Abstract
Infection with Lactococcus garvieae is considered the most important risk factor for the European trout industry, and the losses are approximately 50% of the total production. To improve our understanding of the genetic links among strains originating from different countries, we examined the population structure of L. garvieae by comparing 81 strains isolated from different sources and ecosystems (41 farms in six countries) in which the bacterium is commonly found. Genetic similarities (as assessed with molecular tools, including restriction fragment length polymorphism ribotyping with two endonucleases) were compared with serological data. The combined results reveal that in endemic sites the bacterial population displays a clonal structure, whereas bacterial diversity characterizes sites where the infection is sporadic.
The story of Lactococcus garvieae is an attractive example of how modern aquaculture influences the spread of pathogens and how a new pathogen settles in a new niche. The original description of L. garvieae dates back to 1984, when the organism was isolated from a mastitic udder of a cow in the United Kingdom (7). However, in contrast to its limited importance as a mastitogenic agent, L. garvieae turned out to be the most important risk factor for the Mediterranean European trout industry, causing specific losses that are approximately 50% of the total production (18).
Although fish diseases due to gram-positive cocci have been known in Japan for over 50 years (19), the association between L. garvieae and infected fish was determined only in 1991, when strains collected from diseased fish in Japan during the 1970s were analyzed and (erroneously) described as Enterococcus seriolicida sp. nov. (21). Indeed, subsequent studies indicated that E. seriolicida is a junior synonym of L. garvieae (11, 12). The proposition that throughout the 1970s and 1980s this pathogen and its associated disease, although not monitored, were present in European aquaculture ought to be discarded. L. garvieae induces a fatal hemorrhagic septicemia of trout, and it is inconceivable that an industry that produces over 100,000 tons yearly, as the European trout industry does, would not be aware of such considerable losses (18). The spread of L. garvieae throughout Mediterranean Europe was rapid; L. garvieae infections of trout were recorded in Spain in 1991 (11), and a year later the same pathogen was detected in Italy (18). From that point on, the pathogen and its specific disease rapidly spread throughout the southern part of the European continent, including countries such as Turkey (10) and Portugal (26), as well as the Balkans (this study). The rapid spread of the pathogen is a result of the multiple routes of dissemination and transmission of this pathogen, which include direct spread through movement of infected fish or asymptomatic carriers, as well as horizontal transmission by contaminated water (25, 28). L. garvieae infections of trout have also reached other continents, including Australia (5) and the Middle East (Asia) (this study).
The epidemiological puzzle of how the disease has spread throughout countries and continents is likely to remain unsolved for the most part. In previous work, it was shown that epidemiological markers (restriction fragment length polymorphism [RFLP] ribotyping) could differentiate diverse epidemiological clones; since one of the early isolates, which was collected in Japan some 20 years before the disease was encountered in Europe, was identical to Italian isolates, it was concluded that “potential routes of infection headed from Japan to Italy can be hypothesized” (13). During the last 3 years two additional epidemiological studies were published. By using pulsed-field gel electrophoresis, it was shown that Spanish isolates cluster in two different pulsotypes that are different from the two closely related Italian pulsotypes (28). In that study, the pulsotype of a French isolate was found to be genetically distinct from that of all other fish isolates. These data, which generally substantiated the RFLP data, were not confirmed by random amplified polymorphic DNA analysis, which indicated that 9 of 11 French isolates clustered in the same genogroup as the Italian rainbow trout isolates (26).
In the present work we monitored the population structure of L. garvieae strains from sights included in the original work (13), in addition to strains collected from locations where the pathogen was not present formerly. The epidemiological criteria were amplified and included a molecular approach (RFLP ribotyping) combined with a serological survey. A comparison between previous and current L. garvieae clinical isolates indicated the clonality of this pathogen in endemic areas where the disease is well established and its diversity in sites where the infection has been introduced only recently and is still sporadic.
MATERIALS AND METHODS
Bacterial strains.
All L. garvieae strains included in this study are clinical specimens isolated from viscera (mainly brains and spleens) of infected rainbow trout. A total of 81 strains were collected over a 5-year period from 41 trout farms in six countries. Confirmatory identification was performed by PCR, as previously described (31). The strains used in this study and their geographic origins are listed in Table 1. The Spanish isolates were kindly provided by Jose Francisco Fernandez-Garayzabal (School of Veterinary Medicine, Complutense University of Madrid). The French isolates (14 of which were provided by LD40/Groupement de Défense Sanitaire Aquacole d'Aquitaine, Mont de Marsan, France) were recovered between 1998 and 2003 from 14 farms in different geographical locations. The French isolates represented 18 different outbreaks; reoccurrences at the same site were recorded for only four farms.
