Abstract
Lactobacillus curvatus LTH 1174, a fermented sausage isolate, produces the antilisterial bacteriocin curvacin A. Its biokinetics of cell growth and bacteriocin production as a function of various concentrations of a complex nutrient source were investigated in vitro during laboratory fermentations with modified MRS medium. A modification of the nutrient depletion model (Leroy and De Vuyst, Appl. Environ, Microbiol. 67:4470-4473, 2001) was used to fit the data describing growth and bacteriocin production. Both cell growth and bacteriocin activity were influenced by changes in the complex nutrient source concentration. Standard MRS medium clearly limited the growth of L. curvatus LTH 1174. Higher nutrient concentrations, up to a certain degree, led to improved growth, a higher attainable biomass concentration, and a higher bacteriocin activity in the supernatant. A lower concentration of complex nutrient source caused severe growth inhibition, leading to a lower biomass concentration but a much higher specific bacteriocin production. When examining the separate components of the complex nutrient source, a stimulating effect of bacteriological peptone on growth was found without an adverse effect on bacteriocin production, resulting in increased curvacin A activity. Furthermore, specific depletion of the amino acids tyrosine, serine, and asparagine/aspartic acid was observed for this strain.
Raw sausages consist of raw meat and fat, which are mixed with several additives. They partially owe their microbiological stability and organoleptic properties to fermentation carried out by lactic acid bacteria at the appropriate temperature and air humidity. The basic concept for the manufacturing of raw sausages is an adequate reduction of pH and/or water activity (aw) to inhibit the growth of undesired bacteria. A fast drop in pH is usually achieved by adding fermentable carbohydrates to the sausage batter together with the lactic acid bacteria starter culture, while the reduction in aw is achieved by the addition of salt and subsequent drying (25, 31). In Europe, fermented sausages are manufactured with starter cultures containing mainly Lactobacillus sakei and Lactobacillus curvatus (16).
The average contamination of meat with Listeria monocytogenes is about 30 to 60%, and the spread of antibiotic resistance genes among this and other pathogenic bacteria is raising new concerns (6, 36, 44). In fermented sausages, L. monocytogenes can even be isolated after sausage fermentation and drying (14, 15, 18, 20). A study concerning the prevalence of L. monocytogenes on the Belgian retail market revealed that in 11.69% of a total of 308 samples of minced cured meat products (including dry fermented sausages), this pathogen was detectable in a 25-g sample (38). Bacteriocin production may be considered an extra hurdle in the production of fermented sausages, contributing to a more stable and safer end product. Hugas et al. (17) have found that L. curvatus LTH 1174 can reduce the counts of artificially added listeriae in fermented sausages to a higher extent than a nonbacteriocinogenic (control) culture.
The starter cultures used to date for the production of fermented sausage are not necessarily the best-suited strains. They either cannot be or are not competitive enough compared with the contaminating lactic acid bacteria naturally present in the raw material. Moreover, too little is known as to what properties a starter or protective culture should display or not (25). However, in the past few years, suppression of pathogens like L. monocytogenes has become a major issue, and much work has been devoted to select strains that are antagonistic to food-borne pathogens (31). L. curvatus LTH 1174, which produces the bacteriocin curvacin A, was shown to be a successful starter strain for German-type sausage fermentations; the strain gained dominance over the fortuitous flora and even over a commercial L. curvatus starter and was able to reduce the listeria counts in fermented sausages (17, 43). Modeling the response of this strain to changing medium conditions will give new insights concerning the nutrient requirements of this interesting sausage starter (32).
De Man, Rogosa, and Sharpe (MRS) medium is usually used to study meat fermentation processes (12, 26, 27). This medium has been developed to support good growth of lactic acid bacteria (9). However, lactic acid bacteria are fastidious with respect to their nutrient requirements, and the growth of some strains is often inhibited in MRS medium because of nutrient limitation (28). This is not the case in sausage batter, a nutrient-rich environment, usually with added carbohydrates, where increases in free amino acids and peptides are evident during ripening of the sausage (8). It is unclear which compound(s) is responsible for the limitation of cell growth in cultivation media, but sugars, amino acids, and vitamins seem to be the most important (3, 19, 24, 30, 35, 39). Consequently, to mimic meat environments, nutrient-rich environments have to be employed. An additional strategy to understand the nutrient requirements of a strain is to model the limitations of the strains when grown in meat simulation media.
The aim of this study was to determine growth inhibition due to nutrient limitation of the bacteriocin producer L. curvatus LTH 1174 active against listeriae when grown in standard MRS and modified MRS medium. The data obtained were fitted with a modified version of the nutrient depletion model (28).
MATERIALS AND METHODS
Microorganisms and media.
L. curvatus LTH 1174, producer of curvacin A (37), and Listeria innocua LMG 13568, which was used as a sensitive indicator organism to determine bacteriocin activity levels, were stored at −80°C in MRS medium (Oxoid, Basingstoke, United Kingdom) and brain heart infusion (BHI) medium (Oxoid), respectively, both of which contained 25% (vol/vol) glycerol as a cryoprotectant. The strains were propagated twice at 30°C for 12 h before experimental use. Solid medium was prepared by adding 1.5% agar (Oxoid) to the broth. The overlays used for estimation of the bacteriocin titers were prepared with 0.7% agar.
Fermentation experiments.
