Abstract
Lactococcus lactis grows homofermentatively on glucose, while its growth on maltose under anaerobic conditions results in mixed acid product formation in which formate, acetate, and ethanol are formed in addition to lactate. Maltose was used as a carbon source to study mixed acid product formation as a function of the growth rate. In batch and nitrogen-limited chemostat cultures mixed acid product formation was shown to be linked to the growth rate, and homolactic fermentation occurred only in resting cells. Two of the four lactococcal strains investigated with maltose, L. lactis 65.1 and MG1363, showed more pronounced mixed acid product formation during growth than L. lactis ATCC 19435 or IL-1403. In resting cell experiments all four strains exhibited homolactic fermentation. In resting cells the intracellular concentrations of ADP, ATP, and fructose 1,6-bisphosphate were increased and the concentration of Pi was decreased compared with the concentrations in growing cells. Addition of an ionophore (monensin or valinomycin) to resting cultures of L. lactis 65.1 induced mixed acid product formation concomitant with decreases in the ADP, ATP, and fructose 1,6-bisphosphate concentrations. ADP and ATP were shown to inhibit glyceraldehyde-3-phosphate dehydrogenase, lactate dehydrogenase, and alcohol dehydrogenase in vitro. Alcohol dehydrogenase was the most sensitive enzyme and was totally inhibited at an adenine nucleotide concentration of 16 mM, which is close to the sum of the intracellular concentrations of ADP and ATP of resting cells. This inhibition of alcohol dehydrogenase might be partially responsible for the homolactic behavior of resting cells. A hypothesis regarding the level of the ATP-ADP pool as a regulating mechanism for the glycolytic flux and product formation in L. lactis is discussed.
Lactococcus lactis is a lactic acid bacterium that is used as a starter culture in the dairy industry. L. lactis has a rather simple and well-characterized metabolism and converts sugars mainly into lactic acid. In recent years L. lactis has also been evaluated as organism for production of industrial lactic acid (23, 38) and has been subjected to metabolic engineering to reroute its metabolism towards novel products (13, 18, 22, 25, 26). To take full advantage of bacterial processes, it is very important to understand which intracellular and extracellular factors influence the metabolic rate and product formation. L. lactis is an attractive model organism for studies of glycolysis and pyruvate metabolism, since oxidative phosphorylation normally does not occur and more than 90% of the carbon source is recovered as fermentation by-products, mainly lactate. In glycolysis glyceraldehyde-3-phosphate dehydrogenase (GAPDH) converts NAD+ to NADH, which must be regenerated for continued carbon catabolism. Lactate dehydrogenase (LDH) regenerates NAD+ by converting the end product of glycolysis, pyruvate, to lactate. An alternative way for lactococci to regenerate NAD+ is by production of ethanol by alcohol dehydrogenase (ADH), which is part of the pyruvate formate-lyase (PFL) pathway. The PFL pathway is active under anaerobic conditions and produces formate, acetate, and ethanol. The first enzyme in the pathway, PFL, converts pyruvate to formate and acetyl coenzyme A, which is further metabolized to either ethanol or acetate. PFL is inactive in the presence of oxygen and at a low pH (24, 35). With an active PFL pathway, three molecules of ATP are conserved per hexose molecule catabolized, compared with the two ATP molecules conserved per hexose molecule when LDH is used. The extra ATP comes from the production of acetate, which is catalyzed by acetate kinase. Since no NAD+ is regenerated in the acetate formation pathway, one ethanol molecule must be formed for every acetate molecule to regenerate two NAD+ molecules and maintain the redox balance. It is important to note that LDH under anaerobic conditions competes with two enzymes for its substrates; it competes with PFL for pyruvate and with ADH for NADH.
The shift from mixed acid to homolactic fermentation has been ascribed to the level of PFL (33, 34), as well as inhibition of PFL by glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (16, 33, 34, 50). These intracellular metabolites accumulate together with fructose 1,6-bisphosphate (FBP) during high glycolytic flux (16). It has also been proposed that FBP and orthophosphate (Pi) have controlling effects on LDH and particularly on pyruvate kinase (10, 11). The concentrations of FBP and Pi vary inversely (31, 52), and FBP activates whereas Pi is inhibits. The direct effect of FBP and Pi on product formation has been questioned (16) since the intracellular concentrations of FBP in general are sufficient to ensure full activation of LDH (11). The redox charge and activity of GAPDH have also been put forward as possible regulators of product formation, as well as of glycolytic flux, in L. lactis (14, 16, 41). This hypothesis has been disproved in other studies in which the enzymatic level of GAPDH has been altered (37, 48).
L. lactis displays homolactic fermentation when it is grown with excess glucose. However, in glucose-limited chemostat cultures at low dilution rates mixed acid product formation occurs (33, 47, 49). Similarly, when fermenting sugars that are metabolized slowly, such as galactose and maltose, L. lactis exhibits pronounced mixed acid product formation (47, 50). The mixed acid product formation with galactose and maltose has been hypothesized to be due to a limitation in the initial metabolism (15, 16). However, the initial pathways for degradation of galactose and maltose are distinctly different (2, 6, 42, 43), making it difficult to suggest where there is a putative limitation. In the initial metabolism of maltose, β-phosphoglucomutase (β-PGM) has a crucial role. Glucose 1-phosphate, derived from cleavage of maltose, is converted by β-PGM into glucose 6-phosphate, which is further metabolized during glycolysis. Although β-PGM is an important enzyme, overproduction of β-PGM in L. lactis 65.1 did not change the product formed from maltose (42).
In maltose-limited chemostat cultures a high degree of mixed acid product formation is observed irrespective of the dilution rate (47), indicating that maltose always is fermented through mixed acid product formation. However, mixed acid product formation from maltose has been shown to decrease at the end of cultivation when the pH is low (42), which can be attributed to inhibition of PFL at low pH (24). Galactose-fermenting L. lactis NCDO 2118 was recently shown to produce less mixed acids when the growth rate in batch mode was limited by means of a defined medium with a low concentration of amino acids (17). In a recent study it was shown that growing and resting cells of L. lactis on maltose had different types and rates of product formation, although the proteome was highly conserved (40). In the present study we characterized the effect of the growth rate on product formation and quantified intracellular metabolites in growing cells versus resting cells. The difference between low metabolic flux and energy limitation was highlighted, and we concluded that only energy limitation per se increased mixed acid product formation. The relationship between large pools of ADP and ATP and the metabolic activity of resting cells is discussed below.