TABLE 1.
L. garvieae strains analyzed in this study
| Strain(s) | Country and year of isolation | Sourcea | Serotype | EcoRI ribotype | HindIII ribotype |
|---|---|---|---|---|---|
| KFP 430 | Israel, 1999 | IF 1; Upper Galilee | I | B | K |
| KFP 24, KFP 30, KFP 36, KFP 442, KFP 457 | Israel, 2000 | IF 1 and IF 2, Upper Galilee | I | A | K |
| KFP 458, KFP 459, KFP 460, KFP 461, KFP 462, KFP 470 | Israel, 2001 | IF 1 to IF 3, Upper Galilee | I | A | K |
| KFP 471, KFP 473, KFP 498 | Israel, 2002 | IF 1 to IF 3, Upper Galilee | I | A | K |
| KFP 17, KFP 505, KFP 508, KFP 511, KFP 524 | Israel, 2003 | IF 1 to IF 3, Upper Galilee | I | A | K |
| 00/5056 | Spain, 2000 | SF 1, northwest | II | C | L |
| 3265 | Spain, 2000 | SF 2 | II | C | L |
| 27/00 | Spain, 2000 | SF 3 | II | C | L |
| 00/5084 | Spain, 2000 | SF 4, west | II | C | L |
| 00/5094 | Spain, 2000 | SF 1, northwest | II | C | L |
| 00/5069 | Spain, 2000 | SF 5, center | II | D | M |
| 111 | Spain, 2001 | SF 2 | II | C | L |
| 409 | Spain, 2001 | SF 3 | II | C | L |
| 01/5611 | Spain, 2001 | SF 6, south | II | C | L |
| 01/5677 | Spain, 2001 | SF 7, west | II | C | L |
| 01/5687 | Spain, 2001 | SF 8, south | II | C | L |
| 01/5695 | Spain, 2001 | SF 9, south | II | C | L |
| 02/6018 | Spain, 2002 | SF 10, south | II | C | L |
| 03/8460 | Spain, 2003 | SF 11, south | II | C | L |
| 03/8545 | Spain, 2003 | SF 12, northwest | II | C | L |
| 03/8566 | Spain, 2003 | SF 13, center | II | C | L |
| 03/8585 | Spain, 2003 | SF 14, west | II | C | L |
| 7898/98 | France, 1998 | FF 1 | I | E | N |
| 8338/98 | France, 1998 | FF 2 | I | E | N |
| 1966/00 | France, 2000 | FF 3 | NTb | F | O |
| 2138/00 | France, 2000 | FF 4 | NT | F | P |
| 3032/01 | France, 2001 | FF 5 | I | G | Q |
| 378 | France, 2001 | FF 6 | III | H | R |
| 2599/02 | France, 2002 | FF 5 | I | G | Q |
| 2976/02 | France, 2002 | FF 7 | I | G | Q |
| 3954/02 | France, 2002 | FF 8 | II | J | L |
| 033 | France, 2002 | FF 9 | I | A | S |
| 297 | France, 2002 | FF 10 | IV | I | T |
| 379 | France, 2002 | FF 11 | I | A | S |
| 2441/03 | France, 2003 | FF 1 | II | C | L |
| 2645/03 | France, 2003 | FF 2 | I | G | Q |
| 2442/03 | France, 2003 | FF 12 | I | G | U |
| 2429/03 | France, 2003 | FF 13 | I | G | Q |
| 1477/03 | France, 2003 | FF 7 | I | G | Q |
| 2747/03 | France, 2003 | FF 14 | II | C | L |
| ITP 01A, ITP 011, ITP 191, ITP 231, ITP 320, ITP 408 | Italy, 2000 | ITF 1 to ITF 3, Po Valley and northern Italy | I | A | K |
| ITP 109, ITP 256, ITP 322, ITP 329, ITP 387, ITP 393, ITP 431, ITP 487 | Italy, 2001 | ITF 1 to ITF 8, Po Valley, Central, northern, and northeastern Italy | I | A | K |
| ITP 054, ITP 099, ITP 109, ITP 401, ITP 256 | Italy, 2002 | ITF 1, ITF 5, Po Valley, central and northern Italy | I | A | K |
| ITP 42, ITP 58, 82921, 82277, 85830 | Italy, 2003 | ITF 1, ITF 2, and ITF 6 to ITF 8, Po Valley, central, northern, and northeastern Italy | I | A | K |
| 053 | Greece, 2002 | II | C | L | |
| 065 | Bulgaria, 2003 | II | C | L |
IF, SF, FF, and ITF indicate different fish farms in Israel, Spain, France, and Italy, respectively. Italian, Spanish, and Israeli strains were collected from sites where the disease is endemic. The French isolates are representatives of 17 different outbreaks; outbreak reoccurrences in the same site were recorded at only three farms.
