Abstract
Human cytomegalovirus UL54 DNA polymerase gene mutations that confer foscarnet resistance in clinical practice typically cluster in the amino terminal 2, palm and finger domains. Exposure to foscarnet in cell culture selected for mutations elsewhere in UL54, including amino acid substitutions S290R in the amino terminal 1 domain and E951D in the palm 2 domain. These are newly confirmed to confer foscarnet resistance and slightly decreased ganciclovir susceptibility. Other emergent substitutions N495K, T552N and T838A are known to confer foscarnet resistance, while additional ones Q783R and V798A only slightly affected susceptibility. An expanded set of domains is involved in foscarnet resistance and its genotypic diagnosis.
Short Communication
The pyrophosphate analog foscarnet sodium (trisodium phosphonoformate, FOS) has been an approved treatment for cytomegalovirus (CMV) retinitis for 25 years, and is widely used without FDA approval for treating CMV infection when first line treatment with ganciclovir (GCV) or valganciclovir is unsuccessful or limited by toxicity (Avery et al., 2016; Bacigalupo et al., 2012). As FOS is commonly considered for treatment of GCV-resistant infection, the viral UL54 DNA polymerase gene mutations that confer FOS resistance and GCV cross-resistance have been extensively studied. FOS resistance mutations encountered in clinical practice are clustered in the DNA polymerase structure domains designated amino terminal 2 (residues 555– 600), palm and finger (residues 696–981) (Liu et al., 2006). Amino acid substitutions are detected at residues Q578, N588, T700, V715, E756, V781, V787, L802, A809, V812 and A834, among others (Campos et al., 2016; Lurain and Chou, 2010; Topalis et al., 2016), typically conferring 3- to 5-fold decreases in FOS susceptibility with variable low-grade cross-resistance to GCV and sometimes to cidofovir (CDV) as well. Rarely, substitutions in the exonuclease region have been implicated in clinical FOS resistance (N495K, D515Y) (Andouard et al., 2016; Ducancelle et al., 2006), or observed after FOS exposure in cell culture (T419M, T552N) (Gilbert et al., 2011; Mousavi-Jazi et al., 2003).
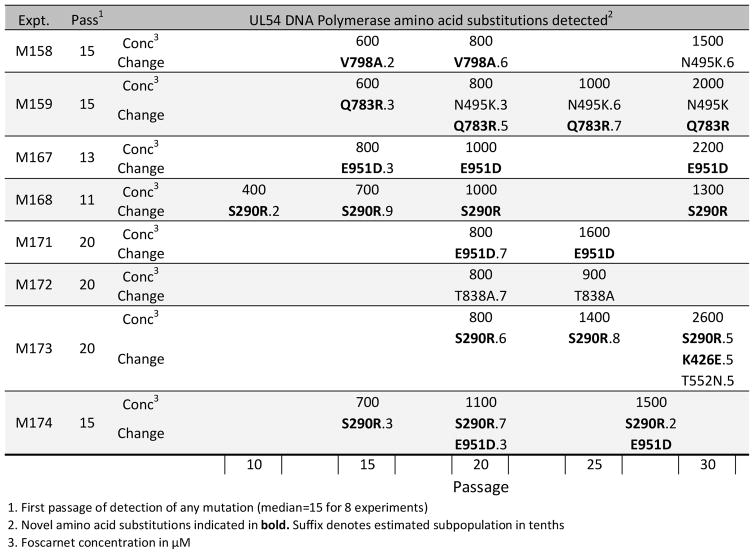
To compare the in vitro evolution of resistance mutations after exposure to the terminase inhibitor letermovir, eight control experiments using an error-prone exonuclease mutant D413del were performed under increasing concentrations of FOS in fibroblast cell culture, which resulted in the selection of UL54 mutations after a median of 15 passages, as recently reported (Chou, 2015). Novel mutations were encountered that required phenotypic characterization. As shown in Fig. 1, mutations were first detected by conventional dideoxy sequencing at 600 to 800 μM concentrations of FOS, about 12 times the concentrations required to inhibit viral growth by 50% (EC50) at baseline. Amino acid substitutions S290R and E951D were detected in 3 experiments each, while K426E, Q783R and V798A were detected in one experiment each, adding to previously reported substitutions N495K (Ducancelle et al., 2006), T552N (Gilbert et al., 2011) and T838A (Springer et al., 2005) which were also detected in this series of experiments. The newly detected substitutions are atypical in that S290R is located in an amino terminal 1 polymerase domain (residues 94–295) not previously linked to drug resistance, and E951D is unusually located within the palm 2 domain (residues 825–981). In the latter domain, most FOS resistance maps to residues up to 841, leaving a single documented resistance marker between 842 and 980, namely V946L, which was selected in vitro under GCV but phenotyped as GCV-susceptible and FOS-resistant at 2.4-fold increased EC50 (Gilbert et al., 2011).
