Abstract
The alterations in the balance of the normal intestinal bacterial flora of chickens exposed to acidified wood-derived litter were analyzed and compared to those of a control group exposed to nonacidified litter. A total of 1,728 broilers were divided into two groups, with six replicates in each. One group was exposed to dry wood-derived litter, and the other was exposed to dry wood-derived litter sprayed with a mixture of sodium lignosulfonate, formic acid, and propionic acid. At five different times, five chickens from each pen were killed and the intestinal contents from ileum and caeca were collected. The samples were diluted and plated onto selective media to identify coliforms, Lactobacillus spp., Clostridium perfringens, and Enterococcus spp. Covariance analysis of bacterial counts showed significantly lower counts for C. perfringens in the caeca and the ileum and for Enterococcus spp. and Lactobacillus spp. in the ileum in chickens exposed to the acidified litter. Lactobacillus spp. showed significantly higher counts in the caeca in chickens exposed to acidified litter. There was no difference between the two litters with regard to coliforms in the ileum and the caeca or to Enterococcus spp. in the caeca. The study shows that exposing the chickens to acidified litter lowers the intestinal bacterial number, especially in the ileum, without negative consequences for the chicken's health or performance. Of special interest are the lower counts of C. perfringens and Enterococcus spp. that might reduce the risk of developing clinical or subclinical necrotic enteritis and growth depression.
Antibiotic growth promoters (AGPs) have been used in animal production worldwide since 1946, when their positive effects were observed for the first time (29). AGPs are antibiotics added to animal feed at subtherapeutic levels to increase growth, improve feed efficiency, and decrease the incidence of diseases (1, 18).
The use of antibiotics over time in human and animal medicine and for growth promotion in animals has caused a large pressure on the microfloras, with the consequent appearance of resistance to these antibiotics among pathogenic bacteria (4, 35, 36, 37, 46). A lot of attention is being focused upon this problem, which has resulted in the banning and/or regulation of the use of AGPs by a number of countries and an increasing interest in organic farming. Development of alternative products and improved management is therefore necessary to eliminate the use of AGPs while achieving the same productivity.
Organic acids (OAs) have increasingly and successfully been supplemented in feed in swine and broiler production. The way of action of OAs seems to be related to a reduction of pH in the upper intestinal tract, interfering with the growth of undesirable bacteria and modifying the intestinal flora (19). OAs also improve the digestibility of proteins and amino acids and the absorption of minerals (30, 31), modulate endocrine and exocrine secretions, and influence the mucosal morphology (33). Whether these effects can be applied to all animal species can be discussed. In chickens fed ad libitum, for instance, pH in the intestinal tract is not altered by the addition of formic or propionic acid (41) and the pH in the proventriculus and the gizzard is very acidic per se (12).
Another aspect to take into consideration is the importance of the normal floras. A well-established normal intestinal flora competes with pathogens and hence decreases the risk of salmonellosis, Clostridium perfringens-associated lesions, campylobacteriosis, or colibacillosis. It is also probable that some specific normal flora compositions are more beneficial than others (3, 5, 9, 17, 38, 42).
In chicken production, litter is a potential reservoir and transmission vehicle for pathogens and potential pathogens (26, 28, 43, 45). Some work has been done to study the effect of acidification of the litter with acids and OAs in attempt to reduce the presence of pathogenic bacteria and improve the environmental conditions of the chicken houses (13, 23). The aim of the present study was to characterize the normal intestinal flora (Lactobacillus spp., Enterococcus spp., coliforms, and C. perfringens) of chickens and analyze the changes in the balance upon exposure to a litter acidified with formic and propionic acid. Special attention is paid to C. perfringens because of its participation in the development of necrotic enteritis (NE) (44).
MATERIALS AND METHODS
Animals, treatment, and diet.
A number (1,728) of 1-day-old healthy male broiler chickens (type Ross 208) from the hatchery Samvirkekylling, Solør, Norway, were aleatorily chosen and arbitrarily separated in 12 pens (5.35 m2), each pen containing 144 animals. The pens were assigned either to the control litter or the acidified litter, alternately. The house had a monitored temperature of 30°C which was reduced a half degree per day to 22°C. A light regimen of 18 h was employed. The relative humidity started at 70% and was decreased to 50% during the testing period.
