Abstract
Escherichia coli O157:H7 is only occasionally isolated from healthy swine, but some experimentally infected animals will shed the organism in their feces for at least 2 months. Potential explanations for the paucity of naturally occurring infections in swine, as compared to cattle, include a lack of animal-to-animal transmission so that the organism cannot be maintained within a herd, a high infectious dose, or herd management practices that prevent the maintenance of the organism in the gastrointestinal tract. We hypothesized that donor pigs infected with E. coli O157:H7 would transmit the organism to naïve pigs. We also determined the infectious dose and whether housing pigs individually on grated floors would decrease the magnitude or duration of fecal shedding. Infected donor pigs shedding <104 CFU of E. coli O157:H7 per g transmitted the organism to 6 of 12 naïve pigs exposed to them. The infectious dose of E. coli O157:H7 for 3-month-old pigs was approximately 6 × 103 CFU. There was no difference in the magnitude and duration of fecal shedding by pigs housed individually on grates compared to those housed two per pen on cement floors. These results suggest that swine do not have an innate resistance to colonization by E. coli O157:H7 and that they could serve as a reservoir host under suitable conditions.
Escherichia coli O157:H7 and other serotypes of Shiga toxigenic E. coli (STEC) cause an estimated 110,000 cases of human illness yearly in the United States (26). Most cases are thought to occur as a result of the ingestion of contaminated food or water, although direct contacts with animals and person-to-person transmission have also been documented (4). Cattle are considered to be the major reservoir of STEC, and the prevalence of E. coli O157:H7 in the U.S. herd ranges from 2 to 28%, depending on the culture techniques used, the age of the animals, and the season in which samples are collected (10, 12, 15, 17, 29, 33). E. coli O157:H7 has also been recovered from other ruminants such as deer (22, 30) and sheep (24). E. coli O157:H7 has occasionally been isolated from nonruminant animals such as wild birds (32) and raccoons (18), but the bulk of the data suggests that the prevalence of STEC is greater in ruminants than it is in other animals.
In the last several years, there have been reports that E. coli O157:H7 has been isolated from healthy swine in Japan, The Netherlands, Sweden, Canada, Norway, and the United States (11, 13, 19, 20, 27; C. L. Gyles, R. Friendship, K. Ziebell, S. Johnson, I. Yong, and R. Amezcua, Proc. 2002 Congr. Int. Pig Vet. Soc., abstr. 191). The prevalence of the organism in these studies is generally low (0.1 to 6%), and no human outbreaks have been specifically traced back to pork, although sausage containing both beef and pork was implicated as the source of human infection in at least one outbreak (28). In Chile, the prevalence of E. coli O157:H7 reported from pigs (10.8%) was greater than that reported from cattle (2.9%), suggesting that swine may be an important source of this organism in some countries (3). Previously, we have shown that some market-weight pigs experimentally infected with E. coli O157:H7 will shed the organism for at least 2 months (2). These animals do not become clinically ill, and the magnitude and duration of fecal shedding of E. coli O157:H7 are reminiscent of those seen in experimentally infected ruminants (6, 7). This suggests that swine have the biological potential to emerge as a reservoir for E. coli O157:H7 and other STEC strains pathogenic for humans. In order for swine to serve as a reservoir host, not only must the organism colonize the gastrointestinal tract of individual animals, it must also be transmitted from colonized animals to naïve animals to be maintained within the herd. In addition, the infectious dose must be of such a magnitude that a natural infection could be perpetuated within the herd. We hypothesized that E. coli O157:H7 would be transmitted from infected donor pigs to naïve pigs at levels that could be sustained in a natural infection. In addition, we determined the infectious dose of in vitro-grown E. coli O157:H7 for 3-month-old pigs and determined whether housing pigs individually on raised decks or in groups on cement floors affected the magnitude and duration of fecal shedding in infected animals.
(A preliminary report of this work was presented at the International Symposium on Shiga Toxin-Producing E. coli, Kyoto, Japan, 2000, and Edinburgh, Scotland, 2003.)
MATERIALS AND METHODS
Experimental design.