To investigate the influence of the complex nutrient source, i.e., bacteriological peptone (Oxoid), Lab Lemco (Oxoid), and yeast extract (VWR International, Darmstadt, Germany), on both growth and bacteriocin production of L. curvatus LTH 1174, fermentations were performed in standard MRS and modified MRS medium. The latter contained 0.5, 2, 4, or 6 times the concentration of each complex nutrient source component of standard MRS medium. Fermentation in standard MRS medium was run as a control fermentation and contained the following components (per liter): 10 g of bacteriological peptone, 8 g of Lab Lemco, 4 g of yeast extract, 0.2 g of MgSO4 · 7H2O, 0.038 g of MnSO4 · H2O, and 1 ml of Tween 80. The fermentation carried out with a double amount of complex nutrient source was performed in triplicate to show the reproducibility of the experiments. Standard deviations were calculated both on the experimental values and on the biokinetic parameters derived from the primary model. Also, three fermentations were carried out whereby the concentration of one of the above-mentioned complex nutrient source components was used at four times its concentration in standard MRS medium.
Fermentations were carried out in a 15-liter laboratory fermentor (BiostatC; B. Braun Biotech International, Melsungen, Germany) as previously described (26). All fermentations were performed at a constant temperature of 25°C and a controlled pH of 5.5. For the preparation of the inoculum, 10 ml of MRS medium was inoculated with 0.5 ml of a freshly prepared L. curvatus LTH 1174 culture and incubated at 30°C for 12 h; 5 ml of this preculture was added to 100 ml of MRS medium. After 13 h of growth at 30°C, this culture was used to inoculate the fermentor. Temperature and pH control as well as agitation were performed on-line (Micro-MFCS for Windows NT; B. Braun Biotech International) (26).
Assays.
At regular intervals, samples were withdrawn aseptically from the fermentor to determine cell dry mass (CDM), bacteriocin activity in cell-free culture supernatant, lactic acid concentration, and residual glucose concentration. Briefly, CDM was determined gravimetrically after membrane filtration (26); the amount of lactic acid produced and the residual glucose concentration were determined by high-performance liquid chromatography (HPLC) (10). The level of bacteriocin activity in the cell-free supernatant was determined by a modified critical dilution method (10) with L. innocua LMG 13568 as the indicator organism (26). The bioavailable bacteriocin activity measured in the cell-free culture supernatant was expressed in arbitrary units (AU) per milliliter or mega-arbitrary units (MAU) per liter. The standard deviations for the CDM, glucose, and lactic acid measurements were 0.11, 0.04, and 0.02 g liter−1, respectively. Modeling of bacterial growth was performed with both CDM measurements and the biomass concentrations obtained from measurements of optical density at 600 nm. Therefore, the optical density values from a series of previously performed fermentations were calibrated against biomass (CDM). A change of one unit of optical density was shown to be equivalent to an increase of 0.26 g of CDM liter−1 (r2 = 0.962).
Analyses of free amino acids were performed in duplicate for all fermentations on selected samples distributed over the course of the fermentation. Proteins were removed from the samples by addition of an isovolume of trichloroacetic acid and subsequent centrifugation (16,060 × g, 15 min). Amino acid analysis was performed with the Waters AccQ•Tag amino acid analysis method (Waters Corporation, Milford, Mass.).
Modeling.
Cell growth was modeled with the equation dX/dt = μmax γi X for t > λ, where X is the biomass concentration, t is the time, λ is the duration of the lag phase, μmax is the maximum specific growth rate, and γi is a dimensionless general inhibition function (see Table 1 for units). During the lag phase, cell growth was set to zero.
TABLE 1.
Equations used for primary model developmenta
| Model | Equation |
|---|---|
| Glucose consumption | dS/dt = −1/YX/S dX/dt − mSX |
| Lactic acid production | dL/dt = −YL/S dS/dt |
| Bacteriocin production | dB/dt = kB dX/dt − kinactXB when X > XB |
Abbreviations: X, biomass concentration (in grams of CDM per liter); t, time (in hours); S, residual glucose concentration (in grams of glucose per liter); YX/S, cell yield coefficient (in grams of CDM per gram of glucose); mS, maintenance coefficient (in grams of glucose per gram of CDM per hour); L, lactic acid production (in grams of lactic acid per liter); YL/S, yield coefficient for the conversion of glucose into lactic acid (in grams of lactic acid per gram of glucose); B, bacteriocin activity in the cell-free culture supernatant (in AU per liter); kB, specific bacteriocin production (in AU per gram of CDM); kinact, apparent rate of bacteriocin inactivation (in liters per gram of CDM per hour); XB, minimum biomass concentration for the onset of bacteriocin production (in grams of CDM per liter).
The inhibition function γi was modeled with a three-step inhibition function based on the nutrient depletion model (28): γi = 1 if X < X1;γi = 1 − I1 (X − X1) if X1 < X < X2; and γi = 1 − I1 (X2 − X1) − I2 (X − X2) if X > X2, where X1 and X2 are the critical biomass concentrations at the start of the consecutive phases of decreasing growth rate and I1 and I2 are the inhibition slope coefficients. The parameters X1, X2, I1, and I2 of the three-step inhibition function used describe the self-inhibition of the cells due to depletion of sugar and other nutrients and to the accumulation of lactic acid.
Primary modeling of glucose consumption, lactic acid production, and bacteriocin production and apparent inactivation was performed both to fit the data as well as to estimate the biokinetic parameters representative for growth and curvacin A production. The equations used are listed in Table 1. They are the same as those reported by Messens et al. (32), except that bacteriocin production was made dependent on a value of XB, i.e., the minimum biomass concentration required for the onset of bacteriocin production due to induction (11, 28, 42).
