Abstract
The distribution of nitrogenase activity in the rice-soil system and the possible contribution of epiphytic cyanobacteria on rice plants and other macrophytes to this activity were studied in two locations in the rice fields of Valencia, Spain, in two consecutive crop seasons. The largest proportion of photodependent N2 fixation was associated with the macrophyte Chara vulgaris in both years and at both locations. The nitrogen fixation rate associated with Chara always represented more than 45% of the global nitrogenase activity measured in the rice field. The estimated average N2 fixation rate associated with Chara was 27.53 kg of N ha−1 crop−1. The mean estimated N2 fixation rates for the other parts of the system for all sampling periods were as follows: soil, 4.07 kg of N ha−1 crop−1; submerged parts of rice plants, 3.93 kg of N ha−1 crop−1; and roots, 0.28 kg of N ha−1 crop−1. Micrographic studies revealed the presence of epiphytic cyanobacteria on the surface of Chara. Three-dimensional reconstructions by confocal scanning laser microscopy revealed no cyanobacterial cells inside the Chara structures. Quantification of epiphytic cyanobacteria by image analysis revealed that cyanobacteria were more abundant in nodes than in internodes (on average, cyanobacteria covered 8.4% ± 4.4% and 6.2% ± 5.0% of the surface area in the nodes and internodes, respectively). Epiphytic cyanobacteria were also quantified by using a fluorometer. This made it possible to discriminate which algal groups were the source of chlorophyll a. Chlorophyll a measurements confirmed that cyanobacteria were more abundant in nodes than in internodes (on average, the chlorophyll a concentrations were 17.2 ± 28.0 and 4.0 ± 3.8 μg mg [dry weight] of Chara−1 in the nodes and internodes, respectively). These results indicate that this macrophyte, which is usually considered a weed in the context of rice cultivation, may help maintain soil N fertility in the rice field ecosystem.
Rice fields are one of the most extensive freshwater ecosystems on Earth, covering about 150 × 106 ha. In spite of the widespread dominance of rice plants, a conspicuous photosynthetic aquatic biomass composed of cyanobacteria, planktonic, filamentous, and macrophytic algae, and vascular macrophytes develops during different phases of rice growth and competes with rice for nutrients and light (23). Nitrogen is a major factor in rice production. However, the efficiency of N fertilizer is one of the lowest efficiencies of all plant nutrients due to large N losses from flooded soils (7). Native soil nitrogen is the main N source for rice, accounting for more than 50% of the N in the rice plant (9, 10, 17), and so long-term sustainability of rice cultivation depends on the use and effective management of internal resources that maintain soil N fertility. The soil N pool is believed to be maintained by biological nitrogen fixation (17, 25). In fact, biological N2 fixation has allowed a stable and moderate yield to be maintained in traditional low-input rice cultivation (25). Among the indigenous nitrogen fixers, N2-fixing cyanobacteria are responsible for most of the biological N2 fixation in rice paddy fields (14, 22, 23, 24, 25, 26, 28). Estimates of the N balance in the presence and absence of light indicate that, on average, photodependent nitrogen fixation contributes two-thirds of the balance (26). A similar value was obtained in daily cycle studies of acetylene-reducing activity in Valencian rice fields (21). Estimates of N fixed by N2-fixing cyanobacteria in Asian rice fields ranged from less than 5 to more than 80 kg of N ha−1 crop−1, with an average of around 30 kg of N ha−1 crop−1 (24). Studies of N2 fixation by cyanobacteria in rice fields have focused on benthic and planktonic communities (14, 22, 28); no attention has been paid to epiphytic cyanobacteria, except in deepwater rice fields in which colonies of N2-fixing cyanobacteria appear to be associated with deepwater rice tillers (16, 29).
Studies of the rice fields of Valencia, Spain, during the last 15 years have revealed abundant benthic and planktonic N2-fixing cyanobacteria at densities similar to those in Asian rice fields (20). Isotope experiments indicated that N fixed by cyanobacteria was readily and rapidly available to rice plants, which allowed a significant reduction in N fertilizer input without a loss of productivity (9). The photodependent nitrogen fixation ranged from 0.23 to 75.5 kg of N ha−1 year−1 in the absence of cyanobacterial blooms (22). However, this activity was not well correlated with the number of N2-fixing cyanobacteria in water and soil (22). A similar lack of correlation was reported for the rice fields of Uruguay (14). To explain this absence of association, the distribution of nitrogenase activity in the rice-soil system and the possible contribution of epiphytic cyanobacteria to rice plants and other macrophytes were studied. The most abundant macrophyte in these rice fields is the macroalga Chara vulgaris, which covers the soil surface almost completely during all or part of the cultivation cycle, depending on the density of rice plants.
