germ plasm (Fig. 1C and D). In embryos that harbored Wolbachia strain wNo, wMa, or wKi, the picture was strikingly different; there were more bacteria in the anterior part of the embryo and fewer bacteria at the pole cells (Fig. 1E and F). This distribution remained constant throughout embryogenesis from the early preblastoderm stage to the late gastrulation stage (Fig. 2), suggesting that there was no movement or preferential cell division.
FIG. 1.
Wolbachia distribution in Drosophila embryos at the syncytial blastoderm stage (mitotic cycles 10 to 13). (A) D. simulans embryo naturally infected with wRi bacteria. (B) Magnified view of the posterior part of the embryo, where pole cells are being formed. (C) Cells of the wSty strain are mainly concentrated in the posterior part of a D. teissieri embryo. (D) Pole plasm is heavily infected with bacteria compared to the rest of the embryo. (E) In a D. simulans embryo transinfected with the wMa strain, most of the bacteria are concentrated in the anterior part of the embryo. (F) Few bacteria are scattered in the pole plasm. The bacteria are green-yellow, and the nuclei are red. The embryos are oriented with the anterior part to the left. (E) Scale bar = 100 μm. (F) Scale bar = 20 μm.
FIG. 2.
Distribution of Wolbachia is conserved during embryogenesis. (A) wRi bacteria are uniformly distributed in a transinfected D. simulans unfertilized egg. (B) The pattern is the same after gastrulation. (C) wSty bacteria are concentrated in the pole plasm in a naturally infected D. teissieri embryo. (D) Bacteria of the same strain migrate along with the pole cells inside the embryo, in the region where gonads are going to be formed. (E) D. simulans embryo infected with wKi at the preblastoderm stage (mitotic cycle 6). The bacteria are concentrated mainly in the anterior part. (F) Late developed embryo of the same strain exhibiting accumulation of bacteria in the head. The embryos are oriented with the anterior part and head to the left. Scale bar = 100 μm.
To quantify the differences described above for every line, we analyzed the fluorescent images of 15 early embryos using confocal microscopy and Scion image analysis software. For each embryo 20 1-μm-thick sections of the whole embryo were obtained and projected onto single images, and the numbers of pixels in clear stained regions were determined. The bacterial numbers are shown in Table 1. The wRi strain exhibited the highest overall density in the embryos irrespective of the host genetic background. Although embryos infected with strain wSty bacteria (Drosophila santomea, Drosophila teissieri, and Drosophila yakuba) had the lowest densities, they exhibited the tightest posterior localization. Embryos infected with group B Wolbachia strains unexpectedly had larger amounts of bacteria anteriorly. We did not observe any significant bacterial growth for any of the strains during the first 13 nuclear divisions, confirming past observations (23). However, considerable intra- and interstrain variation of Wolbachia density was observed, which was established during the first mitotic divisions, and there was no apparent correlation between bacterial numbers and embryonic stages.
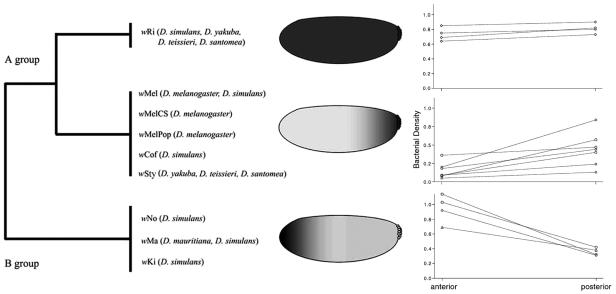
Interestingly, the localization of bacterial strains appears to be congruent with the classification based on the wsp gene sequence (Fig. 3). Bourtzis et al. suggested that the phylogeny of this gene could predict compatibility types for strains (2). However, Poinsot et al. showed that this hypothesis cannot be generalized (33). Veneti et al. were also unable to correlate the number of infected cysts and wsp gene sequences (43). Furthermore, our results showed that the distribution of a given Wolbachia strain does not change after transfer to a new host, implying that the distribution pattern is under bacterial control. In Trichogramma, posterior localization of Wolbachia has also been described. However, when transferred to a naturally uninfected line, Wolbachia did not have a similar posterior localization (32). In addition, with the transinfected line there were successively decreasing numbers of bacteria, which led to loss of infection. The relative contributions of host and Wolbachia factors to bacterial density and distribution remain unclear for this system.