The differential equations were solved numerically with the Euler integration technique in Microsoft Excel 97 (version 8.0a). All parameters needed for modeling were estimated by minimizing the residual sum of squares between experimental and modeled data. For bacteriocin activity, the sum of squares was weighted inversely proportional with the uncertainty of the measurement (i.e., the detection limit in AU of each dilution). Previously, it has been shown that these large-scale and strictly computer-controlled fermentation experiments are highly repeatable, as well as the estimation of biokinetic parameters for a defined set of fermentation conditions. It has been estimated for L. sakei CTC 494 and L. curvatus LTH 1174 that upon repetition, the coefficients of variation of the biokinetic parameters are generally lower than 10%, with the exception of kinact (26, 32, 41, 42). Moreover, inherent biological variation within a treatment was small compared to the large differences observed among experiments under different conditions.
RESULTS
Fermentation profiles.
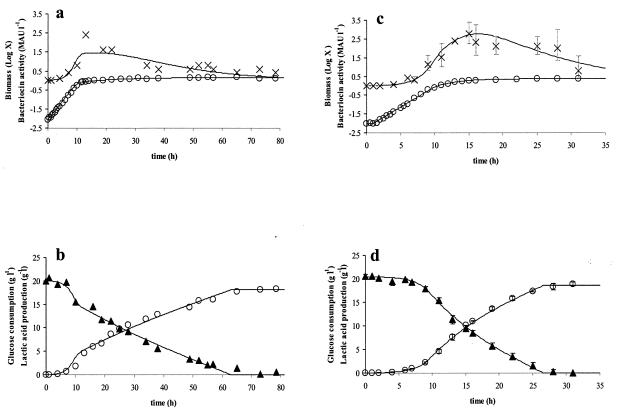
In all cases, similar profiles of biomass, bacteriocin, and lactic acid production were obtained (Fig. 1). Bacteriocin production started concomitantly with growth and increased rapidly when cells were growing exponentially. When cells reached the stationary phase, growth ceased and bacteriocin activity started to decrease as a function of time (Fig. 1a and c). To show the reproducibility of the fermentation experiments, the average of three fermentations with a double amount of complex nutrient source is also represented with standard deviations (Fig. 1c and d).
FIG. 1.
Modeling of biomass (in grams of CDM per liter (○) and bacteriocin production (in MAU per liter) ×) (a and c) and of glucose consumption (in grams of glucose per liter) (▴) and lactic acid formation (in grams of lactic acid per liter) (○) (b and d) for L. curvatus LTH 1174 in the control fermentation in standard MRS medium (a and b) and in MRS medium with twice the amount of the complex nutrient source (c and d) at a controlled temperature of 25°C and a constant pH of 5.5. The symbols represent experimental values; lines are drawn according to the model. For the fermentation with twice the concentration of complex nutrient source, the values are the averages of three fermentations, and the error bars represent the standard deviations.
Influence of the overall complex nutrient concentration.
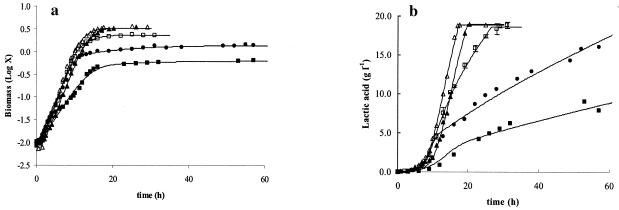
To investigate growth inhibition of L. curvatus LTH 1174 due to nutrient limitation, altered complex nutrient source concentrations, i.e., 0.5, 2, 4, and 6 times the concentrations of standard MRS, were used. The μmax increased with increasing complex nutrient source concentrations until a maximum value of 0.61 h−1 was reached at four times the standard complex nutrient source concentration (Table 2). This value decreased again to 0.51 h−1 when six times the standard concentration of complex nutrient source was used. With increasing concentrations of complex nutrient source, X1 also increased, reaching a maximum value at four times the complex nutrient source concentration. This indicates a later start of the first phase of inhibition with increasing complex nutrient source concentrations. Similarly, the critical biomass concentration X2 increased with increasing complex nutrient source concentrations up to twice the amount of complex nutrient source. At four times the complex nutrient source concentration, X2 was comparable to the value at two times the complex nutrient source concentration, while at six times the standard complex nutrient source concentration, X2 decreased again. With increasing complex nutrient source concentrations, the maximum amount of biomass the strain was able to produce (Xmax) increased from 0.60 to 1.37, 2.32 ± 0.2, 3.22, and 3.30 g of CDM liter−1 for 0.5, 1, 2, 4, and 6 times the standard complex nutrient source concentration, respectively (Fig. 2a).
TABLE 2.
Influence of the complex nutrient source (CNS) on μmax, X1, I1, X2, I2, YX/S, and mS of L. curvatus LTH 1174 in modified MRS medium at a constant temperature of 25°C and a constant pH of 5.5a
| CNS Conc (-fold) | μmax (h−1) | X1 (g of CDM liter−1) | I1 | X2 (g of CDM liter−1) | I2 | YX/S [g of CDM (g of glucose)−1] | mS [g of glucose (g of CDM)−1 h−1] |
|---|---|---|---|---|---|---|---|
| 0.5 | 0.33 | 0.01 | 1.83 | 0.42 | 0.10 | 0.10 | 0.29 |
| 1.0 | 0.47 | 0.29 | 1.67 | 0.85 | 0.14 | 0.17 | 0.18 |
| 2.0 | 0.53 ± 0.02 | 0.42 ± 0.03 | 0.86 ± 0.07 | 1.24 ± 0.06 | 0.27 ± 0.04 | 0.24 ± 0.01 | 0.32 ± 0.07 |
| 4.0 | 0.61 | 0.50 | 0.70 | 1.22 | 0.22 | 0.23 | 0.51 |
| 6.0 | 0.51 | 0.46 | 0.65 | 1.01 | 0.26 | 0.32 | 0.47 |
Standard deviations are indicated where appropriate.
