Abstract
Optimized phenolics from oregano and cranberry extracts were evaluated for antimicrobial activity against Listeria monocytogenes in laboratory media and in beef and fish. The antimicrobial activity increased when oregano and cranberry extracts were mixed at a ratio of 75% oregano and 25% cranberry (wt/wt) with 0.1 mg of phenolic per disk or ml, and the efficacy was further enhanced by lactic acid. The inhibition by phytochemical and lactic acid synergies was most effective when beef and fish slices were stored at 4°C.
Listeria species have been isolated from diverse sources such as soil, decaying vegetable matter, silage, sewage, water, animal feed, fresh and processed meats, raw milk, cheese, slaughterhouse waste, and asymptomatic human and animal carriers (1). Owing to their widespread occurrence, Listeria species have many opportunities to enter food production and processing environments. Consequently, outbreaks and sporadic cases of listeriosis have been traced to different foodstuffs, such as dairy products (7, 11).
Listeria monocytogenes is a psychrotrophic, gram-positive bacterium found in many food products (10) and has been associated with food-borne outbreaks, mostly in North America and Europe (6). Since May 2000, multistate outbreaks of listeriosis linked to L. monocytogenes in deli turkey meat have been identified in 10 states (3). These cases have been associated with four deaths and three miscarriages or stillbirths. Listeriosis is regarded as a major problem because of its mortality rate, particularly in neonates and elderly citizens. Owing to the psychrotrophic character of L. monocytogenes, it has the ability to proliferate at refrigeration temperatures, making it difficult to control in refrigerated foods. Further, L. monocytogenes can tolerate acid pHs as low as 4.4 (2, 8, 16, 19). Antimicrobial strategies to overcome low pH tolerance are essential, and use of natural phytochemicals is promising (23, 24).
Many natural compounds found in dietary plants, such as extracts of herbs and fruit extracts, have been shown to possess antimicrobial activities against L. monocytogenes (5, 12, 14, 17). Thymol and carvacrol, found in oregano extract, have been evaluated and were effective at inhibiting the growth of L. monocytogenes. Approximately 150 to 200 ppm of pure carvacrol or thymol significantly inhibited the growth of L. monocytogenes (23). Further, a variety of herbs and spices have been used in suppressing the growth of L. monocytogenes (15, 17). Numerous other plant extracts have been tested, but results were not always consistent (12, 18, 28). This inconsistency of commercial samples of plant phytochemicals and different varieties of the same herbs may be due to the varying amounts of critical compounds (e.g., phenolic compounds), which could account for differences in antimicrobial potency. Despite the inconsistency, some plant extracts were also found to be effective against L. monocytogenes in meat products (12, 21, 29), for example, rosemary in ready-to-eat sausage (21). Owing to the partial hydrophobic nature of several phenolic phytochemicals, they are able to work more effectively at the interface of the lipid- and water-compatible portions of the meat. This makes them a suitable ingredient to apply in meat systems. A previous study has noted that L. monocytogenes was usually less sensitive to these phytochemical extracts in meat (compared to culture media) and sensitivity also varied with the fat content of the meat (17). Our research investigated whether the sensitivity problem in meat systems can be resolved if the water- and ethanol-extractable phenolic profile is optimized by using an oregano-and-cranberry mixture to enhance antimicrobial efficacy. Therefore, the use of these synergistic, natural, food grade antimicrobial ingredients may provide additional hurdle technology protection beyond that of physical and other chemical treatment methods. Further, owing to consumer interest in natural ingredients and functional foods, a food grade botanical ingredient with water- and ethanol-soluble antioxidant properties would be a preferred choice for hurdle technology. We demonstrate here the potential of using a water-soluble phenolic antioxidant containing oregano and cranberry extracts combined with lactic acid to inhibit L. monocytogenes growth in laboratory medium and muscle foods such as meat and fish.
L. monocytogenes Scott A 4b was used to test the efficacy of antimicrobial extracts from oregano and cranberry. L. monocytogenes Scott A 4b was maintained on slants (tryptic soy agar plus 1.5% yeast extract [TSA]; Difco) at 4°C. Stock cultures were grown in tryptic soy broth plus 1.5% yeast extract (TSB; Difco) at 37°C and shaken for 16 h prior to use. From this, 100 μl was spread plated on the same medium plus agar and then incubated at 37°C for 16 h to obtain a bacterial lawn.