FIG. 3.
Distribution and density of Wolbachia strains used in this study. The phylogeny is based on wsp gene sequences. wRi bacteria are evenly distributed throughout the cortex of the embryo, while wMel, wCof, and wSty bacteria are concentrated mostly in the posterior part of the embryo, where pole cells are formed. Bacteria belonging to the B group are concentrated in the anterior part of the embryo. The lines indicate the relative densities of strains. Note the differences in bacterial density between the posterior and anterior parts of the embryos and different slopes (tightness of localization).
Unlike bacterial distribution, density seems to be independent of the wsp phylogeny and to be strongly influenced by the host. For example, the density of the wMel strain is higher in D. simulans than in D. melanogaster, as observed previously (3, 26). However, host factors are not the only determinants of bacterial density. The wRi strain seems to be able to establish high-level infections irrespective of the host genetic background, while strain wSty bacteria have low replication rates in their native hosts. Finally, the wMa strain does not show any sign of replication preference in D. simulans or Drosophila mauritiana embryos. Interestingly, the virulent popcorn strain (wMelpop) (29), which causes widespread degeneration of tissues and early death due to its massive proliferation in adult flies, behaves like the nonvirulent wMel strain during embryogenesis.
Wolbachia in pole cells, testis infection, and cytoplasmic incompatibility.
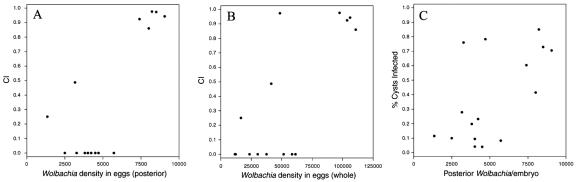
The infection density of Wolbachia and the level of cytoplasmic incompatibility have been studied extensively in the past (1, 2, 3, 5, 6, 9, 10, 11, 12, 15, 19, 21, 25, 30, 33, 34, 43). All of these studies led to the conclusion that the density of bacteria influences the level of CI as far as the bacterial strain infects the sperm cysts of its host and has the genetic machinery to induce it (10, 25, 43). These studies also included measurements of bacterial levels in embryos, gonads, somatic organs, and adults. Although Wolbachia within somatic cells may contribute to unknown host-symbiont interactions, it is clear that bacteria within the germ line have a disproportionate effect on CI. Indeed, a linear regression analysis showed that the total variance in levels of CI between the lines used in this study is explained better by the density of bacteria in the posterior part of the embryo (R2 = 0.559, F1,15 = 17.791, P = 0.00086) than by the total amount of bacteria in the whole embryo (R2 = 0.548, F1,15 = 16.981, P = 0.001039) (Table 1 and Fig. 4A and B).
FIG. 4.
(A) Positive correlation between CI levels and bacterial loads in the posterior part of the embryos. (B) Positive correlation between CI levels and densities of bacteria in the whole embryo. (C) Positive correlation between bacterial loads in the posterior part of the embryos and percentages of infected cysts.
Veneti et al. (43) recently showed that there is a positive correlation between the number of infected sperm cysts and the level of CI using young males (less than 1 day old) since previous studies had clearly shown that strong incompatibility is induced by sperm originating from young males and that weak incompatibility is evident only in sperm from somewhat older males (34, 43). In order to see if differences in Wolbachia growth and proliferation in Drosophila testes could result from differences in Wolbachia distribution earlier in development (e.g., pole cell formation), from active host suppression of bacterial entrance into the testes, from differences in bacterial replication in larval testes, or from a combination of these factors, we performed a linear regression analysis of the number of infected sperm cysts and the number of bacteria in the pole cells of each line (Table 1 and Fig. 4C). The latter data were square root transformed for normalization. We concluded that although there is a statistically significant positive correlation (R2 = 0.349, F1,15 = 7.507, P = 0.016), it seems that factors other than Wolbachia density in pole cells may determine the number of cysts that are infected. For example, the D. simulans Coffs Harbor and D. mauritiana lines have considerably more infected sperm cysts than a linear relationship with bacterial numbers in the pole cells would predict, suggesting that there is tissue-preferential bacterial replication. This may be due to the inability of these strains to induce incompatibility; therefore, the host did not evolve a mechanism to suppress bacterial proliferation in the target tissue of sperm modification.