FIG. 2.
Influence of the complex nutrient source on production of biomass (in grams of CDM per liter) (a) and production of lactic acid (in grams of lactic acid per liter) (b) as a function of time. Standard MRS (•) and MRS with 0.5 (▪), 2 (□), 4 (Δ), or 6 (▴) times the standard complex nutrient source concentration was used. The symbols represent experimental values; lines are drawn according to the model. In the case of twice the amount of complex nutrient source, the values are the averages of three fermentations, and the error bars represent the standard deviations.
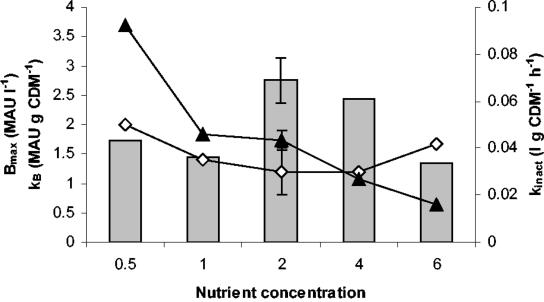
The maximum attainable bacteriocin activity (Bmax) was highest (2.76 ± 0.38 MAU liter−1) when the standard complex nutrient source concentration was doubled and was only slightly lower (2.43 MAU liter−1) at four times the standard complex nutrient source concentration. At concentrations higher than four times the complex nutrient source concentration or lower than two times the complex nutrient source concentration, Bmax showed a decreasing trend (Fig. 3). Compared to the fermentation in standard MRS medium, the specific bacteriocin production (kB) strongly increased when the concentration of complex nutrient source was halved. In contrast, at concentrations above twice the amount of complex nutrient source, kB quickly decreased (Fig. 3). The value obtained at six times the standard complex nutrient source concentration was approximately one fifth of the maximum value of 3.69 MAU (g of CDM)−1, which was reached with half of the standard complex nutrient source concentration. Although higher nutrient concentrations allowed better growth of the strain and subsequently led to higher biomass production, they did not lead to higher bacteriocin activity in the supernatant, mainly due to a lower kB. Bacteriocin induction was unaffected, since the minimum biomass concentration necessary to start bacteriocin production (XB) was zero in all cases, indicating that enough induction factor was present in the inoculum to initiate bacteriocin production from the start of the fermentation.
FIG. 3.
Influence of complex nutrient source concentration on maximum attainable bacteriocin activity (bars), specific bacteriocin production (▴), and bacteriocin inactivation rate (⋄). The values for the fermentation with twice the amount of complex nutrient source are the averages of three experiments for which the error bars represent the standard deviations.
Lactic acid production was slowest for the fermentation with half the concentration of complex nutrient source. With increasing concentrations of complex nutrient source, lactic acid was also produced more rapidly. However, in standard MRS and in MRS medium with half the concentration of complex nutrient source, slowing of lactic acid production was observed when the critical biomass X2 was reached (Fig. 2b). At higher concentrations of complex nutrients, glucose was consumed faster, resulting in faster production of lactic acid; slowing of the lactic acid production curve did not occur (Fig. 2b). Also, at four and six times the concentration of complex nutrient source, the maintenance coefficient increased.
Influence of the separate components of the complex nutrient source.
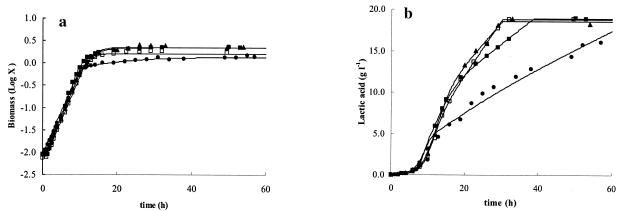
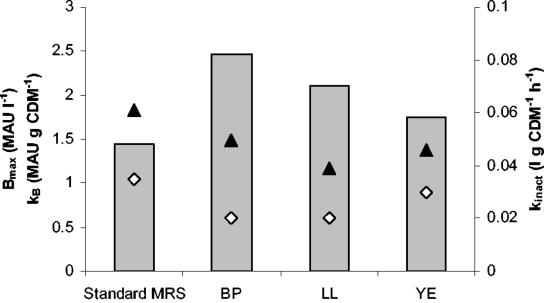
To determine which of the three constituting components of the complex nutrient source (bacteriological peptone, Lab Lemco, or yeast extract) was most crucial for growth and bacteriocin production of L. curvatus LTH 1174, fermentations were performed with an elevated concentration (four times the concentration of standard MRS medium) of each component separately. In the case of the elevated bacteriological peptone concentration, a boost of cell growth was seen, as shown by the μmax of 0.58 h−1 compared to that of the control fermentation (μmax = 0.47 h−1). The μmax obtained was comparable to the value obtained when the concentration of all components was increased fourfold simultaneously (0.61 h−1). With higher amounts of Lab Lemco and yeast extract, μmax was comparable to that of the control fermentation (Table 3). In all cases, the nutrients added resulted in higher values of X1 and Xmax compared to the control fermentation (Fig. 4a). In the case of bacteriological peptone and Lab Lemco, Xmax was comparable (2.20 g of CDM liter−1), but a lower concentration was obtained with the elevated level of yeast extract (1.64 g of CDM liter−1). In addition, higher maintenance was seen in the case of an elevated level of yeast extract, while for bacteriological peptone and Lab Lemco, the maintenance coefficient was comparable to that of the control fermentation (Table 3). In all cases, Bmax was higher than in standard MRS, being 2.46, 2.11, and 1.75 MAU liter−1 for bacteriological peptone, Lab Lemco, and yeast extract, respectively (Fig. 5). However, this was the result of increased biomass, since kB was comparable (bacteriological peptone) or slightly lower (Lab Lemco and yeast extract) than for the control fermentation (Fig. 5).