Water-soluble oregano (Origanum vulgare) and cranberry (Vaccinium macrocarpon) extract powders were obtained from Barrington Chemicals, Harrison, N.Y., and Decas Cranberry Products, Wareham, Mass., respectively. Ten grams of different ratios of an oregano-cranberry powder mixture was added to 100 ml of water to make concentrated stock extracts, which were stored at 4°C. Different ratios of concentrated extracts were then diluted on a total-phenolic basis and filter sterilized before use. The total phenolic contents of the oregano and cranberry powders used were 16 and 5 mg/g (dry-weight [dw] basis), respectively. The melting point of these extracts was in the range of 180 to 200°C. The major phenolic found in these extracts was rosmarinic acid, at a minimum of 5% (dw) of the total phenolics.
Agar diffusion assays were done aseptically with sterile 10-mm-diameter paper disks (Schleicher & Schuell, Inc., Keene, N.H.) to which 100 μl of the test phytochemical extracts was added. Phytochemical saturated disks (three disks per concentration) were placed onto the surface of seeded agar plates. The concentration of test extracts was adjusted such that each disk had only 100 μl of extract mixture with the desired total phenolic concentration (e.g., 0.1, 0.2, 0.3 or 0.4 mg/100 μl). Each experiment consisted of three replicates of various phytochemical concentrations. Each experiment was repeated three times. Statistical analysis was done by analysis of variance (single-factor F test; P < 0.05). Controls consisted of disks without phytochemicals. Treated plates were inverted and immediately placed at 37°C. After 18 h, the diameter of the inhibition zone surrounding each disk was measured. Various initial combinations of oregano and cranberry (Table 1) were evaluated for antimicrobial efficacy. To investigate the phytochemical and lactic acid synergy effects, a 75-to-25% (wt/wt) oregano-and-cranberry mixture was used. The phenolic levels were 0.05, 0.1, 0.15, and 0.2 mg/disk. TSA plates were adjusted with lactic acid to different pH levels (7.0, 6.0, and 5.5).
TABLE 1.
Total phenolic contents of oregano and cranberry in various mixtures
| % Oragano | % Cranberry | Amt (mg) of phenolic/g (dry wt) |
|---|---|---|
| 100 | 0 | 19.1 |
| 90 | 10 | 18.0 |
| 75 | 25 | 16.3 |
| 50 | 50 | 14.1 |
| 25 | 75 | 11.9 |
| 10 | 90 | 9.1 |
| 0 | 100 | 8.2 |
The total phenolic content was determined as gallic acid equivalents (22). This method was originally developed by Chandler and Dodds (4) and later modified for oregano phenolics (25). For this, approximately 50 mg of different ratios of phytochemical powder mixtures was added to glass tubes (150 by 15 mm). Two and one-half milliliters of distilled water was added, and the tubes were kept at −4°C for 72 h until used. After thawing, the sample was homogenized with Tissue-Tearor (Biospec Products, Bartlesville, Okla.) and centrifuged at 13,000 × g for 10 min (25°C). One milliliter of the supernatant fluid was transferred to a test tube to which 6 ml of distilled water and 0.5 ml of Folin-Ciocalteu phenol reagent (Sigma Chemical Co., St. Louis, Mo.) were added. After incubation for 5 min (25°C), 1 ml of 5% Na2CO3 was added. The tubes were mixed well and kept in the dark (25°C) for 1 h. The samples were vortexed, and the A725 was measured. The phenolic content was then calculated from the absorbance versus a gallic acid standard curve.
In broth studies, five ratios of extract mixtures were prepared in 5 ml of TSB (pH adjusted to 7.0). A critical issue was color development by extracts, which had the potential to interfere with absorbance. Therefore, the phenolic level in the broth was reduced to 0.01 mg/ml. The effect of a phenolic level of 0.1 mg/ml was also evaluated. The final concentration in each tube was 0.01 mg of phenolic/ml. The initial inoculation level was ∼103 CFU/ml, and the tubes were shaken at 37°C for 10 h. The A600 was read every 2 h. Similarly, inhibition at 0.1 mg of phenolic/ml was also evaluated by plate counts to avoid potential interference.