The litter was wood shavings from dry pinewood from a local sawmill. The acidified litter was treated with a SoftAcid product (Borregaard LignoTech, Sarpsborg, Norway), a mixture of sodium lignosulfonate, formic acid, and propionic acid. The added mixture constituted 7% of the litter weight and was sprayed on the litter.
The chickens had ad libitum access to water and feed, which was supplemented with the anticoccidial Amprol+ (125 mg of amprolium/kg of body weight and 8 mg of ethopabate/kg). No AGPs were added. The feed was formulated with the intention of promoting subclinical NE and hepatitis associated with a spontaneous C. perfringens infection (M. Kaldhusdal, unpublished data). C. perfringens (100 to 500 CFU/g) was found in the starter feed.
Intestinal sampling and processing.
At 18, 21, 25, 29, and 32 days of age, five animals from each pen were randomly selected for sampling. The chickens were transported for 1.5 h in well-ventilated cartons, each with six chickens from the same pen, without access to food or to water until they were killed.
The chickens were made unconscious by a blow to the head and then killed by cervical dislocation. Then the alimentary tract was immediately dissected, and the intestinal contents were collected from an approximately 30-cm-long segment of the lower ileum measured from the vitelline diverticulum and the ceca (one of the horns) into plastic 50-ml Falcon tubes (Corning Incorporated, Corning, N.Y.); the collected contents were kept on ice until inoculation and incubation. When the chosen section from the lower ileum was empty or contained little material, the contents of the next 30 cm of the lower ileum were collected.
The samples were processed within 10 to 60 min after collection. They were weighed and serially diluted in 0.9% saline, and 0.1 ml of each sample was plated on selective media.
Bacterial counts from intestinal samples.
C. perfringens was grown on 5% blood agar (MERCK 10886) anaerobically at 37°C for 48 h with MERCK Anaerocult A. The colonies appeared as large dome-shaped colonies with a double zone of hemolysis.
Lactobacillus spp. were grown on Rogosa agar (MERCK 5413) anaerobically at 37°C for 48 h with Merck Anaerocult A. The bacteria were enumerated by counting white colonies.
Enterococcus spp. were grown on 5% blood agar (MERCK 10886) aerobically at 37°C for 24 h. The colonies were small (1 mm in diameter) and presented an α-hemolysis.
Coliforms were grown on MacConkey agar (MERCK 5465) aerobically at 37°C for 24 h. These were typical lactose-fermenting colonies.
The macroscopic image of the colonies of the bacteria was confirmed by the microscopic image of the bacteria after Gram staining.
Recording of data from intestinal bacterial counts.
The figures from the bacterial counts were recorded as CFU (40). In a few cases it occurred that the dilution ranges chosen for isolation were either under- or overestimated. When the bacterial concentrations were lower than our minimal detection level, a log10 value 0.1 lower than the log10 value corresponding to the minimal detection level was registered. In cases of high bacterial counts, an absolute maximum was set to the log10 value 10; then the mean value of the largest possible count and 10 was registered.
Litter sampling and processing.
Litter samples were collected from all the pens on days 4, 7, 11, 14, 18, 21, 25, 28, and 32 to measure pH and humidity and to do bacterial counts.
pH was measured using a combined pH electrode (Model Knick Portamess 751 Calimatis, Knick, Germany) in the first dilution of the samples for bacterial count.
For humidity (percent) measurements, the samples were weighed and placed in a heat cabinet at 105°C. After 5 h the samples were weighed again, and the weight loss was calculated according to the following formula:
 |
Bacterial counts.
The samples were serially diluted in 0.9% saline, and 0.1 ml of each was plated on selective media. C. perfringens was counted on tryptose-sulfite-cycloserine (TSC) agar for C. perfringens (OXOID CM587 with the addition of SR88 and SR47) after an incubation period of 24 h in an anaerobic atmosphere at 37°C. The colonies were black with an opaque halo, indicating a lecithinase reaction. When needed for verification, colonies on TSC agar that were suspected to be C. perfringens were plated secondarily on blood agar. Coliforms were isolated on MacConkey agar (Difco 0075-17) after an incubation time of 24 h in an aerobic atmosphere at 37°C.
Performance.
Chicken feed consumption and live weight were recorded per pen on day 32.
Clinical observations.