Figure 1.
Evolution timelines of UL54 mutations
For each of 8 selection experiments (M158–M174), the sequential evolution of UL54 amino acid substitutions during passages 10 to 30 is displayed in order from left to right.
Recombinant phenotyping of the new mutations was performed by mutagenesis of a bacterial artificial chromosome (BAC) clone BD1 (Chou, 2015) derived from standard laboratory strain AD169 as previously described (Chou, 2011). This involved heat-induced conditional recombination in E. coli strain SW105 of a derivative of BD1 lacking the UL54 coding sequence, with a transfer vector containing a Frt-delimited kanamycin selection marker (Kan) adjacent to the mutant UL54 coding region. Following excision of Kan by induction of Flp recombinase, the resulting recombinant BAC was transfected into human foreskin fibroblast cultures to recover infectious CMV, which was checked throughout the UL54 sequence for presence of the intended mutation and absence of other changes. The virus was then phenotyped for susceptibility to GCV, FOS and CDV using secreted alkaline phosphatase (SEAP) activity of the reporter gene in the BAC clone for growth quantitation 6 to 7 days after a low multiplicity inoculum (MOI=0.01 to 0.03, reflected in SEAP activity at 24 hours), as standardized in multiple previous publications (Chou, 2011; Chou et al., 2014; Chou et al., 2005). At least 7 SEAP yield reduction assays over 4 separate setup dates were used to determine mean and standard deviation EC50 values, which were compared with control values obtained with a wild type strain (T4198) and well-known FOS-, CDV- and GCV-resistant mutants containing substitution A809V, A987G (Chou, 2011) or UL97 C592G (Chou, 2010) respectively, to calibrate the level of drug resistance conferred by the new mutations.
In this study, the assay methodology was updated to include a comparison of traditional human foreskin fibroblasts (HFF) and modified retinal epithelial cells as cell culture models for drug susceptibility EC50 assays. There is interest in antiviral assays performed in cells other than fibroblasts, since these are not the cells mainly involved in CMV pathogenesis. There are also practical issues of host cell metabolic changes affecting viral permissiveness after extended passage of primary fibroblast cultures. The continuous retinal epithelial cell line (ARPE-19) is unsatisfactory for the routine propagation of laboratory strains (such as strain AD169), but it was discovered that the stable over-expression of the platelet derived growth factor receptor, as in the transduced cell line ARPE-19α, provides an alternate entry pathway for laboratory CMV strains, allowing fully permissive growth (Vanarsdall et al., 2012). ARPE-19α cells were kindly provided by Dr. Adam Vanarsdall (Oregon Health & Science University) and tested as direct replacements for HFF in the previously standardized SEAP yield reduction EC50 assays. Except for a change in the basal cell culture medium to Dulbecco Minimal Essential Medium (DMEM) with high glucose (4.5 g/L), no other changes in protocol were required for use of ARPE-19α cells.
Susceptibility testing results for the recombinant viruses are shown in the tables. For FOS, the substitutions S290R and E951D individually conferred 2- to 4-fold increased EC50 (Table 1) when assayed in either cell type, similar to increases observed for known FOS-resistant mutants such as A809V, N495K and T552N, which were phenotyped here to show consistency with published data (Chou, 2011; Ducancelle et al., 2006; Gilbert et al., 2011). Substitutions Q783R and V798A slightly decreased FOS susceptibility with EC50 ratio of 1.7. When Q783R was combined with N495K (as found in the selection experiment M159 in Fig. 1), it increased the EC50 as compared with N495K alone. Table 2 shows borderline decreases in GCV susceptibility for mutants containing S290R, T552N, Q783R, V798A and E951D, a phenotype commonly observed in foscarnet-resistant mutants. The GCV EC50 ratio for T552N (1.8-fold in ARPE-19α and 2.6-fold in HFF) compares with the 1.9-fold previously published for this substitution as assayed in HFF (Gilbert et al., 2011), indicating that slight decreases in GCV susceptibility may give EC50 ratios just above or below 2-fold even when performed in the same cell type (HFF). Table 3 shows no significant CDV resistance conferred by any of the mutants being phenotyped. The substitution K426E that appeared late in one experiment (M173) was coincident with the emergence of T552N. Since K426E by itself conferred no drug resistance (Tables 1–3), it probably compensates for other growth-attenuating changes in UL54.