In meat studies, oregano-and-cranberry mixtures (75 and 25%, wt/wt) were used with a total phenolic content of 0.1 mg/ml. Excess fat from sirloin tips was trimmed with a sterile knife. To reduce background contamination, sirloin tips (∼300 g) were placed on a rack, frozen at −20°C for 3 h, and thawed (at 55°C for 30 min). This was repeated three or four times and followed by exposure to UV light (115 V, 0.2 A; Spectroline Co., Westbury, N.Y.) for 15 min. The sirloin tips treated by this method did not alter L. monocytogenes growth compared to the sirloin tips without any treatment. Sirloin tips were then cooled to 4°C. Meat tips were cut into 5-g slices with a sterile knife, placed in sterile petri dishes, and immediately refrigerated at 4°C. Each experiment consisted of three replicates of various phytochemical concentrations. Each experiment was repeated three times. Controls consisted of meat slices treated with water only. Oregano-cranberry mixtures (75 and 25%, wt/wt) were adjusted to the appropriate pH. Individual extracts were prepared to make a final concentration of 0.1 mg of phenolic/ml of solution. Each 5-g meat slice was then dipped into 100 ml of prepared extract solution. Excess solution was removed by placing the dipped slices onto a presterilized paper towel before transferring them to petri dishes for incubation. Treated meat slices in sterile petri dishes were kept in a laminar-flow biological safety cabinet for 15 min. This allowed extra moisture to evaporate before inoculation with test microbes. The treated meat slices were then dipped into 100-ml cell suspensions (∼104 cells per ml). Cell suspensions were prepared from a 16-h culture of L. monocytogenes that had been centrifuged at 10,000 × g and resuspended in 0.1 M phosphate buffer, pH 7.0. The inoculated meat slices were then placed in separate sterile petri dishes. Initial counts were performed after dipping in a 100-ml cell suspension. Inoculated meat slices were then incubated at 37°C for 18 h. The initial tests were performed at 37°C since broth medium studies were done at 37°C. Subsequent experiments were done at 4 and 25°C to mimic typical storage conditions. To enumerate L. monocytogenes, 0.1 M phosphate buffer was added to each stomacher bag (5-g beef slice in 45 ml of phosphate buffer), and the contents were mixed in a Stomacher 400 (Tekmar, Cincinnati, Ohio) for 2 min. Mixtures were serially diluted (10-fold) with 0.1 M phosphate buffer, pH 7.0. Subsequently, 0.1 ml of each diluted mixture was spread on TSA. Plates were incubated at 37°C for 18 h before enumeration.
The skin of cod fish fillets was removed with a sterile knife. Pieces of fish (∼150 g) were frozen and thawed three or four times and exposed to UV light for 15 min to reduce the background flora as described above. The fish fillet was cut into ∼10-g slices. Different ratios of extract mixtures with appropriate pH adjustment were prepared to make a final concentration of 0.1 mg of phenolic/ml of solution. Fish slices (10 g) were then dipped into 100 ml of the prepared solution.
The treated slices were then inoculated by dipping into a 100-ml cell suspension (∼104 cells per ml) and placed in separated preautoclaved petri dishes. The inoculated fish slices were then incubated at 37°C for 18 h and similarly at 25 and 4°C. To enumerate L. monocytogenes cells, 90 ml of 0.1 M phosphate buffer was added to the stomacher bag and mixed for 2 min. L. monocytogenes cells were then enumerated as in the beef study. Each experiment consisted of three replicates of various phytochemical concentrations. Each experiment was repeated three times.