TABLE 3.
Influence of an elevated concentration of bacteriological peptone (BP), Lab Lemco (LL), or yeast extract (YE) on μmax, X1, I1, X2, I2, YX/S, and mS of L. curvatus LTH 1174 in MRS medium at a constant temperature of 25°C and a constant pH of 5.5
| Componenta | μmax (h−1) | X1 (g of CDM liter−1) | I1 | X2 (g of CDM liter−1) | I2 | YX/S [g of CDM (g of glucose)−1] | mS [g of glucose (g of CDM)−1 h−1] |
|---|---|---|---|---|---|---|---|
| Standard MRS | 0.47 | 0.29 | 1.67 | 0.85 | 0.14 | 0.17 | 0.18 |
| BP | 0.58 | 0.39 | 1.28 | 0.91 | 0.26 | 0.21 | 0.18 |
| LL | 0.48 | 0.38 | 1.00 | 0.78 | 0.41 | 0.22 | 0.26 |
| YE | 0.49 | 0.41 | 1.10 | 0.99 | 0.57 | 0.24 | 0.45 |
The concentration of the component of standard MRS medium investigated was quadrupled.
FIG. 4.
Influence of an elevated concentration of the separate components of the complex nutrient source of standard MRS (•), 4× bacteriological peptone (▪), 4× Lab Lemco (▴), or 4× yeast extract (□) on the production of biomass (in grams of CDM per liter) (a) and the production of lactic acid (in grams of lactic acid per liter) (b) as a function of time. The symbols represent experimental values; lines are drawn according to the model.
FIG. 5.
Influence of fourfold-higher concentration of the separate components of the complex nutrient source of standard MRS (BP, bacteriological peptone; LL, Lab Lemco; YE, yeast extract) on maximum attainable bacteriocin activity (Bmax) / (kB) (bars), specific bacteriocin production (kinact) (▴), and bacteriocin inactivation rate (⋄).
Lactic acid production occurred faster than in the control fermentation due to the higher biomass produced in all cases (Fig. 4b). Lactic acid production kinetics were comparable for bacteriological peptone and Lab Lemco and slower for yeast extract due to a biomass concentration lower than that obtained for bacteriological peptone and Lab Lemco.
Amino acid analyses.
To determine the use of free amino acids by L. curvatus LTH 1174, amino acid analyses were performed on samples of each fermentation. For all fermentations studied, the concentration of tyrosine showed a strong decreasing trend, and at some point during the fermentation it was no longer detected in the samples. Tyrosine was the only amino acid that was completely depleted during all fermentations. Additionally, serine and asparagine/aspartic acid were completely used up during fermentation in standard MRS medium, while all other amino acids showed a decreasing trend. For fermentations with a fourfold or sixfold concentration of complex nutrient source, the concentrations of all amino acids apart from tyrosine remained constant. For the fermentation with double the amount of complex nutrient source, tyrosine and serine showed a decreasing trend. For fermentations with a fourfold-higher concentration of either bacteriological peptone, Lab Lemco, or yeast extract, tyrosine was completely depleted, asparagine/aspartic acid and serine showed a decreasing trend, and the concentration of all other amino acids remained constant.
DISCUSSION
Growth of lactic acid bacteria is inhibited not only by the production of metabolites such as lactic acid but also by limited concentrations of indispensable nutrients present in the medium. Even in MRS, a commercial medium developed to support good growth of lactobacilli and often used for studying meat fermentations, inhibition of bacterial growth due to nutrient limitation occurs (28). As a result of the growth-associated character of bacteriocin production, this growth inhibition may lead to a limitation of bacteriocin production.
An increase of the initial complex nutrient source concentration of MRS medium up to four times the standard concentration led to an increase in both the maximum specific growth rate (μmax) and the maximum attainable biomass (Xmax) and resulted in faster lactic acid production, indicating the limitation of nutrients in standard MRS medium. The decreased μmax at six times the standard complex nutrient source concentration may be due to the formation of toxic metabolites, for instance, those formed during sterilization of the medium. Slowing of lactic acid production was not observed at complex nutrient source concentrations higher than the concentration in standard MRS medium. The biomass concentration at which the two phases of inhibition started (X1 and X2) increased up to a fourfold increase in complex nutrient source concentration. Hence, the cells were able to grow faster and could reach a higher biomass before certain nutrients became limiting. A similar observation for increasing values of X1 and X2 with increasing nutrient concentrations has been made for L. sakei CTC 494 (28).
In the case of doubling the complex nutrient source concentration of standard MRS medium, the higher μmax and Xmax values reached also led to maximum bacteriocin activity in the cell-free culture supernatant (Bmax), since the specific bacteriocin production (kB) was comparable to that obtained in standard MRS medium. In contrast, at higher complex nutrient source concentrations, Bmax did not increase further because of a counterbalance due to a decreased kB with increasing nutrient concentrations. This is in accordance with the results obtained by Aymerich et al. (2), who showed that doubling the concentration of the separate components of standard MRS medium did not significantly alter bacteriocin production, while concentrations higher than threefold even had a detrimental effect on bacteriocin production. It has also been shown for Lactococcus lactis subsp. lactis ATCC 11454 that the relative nisin production rate was not directly proportional to the specific growth rate, since low nutrient concentrations supported a relatively higher specific nisin production rate than higher nutrient concentrations (21). Likewise, for Lactobacillus amylovorus DCE 471, specific bacteriocin production was reduced if the complex nitrogen source of the MRS medium was increased (5).