MATERIALS AND METHODS
Organisms and culture media.
The following four lactococcal strains were used: L. lactis subsp. lactis 65.1, a dairy strain obtained from the Swedish Dairies Association, Malmö, Sweden; L. lactis subsp. lactis ATCC 19435; L. lactis subsp. lactis IL-1403 (9); and L. lactis subsp. cremoris MG 1363 (19). L. lactis 65.1 was recently determined to be an L. lactis subsp. lactis strain by 16S rRNA sequencing (carried out by Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) and by API analysis (BioMerieux, Marcy l'Etoile, France). The organisms were precultured in a complex medium containing (per liter) 10 g of maltose, 10 g of tryptone, 2 g of yeast extract, 2.5 g of KH2PO4, 2.5 g of K2HPO4, and 0.5 g of MgSO4 · 7H2O. The medium used for growth in batch cultures was a modified SD3 medium (55), in which 5 g of tryptone (Merck, Darmstadt, Germany) per liter replaced 5 g of Casamino Acids per liter as the nitrogen source. Tryptone, which consists of peptone from pancreatically digested casein, was found in an initial study to improve the growth of strain L. lactis 65.1 because of the presence of oligopeptides. Maltose or glucose was added to the SD3 medium at a level of 10 g/liter unless indicated otherwise. Maltose, glucose, and MgSO4 · 7H2O were autoclaved separately. Solutions of nucleic acid bases, trace elements, and vitamins were filter sterilized. In resting cell experiments the following nitrogen sources were omitted from the SD3 medium: tryptone, uracil, adenine, guanine, and yeast nitrogen base. In the experiments in which intracellular Pi was quantified, the concentrations of KH2PO4 and K2HPO4 were decreased to 0.25 g/liter and the concentration of yeast nitrogen base was decreased to 1.6 g/liter to reduce the background values for Pi.
Cultivation conditions.
Fermentation was performed at 30°C in a fermentor (Applikon, Schiedam, The Netherlands) with a working volume of 1 liter. The agitation speed was 200 rpm, and the pH was controlled at pH 6.5 by automatic addition of sodium hydroxide (3 M). The headspace was continuously flushed with nitrogen gas (0.1 liter/min). The inoculum was grown overnight, harvested in the early stationary phase by centrifugation (6,300 × g, 10 min), and resuspended in SD3 medium. The inoculum size was about 5% (vol/vol). The cultures for the resting cell experiments were inoculated with cells from exponentially growing cultures that had been washed once in SD3 medium without nitrogen sources (see above).
Measurement of growth, sugars, and products.
Samples taken from the fermentor were used to monitor cell growth, sugar consumption, and product formation. Cell growth was measured by measuring the optical density at 620 nm (U1100; Hitachi, Tokyo, Japan). Cells were removed from the samples by centrifugation and membrane filtration (pore size, 0.2 μm), and the filtrates were assayed for substrates and products. Maltose, lactate, formate, acetate, and ethanol contents were determined with a high-performance liquid chromatography system. The compounds were separated on an Aminex HPX-87H column (Bio-Rad, Hercules, Calif.) at 45°C and were detected with a refractometer (RID-6A; Shimadzu, Kyoto, Japan). The mobile phase was 5 mM sulfuric acid, and the flow rate was 0.6 ml/min. Standards were injected separately before the samples. Quantification was performed by computer integration (Gilson UniPoint) of the area under each chromatographic peak. The percentage of lactate was calculated as follows:
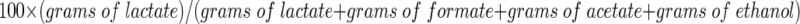 |
(1) |
Measurement of intracellular metabolites and dry weight.
Cell samples (2 ml) used for determination of intracellular metabolite concentrations were collected on 0.2-μm-pore-size filters (Gelman Sciences, Ann Arbor, Mich.), which were immediately frozen in liquid nitrogen and stored in a freezer at −80°C until they were analyzed. Before analysis of FBP and Pi the frozen cells were disrupted by addition of 0.6 M perchloric acid, incubated for 10 min with vigorous vortexing, and neutralized with 3 M sodium hydroxide. Before analysis of ADP and ATP the frozen cells were disrupted by 5 min of treatment at 100°C with periodic vigorous vortexing. For each metabolite three independent filters per cultivation were prepared, and the extraction liquid from each filter was analyzed at least in duplicate.
FBP was quantified enzymatically by using a filter fluorometer (TD-700 laboratory fluorometer; Turner Design, Sunnyvale, Calif.) equipped with a 300- to 400-nm excitation filter and a 410- to 600-nm emission filter. The coupled assay, adapted from the assay described by Bergmeyer (5), was carried out in 0.5 M triethanolamine (TEA) buffer (pH 7.2) containing 15 mM MgSO4 and 4 mM EDTA by measuring the consumption of NADH by glycerophosphate dehydrogenase. The sample extraction liquid was added to a reaction mixture containing 10 μM NADH, triose phosphate isomerase (5 U), and glycerophosphate dehydrogenase (5 U) in TEA buffer. FBP aldolase was then added, and the measured difference in fluorescence was recorded. FBP standards were used to prepare a standard curve.
Pi was quantified spectrophotometrically at 660 nm as described previously (21). An ammonium molybdate-malachite green mixture develops a color in the presence of Pi. Pi standards were prepared in 0.01 M perchloric acid. Growth medium was applied to filters to obtain a background value for the extracellular Pi concentration.
The ATP concentrations in sample extraction liquids were measured with a luminometer (1250 luminometer; LKB Wallac, Turku, Finland) by using a luciferase-luciferin assay mixture (Sigma). The sum of the ATP and ADP concentrations was determined after conversion of ADP to ATP by 2 min of incubation in a solution containing TEA (50 mM, pH 7.7), phosphoenolpyruvate (10 μM), MgCl2 (2.5 mM). and pyruvate kinase (2 U). The ADP concentration was calculated by subtracting the ATP concentration from the sum of the ATP and ADP concentrations. Standard curves for ATP, ADP, and mixtures of ATP and ADP corresponded well with each other.