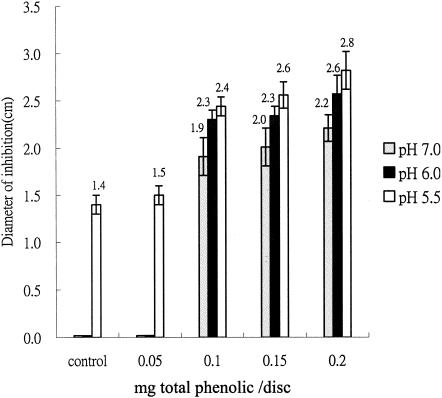
Extracts of oregano and cranberry produced a clear inhibition zone against L. monocytogenes in agar diffusion assays. One-tenth milligram of phenolic per disk was determined from preliminary investigations to be the effective concentration. Optimal antimicrobial activity against L. monocytogenes was observed when the mixture contained 75% oregano with 25% cranberry (wt/wt) (data not shown). Pure cranberry extracts showed better inhibition than pure oregano extracts at pH 7.0 when tested on an equivalent phenolic basis (0.1 mg/disk) (data not shown). This may be due to the acidic character of cranberry phenolics. When the pH was adjusted to 6.0, pure oregano extracts on an equivalent phenolic basis were more effective at inhibiting L. monocytogenes growth than were pure cranberry extracts. Our results, however, show that mixtures of oregano and cranberry (75 and 25%, wt/wt) had the best inhibition on both pH 7.0 and pH 6.0 plates (data not shown). The antimicrobial effect of phytochemical extracts was further enhanced by adding lactic acid (Fig. 1). Control plates were also adjusted to pHs 5.5 and 6.0 with lactic acid. The antimicrobial efficacy of the phytochemical extract and lactic acid together was clearly superior to that of lactic acid alone (Fig. 1).
FIG. 1.
Inhibition of L. monocytogenes by oregano and cranberry extracts (75 and 25%, respectively) with pH levels adjusted with lactic acid. Controls consisted of addition of water under the same conditions.
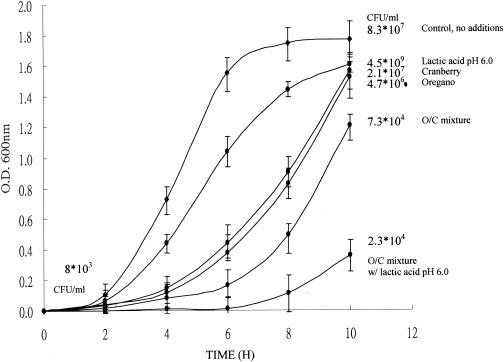
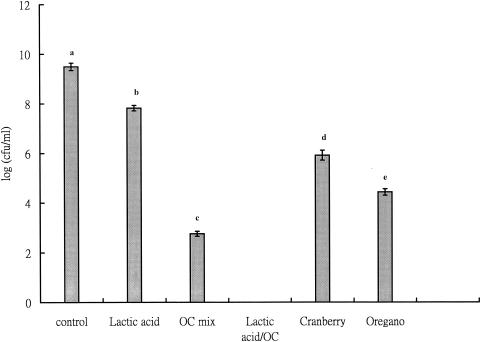
From our previous research, oregano extracts alone can effectively inhibit L. monocytogenes in broth (23). Results here show that the antimicrobial effect of oregano can be enhanced by addition of cranberry extract at an equivalent phenolic concentration and further that the antimicrobial effect of the extract mixture is significantly enhanced in the presence of lactic acid (Fig. 2). In broth studies, phytochemical extracts show not only a longer lag phase but also a lower absorbance at the end of 10 h (Fig. 2). In all cases after 10 h, the population of L. monocytogenes was greater than the initial level (8 × 103/ml) but the tubes with phytochemical extracts had lower cell counts than the control. At 0.01 mg of phenolic/ml, viable cell counts were obtained at the end of incubation (Fig. 2). Decreases of 1.7- and 2.9-fold were observed with cranberry and oregano alone, respectively. The viable cell count was even lower when an oregano-cranberry extract mixture was used (3.8-log decrease compared to the control), and as observed in the agar diffusion study, the viable cell count was even lower when lactic acid was used. In order to compare results from the disk assay, broth studies were also done with 0.1 mg of phenolic/ml. Since such a high phenolic level affected absorbance, plate counts were necessary. Figure 3 shows that the cell counts were even lower as the phenolic concentration was increased to 0.1 mg/ml. An oregano-and-cranberry mixture (optimized to a phenolic content of 0.1 mg/ml; 75 and 25%, wt/wt), when combined with lactic acid (pH 6.0), completely inhibited L. monocytogenes. At 0.01 mg of phenolic per ml, maximum cell counts were lower than in the tubes without treatment (Fig. 2). However, when the phenolic level was increased to 0.1 mg/ml and combined with lactic acid, growth of L. monocytogenes was totally inhibited (Fig. 3).