Wolbachia during oogenesis.
Unlike the behavior during spermatogenesis, Wolbachia's behavior during oogenesis has not been described in detail, although oogenesis is the site of rescue activity and maternal transmission (see reference 41 for a review).
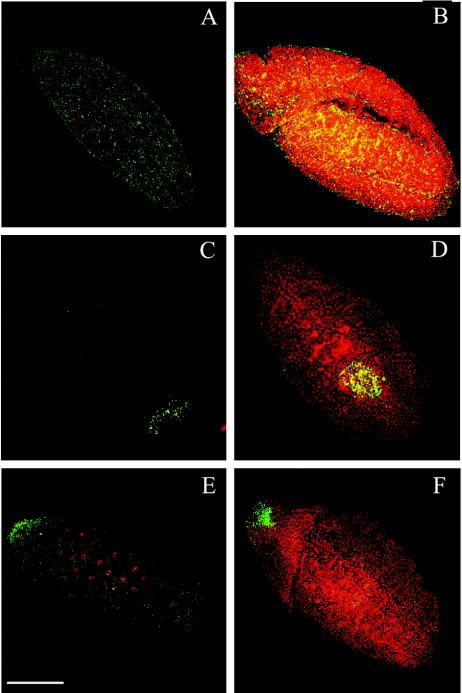
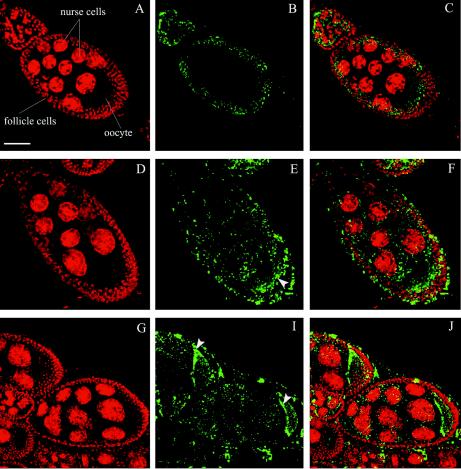
Confocal analysis of Wolbachia-infected ovaries was used to test the possibility that variation of Wolbachia density and distribution within embryos is determined maternally. As shown in Fig. 5, Wolbachia cells were abundant in the ovaries, especially in the early stages (stages 2 to 5). At these stages, bacterial density was so high that detailed observations were impossible. We therefore focused on stages 8 to 11, in which bacterial density was much lower, probably due to a lack of bacterial division. We were unable to monitor bacteria after these stages, as formation of the vitelline membrane prevented entry of the antibody into the developing oocytes. wRi bacteria were present mainly in a thin layer at the basal level of the follicle cells, which covered the oocyte, and were almost absent from the center of the embryo chamber, where the nurse cells were located (Fig. 5A to C). wMel, wCof, and wSty bacteria were present around follicle and nurse cell nuclei, and they accumulated in the posterior part of the oocyte, where the pole plasm formed (Fig. 5D to F). wNo, wMa, and wKi bacteria were also present around follicle and nurse cells but were mainly concentrated at the anterior wall of the oocyte (Fig. 5G to I). Thus, this analysis clearly showed that the distribution of Wolbachia in Drosophila is determined during oogenesis no later than stage 8 to 10 and does not change until late embryogenesis. The observed density in the developing oocyte suggests that Wolbachia undergoes several rounds of division at the beginning of oogenesis, ceasing to divide following the onset of vitellogenesis and probably commencing again, albeit at a lower rate, before embryo laying.
FIG. 5.