MATERIALS AND METHODS
Field assays.
The experimental plots were located in the rice fields surrounding the Albufera Lake nature reserve in Valencia, Spain. Field assays were conducted in June, July, and August at two different places, Sueca and Tancat de Malta, which are 15 km apart. Nitrogen fixation rates were estimated in situ by the acetylene reduction method (30), as previously described (21). During the 1999 cultivation cycle, experiments were conducted in two fields: at the Tancat de Malta site in June and at the Sueca site in July and August. In the 2000 crop season, experiments were conducted at the Sueca site in June, July, and August. Nitrogen fixation rates associated with the different parts of the rice soil system (soil, roots, submerged parts of the rice plants, and Chara) were estimated by measuring acetylene reduction activity (ARA) during the morning (0900 to 1400 h) by field incubation at places where no conspicuous cyanobacterial biomass was observed.
On the first day of each sampling campaign, ARA was measured in situ in 11 incubation chambers that included the unmodified whole system, which was comprised of one rice plant, the macrophyte Chara, the water layer, and the soil. Each component of the whole system was separated from the other components on subsequent days and independently incubated in situ or in vitro to assess nitrogenase activity. In vitro nitrogenase activity was determined in 0.5-liter transparent plastic chambers. These chambers were incubated in the field to maintain the natural temperature and illumination conditions. In the assays conducted in the dark, the plastic chambers were covered with aluminum foil. Additionally, in order to determine the daily variation in the ARA and its possible influence on the calculated rates, control assays were done in three chambers maintained under unmodified conditions in the same place for 4 days. In all cases, the chambers were aerated after completion of the assays in order to prevent accumulation of ethylene and to allow recovery from the exposure to acetylene. Gas samples were collected with double needles in 10-ml Vacutainer vacuum tubes, and their ethylene concentrations were measured by using a Shimadzu gas chromatograph equipped with a flame ionization detector and a Porapak N 80/100 column.
The acetylene reduction method was calibrated by using a bloom of Anabaena sp. that developed in the experimental field sites. Five replicate samples of the bloom were incubated in parallel with acetylene and 15N2 gas for 1 h under field conditions. Control flasks containing the Anabaena bloom with no 15N2 gas were also incubated in parallel. Anabaena samples were dried at 60°C, ground in a mortar, and analyzed for 15N enrichment by using a continuous-flow isotope ratio mass spectrometer.
Microscopic study.
The biomass of Chara was collected from the assay chambers in sterile Whirl Pak bags after the nitrogenase assays, weighed, fixed with 4% paraformaldehyde, and kept in the dark at 4°C until further analyses were performed. Portions of Chara were examined with a fluorescence microscope by using violet and green filters to identify fluorescence from chlorophyll and phycobiliproteins. Additionally, portions of fresh Chara were placed in petri dishes containing N-free BG11 medium. Biological material was incubated at 25°C with continuous light until the growth of cyanobacteria was confirmed with the fluorescence microscope.
Fixed portions of Chara were used for scanning electron microscopy. Fixed samples were washed in distilled water to eliminate any remaining paraformaldehyde. The samples were subsequently dehydrated with increasing concentrations of acetone, mounted on grids, and finally gold coated with an SC502 sputter coater. They were examined with a Philips XL30 scanning microscope at 20 kV. For confocal microscopy, fixed Chara was cut into small portions (length, around 0.5 mm), and these portions were then encased in 5% agarose cubes. Each of the cubes was then placed on a slide, covered with a coverslip, and sealed to avoid desiccation. Chara was observed with a Radiance 2000 confocal scanning laser microscope by using argon (488 and 514 nm) and helio-neon (543 nm) lasers.
Quantification of epiphytic cyanobacteria on C. vulgaris.
Nodal and internodal zones of fixed samples of Chara were separated and analyzed by using a fluorescence stereoscopic microscope with a green filter to quantify epiphytic cyanobacteria. Eleven samples were examined for each of the sampling periods in 2000, and at least 50 pictures per sample were taken with a Leica MZ 125 stereoscopic microscope. The pictures were processed with Leica Qwin image analysis software to determine the percentage of the Chara surface colonized by epiphytic cyanobacteria.