FIG. 2.
Inhibition of L. monocytogenes by oregano and cranberry extracts in TSB at pHs 7.0 and 6.0. O/C, oregano and cranberry at 75 and 25%, wt/wt, respectively. Viable counts were determined at 0 and 10 h. The total phenolic concentration was 0.01 mg/ml. (O.D., optical density.)
FIG. 3.
Synergistic effect of oregano-and-cranberry mixtures on inhibition of L. monocytogenes in broth. The total phenolic concentration was 0.1 mg/ml, and viable cells were monitored after incubation at 37°C for 10 h. Controls consisted of water in place of extracts. O/C mix, oregano-and-cranberry mixture at 75 and 25%, wt/wt, respectively. Values with the same letter are not significantly different. The initial pH was 7.0, except for tubes containing lactic acid (pH 6.0).
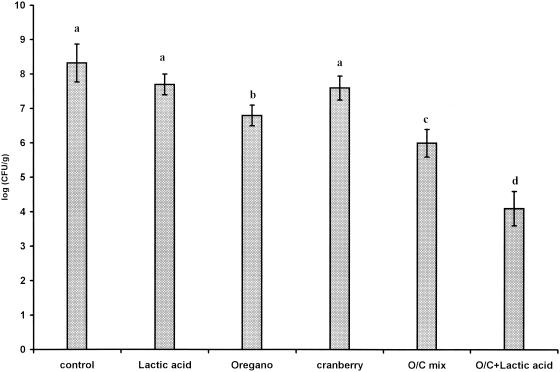
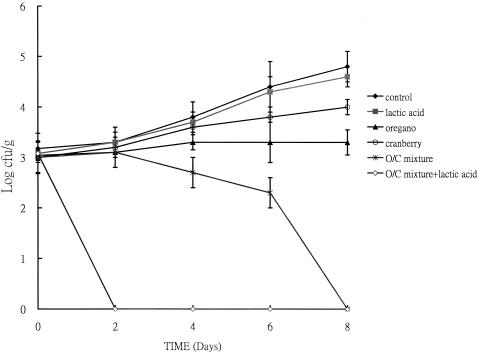
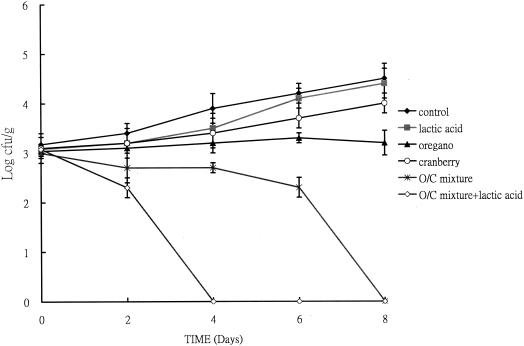
The effect of a mixture of oregano and cranberry (0.1 mg of phenolic/ml) on beef slices and cod fish fillet was determined. After 18 h of incubation, samples treated with phytochemical extracts had no significant difference in cell numbers at pH 7.0 (data not shown), but when the pH was adjusted to 6.0 with lactic acid, differences in viable cell counts were observed in both beef slices (Fig. 4) and fish slices (data not shown). After 18 h, the cell count of beef slices with the oregano-cranberry mixture was 2.8 logs lower than that of slices without any treatment (Fig. 4). In fish slices, cell counts of slices treated with the extract mixture were 2.3 logs lower than slices without any treatment (data not shown). Also, the slices treated with an oregano-cranberry extract mixture had lower viable counts than the slices treated with the individual extracts. For example, final cell numbers were about 1.5 logs lower when beef slices were treated with an oregano-cranberry mixture compared to those of slices treated with pure oregano extract only and 2.5 logs lower compared to those of slices treated with pure cranberry extract only (Fig. 4). In fish fillets, cell counts of slices treated with an oregano-cranberry mixture were 0.8 and 1.6 logs lower compared to those of slices treated with pure oregano and cranberry, respectively (data not shown). The efficacy of extracts and lactic acid together was also tested at 4 and 25°C. For beef slices stored at 4°C for 8 days, cranberry or oregano alone inhibited L. monocytogenes (Fig. 5). A mixture of both reduced the bacterial numbers to less than that of the initial inoculum. The antimicrobial effect was even more significant when extract mixtures were applied at pH 6.0 (adjusted with lactic acid). Similar results were observed with fish slices (Fig. 6).