Dry weight, which was proportional to optical density at 620 nm, was determined by collecting 2 ml of cells from fermentation on 0.2-μm-pore-size filters (Gelman Sciences). The filters were washed three times with 2 ml of water and subsequently dried in a microwave oven for 8 min at 350 W. Intracellular concentrations were calculated on the basis that 1 g (dry weight) of L. lactis cells was equivalent to 1.67 ml of intracellular fluid (51).
Enzyme assays.
Cells were harvested from late-exponential-phase cultures, washed, and disrupted by using glass beads (55). Enzymatic analyses were performed with cell extracts at 30°C by monitoring NADH spectrophotometrically at 340 nm. LDH activity was measured by using a protocol adapted from the protocol described by Garrigues et al. (16) in a reaction mixture containing cell extract in a solution containing TEA (100 mM, pH 7.2), NADH (0.4 mM), MgCl2 (5 mM), FBP (10 mM), and pyruvate (10 mM), which was used to initiate the reaction. GAPDH activity was assayed by a method adapted from the method described by Garrigues et al. (16) by using TEA (100 mM, pH 7.2) containing NAD+ (5 mM), sodium arsenate (40 mM), and glyceraldehyde 3-phosphate (10 mM). ADH activity was assayed by a method adapted from the method described by Even et al. (15) by using a mixture containing Tris-HCl (100 mM, pH 7.2), NADH (0.4 mM), dithiothreitol (2 mM), and acetaldehyde (10 mM). The compounds were added in order of appearance so that the carbon substrate for the corresponding enzyme was added last to initiate the reaction. Inhibition by ATP and ADP was measured by adding 0 to 32 mM ATP or ADP to the reaction mixtures described above immediately prior to addition of the carbon source. The inhibition kinetics could be described adequately with a generally applicable equation described by Han and Levenspiel (20):
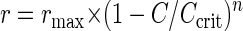 |
(2) |
where r is the enzymatic rate, rmax is the enzymatic rate in the absence of inhibitor, C is the concentration of inhibitor (ADP or ATP), Ccrit is the inhibitor concentration at which the enzyme is totally inhibited, and n is the degree of inhibition. The parameters Ccrit and n were estimated by fitting a line through the data points by using the least-squares method.
Protein concentrations were determined by the Bradford method with serum albumin as the standard (8).
RESULTS
Product formation in growing and resting cells of different strains of L. lactis.
In previous studies fixed acids have been shown to be formed when lactococci metabolize maltose (24, 42, 47). To investigate whether formation of mixed acid products from maltose is related to growth, formation of products by four strains of L. lactis was studied during growth in semidefined medium (55) and in resting cell experiments (Table 1). Resting cell experiments were performed in medium deficient in a nitrogen source. All four strains produced mixed acids during growth, although each strain produced them to a different extent. L. lactis 65.1 and MG1363 showed more pronounced mixed acid product formation than L. lactis ATCC 19435 and IL-1403. For all strains, homolactic fermentation of maltose occurred when the cells were resting. The formation of mixed acid products from maltose was studied in additional experiments by using L. lactis 65.1, because this strain displayed the most pronounced mixed acid product formation.
TABLE 1.
Product formation and specific growth rates for L. lactis strains growing on maltosea
| Strain | % of lactate in exponential phaseb | % of lactate at the end of fermentationc | μmax (h−1)d |
|---|---|---|---|
| L. lactis subsp. cremoris MG1363 | 14 | 51 | 0.65 |
| L. lactis subsp. lactis 65.1 | 11 | 55 | 0.58 |
| L. lactis subsp. lactis ATCC 19435 | 61 | 80 | 0.52 |
| L. lactis subsp. lactis IL-1403 | 57 | 88 | 0.62 |
For all strains lactate was the sole product detected in resting cell experiments.
The percentage of lactate was calculated by using equation 1.
Based on the final product concentrations of cultures with an initial maltose concentration of 25 g/liter.
μmax, maximum specific growth rate.
Batch fermentation of glucose and maltose.
Glucose and maltose were fermented by L. lactis 65.1 in pH-controlled batch mode. With glucose as the carbon source the maximum specific growth rate was 1.1 h−1 and lactate constituted 90% of the products formed (Table 2). On maltose the maximum specific growth rate was about one-half that on glucose, and the product formation was shifted to mixed acids, with formate, acetate, and ethanol formed in addition to lactate. The sugar uptake rate (in millimoles of C6 carbon per gram [dry weight] per hour) was lower during maltose fermentation (Table 2), whereas the biomass yields obtained with the two sugars were quite similar. This indicates that maltose fermentation is kinetically, but not thermodynamically, unfavorable compared with glucose fermentation.
TABLE 2.
Sugar consumption and product formation in batch cultures of L. lactis 65.1 grown on glucose and maltosea
| Sugar | Sugar consumption rate (mmol of C6 carbon · g [dry wt] · h−1)b | % of lactatec | μmax (h−1)d | Biomass yield (g [dry wt] · g of sugar−1) |
|---|---|---|---|---|
| Glucose | 24.4 | 90 | 1.1 | 0.20 |
| Maltose | 8.0 | 11 | 0.58 | 0.22 |
pH-controlled cultures with an initial sugar concentration of 10 g/liter were used.
Calculated by determining the consumption of C6 carbon when the cells grew at the maximal growth rate in the presence of about 8 g of sugar per liter.
The percentage of lactate for mid-exponential-phase batch cultures was calculated by using equation 1.
μmax, maximum specific growth rate.
Effect of growth rate.
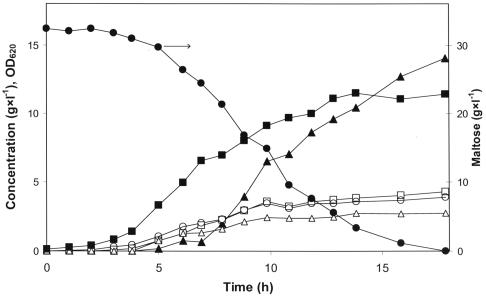
Batch cultures with excess maltose were used to study the product formation in different growth phases (Fig. 1). In these experiments the nitrogen source was the growth-limiting factor and allowed maltose to be present in the batch even after cessation of growth. In the mid-exponential phase only 10 to 20% of the sugar consumed was converted to lactate. In the late exponential phase mixed acid product formation decreased, while lactate formation increased (Fig. 1). When growth ceased, due to depletion of the nitrogen source, the residual maltose was converted into lactate. In a fed-batch experiment, in which the culture was fed with maltose to prolong the stationary phase, the homolactic behavior could be maintained for more than 100 h (data not shown).