Table 1.
Genotypes and Foscarnet Susceptibility Phenotypes
| Strain | Genotype | ARPE-19α cells
|
HFF cells
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| EC501 | SD2 | N3 | Fold4 | EC501 | SD2 | N3 | Fold4 | ||
| T4198 | Wild Type | 43 | 8 | 21 | 57 | 16 | 18 | ||
| T4199 | A809V | 155 | 39 | 23 | 3.6 | 255 | 36 | 18 | 4.4 |
|
| |||||||||
| T4280 | S290R | 97 | 11 | 13 | 2.3 | 124 | 19 | 9 | 2.2 |
| T4291 | K426E | 47 | 18 | 13 | 1.1 | 50 | 9 | 14 | 0.9 |
| T4286 | N495K | 109 | 29 | 11 | 2.6 | 143 | 23 | 9 | 2.5 |
| T4281 | N495K Q783R | 147 | 17 | 10 | 3.5 | 249 | 54 | 8 | 4.3 |
| T4294 | T552N | 108 | 23 | 13 | 2.5 | 180 | 20 | 10 | 3.1 |
| T4287 | Q783R | 71 | 23 | 14 | 1.7 | 99 | 16 | 10 | 1.7 |
| T4269 | V798A | 71 | 9 | 12 | 1.7 | 104 | 25 | 10 | 1.8 |
| T4279 | E951D | 166 | 26 | 17 | 3.9 | 251 | 28 | 9 | 4.4 |
Mean concentration (μM) for 50% SEAP yield reduction
Standard deviation of EC50 values
Number of replicates performed over at least 4 assay dates
Fold change in EC50 compared with wild type (>2 in bold)
Table 2.
Genotypes and Ganciclovir Susceptibility Phenotypes
| Strain | Genotype | ARPE-19α cells
|
HFF cells
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| EC501 | SD2 | N3 | Fold4 | EC501 | SD2 | N3 | Fold4 | ||
| T4198 | Wild Type | 2.4 | 0.78 | 19 | 1.1 | 0.25 | 24 | ||
| T4207 | UL97 C592G | 7.4 | 2.2 | 15 | 3.1 | 3.5 | 0.51 | 13 | 3.0 |
|
| |||||||||
| T4280 | S290R | 4.1 | 1.0 | 9 | 1.7 | 1.7 | 0.51 | 12 | 1.5 |
| T4291 | K426E | 2.5 | 0.68 | 12 | 1.1 | 1.1 | 0.25 | 13 | 1.0 |
| T4286 | N495K | 3.1 | 0.95 | 15 | 1.3 | 1.6 | 0.59 | 9 | 1.4 |
| T4281 | N495K Q783R | 4.1 | 0.39 | 8 | 1.7 | 2.5 | 0.48 | 9 | 2.2 |
| T4294 | T552N | 4.2 | 1.0 | 11 | 1.8 | 3.0 | 0.62 | 7 | 2.6 |
| T4287 | Q783R | 4.4 | 1.8 | 17 | 1.8 | 1.5 | 0.52 | 12 | 1.3 |
| T4269 | V798A | 3.5 | 0.78 | 10 | 1.5 | 1.7 | 0.48 | 13 | 1.5 |
| T4279 | E951D | 4.2 | 1.9 | 14 | 1.8 | 2.5 | 0.76 | 11 | 2.2 |
Mean concentration (μM) for 50% SEAP yield reduction
Standard deviation of EC50 values
Number of replicates performed over at least 4 assay dates
Fold change in EC50 compared with wild type (>2 in bold)
Table 3.