NT, nontypeable.
Italian, Spanish, and Israeli strains were collected from sites where the disease is endemic, with regular seasonal occurrence (when the temperature is above 16°C). The three Israeli farms from which strains were collected have common water sources.
DNA extraction and ribotyping and generation of ribosomal DNA (rDNA) RFLP patterns.
The methods used to lyse gram-positive cocci and to extract their DNA have been described elsewhere (13). Five micrograms of genomic DNA from each isolate was completely digested (16 h at 37°C) with 50 U of restriction enzyme HindIII and 50 U of restriction enzyme EcoRI (Promega, Madison, Wis.) and separated by electrophoresis on a 20-cm-long 1% agarose gel. Southern blotting and all other procedures, including hybridization to a digoxigenin (Boehringer)-labeled 7.5-kb BamHI fragment of pKK3535 comprising the E. coli rRNA B operon (4), were performed as described previously (13).
Computer data analysis.
The rRNA B operon patterns obtained after digestion with HindIII were digitized by using a BIS 202 gel documentation system (DNR). Digitized images were converted and normalized with Phoretix 1D software (Nonlinear). Following normalization we defined a set of bands for each normalized densitometric curve. The similarity between patterns was calculated by using the Dice (Sd) coefficient (9) as implemented in the Phoretix 1D software. Restriction patterns for each locus were considered to be the same if they had the same number of bands with similar molecular sizes. Different restriction patterns for each locus were considered to represent different alleles, and each allele was assigned an arbitrary integer. Dendrograms were produced on the basis of the unweighted average pair group method (UPGMA).
Production of hyperimmune sera.
Isolates were grown in 200 ml of Todd-Hewitt broth (Difco) at 27°C for 4 to 6 h until an optical density (absorbance) at 630 nm of 0.6 was achieved. Bacterial cells were collected by centrifugation (3,000 × g for 20 min) and inactivated with formalin (3% for 24 h). Then the bacterial cells were collected by centrifugation (3,000 × g for 20 min), and each pellet was washed three times in phosphate-buffered saline (PBS). Finally, the bacterial pellet was resuspended in PBS to an optical density (absorbance) at 630 nm of 1.4 (equivalent to 109 CFU/ml). Hens were immunized subcutaneously with biweekly doses of 30 μl of formalin-killed bacteria emulsified in 70 μl of incomplete Freund's adjuvant (Sigma). Serum samples for measurement of antigen-specific immunoglobulin titers were prepared from blood.
Measurement of immunoglobulin titers.
Immulon 4 (Dynex, Chantilly, Va.) plates were coated overnight at 4°C with 100 μl of a formalin-free bacterial suspension (diluted in PBS to an optical density at 630 nm of 0.6), washed three times with PBST (PBS containing 0.1% Tween 20) and blocked with PBS containing 0.5% bovine serum albumin at room temperature (RT) for 30 min. The plates were then washed once with PBS, and serum samples (twofold dilutions from 1/500 to 1/32,000) in blocking buffer were added. After 1 h of incubation at 37°C, the plates were washed three times with PBST, and alkaline phosphatase-conjugated polyclonal goat anti-hen immunoglobulin G (final concentration in blocking buffer, 1/1,000; Sigma) was added. The plates were incubated at 37°C for 1 h and washed three times with PBST, and the substrate 4-methylumbelliferyl phosphate (50 μg/ml; 50 μl/well) was added. Fluorescence was read with a MicroFLUOR enzyme-linked immunosorbent assay (ELISA) reader (Dynatech Laboratories, Inc.).
Dot blot assay for serotyping.