The chickens were inspected each day, and the number of dead birds was registered. At slaughter the number of discarded chickens was registered.
Statistical analysis.
Due to skewing in the distribution, statistical analyses of intestinal bacterial counts were performed on logarithmic transformed data. The results were backward transformed and expressed as mean values and 95% confidence intervals constructed by using the Student procedure (2). Additionally, standard deviations (SD) were determined. Comparisons of groups with assumed continuously distributed variables were performed by using analysis of variance with pen and age as covariates. Comparisons of groups on categorical variables were performed by using contingency table analysis (20). Differences were considered significant when the P values were less or equal to a level of 5%.
RESULTS
Intestinal bacterial counts.
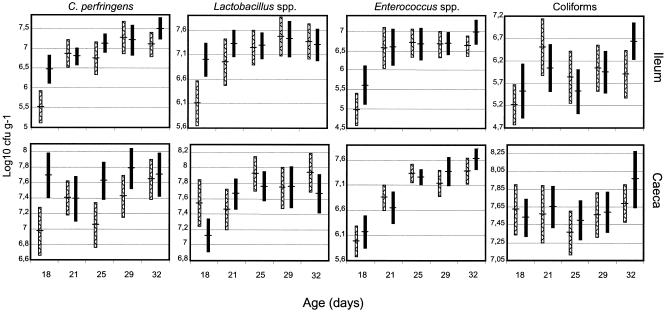
The sizes of the bacterial populations in the ileum and caeca of the chickens exposed to the acidified and control litter are presented in Fig. 1.
FIG. 1.
Log 10 CFU of the different types of bacteria in caeca and ileum per gram of intestinal contents in the acidified litter (hatched bars) and the control litter (solid bars). Values are expressed as means with 95% confidence intervals.
Ileum. (i) C. perfringens.
Chickens on both litters harbored a number of C. perfringens bacteria in the intestines that increased with time. The acidified group had much lower counts (5.52 CFU/g) at day 18 than the control group (6.47 CFU/g), but these counts increased noticeably at day 21. In total the acidified group had lower counts (P < 0.01) and specifically at 18 (P < 0.01) and 32 (P = 0.06) days of age.
(ii) Enterococcus spp.
The counts increased rapidly in both groups from day 18 to day 21 and remained stable for the rest of the testing period. The acidified group had an overall lower (P ≤ 0.05) number of CFU/gram, with lower counts at 18 (P = 0.06) and 32 (P = 0.08) days of age.
(iii) Lactobacillus spp.
The counts in both groups increased with time. The acidified group had overall significantly lower counts (P < 0.01). On day 18, a significant difference (P < 0.01) was observed between the two groups, with means of 6.11 CFU/g for the acidified group and 7 CFU/g for the control group. The counts increased rapidly in the acidified group up to day 21, reaching the level of 6.95 CFU/g, and reached levels similar to those of the control group at 25 days.
(iv) Coliforms.
No difference between the two groups was observed. There was an increase in the number of CFU/gram from day 18 to day 21, followed by a decrease at day 25 and an increase the last two sampling days. The difference between the counts determined on the first and the last sampling days was higher for the control group than for the acidified group.
Caeca. (i) C. perfringens.
The development of counts of C. perfringens populations of the acidified and the control groups showed different patterns. At day 18 the acidified group had a count of 6.97 CFU/g; the count oscillated first up and then down and increased in the last two sampling days, getting close to the level of the control group (7.64 CFU/g). The control group had a higher count (7.69 CFU/g) at day 18; the count oscillated first down and then up and down again to end up at 7.7 CFU/g, very close to the level from day 18. In general the acidified group had lower counts (P < 0.01) than the control group through the whole testing period. These differences were significant specifically at 18 (P < 0.01), 25 (P < 0.01), and 29 (P = 0.06) days of age.
(ii) Enterococcus spp.
No difference between the groups was observed. A rapid and continuous increase in the number of CFU/gram was observed in both groups.
(iii) Lactobacillus spp.
The counts increased with time in both groups. The acidified group had overall significantly higher (P ≤ 0.05) counts, but on day 18 only there was a significant difference (P ≤ 0.05) between the two groups, with means of 7.54 CFU/g for the acidified group and 7.12 CFU/g for the control group.
(iv) Coliforms.