FIG. 1.
Maltose consumption and product formation in pH-controlled batch fermentation of L. lactis 65.1. Symbols: ▪, optical density at 620 nm (OD620); •, maltose concentration; ▴, lactate concentration; □, formate concentration; ○, acetate concentration; ▵, ethanol concentration.
Chemostat culture permits comparison of product formation at different growth rates since the dilution rate equals the growth rate. Intermediate growth rates were studied in nitrogen-limited chemostat cultures with maltose as the carbon source, and there was increased lactate formation at lower growth rates (Table 3). Addition of the antibiotic chloramphenicol at a low concentration (5 mg/ml) was used as an alternative method to decrease the growth rate. In response the percentage of lactate increased. Chloramphenicol (300 mg/liter) was also used to arrest the growth in a medium that normally supports growth. After growth arrest had set in, lactate constituted 90% of the products (Table 3). These results indicate that the shift towards homolactic fermentation was due to the decreased growth rate and not to the nitrogen content in the medium.
TABLE 3.
Growth rates and product formation in cultures of L. lactis 65.1a
| Growth rate (h−1) | Sugar consumption rate (mmol of C6 carbon · g [dry wt] · h−1) | % of lactate | Cultivation mode | Growth restriction |
|---|---|---|---|---|
| 0.58 ± 0.4 | 8.0 ± 0.9b | 11 ± 0.5 | Batch | None |
| 0.36 | 8.6 | 27 | Chemostat | Nitrogen |
| 0.13 | 4.2 | 48 | Chemostat | Nitrogen |
| 0.12 | NAc | 45 | Batch | Chloramphenicol (5 mg liter−1) |
| 0 | 3.2 | 90 | Batch | Chloramphenicol (300 mg liter−1) |
| 0 | 2.8 | 100 | Resting cells | Nitrogen |
Maltose was present in excess (at a concentration between 8 and 14 g/liter) in all experiments.
Calculated from values for mid-exponential batch cultures.
NA, not analyzed.
Effect of maltose concentration.
A maltose-limited chemostat culture at a dilution rate 0.1 h−1 exhibited a distinct mixed acid product formation; only 12% lactate was formed despite the relatively low dilution rate of the culture (data not shown). The residual maltose concentration at steady state was 0.9 g/liter, and a subsequent experiment was conducted to see whether the maltose concentration per se influenced product formation. The product formation by resting cells was assessed at maltose concentrations between 0.8 and 44 g/liter. Only lactate was formed at maltose concentrations above 4 g/liter, whereas at concentrations below 4 g/liter mixed acids were also formed (data not shown), which might have reflected sugar limitation caused by low substrate affinity of the initial maltose-metabolizing pathway.
Product formation in relation to intracellular metabolites.
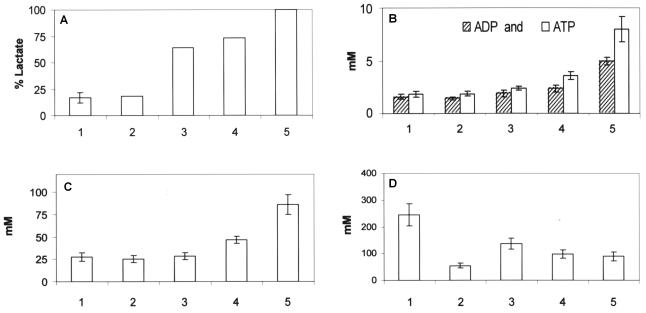
Intracellular concentrations of ADP, ATP, FBP, and Pi were quantified in growing and resting cells metabolizing maltose in order to investigate the relationship among growth, the concentration of intracellular metabolites, and product formation. It was found that growing cells had lower concentrations of ADP, ATP, and FBP and higher concentrations of Pi than resting cells (Fig. 2). L. lactis 65.1 is capable of metabolizing arginine and thereby gaining ATP (12). When arginine (30 mM) was added to a batch culture growing on maltose, the biomass yield from maltose increased 25% and the specific growth rate increased approximately 15% (data not shown). The intracellular Pi concentration was lower when arginine was added; however, lactate formation and the intracellular concentrations of ADP, ATP, and FBP were unchanged (Fig. 2).
FIG. 2.
Percentage of lactate (A) and concentrations of ADP and ATP (B), FBP (C), and Pi (D) in L. lactis 65.1 cells metabolizing 10 g of maltose per liter. The culture conditions were as follows: mid-exponential growth (n = 6) (bars 1), mid-exponential growth with 30 mM arginine (n = 3) (bars 2), resting cells with 0.5 μM monensin (n = 3) (bars 3), resting cells with 2 μM valinomycin (n = 3) (bars 4), and resting cells (n = 6) (bars 5), where n is the number of cell samples analyzed for intracellular metabolites.
The ionophores monensin and valinomycin can be used to increase energy spilling by the cells, since they induce ATPase activity (7). Addition of monensin (0.5 μM) or valinomycin (2 μM) resulted in 50% growth inhibition of L. lactis 65.1 growing on maltose. When the same concentrations were used for resting cells, they resulted in increased maltose consumption rates (37% for monensin and 22% for valinomycin) and decreased percentages of lactate and intracellular concentrations of ADP, ATP, and FBP (Fig. 2).
Activities of GAPDH, LDH, and ADH.