The dot blot assay was carried out as described elsewhere (16), with modifications in the antigen composition. Bacteria were grown in 50 ml of Todd-Hewitt broth as previously described and were collected by centrifugation (3,000 × g for 20 min). Specific group polysaccharide (PS) antigens were extracted by exposure of a bacterial pellet to 500 μl of 0.2 N HCl for 120 min at 50°C (22). The extracts were neutralized with NaOH and centrifuged (10,000 × g for 20 s), and 50 μl of the supernatant was spotted (dot blot; Bio-Rad) onto nitrocellulose membranes (Bio-Rad). The membranes were dried for 30 min at RT and blocked for 1 h at RT with 5% skim milk in PBS. The membranes were then divided into strips and washed three times with PBS containing 0.05% Tween 20; a 1:1,000 dilution (for serum against strain ITP 054) or a 1:5,000 dilution (for all other sera) of each antiserum in PBS containing 1% skim milk was added to the corresponding strip and incubated for 2 h at 37°C on a rocking platform. After the strips were washed three times in PBS containing 0.05% Tween 20, a 1:5,000 dilution of goat anti-chicken immunoglobulin G (heavy plus light chains)-peroxidase conjugate (Sigma) in PBS containing 1% skim milk was added, and the mixture was incubated for 1 h at RT (different dilutions of sera and conjugate were tested before the final dilutions were selected). After the preparations were washed once in PBS containing 0.05% Tween 20 and twice in PBS, the reaction was developed with a 1:33 dilution of a 5-mg/ml solution of nitroblue tetrazolium plus a 1:33 dilution of a 2.5-mg/ml solution of BCIP (5-bromo-4-chloro-3-indolylphosphate) in 0.1 M Tris buffer (KPL, Gaithersburg, Md.). A dark color of spots, often surrounded by a halo, indicated a positive reaction. These results clearly contrasted with the smaller, weaker spots given by the negative strains. The reactions were stopped with distilled water.
Adsorption.
Serum against isolate 27/00 was adsorbed as described by Musher et al. (24). Formalin-killed L. garvieae 054 cultures were centrifuged (3,000 × g for 20 min), and each pellet (containing 109 CFU equivalents) was washed three times in PBS and suspended in 1 ml of the serum (against isolate 27/00) to be adsorbed. This suspension was kept at 8°C on a rocking platform for 30 min. Serum was then collected by centrifugation, and this procedure was repeated two more times. After the third adsorption, sera were filtered (pore size, 0.22 μm; Gelman Sciences, Ann Arbor, Mich.) and stored at −20°C.
RESULTS
Genetic structure of the L. garvieae population as established by rDNA RFLP analysis.
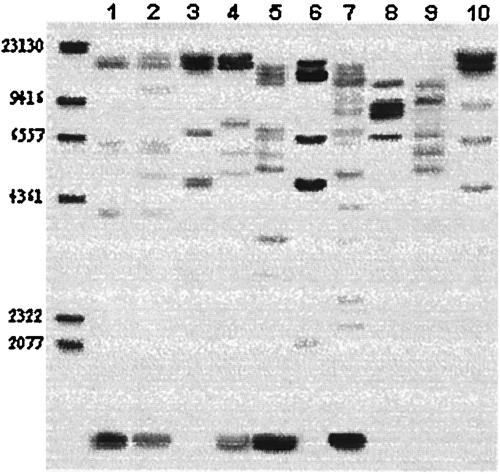
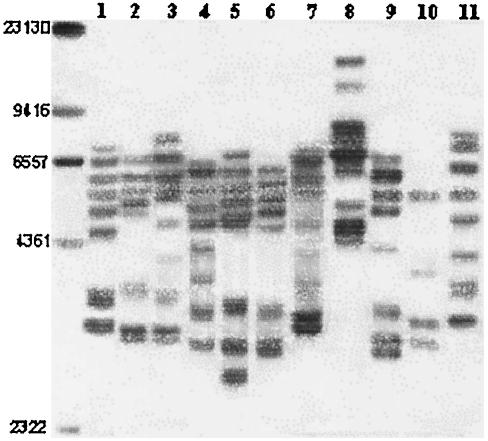
Previous studies have shown that RFLP ribotyping following genomic digestion with the restriction enzymes EcoRI and HindIII can effectively discriminate L. garvieae strains; for ribotype designations we used the criteria described previously (13). A total of 21 different restriction patterns were observed; 10 different patterns characterized by 5 to 13 bands were identified in the EcoRI blots (Fig. 1), and 11 different patterns characterized by 4 to 10 bands were detected in the HindIII blots (Fig. 2).
FIG. 1.
RFLP ribotyping of EcoRI digests. The numbers on the left indicate sizes (in base pairs). Lane 1, Italian isolate 01A; lane 2, Israeli isolate 430; lane 3, Spanish isolate 3265; lane 4, Spanish isolate 00/5069; lane 5, French isolate 7898/98; lane 6, French isolate 1966/00; lane 7, French isolate 3032/01; lane 8, French isolate 378; lane 9, French isolate 297; lane 10, French isolate 3954/02.