No difference between the groups was observed. The counts followed the same patterns as in the ileum, but the oscillations were smaller. The difference between the counts made at day 18 and day 32 is higher for the control group than for the acidified group.
Litter parameters.
Levels of litter pH, humidity, coliforms, and C. perfringens in the litter are shown in Table 1.
TABLE 1.
Mean values of pH and humidity and C. perfringens and coliform countsa
| Day | pH (SD)c
|
% Humidity (SD)d
|
Log10 of C. perfringens CFU/g (SD)e
|
Log10 of coliform CFU/g (SD)f
|
||||
|---|---|---|---|---|---|---|---|---|
| Acidified | Control | Acidified | Control | Acidified | Control | Acidified | Control | |
| 1 | 2.8b | 4.9b | 22.42b | 14.64b | NDg | ND | ND | ND |
| 4 | 5.2 (0.58) | 5.9 (0.24) | 14.5 (2.58) | 16.1 (3.45) | 4.65 (1.21) | 5.49 (0.46) | 5.61 (1.29) | 6 (0) |
| 7 | 5.8 (0.53) | 5.9 (0.2) | 15.7 (11.26) | 17.7 (10.19) | 4.1 (0.97) | 5.38 (0.66) | 6 (1.81) | 6.85 (0.23) |
| 11 | 5.9 (0.29) | 5.9 (0.09) | 17.6 (10.38) | 22.1 (6.6) | 5.62 (0.86) | 6.07 (1.04) | 6.9 (1.35) | 7.12 (1.07) |
| 14 | 6 (0.19) | 5.9 (0.11) | 26.8 (8.74) | 31.9 (13.52) | 5.97 (0.47) | 6.11 (0.38) | 7.04 (1.57) | 6.37 (1.38) |
| 18 | 6.2 (0.33) | 6.5 (1.37) | 35.1 (9.95) | 38.6 (10.34) | 5.89 (1.01) | 6.06 (0.47) | 6.71 (0.63) | 6.58 (0.79) |
| 21 | 6.8 (0.71) | 6.8 (1.0) | 39.2 (7.42) | 36.9 (9.51) | 5.12 (0.69) | 5.37 (1.0) | 6.73 (1.24) | 6.44 (0.14) |
| 25 | 7.9 (0.74) | 7.9 (0.75) | 35.4 (5.44) | 36.7 (4.4) | 5.98 (0.32) | 6.16 (0.81) | 6.09 (1.0) | 5.75 (2.16) |
| 28 | 8.4 (0.41) | 8.4 (0.48) | 29.6 (38.19) | 38.313.0) | 5.92 (1.28) | 6.16 (0.81) | 3.95 (1.92) | 4.4 (2.51) |
The variables were measured in control and acidified-litter samples at various days. Acidified, acidified group; Control, control group.
Measurement taken before the litter was divided among pens.
P, 0.002.
P, 0.78.
P, 0.016.
P, 0.98.
ND, not done.
(i) pH.
In both litters the pH reached the same level at day 7. The acidified litter pH was significantly (P < 0.01) lower than the control litter pH, but when the data from day 1 are ignored, the remaining data show that the acidified litter had no effect compared to the control litter.
(ii) Humidity.
The humidity increased in both litters and dropped down at the end of the observed time for the acidified litter. There was not a significant difference between the litters.
(iii) C. perfringens.
Both litters showed the same development in bacterial counts, but there was a significantly (P ≤ 0.05) lower number for the acidified litter. The number of CFU/gram increased with time and stabilized at values around 5.9 CFU/g for the acidified litter and 6.1 CFU/g for the control litter at day 14, except for the remarkable fall at day 21.
(iv) Coliforms.
No difference between the groups was observed. In both litters the counts increased until day 11, and they were maintained at the same levels until day 21, when they decreased rapidly.
Performance.
The chickens exposed to the acidified litter had a higher (P > 0.05) live weight at day 32 and had consumed a larger (P < 0.01) amount of feed through the testing period than the chickens in the control group. These results showed no significant difference in the feed conversion rates (FCR) between the two groups (Table 2). The carcasses of the acidified group after processing were significantly (P < 0.01) heavier, with a mean weight of 1,097 g, than the ones from the control group, with a mean weight of 1,070 g.
TABLE 2.
