Abstract
Although researchers have long hypothesized a relationship between attention and anxiety, theoretical and empirical accounts of this relationship have conflicted. We attempted to resolve these conflicts by examining relationships of attentional abilities with responding to predictable and unpredictable threat, related but distinct motivational process implicated in a number of anxiety disorders. Eighty-one individuals completed a behavioral task assessing efficiency of three components of attention – alerting, orienting, and executive control (Attention Network Test - Revised). We also assessed startle responding during anticipation of both predictable, imminent threat (of mild electric shock) and unpredictable contextual threat. Faster alerting and slower disengaging from non-emotional attention cues were related to heightened responding to unpredictable threat, whereas poorer executive control of attention was related to heightened responding to predictable threat. This double dissociation helps to integrate models of attention and anxiety and may be informative for treatment development.
Keywords: Attention, Executive Functioning, Cognition, Anxiety, Fear, Predictability, Startle
1. Introduction*
Researchers have long posited a relationship between attention and anxiety (e.g., Daly et al., 1989; Masters & Johnson, 1970; Wine, 1971). Although a large literature indicates that anxiety is associated with biased attention towards emotional (i.e., threat-related) stimuli (Bar-Haim et al., 2007), it is not clear whether and in what way anxiety is related to general attention to non-emotional stimuli. Better characterizing the latter relationship has the potential to improve our understanding of anxiety disorder etiology and has implications for treatments such as attention bias modification and cognitive remediation.
One prominent model posits that attention consists of three processes – alerting, orienting, and attentional control – subserved by separable but interacting brain networks (Posner & Petersen, 1990; Petersen & Posner, 2012). Alerting consists of vigilance and response readiness – either tonic (i.e., sustained over long periods) or phasic (i.e., a temporary increase in readiness in response to a warning signal). The alerting network consists of the locus coeruleus and its noradrenergic projections to widespread cortical and subcortical regions (Fan et al., 2005; Marrocco et al., 1994; Petersen & Posner, 2012). Orienting consists of selection or prioritization of some sensory inputs over others for processing. Orienting includes both engaging attention with selected input and disengaging from previously attended input. Engaging is subserved by a dorsal orienting network including superior parietal lobule and frontal eye fields, whereas disengaging is subserved by a ventral orienting network including temporoparietal junction and middle and inferior frontal gyri (Corbetta & Shulman, 2002; Petersen & Posner, 2012). Finally, attentional control (or executive control of attention) is the effortful process of allocating attention in the face of competing or conflicting demands, such as when a habitual, automatic, or otherwise dominant response to a stimulus must be withheld to attend to an alternative stimulus. This component of attention is closely linked to emotion regulation and other self-regulatory abilities (Posner & Rothbart, 2013; Zelazo & Cunningham, 2007). Attentional control is subserved by cingulo-opercular and frontoparietal networks, in which the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (dlPFC), respectively, play key roles (Matsumoto & Tanaka, 2004; Petersen & Posner, 2012).
Most research on attention and anxiety has examined differences in how anxious people attend to threat-related or other emotional stimuli – a form of emotional or “hot” cognition (Metcalfe & Mischel, 1999). This research indicates that individuals with anxiety disorders or high trait anxiety demonstrate biased attention towards threat cues, particularly when these cues are presented briefly or subliminally (Bar-Haim et al., 2007; Mathews & MacLeod, 2005). This bias may result from facilitated engaging with threat, impaired disengaging from threat, or both (Armstrong & Olatunji, 2012; Yiend, 2010).
These findings are most frequently interpreted as indicating that, because anxious individuals are preoccupied with threat, their attention systems prioritize threat cues for faster and longer processing. However, attention bias findings could also reflect that anxiety is associated with broader differences in how attention networks function, even in processing non-emotional stimuli (Bishop, 2009). That is, anxious individuals may engage with threat cues more quickly and dwell on them for longer not only because these cues are more salient for them, but also because they attend more quickly to or disengage more slowly form all salient cues, regardless of emotional content. Anxious individuals may also have diminished ability to inhibit automatic attention towards salient cues (i.e., diminished attentional control). Indeed, the relationship between anxiety and biased attention to threat is more pronounced in individuals with low self-reported attentional control (Bardeen & Orcutt, 2011; Derryberry & Reed, 2002). In line with these possibilities, several more recent theories propose relationships between anxiety and aspects of general, non-emotional, or “cold” attention. These theories also specify how anxiety is related to different attention networks, whereas the tasks used in “hot” attention bias studies generally cannot cleanly separate effects due to alerting, orienting, and attentional control.