FIG. 2.
RFLP ribotyping of HindIII digests. The numbers on the left indicate sizes (in base pairs). Lane 1, Italian isolate 01A; lane 2, Spanish isolate 3265; lane 3, Spanish isolate 00/5069; lane 4, French isolate 7898/98; lane 5, French isolate 1966/00; lane 6, French isolate 2130/00; lane 7, French isolate 3032/01; lane 8, French isolate 378; lane 9, French isolate 379; lane 10, French isolate 297; lane 11, French isolate 2442/03.
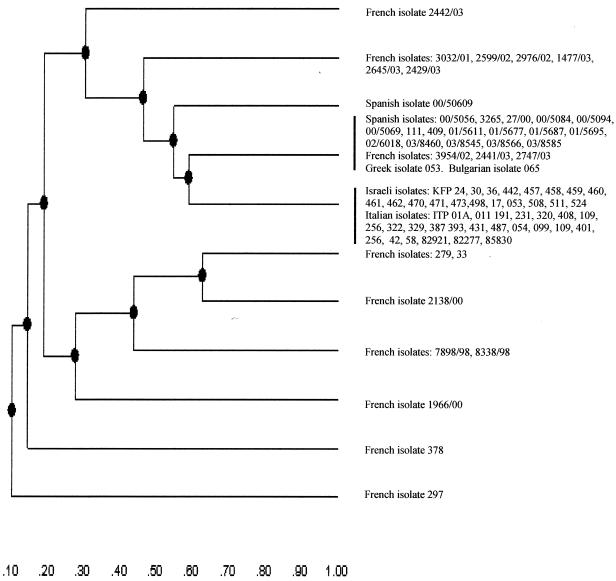
By combining the results obtained with the two enzymes the number of ribotypes detected for the strains was 13. Interestingly, whatever endonuclease was used, the rDNA RFLP analysis of strains originating from Italy and Spain (where the pathogen and its associated disease have been established for more than 10 years) grouped in two phylogenetic groups with no apparent internal differences; the only exception was Spanish isolate 00/5069 (level of similarity with other Spanish strains, 0.54). The genetic relationships of these groups (following digestion with HindIII) are shown in a dendrogram constructed by the UPGMA method in Fig. 3, and the results confirmed the existence of two major epidemiological divisions (level of similarity, 0.58) and the clonality of each division (level of similarity, 1.0). A similar result was obtained for the Israeli strains that grouped in the same epidemiological division as the Italian strains (level of similarity, 1.0). The Greek and Bulgarian isolates were identical to the Spanish isolates and therefore clustered in the same epidemiological rank. However, since only one isolate from each of these countries was examined, no definitive conclusions could be drawn.
FIG. 3.
Dendrogram showing the genetic relationships among the L. garvieae rDNA RFLP patterns after complete digestion with HindIII. The dendrogram was constructed by computer analysis with the Phoretix 1D software (Nonlinear) package by using the UPGMA.
In contrast to the tight relationships among Spanish isolates and between Italian and Israeli isolates, the rDNA RFLP analysis of the French isolates revealed a high degree of heterogeneity, as shown by the high number of ribotypes (nine different combinations) and the low levels of similarity, which ranged from 0.1 (strain 297) to 0.58 (strains 3954/02, 2441/03, and 2747/03, which were identical to the Spanish group [level of similarity, 1.0]).
Computer analysis of the rRNA B operon patterns obtained after digestion with EcoRI substantiated these results (data not shown) and demonstrated the clonality (level of similarity, 1.0) of each division (level of similarity between divisions, 0.30) and the heterogeneity of the French isolates, for which the levels of similarity ranged from 0.08 (strain 297) to 0.58 (strains 2441/03 and 2747/03, which are identical to the Spanish group [level of similarity, 1.0]).
As determined by a comparison of the two dendrograms, the only exception in the two major genetic groups was the first Israeli isolate, KFP 430, which appeared to be a subtype (level of similarity, 0.79) of the Italian-Israeli division.