Distribution of Wolbachia is established during oogenesis, when oocytes start to form (stages 8 to 10). (A to C) wRi bacteria (green) are concentrated at the basal level of the follicle cells but are not present around nurse cells during D. simulans oogenesis. (D to F) wMel bacteria are scattered around follicle and nurse cells and localize in the posterior part of the oocyte during D. melanogaster oogenesis (arrowhead). (G to I) wNo bacteria are present around follicle and nurse cells, but they are concentrated at the anterior border of the oocytes (arrowheads) during D. simulans oogenesis. Scale bar = 30 μm.
Spatial differences in Wolbachia numbers could play an important role in the rescue mechanism. Wolbachia density and distribution in the embryo could be directly correlated with rescue activity, but this has yet to be examined systematically.
Wolbachia: an additional cargo for cytoskeleton?
Identification of bacterial and host factors required for posterior, anterior, or cortical localization would add tremendously to our understanding of the Wolbachia-host interaction. It is striking that there are a number of Drosophila mRNAs specifying the anterior-posterior axis of the embryo that show the same localization as Wolbachia. The distribution of wMel bacteria in oocytes resembles that of oscar mRNA, while wNo seems to colocalize with bicoid mRNA (38). Localization of many transcripts depends on microtubule-based motors (35), and a previous study (7) showed that the same machinery drives specific accumulation of maternal RNAs in the oocyte and apical transcript localization in blastoderm embryos. It has been found that Wolbachia associates with astral microtubules (8, 23), which together with other cytoskeletal elements play an important role in compartmentalization and localization of transcripts in cellularizing embryos. Wolbachia could thus be just an additional cargo for the cytoskeletal system that transports transcripts. Tram et al. suggested that the proteins dynein and kinesin are candidate Wolbachia transporters (41). Different Wolbachia strains could present different proteins on the outer surface with specific affinity to different motor protein complexes. It is intriguing to speculate that the wsp gene product itself might be a candidate for such interactions, since it is an outer membrane protein and is under positive selection in parasites (22).
Evolutionary implications.
Theory suggests (42) that CI levels, transmission efficiency, and fitness cost, the three key factors that are thought to determine the evolution of Wolbachia CI types, may be linked through bacterial density. If these factors do not interfere, host-symbiont coevolution is expected to lead to low CI levels, low fitness costs, and high transmission efficiency and therefore to low density in the male germ line, high density in the ovaries, and limited overall density of the intracellular bacteria. Our observations are in agreement with this model, if we assume that D. yakuba, D. teissieri, D. santomea, and D. melanogaster have evolved long-term associations with Wolbachia which cause low to undetectable levels of CI but target the host germ line to ensure vertical transmission, while wRi infection is more recent, exhibiting a high replication rate, high CI levels, and imperfect maternal transmission, at least in nature (17). In addition, mitochondrial data support the longer association of Wolbachia with D. melanogaster than with D. simulans (37). wCof remains the most puzzling strain due to the moderate overall bacterial numbers, the loose posterior localization in embryos, and the high replication rate in testes that do not induce CI. One could expect different selection pressures to act on a host that is infected with a strain that has lost the ability to induce CI.
The surprising observation that wNo, wMa, and wKi bacteria are concentrated at the anterior part of the embryo needs further investigation. The high concentration in the head of the embryos suggests the exciting possibility that these bacteria might modify the behavior of the flies (36). Dettman et al. proposed a link between the microtubule cytoskeleton in embryogenesis and a behavioral phenotype of Drosophila larvae (13), which makes this assumption worth being tested. It remains to be seen if these bacteria provide a benefit to their hosts, having developed a mutualistic relationship with their hosts, or if the infections are transient due to imperfect maternal transmission, the absence or low levels of CI, and/or a high fitness cost. It should be mentioned that these strains, even though they are present at higher concentrations in the anterior part of the embryos, are present at significant levels in the posterior part as well, which might be sufficient for transmission to the next generation. Laboratory data support the second hypothesis, as such infections are frequently lost and require selection for maintenance. However, immunofluorescence experiments with the selected lines showed nearly perfect (>99%) maternal transmission for every strain used in this study (data not shown).
Acknowledgments
This research was supported in part by European Union grant QLK3-2000-01079 to K.B.