Epiphytic cyanobacteria on Chara were quantified with a fluorometer. This instrument was able to distinguish the chlorophyll a concentrations associated with four different algal groups in mixed samples. Three replicates each of 11 samples were examined for each of the sampling periods in 2000. Nodal and internodal zones were separated, weighed, and cut with a blade to prepare suspensions in 25 ml of distilled water. Pieces of Chara in the suspension were prevented from precipitating by magnetic stirring. Epiphytic cyanobacteria were then quantified by determining the cyanobacterial chlorophyll a concentration.
RESULTS
Nitrogen fixation in the rice soil system.
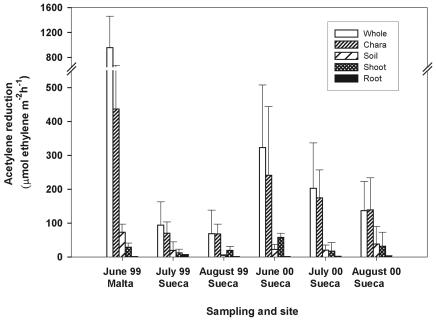
Consistent with previous studies (6, 21), the maximum ARA in the whole system was recorded in June, when rice plants were at the tillering stage (Fig. 1). The average whole-system acetylene reduction rates for each sampling period ranged from 69 to 958 μmol m−2 h−1. Differences between the mean whole-system rates (288 ± 324 μmol m−2 h−1 for all sampling periods) and the sum of the mean rates for the different parts of the system (251 ± 165 μmol m−2 h−1 for all sampling periods) were not significant (paired t test; n = 66). Calibration of the acetylene reduction rates with the 15N2 reduction rates in Valencian rice fields gave a ratio of 3.9 mol of acetylene reduced per mol of N2. Assuming that on average 1 h of activity during the morning represented 4.6% of the total daily activity (21) and that there were 90 days of measurable N2-fixing activity during the crop growth season, we estimated that the average N2 fixation rate was 40.5 kg of N ha−1 crop−1.
FIG. 1.
Acetylene reduction rates (means ± standard deviations; n = 11) associated with the different parts of the rice soil system at two sites in a Valencian rice field during the 1999 and 2000 crop seasons.
The largest proportion of photodependent N2 fixation was associated with Chara plants in both years and at both locations, and the mean values ranged from 68 to 437 μmol m−2 h−1 in the different sampling periods (Fig. 1). The nitrogen fixation rates for Chara always represented more than 45% of the global nitrogenase activity measured in the rice field. The mean acetylene reduction rates associated with Chara (196 ± 146 μmol m−2 h−1 for all sampling periods) accounted for 68% of the average activity of the whole system and 78% of the sum of the mean rates for the different parts of the system. The estimated mean N2 fixation rate associated with Chara was 27.53 kg of N ha−1 crop−1. A positive correlation (r2 = 0.118; P < 0.05; n = 33) was found between the biomass of Chara (on average, 4,840 ± 1,613 kg [fresh weight] ha−1 in June 2000, 7,145 ± 3,543 kg [fresh weight] ha−1 in July 2000, and 6,682 ± 3,001 kg [fresh weight] ha−1 in August 2000) and ARA. The estimated mean N2 fixation rates for the other parts of the system for all sampling periods were as follows: soil, 4.07 kg of N ha−1 crop−1; submerged parts of rice plants, 3.93 kg of N ha−1 crop−1; and roots, 0.28 kg of N ha−1 crop−1.
The mean nitrogenase activity in Chara was significantly lower in the dark (22 ± 15 μmol m−2 h−1 for all sampling periods in 1999) than in the light (191 ± 99 μmol m−2 h−1 for all sampling periods in 1999), indicating that most of the N2 fixation associated with Chara was photodependent and probably due to epiphytic N2-fixing cyanobacteria on Chara.
Microscopic study.