L. garvieae serotypes.
The specificity of each antiserum was determined by ELISA; sera were collected when the ELISA value was more than 1.8 optical density units (for sera against ITP 054 diluted 1:4,000) or more than 1.0 optical density unit (for sera against strains 27/00, 378, and 279 diluted 1:16,000). The optical density of the control sera was 0.1. Dot blotting was performed with all L. garvieae isolates included in this study, which represented clinical isolates collected from different outbreaks in five Mediterranean countries. With antiserum to strain 27/00 cross-reactions with heterologous serogroups or serotypes (SGTs) occurred, giving intermediate results that were difficult to interpret. Antibodies against surface antigens common to all lactococci, such as cell wall polysaccharide, could have been responsible for these cross-reactions. Absorption of the sera with the heterologous lactococcal strains rendered the sera highly specific. In dot blot typing, the homologous strain used for antiserum production was included as a positive control; the results were interpreted by comparison with the controls. A dark color of the spots, often surrounded by a halo, indicated a positive reaction. These results clearly contrasted with the smaller, weaker spots obtained with the negative strains (data not shown).
The largest SGT included all Israeli and Italian isolates (serotype I strains); sera raised against Italian strain ITP 054 reacted positively with all Italian and Israeli strains. The only exception was Italian isolate ITP 109, which showed negative dot blot reactions even when the homologous antiserum was used. Surprisingly, although serum raised against ITP 109 recognized the heterologous Italian and Israeli PS extracts, they did not recognize the PS extracted from the homologous isolate. This raised the question of the specificity of the dot blot assay with PS of ITP 109 as the antigen. The possible explanation that parts of PS that are essential for binding are not surface exposed even in heat-treated bacteria was discarded when binding of homologous sera (as well as sera raised against other Italian and Israeli isolates) was proven to be dose dependent. When the quantity of PS extracted from ITP 109 was tripled, specific recognition of the homologous PS occurred (data not shown). We therefore hypothesized that the quantity of PS synthesized by this isolate is less than the quantity of PS synthesized by other strains. The correlation between serotyping and ribotyping was complete; the serological method placed all serotype I strains in the expected epidemiological division. The two French isolates that were most closely related genetically to the Italian and Israeli isolates (isolates 379 and 33; genetic similarity, 0.87) were also included in the same SGT. This demonstrates the value of serotyping as a discriminative tool, although ribotyping is superior for strain-to-strain differentiation.
The second SGT encompassed all of the Spanish isolates, plus the Greek and Bulgarian strains, which clustered in a tight genetic division (level of similarity, 1.0). The nonspecific binding of the sera with Spanish isolate 27/00 (used at a dilution of 1:1,000) decreased considerably when they were adsorbed with Italian isolate ITP 054, confirming the specificity of the reaction.
The heterogeneity of the French isolates was confirmed by serological means. French isolates were found to group with the Italian-Israeli SGT, as well as with the Spanish SGT; isolates 297 and 378 (levels of genetic similarity, 0.1 and 0.14, respectively) did not group in any SGT but were members of two distinct serotypes. Strains 1966/00 and 2138/00 (level of internal similarity as determined by EcoRI typing, 1.0) were serologically nontypeable.
DISCUSSION
In recent years population genetic studies of many species of human and veterinary bacterial pathogens have provided valuable frameworks for understanding the ecology, pathogenicity, and epidemiology of these bacteria. Moreover, analysis of data from such studies often has provided insight into the phylogeny and the impact of recombination on the evolution of individual species. Thus, we used rDNA RFLP analyses combined with a serological survey to define the genetic diversity and structure of the population features of L. garvieae strains isolated from infected fish. The combination of rDNA RFLP analysis, which examines stable regions of the genome, with serotype pervasiveness is an original and powerful tool for identifying genetic events that have occurred in L. garvieae genomes.
All of our concordant results seem to confirm that the L. garvieae population is divided into two major groups. The first group encompasses Italian and Israeli serotype I isolates, while the second includes Spanish, Greek, and Bulgarian serotype II isolates. Overall, a clear correlation between serotypes and ribotypes was observed (Fig. 3), although related (but not identical) ribotypes can belong to the same serotype, indicating the higher discriminatory power of molecular typing methods than of serotyping. This might be because it is possible for different strains to have similarities in portions of their genomes that encode serotype-specific antigens but have differences in other portions of their genomes (e.g., rRNA genes). Compared to the French isolates, the greater genetic homogeneity in each of the two defined divisions (Spanish-Greek and Italian-Israeli divisions) suggests that a process of evolution has already occurred, leading to a clonal distribution of strains that probably evolved from ancestor strains (27). In contrast, the genetic heterogeneity among French isolates suggests that in France a similar process of evolution has not taken place. The underlying reasons for the observed differences in the L. garvieae population and subpopulation structure in France are unclear at this time, but the differences are likely to be the combined result of recombinational events and ecological factors (6, 14, 15).