These theories are broadly consistent in predicting that trait anxiety is associated with abnormalities in attentional control, but differ in their predictions regarding orienting and alerting. Specifically, Eysenck and colleagues' attentional control theory (Eysenck et al., 2007; Eysenck & Derakshan, 2011) posits that trait anxiety causes less efficient attentional shifting (i.e., orienting) and distractor inhibition (i.e., attentional control) as processing demands increase. Bishop (2009), based on Lavie's (2000, 2005) load theory of selective attention, also maintains that anxiety is associated with poorer attentional control, but during conditions of low perceptual load. Sylvester and colleagues (2012) propose that anxiety is associated with increased functioning of the cingulo-opercular network and reduced functioning of the fronto-parietal network (both associated with executive control of attention), as well as increased functioning of the ventral attention network (associated with orienting and stimulus-driven alerting). According to their account, anxious individuals detect and orient towards task-irrelevant stimuli more easily due to overactivity of the ventral attention network. They are also less able to inhibit these alerting and orienting responses due to dysfunction in executive control networks. Thus, all three of these models predict that individuals high in anxiety will demonstrate poorer attentional control. Their predictions regarding anxiety's relationship with orienting are more divergent: Eysenck's model predicts poorer orienting, Sylvester's predicts enhanced orienting and alerting, and Bishop makes no prediction.
Consistent with these predictions, a number of studies have reported that trait anxiety and anxiety disorders are associated with lower self-reported attentional control (Armstrong et al., 2011; Reinholdt-Dunne et al., 2013) and less efficient performance on behavioral measures of attentional control (Bishop, 2009; Pacheco-Unguetti et al., 2010, 2011). However, this relationship has not been universally observed. Some studies have reported that trait anxiety or related constructs (e.g., behavioral inhibition) are not associated with attentional control, and have instead reported relationships with orienting (Garner et al., 2012; Moriya & Tanno, 2009; Tull et al., 2012) or alerting (Dennis et al., 2008; Garner et al., 2012).
All of these studies examined relationships of attention with self-reported anxiety or similar traits. This raises two potential explanations for the inconsistency of findings. First, trait anxiety and related concepts are broad, heterogeneous constructs; it may be that different subcomponents of trait anxiety are related to attention in different ways, producing inconsistencies in the literature. Focusing on more specific affective processes that underlie broad trait anxiety may clarify this issue. Second, cognition and self-report are relatively distal levels of analysis, making it difficult to consistently find relationships between them (Kendler, 2005; Lilienfeld, 2007). More robust relationships may be found by examining constructs at a level of analysis more proximal to cognition, such as psychophysiology.
To address both of these concerns, the present study examined two well-validated motivational processes underlying broad trait anxiety – sensitivity to predictable, certain, imminent harm (often labeled “fear”), and sensitivity to unpredictable, uncertain, contextual threat (often labeled “anxiety,” Barlow, 2000; Davis, 2006; Davis et al., 2010; Gray & McNaughton, 2000; Grillon, 2002). These processes are often assessed by measuring eye blink acoustic startle response during anticipation of cued (i.e., predictable) and uncued (i.e., unpredictable) aversive stimuli, respectively (Schmitz & Grillon, 2012). Phasic responding to predictable threat cues is subserved by a circuit including the medial central amygdala (CeA) and projecting primarily to the hypothalamus and brainstem nuclei. Tonic responding to unpredictable threat contexts is subserved by a circuit including the lateral bed nucleus of the stria terminalis (BNST) and lateral CeA (Alvarez et al., 2011; Davis, 2006; Davis et al., 2010; Somerville et al., 2013).
In addition to their unique neuroanatomical correlates, the discriminant validity of these processes is supported by their differential response to pharmacological challenge (Grillon et al., 2015; Grillon et al., 2006; Moberg & Curtin, 2009) and differential familial/genetic associations (Sarapas et al., 2012; Nelson et al., 2013). Most importantly, the two processes have discriminant validity for different forms of psychopathology. Exaggerated responding to predictable threat has been associated with specific phobia (McTeague et al., 2012) and suicidality (Ballard et al., 2014), whereas exaggerated responding to unpredictable threat is associated with posttraumatic stress disorder (Grillon et al., 2009). Given their unique physiological mechanisms and clinical correlates, examination of these two motivational processes may clarify whether different aspects of attention are related to different psychopathology-related processes and outcomes. Indeed, several lines of evidence suggest that predictable vs. unpredictable threat responding may be differentially related to components of attention.
First, the processes are relevant to situations with somewhat different cognitive demands. Unpredictably threatening contexts require sustained vigilance for potential danger and the ability to rapidly detect and orient towards danger when it appears. Therefore, individual differences in attentional abilities that promote vigilance (i.e., alerting and orienting) may be associated with responding to unpredictable threat. In contrast, these abilities may be less relevant when stressors are predictable, as the reliable information about the timing and location of stressors make sustained vigilance unnecessary. Instead, predictable stressors afford individuals the opportunity to make effortful preparatory responses such as emotion regulation, self-distraction, or reappraisal (Perkins, 1955, 1968; Imada & Nageishi, 1982; Sheppes et al., 2015). Given that executive control of attention is closely linked to emotion regulation (Petersen & Posner, 2012; Posner & Rothbart, 2013; Zelazo & Cunningham, 2007), this aspect of attention may be more closely related to responding to predictable threat.