All these results are in accordance with the findings of De Vuyst et al. (10), who suggested that a low specific growth rate or unfavorable growth conditions (stress conditions) in general might stimulate bacteriocin production. In the case of L. curvatus LTH 1174, a lower specific growth rate as a result of stress due to nutrient limitation also induced higher specific bacteriocin production. With Enterococcus faecium RZS C5, maximum bacteriocin activity was observed with 25% of the complex nutrient source concentration of standard MRS medium. However, kB was unaffected, and it was hypothesized that the higher activity was due to a lower adsorption of the bacteriocin molecules onto the bacterial cells, since Xmax decreased by 30% (29).
When the bacteriological peptone concentration of standard MRS medium was increased fourfold, bacterial growth was boosted while specific bacteriocin production was unaffected. Although a comparable amount of biomass was produced with the fourfold increased concentration of Lab Lemco, kB and hence Bmax were lower. Both bacteriological peptone and Lab Lemco are of animal origin. However, bacteriological peptone is obtained through an enzymatic treatment of meat, while the latter is a conventional meat extract. When comparing the molecular mass distribution, a greater amount of smaller peptides are found in bacteriological peptone compared to Lab Lemco (4). It seems that bacteriological peptone contains compounds that stimulate growth by L. curvatus LTH 1174 without adversely affecting bacteriocin production. Bacteriological peptone also provided the best growth of Pediococcus damnosus NCFB 1832, followed by meat extract, and in both cases pediocin PD-1 activity was highest (34). Plantaricin 423 activity also reached the highest titers when Lactobacillus plantarum 423 was grown in MRS medium supplemented with 1.7% (wt/vol) bacteriological peptone (40). In contrast, the plantaricin production rate by L. plantarum TMW 1.25 was not affected by altering the nitrogen source of MRS medium (22).
The fourfold increased amount of yeast extract seemed to offer little additional nutrients necessary for growth, since the μmax value obtained was comparable to the value in standard MRS medium and Xmax was only slightly higher. When comparing the results obtained for elevated levels of Lab Lemco and yeast extract, an identical μmax was obtained. In the case of yeast extract, however, the maintenance coefficient was higher, and subsequently less energy was available for growth and bacteriocin production. This resulted in a lower Xmax and, due to the growth-associated character of bacteriocin production, a lower Bmax. Since kB was even slightly lower in the case of Lab Lemco compared to yeast extract, the higher bacteriocin activity was the result of a higher cell number attained in the case of the medium enriched with Lab Lemco. The relatively large amounts of growth factors, free amino acids, and short peptides and an overall higher content of amino nitrogen present in yeast extract did not seem to have a strong stimulatory effect on the growth of L. curvatus LTH 1174. This may be due to the adaptation of this strain to the nutrient spectrum present in its natural environment, fermented sausage. In contrast, sakacin P production by L. sakei CCUG 42687 was increased by addition of yeast extract and to a lesser extent by tryptone (1). Also, in the case of L. lactis subsp. lactis A164, biomass production was stimulated and bacteriocin activity was maximal when the basal medium without a nitrogen source present was supplemented with 3% yeast extract (7).
Amino acid analyses further revealed that only serine, tyrosine, and asparagine/aspartic acid were completely depleted with L. curvatus LTH 1174 in standard MRS. In view of the generation of aromatic compounds during sausage fermentation, the branched-chain amino acids, such as leucine, isoleucine, and valine, as well as phenylalanine or methionine are most important. They can lead to the production of aldehydes, alcohols, and acids which have a very low threshold value (33). Hence, the amino acid metabolism of L. curvatus LTH 1174 most probably will not contribute to the final sausage aroma. This has been shown previously for a strain of L. curvatus which demonstrated very low leucine metabolism (23). For Lactobacillus johnsonii La1, it has been shown that alanine, serine, isoleucine, and cysteine are the most important amino acids for its growth in milk (13).
Aasen et al. (1) found aspartic acid, asparagine, arginine, serine, and glycine to be completely consumed by L. sakei CCUG 42687. However, supplementing the medium with asparagine had no effect on the growth rate or sakacin P production by this strain (3). A study with L. sakei Lb790/pMLS114, a bacteriocin-negative strain transformed with a plasmid containing the sakacin P gene cluster, indicated complete consumption of the amino acids serine, asparagine, and aspartic acid. Bacteriocin production ceased when serine was depleted, while slow growth continued until the two other amino acids were depleted. Supplementing the medium with these limiting amino acids led to an increase in sakacin P production from 34 to more than 100 mg/liter, while cell mass only increased about 20% (I. M. Aasen, T. Møretrø, and L. Axelsson, Eurolab Conference, 2001, abstract, p. 160).
In this paper, it has been shown that the bacteriocin-producing meat isolate L. curvatus LTH 1174 was limited in its growth in standard MRS medium due to nutrient depletion. It is clear that this strain is adapted to a meat environment, since the nutrient spectrum from meat-derived components stimulated biomass production and resulted in higher bacteriocin titers. In contrast, yeast extract did not stimulate growth or bacteriocin production. This study shows that starter cultures to be applied in a certain food matrix should preferably be isolates from their natural environment. In addition, this study shows that nutrient-rich media should be used to mimic food fermentation processes, in particular with respect to bacteriocin production. Although such experiments are carried out in vitro, they will contribute to a better understanding of the behavior of the strain in a food environment.