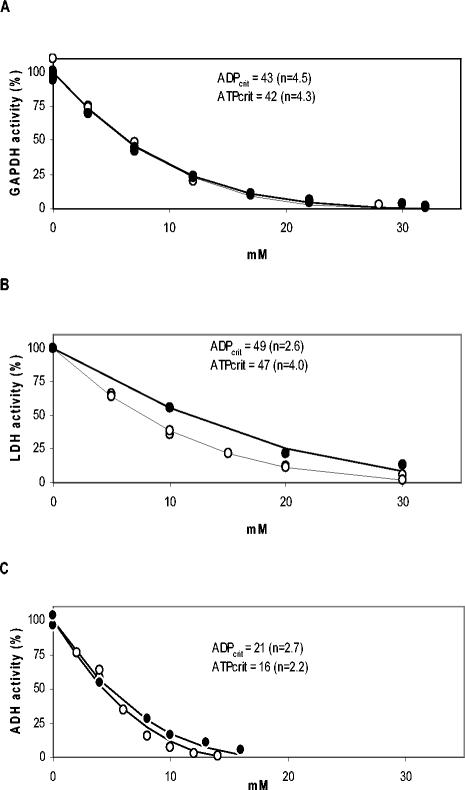
ADP and ATP have been found to inhibit dehydrogenases in different organisms (1, 32, 36, 56). Since L. lactis 65.1 had elevated concentrations of ADP and ATP, we explored the possible impact of ADP and ATP on dehydrogenases relevant for the glycolytic flux. The effects of ADP and ATP on the in vitro activities of GAPDH, LDH, and ADH were assessed (Fig. 3). The critical concentrations of ADP and ATP at which the activity was totally inhibited were lower for ADH than for GAPDH and LDH. Although Mg2+ is able to bind to the phosphate moiety of adenine nucleotides, it did not relieve the inhibition of LDH by ATP (data not shown), probably because mainly the adenine moiety is responsible for the inhibition (56). The in vitro activities of the three dehydrogenases were assayed at the concentrations of ADP and ATP that were found to be present in growing and resting cells, respectively, and were compared with the maximal in vitro activities measured in the absence of ADP and ATP. GAPDH, LDH, and ADH were inhibited 28, 39, and 39%, respectively, by the concentrations of ADP and ATP present in growing cells. Under the conditions present in resting cells GAPDH, LDH, and ADH were inhibited 82, 71, and 95%, respectively. Thus, ADH was the enzyme that was most inhibited, and only 5% of the maximal activity was left under resting cell conditions.
FIG. 3.
Inhibition kinetics for dehydrogenase in vitro activity as a function of ADP and ATP. (A) GAPDH; (B) LDH; (C) ADH. The inhibitors were ATP (○) and ADP (•). ADPcrit and ATPcrit are the inhibitor concentrations at which the enzyme was totally inhibited, and n is the degree of inhibition. The values for these parameters were estimated by fitting equation 2 through the data points.
In vivo enzymatic activities can be controlled not only by the concentrations of metabolites but also by the concentrations of enzymes. We determined the concentrations of GPDH, LDH, and ADH in growing and resting cells to see whether the levels of the enzymes were changed. The concentrations of the enzymes GAPDH and LDH were constant, and 68% of the ADH enzyme remained even after 3 h of nongrowth (data not shown). This indicates that the dehydrogenase activities in resting cells of L. lactis 65.1 are regulated at the enzyme level.
DISCUSSION
When L. lactis 65.1 was actively growing on maltose, the PFL pathway was the dominant pathway for conversion of NADH and pyruvate, which is in agreement with previous studies (42, 47). Mixed acid product formation on maltose was shown to be linked to the growth rate, with homolactic fermentation occurring only when the cells were resting. Other lactococcal strains exhibited the same phenomenon but each to a different extent since some strains (e.g., L. lactis IL-1403) produce relatively small amounts of mixed acids (15). Very recently, a proteomic approach was used to determine the protein concentrations in growing and resting cells of L. lactis 65.1 and IL-1403 (40). It was shown that the protein levels were almost invariant after 3 h in the resting state, indicating that the observed differences in metabolic rate and product formation were due to regulation at the enzyme level.
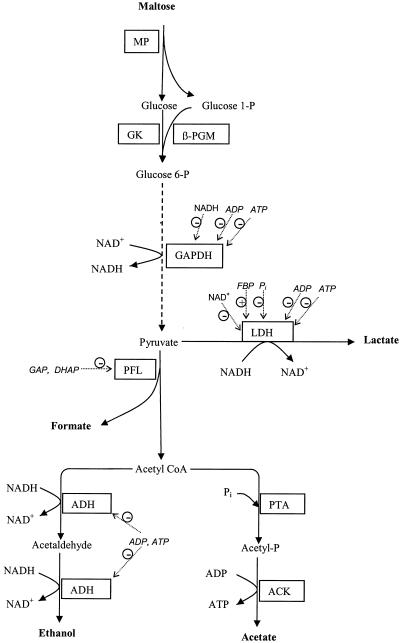
The present work on L. lactis 65.1 shows that the concentrations of ADP and ATP are higher in resting cells than in growing cells. Both ADP and ATP inhibit dehydrogenases (1, 32, 36, 56), and we found that GAPDH, LDH, and especially ADH were inhibited. GAPDH (37) and LDH (54) are subject to product inhibition by NADH and NAD+, respectively (Fig. 4), and ATP has been shown to be a competitive inhibitor (32, 56). Thus, to avoid overestimation of the inhibition by ADP and ATP, the in vitro assays were performed under optimal conditions, i.e., in the absence of product and in the presence of saturating concentrations (11, 16) of the substrates. Only 5% of the maximal in vitro ADH activity was left in the presence of the ADP and ATP concentrations determined to be present in resting cells. Inhibition of ADH may contribute to the homolactic fermentation behavior of resting cells, since ADH activity is essential for the mixed acid product formation pathway (Fig. 4). In Bacillus subtilis phosphotransacetylase (EC 2.3.1.8), the enzyme preceding acetate kinase, is inhibited by ADP and ATP (44). Although the latter finding has not been obtained for lactococci, inhibition of either phosphotransacetylase or ADH or both enzymes could be a way for the cell to block the branches of the mixed acid product formation pathway (Fig. 4).
FIG. 4.
Schematic pathway for metabolism of maltose to anaerobic fermentation end products. A plus sign in a circle and a minus sign in a circle indicate metabolites that activate and inhibit dehydrogenase activities in L. lactis, respectively. MP, maltose phosphorylase; GK, glucokinase; PTA, phosphotransacetylase; ACK, acetate kinase; GAP, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; CoA, coenzyme A.