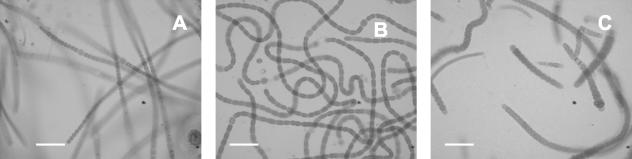
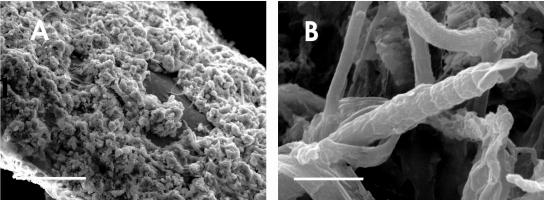
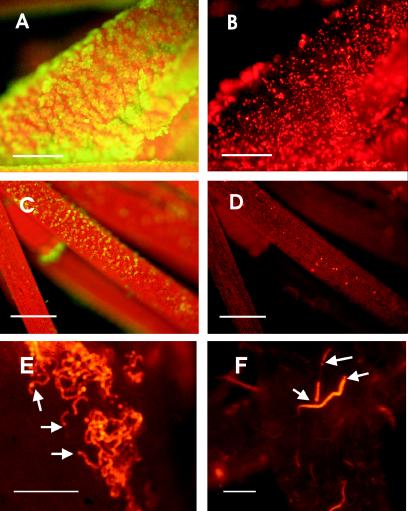
The presence of N2-fixing cyanobacteria belonging to genera such as Calothrix, Nostoc, and Anabaena was evident after samples of Chara collected from the rice field were cultured in N-free BG11 medium (Fig. 2). Scanning electron micrographs showed that the Chara surface was covered with carbonates (Fig. 3A). Calcium- and magnesium-rich carbonate aggregates are typical of members of the Charophyta (5), whose common names, stoneworts and brittleworts, are derived from this characteristic. Filamentous cyanobacteria were present among the carbonate particles (Fig. 3B). Observation of C. vulgaris by fluorescence microscopy with a violet filter also revealed carbonate aggregates (Fig. 4A). Under a green filter, several types of unicellular and filamentous cyanobacteria were observed on the carbonate surface of this macrophyte (Fig. 4B); these cyanobacteria included different species of heterocystous cyanobacteria, such as Nostoc sp. (Fig. 4E) and Calothrix sp. (Fig 4F). However, the carbonate particles progressively disappeared when C. vulgaris was maintained under laboratory conditions (Fig. 4C). Under these conditions, cyanobacteria were almost entirely absent from the surface of Chara (Fig. 4D).
FIG. 2.
Optical micrographs of the main genera of N2-fixing cyanobacteria that grew in petri dishes after field samples of Chara were grown in N-free BG11 medium. (A) Anabaena sp. (B) Nostoc sp. (C) Calothrix sp. Scale bars = 10 μm.
FIG. 3.
Scanning electron micrographs of Chara. (A) Carbonate aggregates were abundant in Chara from field samples. Scale bar = 50 μm. (B) Filamentous epiphytic cyanobacteria attached to the Chara surface. Scale bar = 5 μm.
FIG. 4.
Micrographs of Chara viewed under the fluorescence microscope with a violet (A and C) or green (B, D, E, and F) filter. The violet excitation distinguished carbonate particles (green fluorescence) from Chara chloroplasts (red), while the green excitation distinguished cyanobacterial phycobiliproteins (bright red). Carbonate aggregates were abundant in Chara from field samples (A) but not when the organism was maintained in the laboratory (C). Epiphytic cyanobacteria were abundant on the Chara surface with carbonate particles (B) but were scarce in sections without carbonate (D). Heterocystous cyanobacteria were observed on Chara from field samples (E and F). The arrows indicate heterocysts. (A, B, E, and F) Scale bar = 40 μm. (C and D) Scale bar = 150 μm.
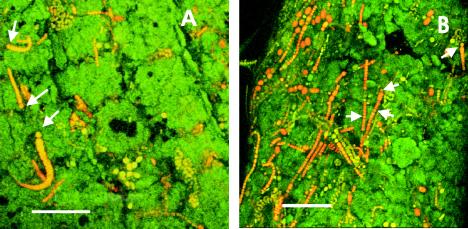
Better evidence for the presence of epiphytic cyanobacterial microcolonies on Chara was obtained by confocal scanning laser microscopy (Fig. 5). The three-dimensional reconstructions clearly identified epiphytism as the only relationship between Chara and cyanobacteria, since no cyanobacterial cells were observed inside the Chara structures.
FIG. 5.