Genotypes and Cidofovir Susceptibility Phenotypes
| Strain | Genotype | ARPE-19α cells
|
HFF cells
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| EC501 | SD2 | N3 | Fold4 | EC501 | SD2 | N3 | Fold4 | ||
| T4198 | Wild Type | 0.55 | 0.19 | 19 | 0.17 | 0.03 | 21 | ||
| T4208 | A987G | 4.7 | 1.7 | 19 | 8.5 | 0.86 | 0.22 | 20 | 5.0 |
|
| |||||||||
| T4280 | S290R | 0.76 | 0.11 | 11 | 1.4 | 0.22 | 0.05 | 8 | 1.3 |
| T4291 | K426E | 0.55 | 0.19 | 17 | 1.0 | 0.16 | 0.04 | 8 | 0.9 |
| T4286 | N495K | 0.55 | 0.17 | 12 | 1.0 | 0.21 | 0.05 | 12 | 1.2 |
| T4281 | N495K Q783R | 0.66 | 0.08 | 9 | 1.2 | 0.22 | 0.07 | 8 | 1.3 |
| T4294 | T552N | 0.56 | 0.14 | 10 | 1.0 | 0.29 | 0.06 | 9 | 1.7 |
| T4287 | Q783R | 0.49 | 0.16 | 12 | 0.9 | 0.16 | 0.03 | 8 | 0.9 |
| T4269 | V798A | 0.60 | 0.09 | 8 | 1.1 | 0.17 | 0.05 | 14 | 1.0 |
| T4279 | E951D | 0.65 | 0.14 | 11 | 1.2 | 0.18 | 0.01 | 9 | 1.0 |
Mean concentration (μM) for 50% SEAP yield reduction
Standard deviation of EC50 values
Number of replicates performed over at least 4 assay dates
Fold change in EC50 compared with wild type
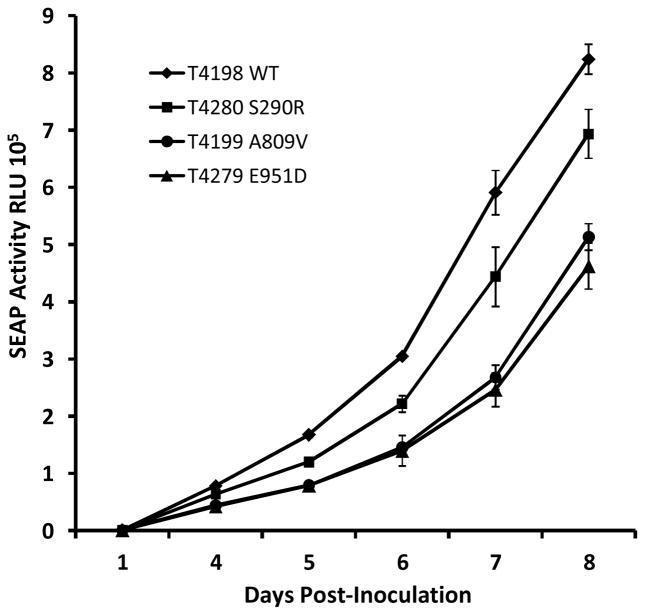
The growth fitness of the atypical mutants S290R and E951D in HFF culture was compared with wild type virus and the well-known growth-attenuated FOS-resistant mutant A809V, as in previous studies of UL54 mutants (Chou, 2011; Chou et al., 2016). Based on achieved culture supernatant SEAP activity at 6 to 7 days for closely matched low multiplicity viral inocula and cell culture conditions, the E951D mutant was growth attenuated to the same extent as A809V while the S290R mutant was intermediate in attenuation between wild type and A809V or E951D (Fig. 2).
Figure 2.
Comparative growth curves of selected UL54 mutants
Viral growth was monitored by culture supernatant SEAP activity over multiple cycles of replication in HFF monolayers after closely matching low input multiplicity of infection (mean 24 hour SEAP activity for the 4 strains ranged from 935 to 967). Data points and error bars represent the mean and standard deviation of 4 simultaneous replicates in 24-well culture plates.
The previously known or newly described UL54 FOS resistance mutations that appeared in these in vitro selection experiments are not among those most commonly encountered in vivo (Kleiboeker et al., 2014; Lurain and Chou, 2010), although N495K and T838A have been found in clinical specimens (Ducancelle et al., 2006; Springer et al., 2005). Likely reasons include prior exposure to GCV in most FOS-treated patients, differences in effective intracellular drug concentrations, and the viral strains (error-prone exonuclease mutant versus wild type) and culture conditions for in vitro selection.