The homolactic behavior of resting L. lactis 65.1 cells metabolizing maltose was disrupted by addition of the ionophore monensin. When monensin was added, the concentrations of ADP and ATP decreased, the glycolytic flux increased, and mixed acid product formation was initiated. This indicates that homolactic fermentation of maltose was easily disrupted when the ATP demand increased. When monensin was added to Streptococcus bovis metabolizing glucose, the intracellular ATP level decreased, due to increased ATPase activity, and this resulted in an increased glycolytic flux (7). An increased glycolytic flux was also observed in resting, glucose-metabolizing L. lactis cells when F1-ATPase was overproduced (29). However, in that study no increase in formate formation was observed, possibly because glucose metabolism results in lower levels of the enzymes in the PFL pathway, including PFL (4, 34), ADH (3, 40), and phosphotransacetylase (40).
We found that the intracellular concentrations of FBP and Pi were also altered in resting cells of L. lactis 65.1 compared with growing cells. These compounds are known signal molecules (53) and act both as allosteric effectors (Fig. 4) and as substrates and products for various glycolytic enzymes. Both compounds also take part in the regulation of phosphotransferase uptake systems and in catabolite repression (for a review see reference 46). The concentrations of Pi and FBP vary inversely (7, 37, 52), and for decades these compounds have been recognized as effectors of pyruvate kinase and LDH (10, 11). A recent study showed that the extents of inhibition by Pi and activation by FBP of LDH vary in different lactococcal strains commonly used in research (54). Since Pi and FBP apparently compete for the same allosteric site (27), the inhibition of LDH by Pi is most likely overcome by the presence of the high concentrations of FBP found in the present study and other studies (11, 16).
In the present study we determined the sugar uptake rate and used it as an estimate of the glycolytic flux, which was possible since approximately 90% of the sugar consumed by L. lactis during batch growth ends up as fermentation end products (39). Low glycolytic flux has commonly been ascribed to be the cause of mixed acid product formation (15, 16, 28, 48). Indeed, during batch growth on maltose, trehalose, and galactose, the glycolytic fluxes are lower than those during growth on glucose (30, 47, 50). However, the glycolytic flux governs product formation only when the flux cannot meet the anabolic demand (17). In response to increased ATP demand the glycolytic flux increases, but if the latter is restricted, mixed acid product formation occurs, and three ATP molecules per hexose sugar molecule metabolized is recovered, compared with two ATP molecules per hexose sugar molecule under homolactic conditions. In the present study homolactic maltose fermentation was achieved only with resting cells, in which the demand for ATP was low.
Regulation of glycolytic flux in Lactococcus is still not completely understood. When GAPDH was inhibited by iodoacetate in L. lactis ML3 and NCDO 2118, the glycolytic flux was shown to be controlled to a great extent by GAPDH (14, 41). In contrast, GAPDH of L. lactis MG1363 was shown to control the flux only when the enzyme level was less than 25% of the wild-type level (48). When the expression levels of phosphofructokinase, pyruvate kinase, and LDH were modulated individually in L. lactis MG 1363, none of these enzymes was found to limit the glycolytic flux, and it was therefore concluded that the control most likely is distributed over several steps (28). The levels of glycolytic enzymes have been shown to be highly conserved when the cells go from the growing state to the resting state (40), even if the glycolytic flux is threefold lower in resting cells than in growing cells. This inconsistence between enzyme concentration and enzyme activity indicates that the glycolytic flux in lactococci is controlled by metabolites. Experiments with resting cells in which ATPase activity has been elevated (29) or an ionophore has been added (this study) indicate that ADP and ATP are involved in the regulation.
Thus, we propose a regulation mechanism governed by the cellular quantity of ADP plus ATP, by which resting L. lactis cells can slow the glycolytic flux down. ADP and ATP play central roles in the metabolic network and influence several steps of glycolysis, since they are substrates and products of kinases and inhibitors of dehydrogenases. The main function of ATP is as a free-energy donor to drive, e.g., transport and biosynthesis of molecules, and ATP is continuously regenerated from ADP by substrate level phosphorylation. ATP is invested in the upper part of the glycolysis pathway to generate a surplus in the lower part. In addition to this, both nucleotides are also used as precursors in the synthesis of DNA and RNA, which constitute approximately 3 and 8% of the dry weight of L. lactis cells, respectively (39). The intracellular concentrations of ADP and ATP in growing cells are tightly controlled at levels optimal for the cellular reactions (homeostatic control). In the present study arginine was added to growing L. lactis 65.1 cells as an extra source of ATP, which increased the growth rate and biomass yield (in grams [dry weight] per gram of maltose). Arginine metabolism did not change the concentrations of ADP and ATP, however, which reflects the ability of growing cells to maintain homeostasis. Major changes in the environmental conditions, such as amino acid starvation, put constraints on the cells and force them from the normal growth state. Without amino acids lactococci cannot proliferate; hence, ADP and ATP are required neither as anabolic precursors nor for excessive ATP-requiring processes, such as protein synthesis (45). Amino acid starvation may thus easily result in elevated levels of both ADP and ATP in the cells, as observed in resting cells (Fig. 2). In the present study the enzymatic levels of GAPDH and LDH remained constant, even when the glycolytic flux was approximately three times lower. The in vivo GAPDH and LDH activities, however, were estimated to be decreased four- and twofold, respectively, due to inhibition of ADP and ATP. The two dehydrogenases have reciprocal actions; NAD+ is a substrate of GAPDH, while it is a product of LDH. Since both dehydrogenases were inhibited, they may together be responsible for the lower glycolytic flux in resting cells than in growing cells, which has been observed during metabolism of maltose (this study) and glucose (29, 48, 49).
REFERENCES
- 1.Alvarado, A., and M. E. Flores. 2003. Characterization and regulation of NADP+-isocitrate dehydrogenase from Saccharopolyspora erythraea. Biotechnol. Lett. 25:1175-1178. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, U., F. Levander, and P. Rådström. 2001. Trehalose-6-phosphate phosphorylase is part of a novel metabolic pathway for trehalose utilization in Lactococcus lactis. J. Biol. Chem. 276:42707-42713. [DOI] [PubMed] [Google Scholar]
- 3.Arnau, J., F. Jorgensen, S. M. Madsen, A. Vrang, and H. Israelsen. 1998. Cloning of the Lactococcus lactis adhE gene, encoding a multifunctional alcohol dehydrogenase, by complementation of a fermentative mutant of Escherichia coli. J. Bacteriol. 180:3049-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnau, J., F. Jorgensen, S. M. Madsen, A. Vrang, and H. Israelsen, H. 1997. Cloning, expression, and characterization of the Lactococcus lactis pfl gene, encoding pyruvate formate-lyase. J. Bacteriol. 179:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmeyer, H. U. 1974. Methods of enzymatic analysis, vol. 3, p. 1314-1319. Academic Press, Inc., New York, N.Y.