Micrographs of field samples of Chara viewed under the confocal scanning laser microscope. Microcolonies of unicellular and filamentous cyanobacteria were present on the surfaces of internodes (A) and verticiles (B) of Chara. The arrows indicate heterocysts. (A) Scale bar = 100 μm. (B) Scale bar = 70 μm.
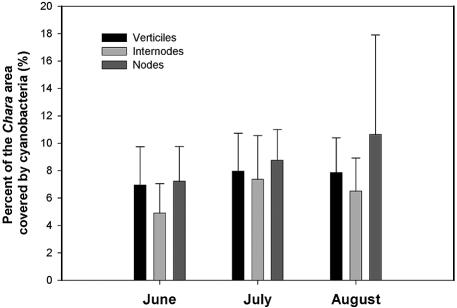
Quantification of epiphytic cyanobacteria on C. vulgaris.
The amount of the surface of Chara covered by epiphytic cyanobacteria was quantified, and the differential colonization of various parts of the algae was determined by using a fluorescence stereoscopic microscope and image analysis. The percentages of the surface area of Chara covered by cyanobacteria were 5.8% ± 4.3% for samples collected in June 2000, 7.5% ± 5.7% for samples collected in July 2000, and 6.9% ± 4.5% for samples collected in August 2000 (Fig. 6). The differences between sampling periods were not significant (as determined by analysis of variance; n = 400). The green alga Chara has an axis divided into nodes and internodes, and each node comprises several verticiles. Stereoscopic analysis revealed that on average, cyanobacteria were significantly more abundant in nodes than in internodes, covering 8.4% ± 4.4% and 6.2% ± 5.0% of the surface area, respectively (P < 0.001, as determined by the Mann-Whitney rank sum test; n = 54) (Fig. 6). Epiphytic cyanobacteria were also quantified by using a fluorometer, which made it possible to discriminate which algal groups were the origin of chlorophyll a. The concentration of chlorophyll a from green algae (Chara and epiphytic green algae) was 24.63 ± 7.19 mg m−2, while the concentrations of chlorophyll a from epiphytic cyanobacteria and diatoms were 5.54 ± 4.12 and 0.13 ± 0.30 mg m−2, respectively (Table 1). No significant differences between sampling periods were found (as determined by analysis of variance; n = 33). Chlorophyll a measurement confirmed that cyanobacteria were significantly more abundant in nodes than in internodes; the concentrations of chlorophyll a in the nodes and internodes were 17.2 ± 28.0 and 4.0 ± 3.8 μg mg (dry weight) of Chara−1, respectively (P = 0.004, as determined by the Mann-Whitney rank sum test; n = 33).
FIG. 6.
Differential colonization of Chara by epiphytic cyanobacteria in field samples collected during the 2000 crop season in Valencian rice fields. The values are percentages of the surface area (means ± standard deviations; n = 54).
TABLE 1.
Concentrations of chlorophyll a from photosynthetic organisms associated with C. vulgaris in field samples collected during the 2000 crop season from Valencian rice fields
| Sampling period | Chlorophyll a concn (mg m−2)a
|
||
|---|---|---|---|
| Chara + green algae | Cyanobacteria | Diatoms | |
| June | 18.32 ± 9.07 | 5.39 ± 4.32 | 0.24 ± 0.53 |
| July | 32.46 ± 15.57 | 5.96 ± 5.21 | 0.16 ± 0.53 |
| August | 23.10 ± 12.20 | 5.27 ± 2.84 | 0.0 ± 0.00 |
n = 33.
DISCUSSION
Chara is one of the main macrophytes found in rice fields throughout the world, and the biomass of this organism ranges from 2.5 to 15 tons (fresh weight) ha−1 (19, 23, 31, 32). The biomass in the Valencian rice fields fell within this range. Submerged macrophytes, such as Chara, play a complex ecological and agronomic role in rice fields. They are often considered detrimental, especially in directly seeded rice, since they compete with the rice plants for nutrients and light (11, 19, 23). In addition, Chara may favor N loss since its photosynthetic activity increases the floodwater pH and, consequently, NH3 volatilization (23). However, it has also been suggested that Chara and other aquatic photosynthetic organisms may have beneficial effects, since they contribute to the maintenance of soil fertility by immobilizing and recycling floodwater nutrients (13, 23, 33). Our results strongly support the idea that Chara may contribute to the maintenance of soil fertility, not only by immobilizing and recycling floodwater nutrients, as suggested previously, but also through its associated nitrogen fixation.