Pigs were obtained from a commercial source at 3 months of age and housed in pens with cement floors at two pigs per pen under animal biosafety level 2 conditions. The pigs were acclimated to an antibiotic-free feed (Lean Grow 100, Land ′O Lakes, Arden Hills, Minn.) for 2 weeks prior to inoculation and had free access to chlorinated city water via nipple drinkers. Fecal samples were collected from each animal once prior to inoculation and screened with sorbitol-MacConkey agar and immunomagnetic beads to ensure that pigs were not naturally colonized by E. coli O157:H7.
The bacterial inoculum was grown as previously described (31). Briefly, individual strains were grown in tryptic soy broth (TSB) overnight at 37°C with shaking (200 rpm), concentrated 10-fold, and frozen at −80°C in glycerol. The inoculum was thawed and diluted in TSB just prior to inoculation. All of theinoculum doses were confirmed by direct plate counts. Prior to inoculation, pigs were physically separated by closing a gate within the pen. Each pig was orally inoculated with E. coli O157:H7 strain 86-24 (all experiments) and strain 3081(housing experiment only) by adding the organism to a small amount of food placed in individual pans. Both strain 86-24 and 3081 produce intimin and Shiga toxin 2 (Stx2) and are pathogenic in animal models of STEC disease (8, 9). Strain 3081 also produces Stx1. Pigs were observed until the inoculum was consumed, and then the gates between them were opened.
Fecal samples were collected on days 2, 3, 4 (initial period), 14, 15, 16 (2 weeks), 58, 59, and 60 (2 months; housing experiment only) postinoculation (p.i.) and cultured for the inoculum strain(s) as previously described (6). Briefly, samples (5 g) were serially diluted 10-fold in phosphate-buffered saline and directly plated in triplicate onto dulcitol-MacConkey agar containing streptomycin (100 μg/ml) and nalidixic acid (20 μg/ml) for recovery of strain 86-24 and onto sorbitol-MacConkey agar containing kanamycin (30 μg/ml) and ampicillin (100 μg/ml) for recovery of strain 3081. The detection limit of direct plating was ≥50 CFU/g. Enrichment broth (TSB with 0.02% bile and antibiotics) was inoculated with 10 g of feces, incubated overnight at 37°C, concentrated with immunomagnetic beads (Dynabeads, Dynal, Oslo, Norway), and plated onto the selective medium described above. The detection limit of the enrichment procedure was approximately 3 CFU/100 g. Colonies recovered on selective medium were confirmed as E. coli O157:H7 by using a commercial latex agglutination kit specific for the O157 lipopolysaccharide (Oxiod). Data from individual pigs were averaged together by using geometric means of bacterial counts collected during each 3-day time period. Samples that were positive by enrichment only were recorded as 49 CFU/g.
Pigs were necropsied at the completion of each study, and 5-cm sections of tissue and adhering content were cultured for the inoculum strains by direct plating and enrichment culture with immunomagnetic concentration (as described above). The tissues collected from all pigs were tonsil, stomach, jejunum, ileum, cecum, spiral colon, distal colon, and rectal content. In addition, the rectal-anal junction was collected from 16 pigs and buccal cell scrapings were collected from 8 pigs.
Transmission experiment.
After a 2-week adaptation period, donor pigs (20 total) were inoculated with 106 to 108 CFU of E. coli O157:H7 strain 86-24. This inoculum dose resulted in fecal shedding by the donor pigs that ranged from <50 to 107 CFU/g 3 days after inoculation, at which time the donors were moved into a different room and penned with a naïve pig. Both the donor and the naïve pigs shared food and water sources and remained in the same pen together for the remainder of the experiment. Individual fecal samples were collected during the initial period and at 2 weeks postexposure from both donor and naïve pigs. The pigs were necropsied at 2 weeks postexposure.
One group of donor animals (four pigs given an inoculum of 106 CFU) did not shed E. coli O157:H7 past 4 days p.i., nor did any transmission occur to the four naïve pigs paired with this group of donors. These eight pigs were then reinoculated with a higher dose (108 CFU), and all four animals shed the inoculum strain for 2 weeks. E. coli O157:H7 was not recovered at any time from one other donor pig; therefore, data from this animal and its naïve pen mate was not included in the final analysis.
Infectious dose experiment.