FIG. 4.
Synergistic effect of oregano-and-cranberry mixture on inhibition of L. monocytogenes on beef slices at pH 6.0. The total phenolic concentration was 0.1 mg/ml. Slices were incubated at 37°C for 18 h. O/C, oregano and cranberry at 75 and 25%, wt/wt, respectively. Values with the same letter are not significantly different. Controls were beef slices in which antibacterial agents were replaced with the same volume of water. The initial count of L. monocytogenes cells was ∼103/g.
FIG. 5.
Synergistic effect of an oregano-and-cranberry mixture on inhibition of L. monocytogenes on beef slices, pH 6.0. Slices were incubated at 4°C for 8 days. The total phenolic concentration was 0.1 mg/ml. O/C, oregano-and-cranberry mixture at 75 and 25%, wt/wt, respectively. Controls were beef slices in which antibacterial agents were replaced with the same volume of water.
FIG. 6.
Synergistic effect of an oregano-and-cranberry mixture on inhibition of L. monocytogenes on fish slices, pH 6.0. Slices were incubated at 4°C for 8 days. The total phenolic concentration was 0.1 mg/ml. O/C, oregano-and-cranberry mixture at 75 and 25%, wt/wt, respectively. Controls were fish slices in which antibacterial agents were replaced with the same volume of water.
The antimicrobial effects of many plant extracts have been well studied (5, 9, 13). These natural products are widely used in food preparation and are classified as generally regarded as safe. Thus, the use of such naturally occurring plant products may provide a potential additional barrier (hurdle technology) to inhibit the growth of food-borne pathogens in food products. However, little information exists regarding the use of such phenolic-optimized antimicrobial combinations in foods. The effects of phenolic-based plant extracts and acid interaction with various foods may not be the same for all extracts. Synergistic effects of combined plant extracts provide a wide range of phenolic diversity, significantly increasing or decreasing antimicrobial efficacy. Therefore, a specific phenolic profile can be designed for different foods. Also, the diversity of phenolic types greatly increases functionality for health and wellness (e.g., antioxidants and for oxidation-linked diseases).
Oregano and cranberry, useful botanicals generally regarded as safe for food flavoring and as functional ingredients, are known for their antimicrobial activity linked to the phenolic moiety and therefore are suitable as antimicrobial natural extracts when effectively combined with lactic acid. The partial hydrophobic nature of their phenolic constituents allows for accumulation and attachment to the bacterial cytoplasmic membrane, where inhibitory effects may eventually lead to cell death. In this study, the effects of simultaneous treatment with an oregano-cranberry extract mixture and lactic acid on the inhibition of L. monocytogenes were demonstrated.
Acidification of foods with short-chain organic acids, either by fermentation or by deliberate addition, is an important and widespread mechanism for controlling food-borne pathogens in a variety of foods. However, a number of studies have demonstrated that L. monocytogenes is more acid tolerant than most food-borne pathogens, although the sensitivity of the organism to organic acids varies with the nature of the acidulant used (27). The optimum pH for L. monocytogenes growth is 7 to 8 but they will grow in the pH range of 5 to 10. Recent studies have shown that L. monocytogenes may survive and grow in material with a pH as low as 4.4 (2). The innate resistance of L. monocytogenes to many of the acid food preservation systems that are effective against other food-borne pathogens has prompted research aimed at developing combination systems for more effective control of this pathogen (20). Therefore we tested the phenolic phytochemical synergies of oregano-and-cranberry extract mixtures following acidification with lactic acid. As shown here, this combination is more effective than when each is used alone.