Acknowledgments
We acknowledge financial support from the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT), in particular the STWW project Functionality of Novel Starter Cultures in Traditional Fermentation Processes. Also, this work was supported by the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders, and different food companies.
The technical assistance of Vincent Schrijvers and Kristof Verbrugghe was greatly appreciated. L. curvatus LTH 1174 was kindly provided by W. P. Hammes (Institut für Lebensmitteltechnologie, Universität Hohenheim, Stuttgart, Germany).
REFERENCES
- 1.Aasen, I. M., T. Møretrø, T. Katla, L. Axelsson, and I. Storrø. 2000. Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl. Microbiol. Biotechnol. 53:159-166. [DOI] [PubMed] [Google Scholar]
- 2.Aymerich, M. T., M. G. Artigas, M. Garriga, J. M. Montfort, and M. Hugas. 2000. Effect of sausage ingredients and additives on the production of enterocins A and B by Enterococcus faecium CTC 492. Optimization of in vitro production and anti-listerial effect in dry fermented sausages. J. Appl. Microbiol. 88:686-694. [DOI] [PubMed] [Google Scholar]
- 3.Benthin, S., and J. Villadsen. 1996. Amino acid utilization by Lactococcus lactis subsp. cremoris FD1 during growth on yeast extract or casein peptone. J. Appl. Bacteriol. 80:65-72. [Google Scholar]
- 4.Bridson, E. Y. 1998. The Oxoid manual, 8th ed. Oxoid, Basingstoke, United Kingdom.
- 5.Callewaert, R., and L. De Vuyst. 2000. Bacteriocin production with Lactobacillus amylovorus DCE 471 is improved and stabilized by fed-batch fermentation. Appl. Environ. Microbiol. 66:606-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charpentier, E., and P. Courvalin. 1999. Antibiotic resistance in Listeria spp. Antimicrob. Agents Chemother. 42:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheigh, C.-I., H.-J. Choi, H. Park, S.-B. Kim, M.-C. Kook, T.-S. Kim, J.-K. Hwang, and Y.-R. Pyun. 2002. Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J. Biotechnol. 95:225-235. [DOI] [PubMed] [Google Scholar]
- 8.Dainty, R., and H. Blom. 1995. Flavour chemistry of fermented sausages, p. 176-193. In G. Campbell-Platt and P. E. Cook (ed.), Fermented meats. Blackie Academic & Professional, London, United Kingdom.
- 9.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 22:130-135. [Google Scholar]
- 10.De Vuyst, L., R. Callewaert, and K. Crabbé. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817-827. [DOI] [PubMed] [Google Scholar]
- 11.Diep, D. B., L. Axelsson, C. Grefsli, and I. F. Nes. 2000. The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiology 146:2155-2160. [DOI] [PubMed] [Google Scholar]
- 12.Doβmann, M. U., R. F. Vogel, and W. P. Hammes. 1996. Mathematical description of the growth of Lactobacillus sake and Lactobacillus pentosus under conditions prevailing in fermented sausages. Appl. Microbiol. Biotechnol. 46:334-339. [DOI] [PubMed] [Google Scholar]
- 13.Elli, M., R. Zink, R. Reniero, and L. Morelli. 1999. Growth requirements of Lactobacillus johnsonii in skim and UHT milk. Int. Dairy J. 9:507-513. [Google Scholar]
- 14.Encinas, J.-P., J. J. Sanz, M. L. Garcia-Lopez, and A. Otero. 1999. Behaviour of Listeria spp. in naturally contaminated chorizo (Spanish fermented sausage). Int. J. Food Microbiol. 46:167-171. [DOI] [PubMed] [Google Scholar]
- 15.Glass, K. A., and M. P. Doyle. 1989. Fate of Listeria monocytogenes in processed meat products during refrigerated storage. Appl. Environ. Microbiol. 55:1565-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammes, W. P., and C. Hertel. 1996. Selection and improvement of lactic acid bacteria used in meat and sausage fermentation. Lait 76:159-168. [Google Scholar]
- 17.Hugas, M., B. Neumeyer, F. Pagés, M. Garriga, and W. P. Hammes. 1996. Comparison of bacteriocin producing lactobacilli on Listeria growth in fermented sausages. Fleischwirtschaft. 76:649-652. [Google Scholar]
- 18.Johnson, J. L., Doyle, M. P., and Cassens, R. G. 1990. Listeria monocytogenes and other Listeria spp. in meat and meat products: a review. J. Food Prot. 53:81-91. [DOI] [PubMed] [Google Scholar]
- 19.Juillard, V., D. Le Bars, E. R. S. Kunji, W. N. Konings, J.-C. Gripon, and J. Richard. 1995. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl. Environ. Microbiol. 61:3024-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juntilla, J., J. Hirn, P. Hill, and E. Nurmi. 1989. Effect of different levels of nitrite and nitrate on the survival of Listeria monocytogenes during the manufacture of fermented sausage. J. Food Prot. 52:158-161. [DOI] [PubMed] [Google Scholar]
- 21.Kim, W. S., R. J. Hall, and N. W. Dunn. 1997. The effect of nisin concentration and nutrient depletion on nisin production of Lactococcus lactis. Appl. Microbiol. Biotechnol. 48:449-453. [DOI] [PubMed] [Google Scholar]
- 22.Klostermaier, P., C. Heiko Scheying, M. Ehrmann, and R. F. Vogel. 1999. Mathematical evaluation of plantaricin formation supports an auto-induced production mechanism. Appl. Microbiol. Biotechnol. 51:462-469. [Google Scholar]
- 23.Larrouture, C., V. Ardaillon, M. Pépin, and M. C. Montel. 2000. Ability of meat starter cultures to catabolize leucine and evaluation of the degradation products by using an HPLC method. Food Microbiol. 16:563-570. [Google Scholar]
- 24.Lauret, R., F. Morel-Deville, F. Berthier, M. Champomier-Verges, P. Postma, S. D. Ehrlich, and M. Zagorec. 1996. Carbohydrate utilization in Lactobacillus sake. Appl. Environ. Microbiol. 62:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leistner, L. 1995. Stable and safe fermented sausages world-wide, p. 160-175. In G. Campbell-Platt and P. E. Cook (ed.), Fermented meats. Blackie Academic & Professional, London, United Kingdom.