We thank Daniel St. Johnston, Christos Delidakis, Stefan Oehler, Greg Hurst, and William Sullivan for critical reading of the manuscript and Filipa Vala for help with the statistics.
REFERENCES
- 1.Bourtzis, K., A. Nirgianaki, G. Markakis, and C. Savakis. 1996. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics 144:1063-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourtzis, K., S. L. Dobson, H. R. Braig, and S. L. O'Neill. 1998. Rescuing Wolbachia have been overlooked. Nature 391:852-853. [DOI] [PubMed] [Google Scholar]
- 3.Boyle, L., S. L. O'Neill, H. M. Robertson, and T. L. Karr. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796-1799. [DOI] [PubMed] [Google Scholar]
- 4.Breeuwer, J. A. J., and J. H. Werren. 1990. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 346:558-560. [DOI] [PubMed] [Google Scholar]
- 5.Breeuwer, J. A. J., and J. H. Werren. 1993. Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bressac, C., and F. Rousset. 1993. The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J. Invertebr. Pathol. 61:226-230. [DOI] [PubMed] [Google Scholar]
- 7.Bullock, S. L., and D. Ish-Horowicz. 2001. Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature 414:611-616. [DOI] [PubMed] [Google Scholar]
- 8.Callaini, G., M. G. Riparbelli, and R. Dallai. 1994. The distribution of cytoplasmic bacteria in the early Drosophila embryo is mediated by astral microtubules. J. Cell Sci. 107:673-682. [DOI] [PubMed] [Google Scholar]
- 9.Clancy, D. J., and A. A. Hoffmann. 1998. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol. Exp. Appl. 86:13-24. [Google Scholar]
- 10.Clark, M. E., and T. L. Karr. 2002. Distribution of Wolbachia within Drosophila reproductive tissue: implications for the expression of cytoplasmic incompatibility. Integ. Comp. Biol. 42:332-339. [DOI] [PubMed] [Google Scholar]
- 11.Clark, M. E., Z. Veneti, K. Bourtzis, and T. L. Karr. 2002. The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila. Mech. Dev. 111:3-15. [DOI] [PubMed] [Google Scholar]
- 12.Clark, M. E., Z. Veneti, K. Bourtzis, and T. L. Karr. 2003. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech. Dev. 120:185-198. [DOI] [PubMed] [Google Scholar]
- 13.Dettman, R. W., F. R. Turner, H. D. Hoyle, and E. C. Raff. 2001. Embryonic expression of the divergent Drosophila beta3-tubulin isoform is required for larval behavior. Genetics 158:253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 15.Giordano, R., S. L. O'Neill, and H. M. Robertson. 1995. Wolbachia infections and the expression of cytoplasmic incompatibility in Drosophila sechellia and D. mauritiana. Genetics 140:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadfield, S. J., and J. M. Axton. 1999. Germ cells colonized by endosymbiotic bacteria. Nature 402:482. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, A. A., M. Turelli, and L. G. Harshman. 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126:933-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann, A. A., M. Turelli, and G. M. Simmons. 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40:692-701. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, A. A., D. Clancy, and J. Duncan. 1996. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76:1-8. [DOI] [PubMed] [Google Scholar]
- 20.Holden, P. R., P. Jones, and J. F. Brookfield. 1993. Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet. Res. 62:23-29. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, T., H. Ishikawa, and T. Sasaki. 2003. Infection density of Wolbachia and level of cytoplasmic incompatibility in the Mediterranean flour moth, Ephestia kuehniella. J. Invert. Pathol. 84:1-5. [DOI] [PubMed] [Google Scholar]
- 22.Jiggins, F. M., G. D. Hurst, and Z. Yang. 2002. Host-symbiont conflicts: positive selection on an outer membrane protein of parasitic but not mutualistic Rickettsiaceae. Mol. Biol. Evol. 19:1341-1349. [DOI] [PubMed] [Google Scholar]
- 23.Kose, H., and T. L. Karr. 1995. Organization of Wolbachia pipientis in the Drosophila fertilized egg and embryo revealed by an anti-Wolbachia monoclonal antibody. Mech. Dev. 51:275-288. [DOI] [PubMed] [Google Scholar]