Mean values of live weight, feed intake, and FCR measured penwise at day 32, and the P-value
| Group | Kg of weight (SD)a | Kg of feed intake (SD)b | FCR in kg of feed intake/kg of weight gain (SD)c |
|---|---|---|---|
| Acidified | 186.5 (13.8) | 338.9 (14) | 1.82 (0.09) |
| Control | 183.8 (2.9) | 327.6 (4.1) | 1.78 (0.03) |
P, 0.41.
P, 0.004.
P, 0.15.
Mortality and discarding.
The mortality during the entire growing period was 18 animals (2.1%) in the control group and 21 animals (2.4%) in the acidified group. A total of seven carcasses (0.8%) in the control group and three (0.4%) in the acidified group were discarded at slaughter.
DISCUSSION
In the ileum, C. perfringens, Enterococcus spp., and Lactobacillus spp. had lower counts in the group exposed to the acidified litter compared to the control group results. In the caeca, only C. perfringens had lower counts whereas Lactobacillus spp. had higher counts in the group exposed to the acidified litter. The most noticeable differences between the groups were observed in the ileum between day 18 and day 21. If the pH had been acidic longer than it actually was (14 days), it would probably have been possible to observe a longer period of difference. It was also at day 14 that the concentrations of C. perfringens in the litters stabilized. It is suspected that the fall in the concentration of C. perfringens in the litter observed at day 21 is due to some methodological failure. The pH levels in the litter of both groups became equal at day 7. All this suggests that the qualities of the litter could be maintained by replacing the used litter with fresh litter. Therefore, the acidified litter should be changed before day 7 to keep its acidification effect. To maintain the effect of the product on C. perfringens, the acidified litter should be changed before day 14. It would have been of great interest to analyze intestinal samples from younger chickens to study the development of the intestinal flora at earlier stages of growth, and when the pH is still acid. Since the flow of digesta into the caeca is just 18.3 to 24.7% of the dry matter and 16.6 to 26.4% for water (39) it is natural that the acidifying product, when applied to the litter, has a larger effect on the ileal microflora than on the caecal microflora. This study was not designed to explain which mechanisms are active when the OAs inhibit growth of bacteria such as C. perfringens and Lactobacillus spp., which produce OAs themselves and tolerate acid environments. However, Thompson and Hinton observed a decrease in the amount of lactic acid in the crop when the concentrations of formic and propionic acid were increased (41), which suggested a decrease in lactic acid bacteria. This could be an explanation for the lower counts of Lactobacillus spp. in the ileum in the group exposed to the acidified litter. A similar effect could explain the reduced growth of C. perfringens. A potential separate effect on the growth of bacteria exerted by the wood-derived component lignosulfonate in the product should be studied.
The lower counts of C. perfringens in the ileum and caeca and of Enterococcus spp. in the ileum are of particular interest. Similar results were obtained after a similar experiment with another acidifying product based on formic acid (M. Novoa Garrido, unpublished data). The presence of large amounts of C. perfringens types A and C in the intestine is a cofactor in the development of clinical and subclinical NE (6, 7, 15, 16, 22, 32, 44). Besides, it has been proved that C. perfringens and Enterococcus faecium have a high level of bile salt hydrolase activity (21) which causes growth depression in chickens (8).
The importance of the intestinal flora in controlling intestinal invasion by pathogens such as C. perfringens, Campylobacter jejuni subsp. jejuni, Salmonella spp., and pathogenic strains of Escherichia coli is well known (27, 37a). Therefore one should be cautious when manipulating the flora balance in the intestine (9, 10). A reduction of several bacterial groups has been observed as well in other experiments in which chickens have been fed with OAs (14). The fact that the ileum in the group exposed to the acidifying product presents lower counts of Lactobacillus spp. than in the control group is not a desired effect because of the regulating and protecting role of these bacteria (9).
Because of the high concentrations of C. perfringens found in the caeca in both groups, mortality and lesions due to NE could have been expected (15, 24). However, mortality and discarding at slaughter was low in both groups, indicating that the chickens in the study had good health despite the alteration in the intestinal flora and the feed and the high density in the pens. One can speculate whether the chickens developed an early and exacerbated central and/or local immune response due to an early exposure to C. perfringens which protected them from NE (11, 25).