- 26.Leroy, F., and L. De Vuyst. 1999. Temperature and pH conditions that prevail during fermentation of sausages are optimal for production of the antilisterial bacteriocin sakacin K. Appl. Environ. Microbiol. 65:974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroy, F., and L. De Vuyst. 1999. The presence of salt and curing agent reduces bacteriocin production by Lactobacillus sakei CTC 494, a potential starter culture for sausage fermentation. Appl. Environ. Microbiol. 65:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroy, F., and L. De Vuyst. 2001. Growth of the bacteriocin-producing Lactobacillus sakei CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: a nutrient depletion model for the growth of lactic acid bacteria. Appl. Environ. Microbiol. 67:4470-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leroy, F., S. Vankrunkelsven, J. De Greef, and L. De Vuyst. 2003. The stimulating effect of a harsh environment on the bacteriocin activity by Enterococcus faecium RZS C5 and dependency on the environmental stress factor used. Int. J. Food Microbiol. 83:26-38. [DOI] [PubMed] [Google Scholar]
- 30.Loubière, P., M. Cocaign-Bousquet, J. Matos, G. Goma, and N. D. Lindley. 1997. Influence of end-products inhibition and nutrient limitations on the growth of Lactococcus lactis subsp. lactis. J. Appl. Microbiol. 82:95-100. [Google Scholar]
- 31.Lücke, F.-K. 1998. Fermented sausages, p. 441-483. In B. J. B. Wood (ed.), Microbiology of fermented foods. Blackie Academic & Professional, London, United Kingdom.
- 32.Messens, W., J. Verluyten, F. Leroy, and L. De Vuyst. 2003. Modelling growth and bacteriocin production by Lactobacillus curvatus LTH 1174 in response to temperature and pH values used for European sausage fermentation processes. Int. J. Food Microbiol. 81:41-52. [DOI] [PubMed] [Google Scholar]
- 33.Montel, M. C., F. Masson, and R. Talon. 1998. Bacterial role in flavour development. Meat Sci. 49(Suppl. 1):S111-S123. [PubMed] [Google Scholar]
- 34.Nel, H. A., R. Bauer, E. J. Vandamme, and L. M. T. Dicks. 2001. Growth optimization of Pediococcus damnosus NCFB 1832 and the influence of pH and nutrients on the production of pediocin PD-1. J. Appl. Microbiol. 91:1131-1138. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Barba, J. L., and R. Jiménez-Díaz. 1994. Vitamin and amino acid requirements of Lactobacillus plantarum strains isolated from green olive fermentations. J. Appl. Bacteriol. 76:350-355. [DOI] [PubMed] [Google Scholar]
- 36.Teuber, M. 1999. Spread of antibiotic resistance with food-borne pathogens. Cell. Mol. Life Sci. 56:755-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curvacin-A from Lactobacillus curvatus LTH 1174 and sakacin-P from L. sake LTH 673. Syst. Appl. Microbiol. 14:460-468. [Google Scholar]
- 38.Uyttendaele, M., P. De Troy, and J. Debevere. 1999. Incidence of Listeria monocytogenes in different types of meat products on the Belgian retail market. Int. J. Food Microbiol. 53:75-80. [DOI] [PubMed] [Google Scholar]
- 39.Van Niel, E. W. J., and B. Hahn-Hägerdal. 1999. Nutrient requirements of lactococci in defined growth media. Appl. Microbiol. Biotechnol. 52:617-627. [Google Scholar]
- 40.Verellen, T. L. J., G. Bruggeman, C. A. Van Reenen, L. M. T. Dicks, and E. J. Vandamme. 1998. Fermentation optimization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum 423. J. Ferm. Bioeng. 86:174-179. [Google Scholar]
- 41.Verluyten, J., W. Messens, and L. De Vuyst. 2003. The curing agent sodium nitrite, used in the production of fermented sausages, is less inhibiting to the bacteriocin-producing meat starter culture Lactobacillus curvatus LTH 1174 under anaerobic conditions. Appl. Environment. Microbiol. 69:3833-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verluyten, J., W. Messens, and L. De Vuyst. 2004. Sodium chloride reduces production of curvacin A, a bacteriocin produced by the Lactobacillus curvatus strain LTH 1174 originating from fermented sausage. Appl. Environment. Microbiol. 70:2271-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel, R. F., B. S. Pohle, P. S. Tichaczek, and W. P. Hammes. 1993. The competitive advantage of Lactobacillus curvatus LTH 1174 in sausage fermentations is caused by formation of curvacin A. Syst. Appl. Microbiol. 15:457-462. [Google Scholar]
- 44.White, D. G., S. Zhao, S. Simjee, D. D. Wagner, and P. F. McDermott. 2002. Antimicrobial resistance of foodborne pathogens. Microbes Infect. 4:405-412. [DOI] [PubMed] [Google Scholar]