Variations in the serologic prevalence of streptococcus (group A) strains in human populations have been reported previously (1, 17). These variations could have been due to either a gradual process that occurred over several years (29) or a rapid and complete replacement of one serotype with another, as described for semiclosed civilian populations (1, 20, 23). It has been suggested that changes in serotype prevalence and stability are the consequence of two key factors, population dynamics and immune pressure (8). If this hypothesis is correct, it may well be extended to farmed fish populations that resemble semiclosed communities. Indeed, the rapid emergence of a novel serotype of the fish pathogen Streptococcus iniae and the complete replacement of the previous serotype following selective pressure that was applied through massive vaccination (2) substantiate this theory. Likewise, the limited selective pressure induced by specific vaccination against L. garvieae (currently available vaccines are partially effective, and only a small part of the entire population is actually vaccinated) also appears to support this theory. Indeed, over the 5-year period in which this study was carried out, L. garvieae capsular (and clonal) stability was preserved in endemic areas, and there were no variations in this major virulence factor (3) involved in resistance to opsonophagocytosis (30) and in avoiding complement-mediated serum killing (3). In contrast, serotype heterogeneity (which correlates with clonal diversity) characterizes sites where the organism has been introduced only recently, the disease is still sporadic, and the pathogen is still adapting to its new niche (France).
Acknowledgments
This work was supported by EU funding (grant QLK2-CT-2000-01049).
REFERENCES
- 1.Anthony, E., L. Kaplan, L. Wannamaker, and S. Chapman. 1976. The dynamics of streptococcal infections in a defined population of children: serotypes associated with skin and respiratory infections. Am. J. Epidemiol. 104:652-666. [DOI] [PubMed] [Google Scholar]
- 2.Bachrach, G., A. Zlotkin, A. Hurvitz, D. L. Evans, and A. Eldar. 2001. Recovery of Streptococcus iniae from diseased fish previously vaccinated with a Streptococcus vaccine Appl. Environ. Microbiol. 67:3756-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes, A. C., C. Guyot, B. G. Hansen, K. Mackenzie, M. T. Horne, and A. E. Ellis. 2001. Resistance to serum killing may contribute to differences in the abilities of capsulate and non-capsulated isolates of Lactococcus garvieae to cause disease in rainbow trout (Oncorhynchus mykiss L.). Fish Shellfish Immunol. 12:155-168. [DOI] [PubMed] [Google Scholar]
- 4.Brosius, J., A. Ulrich, M. A. Raker, A. Gray, T. J. Dull, R. R. Gutell, and H. F. Noller. 1981. Construction and fine mapping of recombinant plasmids containing the rRNB ribosomal RNA operon of E. coli. Plasmid 6:112-118. [DOI] [PubMed] [Google Scholar]
- 5.Carson, J., N. Gudkovs, and B. Austin. 1993. Characteristics of an Enterococcus-like bacterium from Australia and South Africa, pathogenic for rainbow trout (Oncorhynchus mykiss Walbaum). J. Fish Dis. 6:381-388. [Google Scholar]
- 6.Coenye, T., and J. J. LiPuma. 2002. Population structure analysis of Burkholderia cepacia genomovar III: varying degrees of genetic recombination characterize major clonal complexes. Microbiology 149:77-88. [DOI] [PubMed] [Google Scholar]
- 7.Collins, M. D., F. A. E. Farrow, B. A. Phillips, and O. Kandler. 1984. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J. Gen. Microbiol. 129:3427-3431. [DOI] [PubMed] [Google Scholar]
- 8.Dale, J. B., and S. T. Shulman. 2002. Dynamic epidemiology of group A streptococcal infections. Lancet 359:889. [DOI] [PubMed] [Google Scholar]
- 9.Dice, L. R. 1945. Measures of the amount of ecological association between species. Ecology 26:297. [Google Scholar]
- 10.Diler, O., S. Altun, A. K. Adiloglu, A. Kubilay, and B. Isikli. 2002. First occurrence of streptococcosis affecting farmed rainbow trout (Oncorhynchus mykiss) in Turkey. Bull. Eur. Assoc. Fish Pathol. 22:21-26. [Google Scholar]