We clearly showed the importance of Chara in nitrogen fixation in rice fields. The associated activity of this organism is an order of magnitude greater than the activity in the other parts of the system. This activity was different in different sampling periods and locations but always represented more than 45% of the global nitrogenase activity measured in the rice field; in some cases it represented more than 90%. These findings led to a new and unexpected view of nitrogen fixation in rice fields, since nitrogen fixation associated with macrophytes other than the rice plant has never been taken into consideration previously. If the observed rates of nitrogen fixation were extended to the total surface of the Valencian rice fields (13,500 ha), the amount of nitrogen incorporated through Chara and its associated nitrogen fixation would be 372 tons of N per crop season, which represents a significant N input for the ecosystem and must be taken into account for effective management and sustainability. Isotope experiments in Valencian rice fields showed that rice plants incorporated about 117 kg of N ha−1 from the soil N pool, which was between 69 and 85% of the total N incorporated by fertilized plants (9). The amount of N fixed by epiphytic cyanobacteria on Chara in one crop period represented 23.5% of the N removed from the soil by the crop, indicating the agronomic potential for this macrophyte. After mineralization, this N should replenish the soil N pool and become available to rice plants in the same crop season or, more probably, in the next crop season, since most Chara mineralization takes place after the soil dries out at the end of the crop season.
Previous studies on epiphytic cyanobacteria in rice fields have been performed only in deep-water rice fields, where these organisms are associated with deep-water rice tillers (2, 34, 35). In shallow-water rice fields only benthic and planktonic communities have been considered (14, 20, 27). However, our results reveal the consistent presence of epiphytic cyanobacteria on the macrophyte Chara. On average, 7% of the Chara surface was covered by cyanobacterial microcolonies. Statistically significant differences were found in the distribution of epiphytic cyanobacteria on Chara plants. Cyanobacteria were more abundant in nodes than in internodes, probably because the nodes are more protected than the internodes as they are surrounded by verticiles. This morphology could help retain epiphytes, providing protection and physical support. Our results indicate that previous studies based on the quantification of cyanobacteria in soil and water may have underestimated the presence of these microorganisms in rice fields. Furthermore, from a limnological point of view, these findings reveal a previously unreported complex relationship among the different components of this agroecosystem. The biomass of epiphytic cyanobacteria on Chara seems to be sufficient to account for the observed rates of nitrogen fixation associated with Chara since when nitrogen fixation rates are expressed in terms of cyanobacterial chlorophyll concentrations, the values (on average, 57 ± 47 μmol mg of chlorophyll−1 h−1) are in the range of values usually found in laboratory cultures and in cyanobacterial blooms (e.g., 49 ± 37 μmol mg of chlorophyll−1 h−1 for an Anabaena bloom that developed in the rice field studied). Furthermore, the presence of heterocystous cyanobacteria on the Chara surface (Fig. 4E and F and 5) and the fact that N2 fixation associated with Chara was largely photodependent support the idea that the N2 fixation in Chara was mainly due to epiphytic cyanobacteria.
In addition to cyanobacteria, fluorometric measurements also demonstrated the presence of epiphytic diatoms on Chara, although the amounts were smaller than the amounts of cyanobacteria. This is consistent with results of studies in India that showed that a higher diversity of cyanobacteria than of diatoms was associated with cortical species of Chara (1). It could not be determined whether epiphytic green algae were present since fluorometric measurements do not allow these organisms to be discriminated from Chara.
Micrographic studies showed that field samples of Chara were covered by carbonate aggregates that arose from calcite precipitation during periods of intensive photosynthesis (15). When Chara was grown under laboratory conditions, carbonate particles progressively disappeared, probably as a result of a lower photon flux density and hence less photosynthetic activity in the laboratory than in the field. Chara plants were practically devoid of epiphytic cyanobacteria under these conditions. This suggests that the presence of calcium carbonate particles is related to the establishment of epiphytic cyanobacteria. Previous studies in Valencian rice fields revealed that water hardness and calcium content are positively correlated with the abundance of cyanobacteria in soil and water (20).