Previous research also indicated the effectiveness of the essential oils from plants can be reduced in foods owing to the presence of components, such as proteins and fats, that immobilize and inactivate the essential oil components (26). Our work shows that the antimicrobial activity of an oregano-and-cranberry mixture used at the same phenolic concentration was superior at inhibiting L. monocytogenes than were the individual extracts. When different extract ratios based on phenolic content were used, the numbers of viable L. monocytogenes bacteria decreased, indicating their susceptibility to specific ratios of extract mixtures. This difference may be due to the phenolic type of one specific extract that may cause membrane damage, making cells more sensitive to hyperacidification by a different set of phenolics. Damage to the cell membrane might explain the observed effects, since low-pH exposures can cause sublethal injury to cell membranes, causing disruption of proton motive force, owing to loss of H+-ATPase. This could make the bacteria more susceptible to phenolic antimicrobial compounds found in oregano-cranberry extract mixtures, which are more enhanced in diphenolics than in cranberry alone. Further, we postulate that phenolic-containing extracts themselves at higher concentrations may create a low-pH microenvironment owing to proton donation and cell membrane disruption owing to stacking, which is likely more effective than a low pH alone. If this is further confirmed, then this is an excellent strategy to design the right plant extracts with a specific phenolic profile and pH conditions to attack food-borne bacterial pathogens in a food matrix. However, the exact mechanisms of cellular damage by phenolics at low pH have not been elucidated. This will be further investigated in our laboratory.
REFERENCES
- 1.Abrahim, A., A. Papa, N. Soultos, I. Ambrosiadis, and A. Antoniadis. 1998. Antibiotic resistance of Salmonella spp. and Listeria spp. isolates from traditionally made fresh sausages in Greece. J. Food Prot. 61:1378-1380. [DOI] [PubMed] [Google Scholar]
- 2.Barker, C., and S. F. Park. 2001. Senitization of Listeria monoytogenes to low pH, organic acid, and osmotic stress by ethanol. Appl. Environ. Microbiol. 67:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Multistate outbreak of listeriosis—United States, 2000. Morb. Mortal. Wkly. Rep. 49:1129-1130. [PubMed] [Google Scholar]
- 4.Chandler, S. F., and J. H. Dodds. 1983. The effect of phosphate nitrogen and sucrose on the production of phenolics and socosidine in callus cultures of Solanum tuberosum. Plant Cell Rep. 2:105-108. [DOI] [PubMed] [Google Scholar]
- 5.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, J. L. 1989. A perspective on listeriosis. Food Technol. 43:52-59. [Google Scholar]
- 7.Dalton, C. B., C. C. Austin, J. Sobel, P. S. Hayes, W. F. Bibb, L. M. Graves, B. Swaminathan, M. E. Proctor, and P. M. Griffin. 1997. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N. Eng. J. Med. 336:100-105. [DOI] [PubMed] [Google Scholar]
- 8.Davis, M. J., P. J. Coote, and C. P. O'Byrne. 1996. Acid tolerance in Listeria monocytogenes: the adaptive acid tolerance response (ATR) and growth-phase-dependent acid resistance. Microbiology 142:2975-2982. [DOI] [PubMed] [Google Scholar]
- 9.Dean, S. G., and G. Richie. 1987. Antimicrobial properties of plant essential oils. Int. J. Food Microbiol. 5:165-180. [Google Scholar]