After a 2-week acclimation period, groups of eight pigs were inoculated with either 1.7 × 102, 6 × 103, or 4 × 104 CFU of E. coli O157:H7 strain 86-24. Fecal samples from individual pigs were collected during the initial period and at 2 weeks p.i. and cultured for the inoculum strain as described above. All of the pigs were necropsied at 2 weeks p.i. Since E. coli O157:H7 was not ever recovered from the eight pigs inoculated with the lowest dose (1.7 × 102 CFU), these pigs were reinoculated with the 4 × 104 CFU dose 4 weeks after the initial challenge.
Housing experiment.
Following the 2-week acclimation period, pigs were randomly divided into two groups. Group 1 (eight pigs) was moved onto individual decks, and feed was delivered in a trough. Group 2 (seven pigs) was housed two per pen on cement floors and fed on the floor. Pigs in each group were housed in separate rooms of the animal facility. Pigs were dually inoculated with 1010 CFU each of E. coli O157:H7 strains 86-24 and 3081. Fecal samples were collected during the initial period, 2 weeks p.i., and at 2 months p.i. and cultured for the inoculum strains by both direct plating and enrichment culture (as described above). Antibiotics were not added to the enrichment broth in this experiment. All pigs were necropsied at 2 months p.i. Differences in the magnitude of fecal shedding due to the housing arrangement were evaluated with a repeated-measures analysis of variance.
RESULTS
Transmission of E. coli O157:H7 among swine.
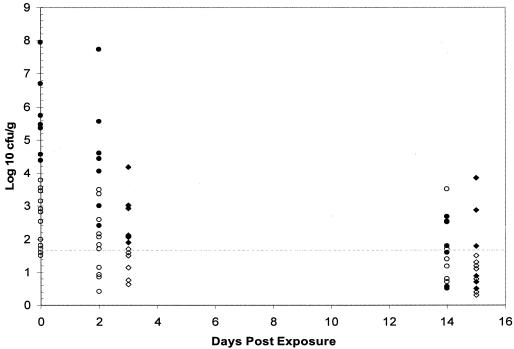
E. coli O157:H7 was transmitted to three of eight naïve pigs when the donor pigs were shedding ≤102 CFU/g in their feces and to three of four naïve pigs exposed to donors shedding 103 CFU/g (Table 1). All of the naïve pigs (seven of seven) that were exposed to donors shedding ≥104 CFU/g of feces became culture positive for E. coli O157:H7. During this initial period (days 2 to 4 postexposure), the magnitude of fecal shedding by the naïve pigs generally reflected that of the donor pigs to which they were exposed (Fig. 1). Naïve pigs exposed to donors shedding low levels (<104 CFU/g) of E. coli O157:H7 shed low levels themselves. Naïve pigs exposed to donors shedding higher numbers (≥104 CFU/g) of E. coli O157:H7 shed higher levels. At 2 weeks postexposure, E. coli O157:H7 was recovered from the feces of 13 of 19 naïve pigs and from 14 of 19 donor pigs. The majority of pigs in both groups were positive only by enrichment culture (<50 CFU/g), but a few individuals continued to shed ≥103 CFU/g of feces.
TABLE 1.
Transmission of E. coli O157:H7 between inoculated donors and naïve swine
| Time postexposure | No. of pigs shedding/no. exposed by CFU/ga
|
||||||
|---|---|---|---|---|---|---|---|
| <102 | 102 | 103 | 104 | 105 | 106 | 107 | |
| 3 days | 2/4 | 1/4 | 3/4 | 2/2 | 3/3 | 1/1 | 1/1 |
| 2 weeks | 2/4 | 2/4 | 3/4 | 2/2 | 2/3 | 1/1 | 1/1 |
The results represent the number of naïve pigs shedding/number of naïve pigs exposed in terms of the CFU per gram shed by the inoculated donor on day 0. Day 0 is the day that the inoculated donors were moved into a different room and penned with a naïve pig.
FIG. 1.
E. coli O157:H7 shed by inoculated donor pigs and naïve pigs exposed to those donors. Day 0 is the day that infected donors were moved into a different room and penned with a naïve pig. •, donor pigs shedding ≥104 CFU/g; ○, donor pigs shedding <104 CFU/g; ♦, naïve pigs exposed to donors shedding ≥104 CFU/g; ⋄, naïve pigs exposed to donors shedding <104 CFU/g. Values below the dotted line were positive by enrichment culture only (<50 CFU/g).