- 24.Lachaise, D., M. Harry, M. Solignac, F. Lemeunier, V. Benassi, and M. L. Cariou. 2000. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from Sao Tome. Proc. R. Soc. Lond. Ser. B Biol. Sci. 267:1487-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2001. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. R. Soc. Lond. Ser. B Biol. Sci. 268:2565-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merçot, H., B. Llorente, M. Jacques, A. Atlan, and C. Montchamp-Moreau. 1995. Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics 141:1015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merçot, H., and D. Poinsot. 1998. Rescuing Wolbachia have been overlooked and discovered on Mount Kilimanjaro. Nature 391:853. [DOI] [PubMed] [Google Scholar]
- 29.Min, K. T., and S. Benzer. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA. 94:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noda, H., Y. Koizumi, Q. Zhang, and K. Deng. 2001. Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem. Mol. Biol. 31:727-737. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill, S. L., and T. L. Karr. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348:178-180. [DOI] [PubMed] [Google Scholar]
- 32.Pintureau, B., S. Grenier, B. Boleat, F. Lassabliere, A. Heddi, and C. Khatchadourian. 2000. Dynamics of Wolbachia populations in transfected lines of Trichogramma. J. Invertebr. Pathol. 76:20-25. [DOI] [PubMed] [Google Scholar]
- 33.Poinsot, D., K. Bourtzis, G. Markakis, C. Savakis, and H. Merçot. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150:227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds, K. T., and A. A. Hoffmann. 2002. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. 80:79-87. [DOI] [PubMed] [Google Scholar]
- 35.Saxton, W. M. 2001. Microtubules, motors, and mRNA localization mechanisms: watching fluorescent messages move. Cell 107:707-710. [DOI] [PubMed] [Google Scholar]
- 36.Sokolowski, M. B. 2001. Drosophila: genetics meets behaviour. Nat. Rev. Genet. 2:879-890. [DOI] [PubMed] [Google Scholar]
- 37.Solignac, M., D. Vautrin, and F. Rousset. 1994. Widespread occurrence of the proteobacteria Wolbachia and partial cytoplasmic incompatibility in Drosophila melanogaster. C. R. Acad. Sci. Paris 317:461-470. [Google Scholar]
- 38.St. Johnston, D. 2001. The beginning of the end. EMBO J. 20:6169-6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stouthamer, R., J. A. J. Breeuwer, and G. D. Hurst. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53:71-102. [DOI] [PubMed] [Google Scholar]
- 40.Stouthamer, R., J. A. J. Breeuwer, R. F. Luck, and J. H. Werren. 1993. Molecular identification of microorganisms associated with parthenogenesis. Nature 361:66-68. [DOI] [PubMed] [Google Scholar]
- 41.Tram, U., P. M. Ferre, and W. Sullivan. 2003. Identification of Wolbachia-host interacting factors through cytological analysis. Microbes Infect. 5:999-1011. [DOI] [PubMed] [Google Scholar]
- 42.Turelli, M. 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48:1500-1513. [DOI] [PubMed] [Google Scholar]
- 43.Veneti, Z., M. E. Clark, S. Zabalou, C. Savakis, T. L. Karr, and K. Bourtzis. 2003. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics 164:545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42:587-609. [DOI] [PubMed] [Google Scholar]
- 45.Wright, J. D., and A. R. Barr. 1981. Wolbachia and the normal and incompatible embryos of Aedes polynesiensis (Diptera: Culicidae). J. Invert. Pathol. 38:409-418. [Google Scholar]
- 46.Zabalou, S., S. Charlat, A. Nirgianaki, D. Lachaise, H. Merçot, and K. Bourtzis. 2004. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics 167:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zchori-Fein, E., R. T. Roush, and D. Rosen. 1998. Distribution of parthenogenesis-inducing symbionts in ovaries and embryos of Aphytis (Hymentoptera: Aphelinidae). Curr. Microbiol. 36:1-8. [DOI] [PubMed] [Google Scholar]