- 6.Bissett, D. L., and R. L. Anderson. 1974. Lactose and d-galactose metabolism in group N streptococci: presence of enzymes for both the d-galactose 1-phosphate and d-tagatose 6-phosphate pathways. J. Bacteriol. 117:318-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bond, D. R., and J. B. Russell. 1998. Relationship between intracellular phosphate, proton motive force, and rate of nongrowth energy dissipation (energy spilling) in Streptococcus bovis JB1. Appl. Environ. Microbiol. 64:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Chopin, A., M. C. Chopin, A. Moillo-Batt, and P. Langella. 1984. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid 11:260-263. [DOI] [PubMed] [Google Scholar]
- 10.Collins, L. B., and T. D. Thomas. 1974. Pyruvate kinase of Streptococcus lactis. J. Bacteriol. 120:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crow, V. L., and G. G. Pritchard. 1977. Fructose 1,6-diphosphate-activated l-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J. Bacteriol. 131:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crow, V. L., and T. D. Thomas. 1982. Arginine metabolism in lactic streptococci. J. Bacteriol. 150:1024-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vos, W. M., and J. Hugenholtz. 2004. Engineering metabolic highways in lactococci and other lactic acid bacteria. Trends Biotechnol. 22:72-79. [DOI] [PubMed] [Google Scholar]
- 14.Even, S., C. Garrigues, P. Loubiere, N. D. Lindley, and M. Cocaign-Bousquet. 1999. Pyruvate metabolism in Lactococcus lactis is dependent upon glyceraldehyde-3-phosphate dehydrogenase activity. Metab. Eng. 1:198-205. [DOI] [PubMed] [Google Scholar]
- 15.Even, S., N. D. Lindley, and M. Cocaign-Bousquet. 2001. Molecular physiology of sugar catabolism in Lactococcus lactis IL1403. J. Bacteriol. 183:3817-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrigues, C., P. Loubiere, N. D. Lindley, and M. Cocaign-Bousquet. 1997. Control of the shift from homolactic acid to mixed-acid fermentation in Lactococcus lactis: predominant role of the NADH/NAD+ ratio. J. Bacteriol. 179:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrigues, C., M. Mercade, M. Cocaign-Bousquet, N. D. Lindley, and P. Loubiere, P. 2001. Regulation of pyruvate metabolism in Lactococcus lactis depends on the imbalance between catabolism and anabolism. Biotechnol. Bioeng. 74:108-115. [PubMed] [Google Scholar]
- 18.Gaspar, P., A. R. Neves, A. Ramos, M. J. Gasson, C. A. Shearman, and H. Santos. 2004. Engineering Lactococcus lactis for production of mannitol: high yields from food-grade strains deficient in lactate dehydrogenase and the mannitol transport system. Appl. Environ. Microbiol. 70:1466-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han, K., and O. Levenspiel. 1988. Extended monod kinetics for substrate, product and cell inhibition. Biotechnol. Bioeng. 32:430-437. [DOI] [PubMed] [Google Scholar]
- 21.Hess, H. H., and J. E. Derr. 1975. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal. Biochem. 63:607-613. [DOI] [PubMed] [Google Scholar]
- 22.Hoefnagel, M. H., M. J. Starrenburg, D. E. Martens, J. Hugenholtz, M. Kleerebezem, S. Van II, R. Bongers, H. V. Westerhoff, and J. L. Snoep. 2002. Metabolic engineering of lactic acid bacteria, the combined approach: kinetic modelling, metabolic control and experimental analysis. Microbiology 148:1003-1013. [DOI] [PubMed] [Google Scholar]
- 23.Hofvendahl, K., and B. Hahn-Hägerdal. 1997. l-Lactic acid production from whole wheat flour hydrolysate using strains of lactobacilli and lactococci. Enzyme Microb. Technol. 20:301-307. [Google Scholar]
- 24.Hofvendahl, K., E. W. J. van Niel, and B. Hahn-Hägerdal. 1999. Effect of temperature and pH on growth and product formation of Lactococcus lactis ssp. lactis ATCC 19435 growing on maltose. Appl. Microbiol. Biotechnol. 51:669-672. [Google Scholar]
- 25.Hols, P., M. Kleerebezem, A. N. Schanck, T. Ferain, J. Hugenholtz, J. Delcour, and W. M. de Vos. 1999. Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat. Biotechnol. 17:588-592. [DOI] [PubMed] [Google Scholar]
- 26.Hugenholtz, J., W. Sybesma, M. N. Groot, W. Wisselink, V. Ladero, K. Burgess, D. van Sinderen, J. C. Piard, G. Eggink, E. J. Smid, G. Savoy, F. Sesma, T. Jansen, P. Hols, and M. Kleerebezem. 2002. Metabolic engineering of lactic acid bacteria for the production of nutraceuticals. Antonie Leeuwenhoek 82:217-235. [PubMed] [Google Scholar]
- 27.Jonas, H. A., R. F. Anders, and G. R. Jago. 1972. Factors affecting the activity of the lactate dehydrognease of Streptococcus cremoris. J. Bacteriol. 111:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koebmann, B. J., H. W. Andersen, C. Solem, and P. R. Jensen. 2002. Experimental determination of control of glycolysis in Lactococcus lactis. Antonie Leeuwenhoek 82:237-248. [PubMed] [Google Scholar]
- 29.Koebmann, B. J., C. Solem, M. B. Pedersen, D. Nilsson, and P. R. Jensen. 2002. Expression of genes encoding F(1)-ATPase results in uncoupling of glycolysis from biomass production in Lactococcus lactis. Appl. Environ. Microbiol. 68:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levander, F., U. Andersson, and P. Rådström. 2001. Physiological role of β-phosphoglucomutase in Lactococcus lactis. Appl. Environ. Microbiol. 67:4546-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmeier-Vogel, E. M., B. Hahn-Hägerdal, and H. J. Vogel. 1986. Phosphorus-31 NMR studies of maltose and glucose metabolism in Streptococcus lactis. Appl. Microbiol. Biotechnol. 25:43-51. [Google Scholar]