Both groups of pigs were necropsied at 2 weeks postexposure, and tissues from the gastrointestinal tract and oral cavity were cultured. E. coli O157:H7 was recovered primarily from the lower gastrointestinal tract of both naïve and donor pigs (Table 2). Two pigs (one donor and one naïve) had comparatively high numbers of E. coli O157:H7 recovered from tonsil tissue: 4 × 106 and 4 × 104 CFU/cm, respectively. These two pigs were not paired together during the experiment, and the magnitude of fecal shedding by each animal at the time of necropsy was considerably less than what was recovered in the tonsil: 63 × 102 and 8 × 102 CFU/g (donor and naïve pigs, respectively).
TABLE 2.
Summary of bacteriological results of tissues from pigs inoculated with E. coli O157:H7
| Tissue | Result for:
|
|||
|---|---|---|---|---|
| 2 wk p.i. (inoculum dose, 1.7-7.0 log10 CFU)a
|
2 mo p.i. (inoculum dose, 10.0 log10 CFU)b
|
|||
| No. positive/total | Mean log10 CFU/g (range) | No. positive/total | Mean log10 CFU/g (range) | |
| Buccal cellsc | 7/8 | 1.7 | NDe | |
| Tonsil | 9/48 | 2.8 (1.7-6.6) | 12/15 | 3.7 (1.7-5.3) |
| Stomach | 11/48 | 1.7 | 5/15 | 1.7 |
| Jejunum | 14/48 | 1.7 (1.7-2.0) | 2/15 | 1.7 |
| Ileum | 15/48 | 2.0 (1.7-4.0) | 11/15 | 2.0 (1.7-3.4) |
| Cecum | 24/48 | 2.6 (1.7-6.0) | 13/15 | 1.8 (1.7-2.7) |
| Spiral colon | 24/48 | 2.2 (1.7-4.0) | 12/15 | 1.9 (1.7-2.6) |
| Distal colon | 20/48 | 2.0 (1.7-5.7) | ND | |
| Rectal-anal junctiond | 10/16 | 2.5 (1.7-4.9) | ND | |
Includes pigs from both transmission and infectious dose experiments.
Pigs from housing experiment.
Buccal cell samples were collected from 8 of 48 pigs.
The rectal-anal junction was collected from 16 of 48 pigs.
ND, not determined.
Infectious dose of E. coli O157:H7 for pigs.
E. coli O157:H7 was recovered at 2 to 4 days p.i. from the feces of six of eight pigs given an inoculum dose of 6 × 103 CFU and eight of eight pigs given an inoculum dose of 4 × 104 CFU. At 2 weeks p.i., all of the pigs in both groups continued to shed E. coli O157:H7. In contrast, E. coli O157:H7 was not recovered from any of the eight pigs during the initial period or at 2 weeks after inoculation with 1.7 × 102 CFU. Pigs were necropsied after 2 weeks p.i., and both the oral cavity and gastrointestinal tract were cultured. The distribution of E. coli O157:H7 in the tissues of these pigs was similar to what was found for those in the transmission experiment (Table 2). Tissue samples from the rectal-anal junction were also collected from these 16 pigs, and buccal cell scrapings were collected from 8 pigs. The magnitude of E. coli O157:H7 (CFU per gram) isolated from the rectal-anal junction was greater than that isolated from the feces in six pigs and was of a similar magnitude in eight pigs. The number of E. coli O157:H7 cells recovered from the feces was greater than that recovered from the rectal-anal junction in one pig.
Effect of housing on shedding of E. coli O157:H7.