Chara is dominant not only in rice fields but also in many mesotrophic and eutrophic lakes and ponds (4, 8, 12), where the Chara biomass can be 478 g (dry weight) m−2 (3). It has been shown that members of the Characeae may directly or indirectly affect nutrient cycling in shallow lakes due to their efficiency in nutrient trapping and because the induced calcite precipitation affects C, Ca, and P availability (15). It has also been reported that Chara beds may influence the redox potential at the sediment-water interface, promoting nitrification-denitrification losses (18). However, the possible influence of Chara on nitrogen fixation in lakes and ponds has never been addressed. It remains to be established whether these epiphytes are able to fix N2 in these ecosystems, as they can in rice fields. If this does prove to be the case, the association of Chara and epiphytic cyanobacteria would have a significant influence on the total input of N and would have to be considered in studies of the N cycle and for effective management and sustainability of these freshwater ecosystems.
Acknowledgments
We thank J. Cambra for taxonomic identification of C. vulgaris, R. Redondo for technical support with the continuous-flow isotope ratio mass spectrometer, M. A. Muñoz for technical support for the confocal microscope, and W. Vincent and F. Fernández del Campo for critical reading of the manuscript.
This work was supported by grants AGF 1997-0303-CO2 and AGL 2001-1626-CO2 from the Spanish Ministry of Science and Technology.
REFERENCES
- 1.Anand, V. K., G. Langer, and K. Anand. 1992. Epiphytic and associated algae of charophytes and their role as biological indicator. Indian J. Ecol. 19:1-4. [Google Scholar]
- 2.Aziz, A., and Q. A. Ahmed. 1992. Occurrence and biomass of algae epiphytic on deepwater rice plants near Sonargaon, Bangladesh. Arch. Hydrobiol. 125:479-486. [Google Scholar]
- 3.Blindow, I. 1992. Long- and short-term dynamics of submerged macrophytes in two shallow eutrophic lakes. Freshwater Biol. 28:15-27. [Google Scholar]
- 4.Blindow, I., A. Hargeby, and G. Anderson. 2002. Seasonal changes of mechanisms maintaining clear water in a shallow lake with abundant Chara vegetation. Aquat. Bot. 72:315-334. [Google Scholar]
- 5.Bold, H. C., and M. J. Wynne. 1985. Introduction to the algae. Prentice-Hall, Inc., Englewood Cliffs, N.J.
- 6.Carreres, R., R. Gonzáles Tomé, J. Sendra, R. Ballesteros, E. Fernández Valiente, A. Quesada, M. Nieva, and F. Leganés. 1996. Effect of nitrogen rates on rice growth and biological nitrogen fixation. J. Agric. Sci. 127:295-302. [Google Scholar]
- 7.De Datta, S. K., and R. J. Buresh. 1989. Integrated nitrogen management in irrigated rice. Adv. Soil Sci. 10:143-169. [Google Scholar]
- 8.Fernández-Alaez, M., C. Fernández-Alaez, and S. Rodríguez. 2002. Seasonal changes in biomass of charophytes in shallow lakes in the northwest of Spain. Aquat. Bot. 72:335-348. [Google Scholar]
- 9.Fernández Valiente, E., A. Ucha, A. Quesada, F. Leganés, and R. Carreres. 2000. Contribution of N2 fixing cyanobacteria to rice production: availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 221:107-112. [Google Scholar]
- 10.George, T., J. K. Ladha, R. J. Buresh, and D. P. Garrity. 1992. Managing native and legume-fixed nitrogen in lowland rice-based cropping systems. Plant Soil 141:69-91. [Google Scholar]
- 11.Guha, P. 1995. Exploring ecological control of Chara. Crop Prot. 14:527-528. [Google Scholar]
- 12.Hart, E. A., and J. R. Lovvorn. 2000. Vegetation dynamics and primary production in saline, lacustrine wetlands of a Rocky Mountain basin. Aquat. Bot. 66:21-39. [Google Scholar]
- 13.Inubushi, K., and I. Watanabe. 1986. Dynamics of available nitrogen in paddy soils. Part II. Mineralized nitrogen of chloroform fumigated soils as a nutrient source for rice. Soil Sci. Plant Nutr. 32:561-577. [Google Scholar]
- 14.Irisarri, P., S. Gonnet, and J. Monza. 2001. Cyanobacteria in Uruguayan rice fields: diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. J. Biotechnol. 91:95-103. [DOI] [PubMed] [Google Scholar]
- 15.Kufel, L., and I. Kufel. 2002. Chara beds acting as nutrient sinks in shallow lakes—a review. Aquat. Bot. 72:249-260. [Google Scholar]
- 16.Kulasooriya, S. A., P. A. Roger, W. L. Barraquio, and I. Watanabe. 1981. Epiphytic nitrogen fixation on deepwater rice. Soil Sci. Plant Nutr. 27:19-27. [Google Scholar]
- 17.Kundu, D. K., and J. K. Ladha. 1995. Efficient management of soil and biologically fixed N2 in intensively-cultivated rice fields. Soil Biol. Biochem. 27:431-439. [Google Scholar]
- 18.Lijklema, L. 1994. Nutrient dynamics in shallow lakes: effects of loading and role of sediment-water interactions. Hydrobiologia 275/276:335-348. [Google Scholar]
- 19.Misra, A., G. K. Patro, and G. C. Tosh. 1976. Studies on chemical control of Chara, p. 265-268. In C. K. Varsheney and J. Rozska (ed.), Aquatic weeds in Southeast Asia. La Hague Publishing, Den Haag, The Netherlands.