Because a large quantity of samples had to be handled, and hence a number of people had to be involved in the laboratory work, it was decided not to handle the samples under anaerobic conditions before incubation. Samples were also plated from 2 to 3 continuous dilutions onto the different media. In the cases in which the sample results were below detectable levels, it was assumed that the value was close to the lower detection limit because the flora normally is well established in chickens at the age at which the samples were collected. The mean between the values corresponding to 75% of the detection level and the detection level values was calculated and transformed into log10; the result was a log10 value 0.1 lower than the log10 value corresponding the minimal detection level. When samples had large bacterial counts, counting the CFU was attempted. Then the mean value between the log10 value corresponding to this count and the log10 value 10 was calculated, in an attempt to use values close to the values that could have been expected. To avoid this problem, at least 3 continuous dilutions should have been plated. However the figures we obtained are similar to those reported in other papers (21).
The results of this study support the results from other studies (13, 23, 34) with respect to the efficiency of litter acidification in reducing the horizontal infection of chickens by pathogenic bacteria. The counts of C. perfringens and Enterococcus spp. in the chicken's intestines are reduced by the use of organic acidifiers in the litter. Because of the lower counts of C. perfringens, it is assumed that the risk of developing clinical or subclinical NE is also reduced. Besides, the lower counts of both C. perfringens and Enterococcus spp. reduce the risk of growth depression and improve the absorption of nutrients in the intestine, implying possible economical results. Finally, an optimal balance in the intestinal normal floras at early stages of growth seems to be beneficial for the health and development of the chickens.
Acknowledgments
We are grateful to all the staff from the Section of Microbiology and Immunology of the Department of Food Safety and Infection Biology at the Norwegian School of Veterinary Science, and to the laboratory staff from the Veterinary Institute in Bergen, Norway, for their invaluable assistance with the laboratory work. We also thank Magne Kaldhusdal (National Veterinary Institute, Oslo, Norway) for practical and theoretical support, Stig Larsen (Norwegian School of Veterinary Science) for his assistance with statistics, and the staff at the hatchery Samvirkekylling, Solør, Kongsvinger, Norway, for their practical help in attending the chickens.
This study has been funded by a grant from the Norwegian Research Council, project number 136326/140.
REFERENCES
- 1.Aarestrup, F. M. 2000. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS Suppl. 101:1-48. [PubMed] [Google Scholar]
- 2.Altman, D. G. 1991. Practical statistics for medical research. Chapman and Hall, London, United Kingdom.
- 3.Barnes, E. M. 1997. Ecological concepts of the anaerobic flora in the avian intestine. Am. J. Clin. Nutr. 30:1793-1798. [DOI] [PubMed] [Google Scholar]
- 4.Berns, J. S. 2003. Infection with antimicrobial-resistant microorganisms in dialysis patients. Semin. Dial. 16:30-37. [DOI] [PubMed] [Google Scholar]
- 5.Craven, S. E., N. J. Stern, N. A. Cox, J. S. Bailey, and M. Berrang. 1999. Cecal carriage of Clostridium perfringens in broiler chickens given mucosal starter culture. Avian Dis. 43:484-490. [PubMed] [Google Scholar]
- 6.Cygan, Z., and J. Nowak. 1974. Nekrotyczne zapalenie jelit u kurczat. Med. Wetery. 30:262-265. [Google Scholar]
- 7.El-Seedy, F. R. 1990. Studies on necrotic enteritis in chickens. Vet. Med. J. 38:407-417. [Google Scholar]