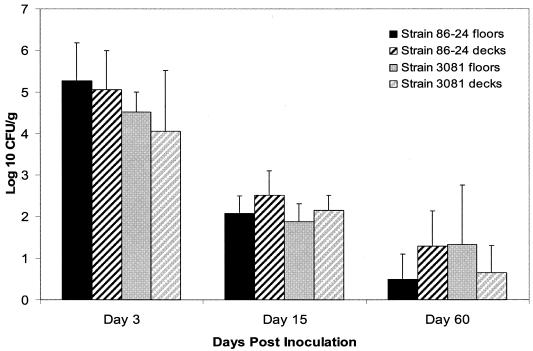
There was no significant difference in the magnitude or duration of fecal shedding of E. coli O157:H7 by pigs housed singly on decks compared to those housed two per pen on cement floors (Fig. 2). The magnitude of shedding ranged from 103 to 107 CFU/g during the initial period, from 50 to 103 CFU/g at 2 weeks p.i., and from undetectable to 104 CFU/g at 2 months p.i. E. coli O157:H7 was recovered from the feces of 10 of 15 pigs at 2 months p.i. Eight of these 10 pigs were shedding both of the inoculum strains, and another 2 pigs were shedding one strain apiece. At necropsy, E. coli O157:H7 was recovered primarily from the tissues of the lower gastrointestinal tract and the tonsils (Table 2). The magnitude (CFU per gram) of E. coli O157:H7 recovered from the tonsils of eight pigs was at least 100× greater than that recovered from the feces of the same pigs. Both E. coli O157:H7 strains were recovered from the tonsils of five of these eight pigs. Since the use of dual-antibiotic selection medium is not definitive, it cannot be excluded that a newly introduced E. coli O157:H7 strain with a similar antibiogram was recovered.
FIG. 2.
Average magnitude of fecal shedding of E. coli O157:H7 by pigs housed one per pen on raised decks and two per pen on cement floors. There was no significant difference between floor types.
DISCUSSION
The transmission of E. coli O157:H7 from infected donor pigs to naïve pigs suggests that this organism has the potential to be sustained in a population of swine once introduced. It is not known why E. coli O157:H7 is only occasionally recovered from U.S. pigs. There apparently is not a biological barrier to the colonization of swine by E. coli O157:H7, since some experimentally infected animals continue to shed the organism in their feces for at least 2 months (2). The relatively high isolation rate of E. coli O157:H7 from swine in Chile also suggests that pigs are a biologically competent host (3). Perhaps the vast majority of swine herds do not come in contact with an infectious dose of E. coli O157:H7. This may be due, in part, to current management practices by swine producers. The infectious dose of E. coli O157:H7 for 3-to 4-month-old pigs in the study reported here was approximately 6 × 103 CFU in vitro-grown bacteria. This is similar to what has been reported for young calves (5 × 103 to 9 × 103 CFU), although lower doses may be infectious to an occasional animal (1). Considerably higher doses of E. coli O157:H7 are required to colonize mature cattle (7) and sheep (6): 107 and 105 CFU, respectively. It is not known why several of the donor pigs given a high inoculum dose (106 CFU) did not shed the strain past 4 days p.i. It is likely that there are some individuals that are more resistant to colonization than others (N. A. Cornick, unpublished observations). Although we did not look for systemic antibody against Stx1 or Stx2 in these pigs, such antibody has not been found in pigs previously (2). Our observation that the magnitude of fecal shedding by the naïve pigs reflected that of the donor pigs (Fig. 1), implies that at least in the early days following exposure, the inoculum dose has an important influence on the magnitude of fecal shedding. At 2 weeks p.i., both pigs shedding >102 CFU of E. coli O157:H7 per g had been exposed to donors shedding ≥104 CFU/g. Collectively, our data suggest that both the inoculum dose and an individuals' susceptibility to colonization are important factors in the persistent shedding of this organism by some animals.
The transmission of E. coli O157:H7 from ruminants to swine has occurred on farms where both species are raised in close contact with one another (11). Pulsed-field gel electrophoresis subtyping of isolates from both pigs and ruminants confirmed that the same strains were recovered from each animal species. Animal management practices were identified on these farms that could have contributed to the transmission. Experimental transmission of STEC, including E. coli O157:H7, has been documented in both calves (1, 5) and sheep (6, 23, 25). Naïve calves exposed to a donor calf inoculated with very low levels of E. coli O157:H7 (2.6 × 102 CFU) shed the organism 10 to 17 days postexposure (1). The transmission of STEC between calves was more efficient when the naïve animals shared a pen with the inoculated donor as opposed to being confined in individual pens (5). However, transmission has been documented between calves that did not have nose-to-nose contact with the inoculated donor (1). Horizontal transmission between individuals within a herd is likely to be an important component in the establishment and maintenance of an animal reservoir.