- 20.Quesada, A., and E. Fernández Valiente. 1996. Relationship between abundance of N2-fixing cyanobacteria and environmental features of Spanish rice fields. Microb. Ecol. 32:59-71. [DOI] [PubMed] [Google Scholar]
- 21.Quesada, A., M. Nieva, F. Leganés, A. Ucha, M. Martín, C. Prosperi, and E. Fernández-Valiente. 1998. Acclimation of cyanobacterial communities in rice fields and response of nitrogenase activity to light regime. Microb. Ecol. 35:147-155. [DOI] [PubMed] [Google Scholar]
- 22.Quesada, A., F. Leganés, and E. Fernández Valiente. 1997. Environmental factors controlling N2 fixation in Mediterranean rice fields. Microb. Ecol. 34:39-48. [DOI] [PubMed] [Google Scholar]
- 23.Roger, P. 1996. Biology and management of the floodwater ecosystem in ricefields. International Rice Research Institute, Manila, The Philippines.
- 24.Roger, P., and S. A. Kulasooriya. 1980. Blue-green algae and rice. International Rice Research Institute, Manila, The Philippines.
- 25.Roger, P., and J. K. Ladha. 1992. Biological N2 fixation in wetland rice fields: estimation and contribution to nitrogen balance. Plant Soil 141:41-55. [Google Scholar]
- 26.Roger, P. A. 1995. Biological N2-fixation and management in wetland rice cultivation. Fertil. Res. 42:261-276. [Google Scholar]
- 27.Roger, P. A., S. Santiago Ardales, P. M. Reddy, and I. Watanabe. 1987. The abundance of heterocystous blue-green algae in rice soils and inocula used for application in rice fields. Biol. Fertil. Soils 5:98-105. [Google Scholar]
- 28.Rother, J. A., and B. A. Whitton. 1989. Nitrogenase activity of blue-green algae on seasonally flooded soils in Bangladesh. Plant Soil 113:47-52. [Google Scholar]
- 29.Rother, J. A., A. Aziz, N. Hye Karim, and B. A. Whitton. 1988. Ecology of deepwater rice-fields in Bangladesh. 4. Nitrogen fixation by blue-green algal communities. Hydrobiologia 169:43-56. [Google Scholar]
- 30.Stewart, W. D. P., G. P. Fitzgerald, and R. Burris. 1967. In situ studies on N2 fixation using the acetylene reduction technique. Biochemistry 58:2071-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaquer, A. 1984. La production algale dans les rizières de Camargue pendant la periode de submersion. Verh. Int. Verein. Limnol. 22:1651-1654. [Google Scholar]
- 32.Vasconcelos, T., M. Tavares, and N. Gaspar. 1999. Aquatic plants in the rice fields of the Tagus Valley, Portugal. Hydrobiologia 415:59-65. [Google Scholar]
- 33.Vlek, P. L. G., and E. T. Crasswell. 1979. Effect of nitrogen source and management on ammonia volatilization losses from flooded rice-soil systems. Soil Sci. Soc. Am. J. 43:352-358. [Google Scholar]
- 34.Whitton, B. A., and H. D. Catling. 1986. Algal ecology of deepwater rice-fields in Thailand. Arch. Hydrobiol. 105:289-297. [Google Scholar]
- 35.Whitton, B. A., A. Aziz, B. Kawecka, and J. A. Rother. 1988. Ecology of deepwater rice-fields in Bangladesh. 3. Associated algae and macrophytes. Hydrobiologia 169:31-42. [Google Scholar]