- 8.Feighner, S. D., and M. P. Dashkevicz. 1987. Subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. Appl. Environ. Microbiol. 53:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukata, T., Y. Hadate, E. Baba, and A. Arakawa. 1991. Influence of bacteria on Clostridium perfringens infections in young chickens. Avian Dis. 35:224-227. [PubMed] [Google Scholar]
- 10.Fukata, T., Y. Hadate, E. Baba, T. Uemura, and A. Arakawa. 1988. Influence of Clostridium perfringens and its toxin in germ-free chickens. Res. Vet. Sci. 44:68-70. [PubMed] [Google Scholar]
- 11.Heier, B. T., A. Løvland, K. B. Soleim, M. Kaldhusdal, and J. Jarp. 2001. A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks. Avian Dis. 45:724-732. [PubMed] [Google Scholar]
- 12.Herpol, C., and G. Van Grembergen. 1967. La signification du pH dans le tube digestif de Gallus domesticus. Ann. Biol. Anim. Biochim. Biophys. 7:33-38. [Google Scholar]
- 13.Ivanov, I. E. 2001. Treatment of broiler litter with organic acids. Res. Vet. Sci. 70:169-173. [DOI] [PubMed] [Google Scholar]
- 14.Izat, A. L., N. M. Tidwell, R. A. Thomas, M. A. Reiber, M. H. Adams, M. Colberg, and P. W. Waldroup. 1990. Effects of a buffered propionic acid in diets on the performance of broiler chickens and on microflora of the intestine and carcass. Poult. Sci. 69:818-826. [DOI] [PubMed] [Google Scholar]
- 15.Kaldhusdal, M., and M. Hofshagen. 1992. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult. Sci. 71:1145-1153. [DOI] [PubMed] [Google Scholar]
- 16.Kaldhusdal, M., M. Hofshagen, A. Løvland, H. Langstrand, and K. Redhead. 1999. Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables. FEMS Immunol. Med. Microbiol. 24:337-343. [DOI] [PubMed] [Google Scholar]
- 17.Kaldhusdal, M., C. Schneitz, M. Hofshagen, and E. Skjerve. 2001. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 45:149-156. [PubMed] [Google Scholar]
- 18.Khachatourians, G. G. 1998. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Can. Med. Assoc. J. 159:1129-1136. [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchgessner, M., and F. X. Roth. 1982. Fumaric acid as a feed additive in pig nutrition. Pig News Inf. 3:259-264. [Google Scholar]
- 20.Kleinbaum, D. G. 2003. Applied regression analysis and other multivariate methods. Duxbury Press, Pacific Grove, Calif.
- 21.Knarreborg, A., R. M. Engberg, S. K. Jensen, and B. B. Jensen. 2002. Quantitative determination of bile salt hydrolase activity in bacteria isolated from the small intestine of chickens. Appl. Environ. Microbiol. 68:6425-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Köhler, V. B., S. Kölbach, and J. Meine. 1973. Untersuchungen zur nekrotischen Enteritis der Hühner. Zeitschrift der wissenschaftlichen gesellschaft für veterinärmedizin. 29:385-391. [Google Scholar]
- 23.Line, J. E. 2002. Campylobacter and Salmonella populations associated with chickens raised on acidified litter. Poult. Sci. 81:1473-1477. [DOI] [PubMed] [Google Scholar]
- 24.Long, J. R., J. R. Pettit, and D. A. Barnum. 1974. Necrotic enteritis in broiler chickens. II. Pathology and proposed pathogenesis. Can. J. Comp. Med. 38:467-474. [PMC free article] [PubMed] [Google Scholar]
- 25.Løvland, A., M. Kaldhusdal, K. Redhead, E. Skjerve, and A. Lillehaug. Maternal vaccination against necrotic enteritis in broilers. Avian Pathol. 33:83-92. [DOI] [PubMed]
- 26.Lu, J., S. Sanchez, C. Hofacre, J. J. Maurer, B. G. Harmon, and M. D. Lee. 2003. Evaluation of broiler litter with reference to the microbial composition as assessed by using 16S rRNA and functional gene markers. Appl. Environ. Microbiol. 69:901-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mead, G. C. 2000. Prospects for “competitive exclusion” treatment to control salmonellas and other foodborne pathogens in poultry. Vet. J. 159:111-123. [DOI] [PubMed] [Google Scholar]
- 28.Montrose, M. S., S. M. Shane, and K. S. Harrington. 1985. Role of litter in the transmission of Campylobacter jejuni. Avian Dis. 29:392-399. [PubMed] [Google Scholar]
- 29.Moore, P. R., A. Evenson, T. D. Luckey, E. McCoy, C. A. Elvehjem, and E. B. Hart. 1946. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 165:437-441. [PubMed] [Google Scholar]