- 10.Farber, J., and P. Peterkin. 1991. Listeria sp. in foods: Listeria monocytogenes, a foodborne pathogen. Microbiol. Rev. 55:493-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilot, P., C. Hermans, M. Yde, J. Gigi, M. Janssens, A. Genicot, P. Andre, and G. Wauters. 1997. Sporadic case of listeriosis associated with the consumption of a Listeria monocytogenes-contaminated ‘Camembert' cheese. J. Infect. 35:195-197. [DOI] [PubMed] [Google Scholar]
- 12.Hao, Y. Y., R. E. Brackett, and M. P. Doyle. 1998. Inhibition of Listeria monocytogenes and Aeromonas hydrophila by plant extracts in refrigerated cooked beef. J. Food Prot. 61:307-312. [DOI] [PubMed] [Google Scholar]
- 13.Jay, J. M., and G. M. Rivers. 1984. Antimicrobial activity of some food flavoring compounds. J. Food Safety 6:129-139. [Google Scholar]
- 14.Kim, J., M. Marshall, and C. Wei. 1995. Antibacterial activity of some essential oil components against five food borne pathogens. J. Agric. Food Chem. 43:2839-2845. [Google Scholar]
- 15.Kouassi, Y., and L. A. Shelef. 1998. Inhibition of Listeria monocytogenes by cinnamic acid—possible interaction of the acid with cysteinyl residues. J. Food Safety 18:231-242. [Google Scholar]
- 16.Kroll, R. G., and R. A. Patchett. 1992. Induced acid tolerance in Listeria monocytogenes. Lett. Appl. Microbiol. 14:224-227. [Google Scholar]
- 17.Larson, A. E., R. R. Y. Yu, O. A. Lee, S. Price, G. J. Haas, and E. A. Johnson. 1996. Antimicrobial activity of hop extracts against Listeria monocytogenes in media and in food. Int. J. Food Microbiol. 33:195-207. [DOI] [PubMed] [Google Scholar]
- 18.Lis-Balchin, M., and S. G. Deans. 1997. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 82:759-762. [DOI] [PubMed] [Google Scholar]
- 19.O'Driscoll, B., C. G. Gahan, and C. Hill. 1996. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh, D. H., and D. L. Marshall. 1993. Antimicrobial activity of ethanol, glycerol monolaurate or lactic acid against Listeria monocytogenes. Int. J. Food Microbiol. 20:239-246. [DOI] [PubMed] [Google Scholar]
- 21.Pandit, V. A., and L. A. Shelef. 1994. Sensitivity of Listeria monocytogenes to rosemary (Rosmarinus officinalis L.). Food Microbiol. 11:57-63. [Google Scholar]
- 22.Perry, P. L., and K. Shetty. 1999. A model for involvement of proline during Pseudomonas-mediated stimulation of rosmarinic acid levels in oregano shoot clones. Food Biotechnol. 13:137-154. [Google Scholar]
- 23.Seaberg, A. C., R. G. Labbe, and K. Shetty. 2003. Inhibition of Listeria monocytogenes by elite clonal extracts of oregano (Origanum vulgare). Food Biotechnol. 17:129-149. [Google Scholar]
- 24.Shetty, K., and R. G. Labbe. 1998. Food borne pathogens, health and role of dietary phytochemicals. Asia Pac. J. Clin. Nutr. 314:270-276. [PubMed] [Google Scholar]
- 25.Shetty, K., O. F. Curtis, R. E. Levin, R. Withowsky, and W. Ang. 1995. Prevention of vitrification associated with in vitro shoot cultures of oregano (Origanum vulgare) by Pseudomonas spp. J. Plant Physiol. 147:447-451. [Google Scholar]
- 26.Smid, E. J., and L. G. M. Gorris. 1999. Natural antimicrobials for food preservation, p. 285-308. In M. S. Rahman (ed.), Handbook of food preservation. Marcel Dekker, New York, N.Y.
- 27.Sorrells, K. M., D. C. Enigl, and J. R. Hatfield. 1989. Effect of pH, acidulant, time and temperature on the growth and survival of Listeria monocytogenes. J. Food Prot. 52:571-573. [DOI] [PubMed] [Google Scholar]
- 28.Stroh, W. H. 1993. New biotechnologies set to impact industrial food preservative market. Genetic Eng. News 13:8. [Google Scholar]
- 29.Ward, S. M., P. J. Delaquis, R. A. Holley, and G. Mazza. 1998. Inhibition of spoilage and pathogenic bacteria on agar and pre-cooked roast beef by volatile horseradish distillates. Food Res. Int. 31:19-26. [Google Scholar]