The recovery of comparatively high numbers of E. coli O157:H7 from the tonsils of some pigs is of interest. Frequently the pigs with the greatest quantity (CFU per gram) of E. coli O157:H7 in the tonsils were shedding 100× less of the organism in their feces. This suggests that the E. coli O157:H7 was replicating and maintaining a population in the tonsil rather than being continually reingested from the environment. The lack of any difference in the magnitude and duration of fecal shedding between animals housed on decks (presumably a cleaner environment) and those housed and fed on cement floors also suggests that E. coli O157:H7 was actually colonizing the gastrointestinal tract of these animals rather than being continuously recycled. STEC strains, including E. coli O157:H7, have been recovered from the oral cavity and tonsils of naturally infected cattle (14, 21; J. T. Gray, D. Smith, R. Moxley, C. Rolfes, L. Hungerford, S. Younts, M. Blackford, D. Bailey, T. Milton, and T. Klopfenstein, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. P-57, p. 524, 2000) and pigs (16). The study by Keen et al. found that the isolation rate of E. coli O157:H7 from the oral cavity and hides of feedlot cattle was actually higher than the isolation rate from feces (21). Experiments to determine the significance of tonsil colonization by E. coli O157:H7 in pigs are warranted.
The transmission of E. coli O157:H7 by donor pigs that were shedding very low levels of the organism in their feces, combined with the relatively low infectious dose, suggests that pigs have the biological potential to emerge as a reservoir for E. coli O157:H7 in the United States. The low prevalence of this organism in the U.S. swine population despite the fact that the serotype has the capacity to colonize and disseminate horizontally could be a lesson to other food animal production systems if the specific management factors contributing to this phenomenon could be further identified.
Acknowledgments
We thank Sheridan Booher, Dianna Jordan, Ilze Matise, and Harley Moon for insightful discussions and assistance. We also thank Carisa Ralph for technical assistance and animal work and Richard Evans for the statistical advice.
This work was supported in part by the U.S. Department of Agriculture National Research Initiative Competitive Grants program 00-02515 and the Iowa State University Healthy Livestock Initiative.
REFERENCES
- 1.Besser, T. E., B. L. Richards, D. H. Rice, and D. D. Hancock. 2001. Escherichia coli O157:H7 infection of calves: infectious dose and direct contact transmission. Epidemiol. Infect. 127:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booher, S., N. A. Cornick, and H. W. Moon. 2002. Persistence of Escherichia coli O157:H7 in experimentally infected swine. Vet. Microbiol. 89:69-81. [DOI] [PubMed] [Google Scholar]
- 3.Borie, C., Z. Monreal, P. Guerrero, M. L. Sanchez, J. Martinez, C. Arellano, and V. Prado. 1997. Prevalencia y caracterizacion de Escherichia coli enterohemorragica aisladas de bovinos y cerdos sanos faenados en Santiago, Chile. Arch. Med. Vet. 29:205-212. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Reported outbreaks of shiga toxin-producing Escherichia coli (including O157:H7) occurring January through December 2001. www.cdc.gov/foodborneoutbreaks/report_pub.htm.
- 5.Cobbold, R., and P. Desmarchelier. 2002. Horizontal transmission of Shiga toxin-producing Escherichia coli within groups of dairy calves. Appl. Environ. Microbiol. 68:4148-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornick, N. A., S. L. Booher, T. A. Casey, and H. W. Moon. 2000. Persistent colonization of sheep by Escherichia coli O157:H7 and other E. coli pathotypes. Appl. Environ. Microbiol. 66:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean-Nystrom, E. A., J. F. L. Pohlenz, H. W. Moon, and A. D. O'Brien. 2000. Escherichia coli O157:H7 causes more-severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect. Immun. 68:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erikkson, E., E. Nerbrink, E. Borch, A. Aspan, and A. Gunnarsson. 2003. Verocytotoxin-producing Escherichia coli O157:H7 in the Swedish pig population. Vet. Rec. 152:712-717. [DOI] [PubMed] [Google Scholar]