- 11.Domenech, A., J. Prieta, J. F. Fernandez-Garayzabal, M. D. Collins, D. Jones, and L. Dominguez. 1993. Phenotypic and phylogenetic evidence for a close relationship between Lactococcus garvieae and Enterococcus seriolicida. Microbiologia 9:63-68. [PubMed] [Google Scholar]
- 12.Eldar, A., C. Ghittino, L. Asanta, E. Bozzetta, M. Goria, M. Prearo, and H. Bercovier. 1996. Enterococcus seriolicida is a junior synonym of Lactococcus garvieae, a causative agent of septicemia and meningoencephalitis in fish. Curr. Microbiol. 32:85-88. [DOI] [PubMed] [Google Scholar]
- 13.Eldar, A., M. Goria, C. Ghittino, A. Zlotkin, and H. Bercovier. 1999. Biodiversity of Lactococcus garvieae strains isolated from fish in Europe, Asia, and Australia. Appl. Environ. Microbiol. 65:1005-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feil, E. J., and B. G. Spratt. 2001. Recombination and the population structures of bacterial pathogens. Annu. Rev. Microbiol. 55:561-590. [DOI] [PubMed] [Google Scholar]
- 15.Feil, E. J., E. C. Holmes, D. E. Bessen, M. S. Chan, N. P. Day, M. C. Enright, R. Goldstein, D. W. Hood, A. Kalia, C. E. Moore, J. Zhou, and B. G. Spratt. 2001. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc. Natl. Acad. Sci. USA 98:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenoll, A., I. Jado, D. Vicioso, and J. Casal. 1997. Dot blot assay for the serotyping of pneumococci. J. Clin. Microbiol. 35:76-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaworzewska, E., and G. Colman. 1988. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol. Infect. 100:257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghittino, C., and M. Prearo. 1992. Report of streptococcosis in rainbow trout (Oncorhynchus mykiss) in Italy: preliminary note. Boll. Soc. It. Patol. Ittica 8:4-11. [Google Scholar]
- 19.Hoshina, T. 1956. An epidemic disease affecting rainbow trout in Japan. J. Tokyo Univ. Fish. 42:35-46. [Google Scholar]
- 20.Kaplan, E. L., J. T. Wotton, and D. R. Johnson. 2001. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet 358:1334-1337. [DOI] [PubMed] [Google Scholar]
- 21.Kusuda, K., K. Kawai, F. Salati, C. R. Banner, and J. L. Freyer. 1991. Enterococcus seriolicida sp. nov., a fish pathogen. Int. J. Syst. Bacteriol. 41:406-409. [DOI] [PubMed] [Google Scholar]
- 22.Lancefield, R. C. 1933. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 57:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, P., and E. Hoiby. 1990. Streptococcal serogroup A epidemic in Norway 1987-1988. Scand. J. Infect. Dis. 22:421-429. [DOI] [PubMed] [Google Scholar]
- 24.Musher, D. M., B. Johnson, and D. A. Watson. 1990. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect. Immun. 58:3871-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Múzquiz, J. L., F. M. Royo, C. Ortega, I. de Blas, I. Ruiz, and J. L. Alonso. 1999. Pathogenicity of streptococcosis in rainbow trout (Oncorhynchus mykiss): dependence on age and diseased fish. Bull. Eur. Assoc. Fish Pathol. 19:114-119. [Google Scholar]
- 26.Ravelo, C., S. B. Magarinos, S. Lopez-Romalde, A. E. Toranzo, and J. L. Romalde. 2003. Molecular fingerprinting of fish-pathogenic Lactococcus garvieae strains by random amplified polymorphic DNA analysis. J. Clin. Microbiol. 41:751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridley, M. 1996. Evolution. Blackwell Science Inc., Cambridge, Mass.
- 28.Vela, A. I., J. Vázquez, A. Gibello, M. M. Blanco, M. A. Moreno, P. Liébana, C. Albendea, B. Alcalá, A. Méndez, L. Domínguez, and J. F. Fernández-Garayzábal. 2000. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks in comparison with isolates of other countries and sources. J. Clin. Microbiol. 38:3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wannamaker, L. 1954. The epidemiology of streptococcal infections, p. 85-112. In M. McCarty (ed.), Streptococcal infections. Columbia University Press, New York, N.Y.
- 30.Yoshida, T., M. Endo, M. Sakai, and V. Inglis. 1997. A cell capsule with possible involvement in resistance to opsonophagocytosis in Enterococcus seriolicida isolated from yellowtail Seriola quinqueradiata. Dis. Aquat. Org. 29:233-235. [Google Scholar]
- 31.Zlotkin, A., Eldar, C. Ghittino, and H. Bercovier. 1998. Identification of Lactococcus garvieae by PCR. J. Clin. Microbiol. 36:983-985. [DOI] [PMC free article] [PubMed] [Google Scholar]