- 32.London, J., and E. Y. Meyer. 1969. Malate utilization by a group D Streptococcus. II. Evidence for allosteric inhibition of an inducible malate dehydrogenase (decarboxylating) by ATP and glycolytic intermediate products. Biochim. Biophys. Acta 178:205-212. [DOI] [PubMed] [Google Scholar]
- 33.Melchiorsen, C. R., N. B. Jensen, B. Christensen, K. V. Jokumsen, and J. Villadsen. 2001. Dynamics of pyruvate metabolism in Lactococcus lactis. Biotechnol. Bioeng. 74:271-279. [DOI] [PubMed] [Google Scholar]
- 34.Melchiorsen, C. R., K. V. Jokumsen, J. Villadsen, H. Israelsen, and J. Arnau. 2002. The level of pyruvate-formate lyase controls the shift from homolactic to mixed-acid product formation in Lactococcus lactis. Appl. Microbiol. Biotechnol. 58:338-344. [DOI] [PubMed] [Google Scholar]
- 35.Melchiorsen, C. R., K. V. Jokumsen, J. Villadsen, M. G. Johnsen, H. Israelsen, and J. Arnau. 2000. Synthesis and posttranslational regulation of pyruvate formate-lyase in Lactococcus lactis. J. Bacteriol. 182:4783-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moritz, B., K. Striegel, A. A. De Graaf, and H. Sahm. 2000. Kinetic properties of the glucose-6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose phosphate pathway flux in vivo. Eur. J. Biochem. 267:3442-3452. [DOI] [PubMed] [Google Scholar]
- 37.Neves, A. R., R. Ventura, N. Mansour, C. Shearman, M. J. Gasson, C. Maycock, A. Ramos, and H. Santos. 2002. Is the glycolytic flux in Lactococcus lactis primarily controlled by the redox charge? Kinetics of NAD+ and NADH pools determined in vivo by 13C NMR. J. Biol. Chem. 277:28088-28098. [DOI] [PubMed] [Google Scholar]
- 38.Nolasco-Hipolito, C., T. Matsunaka, G. Kobayashi, K. Sonomoto, and A. Ishizaki. 2002. Synchronized fresh cell bioreactor system for continuous l-(+)-lactic acid production using Lactococcus lactis IO-1 in hydrolysed sago starch. J. Biosci. Bioeng. 93:281-287. [DOI] [PubMed] [Google Scholar]
- 39.Novak, L., and P. Loubiere. 2000. The metabolic network of Lactococcus lactis: distribution of 14C-labeled substrates between catabolic and anabolic pathways. J. Bacteriol. 182:1136-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmfeldt, J., F. Levander, B. Hahn-Hägerdal, and P. James. Acidic proteome of growing and resting Lactococcus lactis metabolizing maltose. Proteomics, in press. [DOI] [PubMed]
- 41.Poolman, B., B. Bosman, J. Kiers, and W. N. Konings. 1987. Control of glycolysis by glyceraldehyde-3-phosphate dehydrogenase in Streptococcus cremoris and Streptococcus lactis. J. Bacteriol. 169:5887-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian, N., G. A. Stanley, A. Bunte, and P. Rådström. 1997. Product formation and phosphoglucomutase activities in Lactococcus lactis: cloning and characterization of a novel phosphoglucomutase gene. Microbiology 143:855-865. [DOI] [PubMed] [Google Scholar]
- 43.Qian, N., G. A. Stanley, B. Hahn-Hägerdal, and P. Rådström. 1994. Purification and characterization of two phosphoglucomutases from Lactococcus lactis subsp. lactis and their regulation in maltose-utilizing and glucose-utilizing cells. J. Bacteriol. 176:5304-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rado, T. A., and J. A. Hoch. 1973. Phosphotransacetylase from Bacillus subtilis: purification and physiological studies. Biochim. Biophys. Acta 321:114-125. [DOI] [PubMed] [Google Scholar]
- 45.Russell, J. B., and G. M. Cook. 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59:48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. J. Ye. 1996. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology 142:217-230. [DOI] [PubMed] [Google Scholar]
- 47.Sjöberg, A., I. Persson, M. Quednau, and B. Hahn-Hägerdal. 1995. The influence of limiting and nonlimiting growth-conditions on glucose and maltose metabolism in Lactococcus lactis ssp. lactis strains. Appl. Microbiol. Biotechnol. 42:931-938. [Google Scholar]
- 48.Solem, C., B. J. Koebmann, and P. R. Jensen. 2003. Glyceraldehyde-3-phosphate dehydrogenase has no control over glycolytic flux in Lactococcus lactis MG1363. J. Bacteriol. 185:1564-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas, T. D., D. C. Ellwood, and V. M. C. Longyear. 1979. Change from homo-fermentation to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J. Bacteriol. 138:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas, T. D., K. W. Turner, and V. L. Crow. 1980. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J. Bacteriol. 144:672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson, J. 1976. Characteristics and energy requirements of an alpha amino isobutyric acid transport system in Streptococcus lactis. J. Bacteriol. 127:719-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson, J., and D. A. Torchia. 1984. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J. Bacteriol. 158:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a Gram-positive solution. Antonie Leeuwenhoek 82:59-71. [PubMed] [Google Scholar]
- 54.Van Niel, E. W. J., J. Palmfeldt, R. Martin, M. Paese, and B. Hahn-Hägerdal. 2004. Reappraisal of the regulation of lactococcal l-lactate dehydrogenase. Appl. Environ. Microbiol. 70:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Niel, E. W. J., K. Hofvendahl, and B. Hahn-Hägerdal. 2002. Formation and conversion of oxygen metabolites by Lactococcus lactis subsp. lactis ATCC 19435 under different growth conditions. Appl. Environ. Microbiol. 68:4350-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, S. T., and W. C. Deal, Jr. 1969. Metabolic control and structure of glycolytic enzymes. VI. Competitive inhibition of yeast glyceraldehyde 3-phosphate dehydrogenase by cyclic adenosine monophosphate, adenosine triphosphate, and other adenine-containing compounds. Biochemistry 8:2806-2813. [DOI] [PubMed] [Google Scholar]