- 30.Mroz, Z., A. W. Jongbloed, K. H. Partanen, K. Vreman, P. A. Kemme, and J. Kogut. 2000. The effects of calcium benzoate in diets with or without organic acids on dietary buffering capacity, apparent digestibility, retention of nutrients, and manure characteristics in swine. J. Anim. Sci. 78:2622-2632. [DOI] [PubMed] [Google Scholar]
- 31.Omogbenigun, F. O., C. M. Nyachoti, and B. A. Slominski. 2003. The effect of supplementing microbial phytase and organic acids to a corn-soybean based diet fed to early-weaned pigs. J. Anim. Sci. 81:1806-1813. [DOI] [PubMed] [Google Scholar]
- 32.Parish, W. E. 1961. Necrotic enteritis in the fowl. II. Examination of the causal Clostridium welchii. J. Comp. Pathol. 71:394-404. [DOI] [PubMed] [Google Scholar]
- 33.Parttanen, K. H., and Z. Mroz. 199l. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 117-145. [DOI] [PubMed]
- 34.Pope, M. J., and T. E. Cherry. 2000. An evaluation of the presence of pathogens on broilers raised on poultry litter treatment-treated litter. Poult. Sci. 79:1351-1355. [DOI] [PubMed] [Google Scholar]
- 35.Prescott, J. F., W. J. Hanna, R. Reid-Smith, and K. Drost. 2002. Antimicrobial drug use and resistance in dogs. Can. Vet. J. 43:107-116. [PMC free article] [PubMed] [Google Scholar]
- 36.Rerksuppaphol, S., W. Hardikar, P. Midolo, and P. Ward. 2003. Antimicrobial resistance in Helicobacter pylori isolates from children. J. Paediatr. Child Health 39:332-335. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder, C. M., D. G. White, B. Ge, Y. Zhang, P. F. McDermott, S. Ayers, S. Zhao, and J. Meng. 2003. Isolation of antimicrobial-resistant Escherichia coli from retail meats purchased in Greater Washington, DC, USA. Int. J. Food Microbiol. 85:197-202. [DOI] [PubMed] [Google Scholar]
- 37a.Snoeyenbos, G. H. 1979. Role of native intestinal microflora in protection against pathogens, p. 388-392. In Proceedings of the annual meeting of the United States Animal Health Association. United States Animal Health Association, Richmond, Va. [PubMed]
- 38.Soerjadi, A. S., G. H. Snoeyenbos, and O. M. Weinack. 1982. Intestinal colonization and competitive exclusion of Campylobacter fetus subsp. jejuni in young chicks. Avian Dis. 26:520-524. [PubMed] [Google Scholar]
- 39.Son, J. H., D. Ragland, and O. Adeola. 2002. Quantification of digesta flow into the caeca. Br. Poult. Sci. 43:322-324. [DOI] [PubMed] [Google Scholar]
- 40.Swanson, K. M. J., F. F. Busta, E. H. Peterson, and M. G. Johnson. 1992. Colony count methods, p. 75-95. In Compendium of methods for the microbiological examination of foods. American Public Health Association, Washington, D.C.
- 41.Thompson, J. L., and M. Hinton. 1997. Antibacterial activity of formic and propionic acids in the diet of hens on Salmonellas in the crop. Br. Poult. Sci. 38:59-65. [DOI] [PubMed] [Google Scholar]
- 42.Weinack, O. M., G. H. Snoeyenbos, C. F. Smyser, and A. S. Soerjadi. 1981. Competitive exclusion of intestinal colonization of Escherichia coli in chicks. Avian Dis. 25:696-705. [PubMed] [Google Scholar]
- 43.Weinack, O. M., G. H. Snoeyenbos, A. S. Soerjadi-Liem, and C. F. Smyser. 1985. Therapeutic trials with native intestinal microflora for Salmonella typhimurium infections in chickens. Avian Dis. 29:1230-1234. [PubMed] [Google Scholar]
- 44.Williams, R. B., R. N. Marshall, R. M. La Ragione, and J. Catchpole. 2003. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitol. Res. 90:19-26. [DOI] [PubMed] [Google Scholar]
- 45.Willis, W. L., C. Murray, and C. Talbott. 2002. Campylobacter isolation trends of cage versus floor broiler chickens: a one way study. Poult. Sci. 81:629-631. [DOI] [PubMed] [Google Scholar]
- 46.Zhao, S., A. R. Datta, S. Ayers, S. Friedman, R. D. Walker, and D. G. White. 2003. Antimicrobial-resistant Salmonella serovars isolated from imported foods. Int. J. Food Microbiol. 84:87-92. [DOI] [PubMed] [Google Scholar]