The inhibitory effect of FOS on herpesvirus DNA polymerases is conventionally attributed to interference with the pyrophosphate exchange that occurs when an incoming base is added to a growing DNA strand, but the full range of polymerase residues and mutations that affect interaction with FOS, either directly or indirectly through conformational changes, is not well established from structure data or modeling (Andouard et al., 2016; Liu et al., 2006; Shi et al., 2006; Zahn et al., 2011). Based on the prevalent mutations observed to date, residues in the palm and finger domains (residues 696–981) are the most important, along with residues in the amino terminal domain 2 (e.g. Q578, D588). The substitution S290R is located some distance away in amino terminal domain 1 (residues 94–295) (Liu et al., 2006), and may be speculated to affect FOS susceptibility through a transmitted conformational change. Residue S290 is partially conserved among herpesvirus polymerases (as S357 in herpes simplex). Although not well characterized, the amino terminal 1 domain contains residues required for viral viability such as E235 (Chou et al., 2014). Since current clinical genotyping practices do not cover all of this domain (Kleiboeker et al., 2014) it is possible that additional FOS resistance mutations may exist in this part of the polymerase gene. However, some substitutions in this domain including those near S290 (Q229K, D247N, D262N, T271A, D288N and S291P) have been phenotyped and shown to confer no drug resistance (Chevillotte et al., 2010; Chou et al., 2014).
The non-fibroblast ARPE-19α cells were found to be a good substitute for HFFs for drug susceptibility assays, with technical advantages of a continuous cell line that propagates easily and gives equally strong SEAP reporter gene signals for viral growth quantitation. For example, in the assays of the wild type control strain in Table 1, mean SEAP reporter signals at 24 hours (reflecting input viral multiplicity) were 674 for ARPE-19α and 874 for HFF, and the mean achieved SEAP activities were 3.8 x 105 at 6.4 days for ARPE-19α and 3.7 x 105 at 5.6 days for HFF. Although the baseline wild type EC50 values for GCV and CDV were slightly higher in ARPE-19α cells than in HFF, suggesting differences in intracellular phosphorylation of nucleoside analogs, the EC50 ratios of the resistant mutants were similar although they might be slightly above or below a 2-fold cutoff in the case of borderline changes in susceptibility.
In summary, this set of in vitro FOS selection experiments extended the range of CMV UL54 DNA polymerase residues involved in FOS resistance, including those in amino terminal domain 1 and more distal parts of the palm 2 domain, which should be included among those assessed for CMV drug resistance in diagnostic laboratory practice.
Highlights.
CMV DNA polymerase amino acid substitutions S290R and E951D, among others, emerged in vitro to decrease foscarnet susceptibility
The amino terminal 1 structure domain of the CMV DNA polymerase is newly implicated in foscarnet resistance
genotypic testing for foscarnet resistance should consider an increased range of UL54 codons
drug susceptibility phenotyping in a modified retinal epithelial cell line gave similar results as in fibroblasts
Acknowledgments
Gail Marousek, Ronald J. Ercolani and Michelle A. Hendrick provided technical support. The work was supported by NIH grant AI116635 and Department of Veterans Affairs research funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andouard D, Mazeron MC, Ligat G, Couvreux A, Pouteil-Noble C, Cahen R, Yasdanpanah Y, Deering M, Viget N, Alain S, Hantz S. Contrasting effect of new HCMV pUL54 mutations on antiviral drug susceptibility: Benefits and limits of 3D analysis. Antiviral Res. 2016;129:115–119. doi: 10.1016/j.antiviral.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Avery RK, Arav-Boger R, Marr KA, Kraus E, Shoham S, Lees L, Trollinger B, Shah P, Ambinder R, Neofytos D, Ostrander D, Forman M, Valsamakis A. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation. 2016;100:e74–e80. doi: 10.1097/TP.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo A, Boyd A, Slipper J, Curtis J, Clissold S. Foscarnet in the management of cytomegalovirus infections in hematopoietic stem cell transplant patients. Expert Rev Anti Infect Ther. 2012;10:1249–1264. doi: 10.1586/eri.12.115. [DOI] [PubMed] [Google Scholar]