- 12.Faith, N. G., J. A. Shere, R. Brosch, K. W. Arnold, S. E. Ansay, M.-S. Lee, J. B. Luchansky, and C. W. Kaspar. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feder, I. E., M. Wallace, J. T. Gray, P. Fratamico, P. J. Fedorka-Cray, R. Pearce, J. E. Call, R. Perrine, and J. B. Luchansky. 2003. Isolation of Escherichia coli O157:H7 from intact colon fecal samples of swine. Emerg. Infect. Dis. 9:380-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank, G. H., R. E. Briggs, and R. A. Schneider. 1994. Characterization of Escherichia coli isolated from the tonsils of cattle. J. Clin. Microbiol. 32:256-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber, L. P., S. J. Wells, D. D. Hancock, M. P. Doyle, J. Tuttle, J. A. Shere, and T. Zhao. 1995. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J. Am. Vet. Med. Assoc. 207:46-49. [PubMed] [Google Scholar]
- 16.Gill, C. O., and T. Jones. 1998. Control of the contamination of pig carcasses by Escherichia coli from their mouths. Int. J. Food Microbiol. 44:43-48. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington State. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock, D. D., T. E. Besser, D. H. Rice, E. D. Ebel, D. E. Herriot, and L. V. Carpenter. 1998. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev. Vet. Med. 35:11-19. [DOI] [PubMed] [Google Scholar]
- 19.Heuvelink, A. E., J. T. M. Zwartkruis-Nahuis, F. L. A. M. van den Biggelaar, W. J. van Leeuwen, and E. de Boer. 1999. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 from slaughter pigs and poultry. Int. J. Food Microbiol. 52:67-75. [DOI] [PubMed] [Google Scholar]
- 20.Johnsen, G., Y. Wasteson, E. Heir, O. I. Berget, and H. Herikstad. 2001. Escherichia coli O157:H7 in faeces from cattle, sheep and pigs in the southwest part of Norway during 1998 and 1999. Int. J. Food Microbiol. 65:193-200. [DOI] [PubMed] [Google Scholar]
- 21.Keen, J. E., and R. O. Elder. 2002. Isolation of shigatoxigenic Escherichia coli O157 from hide surfaces and the oral cavity of finished beef feedlot cattle. J. Am. Vet. Med. Assoc. 220:756-763. [DOI] [PubMed] [Google Scholar]
- 22.Keene, W. E., E. Sazie, J. Kok, D. H. Rice, D. D. Hancock, V. K. Balan, T. Zhao, and M. P. Doyle. 1997. An outbreak of Escherichia coli O157:H7 infections traced to jerky made from deer meat. JAMA 277:1229-1231. [DOI] [PubMed] [Google Scholar]
- 23.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1996. Escherichia coli O157:H7 in microbial flora of sheep. J. Clin. Microbiol. 34:431-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudva, I. T., C. W. Hunt, C. J. Williams, U. M. Nance, and C. J. Hovde. 1997. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl. Environ. Microbiol. 63:3878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCraig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:Sept.-Oct. [Online.] http://www.cdc.org. [DOI] [PMC free article] [PubMed]
- 27.Nakazawa, M., M. Akiba, and T. Sameshima. 1999. Swine as a potential reservoir of Shiga toxin-producing Escherichia coli O157:H7 in Japan. Emerg. Infect. Dis. 5:Nov.-Dec. [Online.] http://www.cdc.org. [DOI] [PMC free article] [PubMed]
- 28.Paton, A. W., R. M. Ratcliff, R. M. Doyle, J. Seymour-Murray, D. Davos, J. A. Lanser, and J. C. Paton. 1996. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J. Clin. Microbiol. 34:1622-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahn, K., S. A. Renwick, R. P. Johnson, J. B. Wilson, R. C. Clarke, D. Alves, S. McEwen, H. Lior, and J. Spika. 1997. Persistence of Escherichia coli O157:H7 in dairy cattle and the dairy farm environment. Epidemiol. Infect. 119:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sargeant, J. M., D. J. Hafer, J. R. Gillespie, R. D. Oberst, and S. J. A. Flood. 1999. Prevalence of Escherichia coli O157:H7 in white-tailed deer sharing rangeland with cattle. J. Am. Vet. Med. Assoc. 215:792-794. [PubMed] [Google Scholar]
- 31.Sarmiento, J. I., T. A. Casey, and H. W. Moon. 1988. Postweaning diarrhea in swine: experimental model of enterotoxigenic Escherichia coli infection. Am. J. Vet. Res. 49:1154-1159. [PubMed] [Google Scholar]
- 32.Wallace, J. S., T. Cheasty, and K. Jones. 1997. Isolation of Vero cytotoxin-producing Escherichia coli O157 from wild birds. J. Appl. Microbiol. 82:399-404. [DOI] [PubMed] [Google Scholar]
- 33.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]