- Campos AB, Ribeiro J, Boutolleau D, Sousa H. Human cytomegalovirus antiviral drug resistance in hematopoietic stem cell transplantation: current state of the art. Rev Med Virol. 2016;26:161– 182. doi: 10.1002/rmv.1873. [DOI] [PubMed] [Google Scholar]
- Chevillotte M, Ersing I, Mertens T, von Einem J. Differentiation between polymorphisms and resistance associated mutations in the human cytomegalovirus DNA polymerase. Antimicrob Agents Chemother. 2010;54:5004–5011. doi: 10.1128/AAC.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. Recombinant phenotyping of cytomegalovirus UL97 kinase sequence variants for ganciclovir resistance. Antimicrob Agents Chemother. 2010;54:2371–2378. doi: 10.1128/AAC.00186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. Phenotypic diversity of cytomegalovirus DNA polymerase gene variants observed after antiviral therapy. J Clin Virol. 2011;50:287–291. doi: 10.1016/j.jcv.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother. 2015;59:6588–6593. doi: 10.1128/AAC.01623-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Boivin G, Ives J, Elston R. Phenotypic evaluation of previously uncharacterized cytomegalovirus DNA polymerase sequence variants detected in a valganciclovir treatment trial. J Infect Dis. 2014;209:1209–1216. doi: 10.1093/infdis/jit654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Ercolani RJ, Lanier ER. Novel Cytomegalovirus UL54 DNA Polymerase Gene Mutations Selected In Vitro That Confer Brincidofovir Resistance. Antimicrob Agents Chemother. 2016;60:3845–3848. doi: 10.1128/AAC.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Van Wechel LC, Lichy HM, Marousek GI. Phenotyping of cytomegalovirus drug resistance mutations by using recombinant viruses incorporating a reporter gene. Antimicrob Agents Chemother. 2005;49:2710–2715. doi: 10.1128/AAC.49.7.2710-2715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducancelle A, Champier G, Alain S, Petit F, Le Pors MJ, Mazeron MC. A novel mutation in the UL54 gene of human cytomegalovirus isolates that confers resistance to foscarnet. Antiviral therapy. 2006;11:537–540. [PubMed] [Google Scholar]
- Gilbert C, Azzi A, Goyette N, Lin SX, Boivin G. Recombinant phenotyping of cytomegalovirus UL54 mutations that emerged during cell passages in the presence of either ganciclovir or foscarnet. Antimicrob Agents Chemother. 2011;55:4019–4027. doi: 10.1128/AAC.00334-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiboeker S, Nutt J, Schindel B, Dannehl J, Hester J. Cytomegalovirus antiviral resistance: characterization of results from clinical specimens. Transpl Infect Dis. 2014;16:561–567. doi: 10.1111/tid.12241. [DOI] [PubMed] [Google Scholar]
- Liu S, Knafels JD, Chang JS, Waszak GA, Baldwin ET, Deibel MR, Jr, Thomsen DR, Homa FL, Wells PA, Tory MC, Poorman RA, Gao H, Qiu X, Seddon AP. Crystal structure of the herpes simplex virus 1 DNA polymerase. J Biol Chem. 2006;281:18193–18200. doi: 10.1074/jbc.M602414200. [DOI] [PubMed] [Google Scholar]
- Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi-Jazi M, Schloss L, Wahren B, Brytting M. Point mutations induced by foscarnet (PFA) in the human cytomegalovirus DNA polymerase. J Clin Virol. 2003;26:301–306. doi: 10.1016/s1386-6532(02)00046-x. [DOI] [PubMed] [Google Scholar]
- Shi R, Azzi A, Gilbert C, Boivin G, Lin SX. Three-dimensional modeling of cytomegalovirus DNA polymerase and preliminary analysis of drug resistance. Proteins. 2006;64:301–307. doi: 10.1002/prot.21005. [DOI] [PubMed] [Google Scholar]
- Springer KL, Chou S, Li S, Giller RH, Quinones R, Shira JE, Weinberg A. How evolution of mutations conferring drug resistance affects viral dynamics and clinical outcomes of cytomegalovirus-infected hematopoietic cell transplant recipients. J Clin Microbiol. 2005;43:208–213. doi: 10.1128/JCM.43.1.208-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalis D, Gillemot S, Snoeck R, Andrei G. Distribution and effects of amino acid changes in drug-resistant alpha and beta herpesviruses DNA polymerase. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw1875. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanarsdall AL, Wisner TW, Lei H, Kazlauskas A, Johnson DC. PDGF receptor-alpha does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway. PLoS Pathog. 2012;8:e1002905. doi: 10.1371/journal.ppat.1002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn KE, Tchesnokov EP, Gotte M, Doublie S. Phosphonoformic acid inhibits viral replication by trapping the closed form of the DNA polymerase. J Biol Chem. 2011;286:25246–25255. doi: 10.1074/jbc.M111.248864. [DOI] [PMC free article] [PubMed] [Google Scholar]