Second, these differential relationships are supported by structural and functional connectivity studies. For example, locus coeruleus and other noradrenergic nuclei (important for alerting) send strong noradrenergic projections to the BNST (important for unpredictable threat responding; Stamatakis et al., 2014). Animal studies indicate that stress increases norepinephrine levels in the lateral BNST, whereas norepinephrine receptor blockade in this region abolishes anxiety-like behavior following stress (Cecchi et al., 2002). This evidence supports a link between (norepinephrine-mediated) alerting and (BNST-mediated) unpredictable threat sensitivity.
The inferior frontal gyrus (IFG) is a major component of the ventral orienting network. The IFG is important for making go/no-go decisions in ambiguous situations once a certain “set point” of probability is reached. For example, in ambiguously threatening contexts, IFG downregulates activity in the extended amygdala via activation of ventromedial prefrontal cortex (vmPFC),1 but only once the probability of safety reaches a certain threshold (Cha et al., 2016; also see Bach et al., 2009). The level of this “set point” appears to vary between individuals (e.g., anxious individuals require greater certainty that a context is safe before IFG triggers amygdala downregulation, Cha et al., 2016). The involvement of the IFG in both attentional disengaging and sensitivity to ambiguous or unpredictable threat supports a link between these two processes.
The major neural substrates of executive control of attention – the ACC and dlPFC – are reliably associated with emotion regulation (Beauregard et al., 2001; Bush et al., 2000; Ochsner et al., 2002) and show connectivity with the amygdala (Eden et al., 2015; Johansen-Berg et al., 2008; Quaedflieg et al., 2015). However, there is little research on whether these structures have differential connectivity with fear vs. anxiety-related subregions of the amygdala and BNST. Thus, imaging research generally supports a connection between attentional control and defensive responding to threat, but is less informative regarding whether this relationship is specific to responding to predictable vs. unpredictable threat.
Based on this conceptual and neuroanatomical evidence, the present study investigated relationships among individual differences in three aspects of attention (i.e., alerting, orienting, and executive control of attention, as measured by the Attention Network Test - Revised [ANT-R]; Fan et al., 2009) and two aspects of threat responding (i.e., to unpredictable and predictable threat, as measured by startle potentiation during the No Shock-Predictable-Unpredictable [NPU] task; Schmitz & Grillon, 2012). We predicted that greater startle potentiation to unpredictable threat would be associated with faster alerting as well as greater orienting (i.e., faster engaging, slower disengaging, or both). We also hypothesized that greater executive control of attention would be associated with attenuated threat responding, and tentatively predicted that this relationship would be stronger for predictable than for unpredictable threat responding.
As a secondary analysis to evaluate the clinical relevance of our findings, we examined whether defensive responding was associated with self-reported anxiety or intolerance of uncertainty (IU, a transdiagnostic risk factor for anxiety disorders; Carleton, 2012). We also examined whether self-report measures had direct or indirect effects on components of attention.
2. Material and Methods
2.1. Participants
Sample characteristics are presented in Table 1. Participants were 81 undergraduate students who participated to fulfill a course requirement. All participants were right-handed, and none had a history of head injury with greater than 30 s loss of consciousness. Ninety-five participants were initially enrolled. Eight participants were excluded for providing unusable startle data. Six participants whose startle data (n = 4) or attention data (n = 2) fell more than 3 SDs from the mean were also excluded, yielding the final sample of 81. In addition, one participant responded inaccurately to items embedded in self-report questionnaires to check for random responding (e.g., “Please select `very characteristic of me' for this item”), and was therefore excluded from secondary analyses of questionnaire data. Excluded and included participants did not differ on fear- or anxiety-potentiated startle, aspects of attention, or self-report questionnaires. All participants provided informed consent.
Table 1.
Descriptive Statistics for Sample Characteristics, ANT-R, NPU Threat Task, and Questionnaires
| M | SD | Min | Max | Accuracy | |
|---|---|---|---|---|---|
| Sample Characteristics | |||||
|
| |||||
| Age | 19.0 | 1.4 | 18 | 28 | |
| Education (Years) | 11.5 | 0.9 | 12 | 15 | |
| Female (n, %) | 61 | 75.3% | |||
| Race (n, %) | |||||
| African-American | 3 | 3.7% | |||
| Asian | 26 | 32.1% | |||
| Latino | 19 | 23.5% | |||
| White | 29 | 35.8% | |||
| Multiracial | 4 | 4.9% | |||
|
| |||||
| ANT-R | |||||
|
| |||||
| Cue Types (ms) | |||||
| No Cue | 681 | 98 | 487 | 958 | 90.6% |
| Double Cue | 631 | 90 | 479 | 907 | 91.7% |
| Valid Cue | 592 | 86 | 458 | 879 | 92.7% |
| Invalid Cue | 682 | 97 | 511 | 892 | 92.1% |
| Flanker Types (ms) | |||||
| Congruent | 557 | 78 | 442 | 803 | 98.3% |
| Incongruent | 710 | 103 | 527 | 992 | 85.7% |
| Attention Score | |||||
| Alerting | 50 | 27 | −20 | 111 | |
| Engaging | 39 | 22 | −13 | 90 | |
| Disengaging | −51 | 31 | −131 | 19 | |
| Executive Control | −153 | 50 | −304 | −64 | |
|
| |||||
| NPU threat task | |||||
|
| |||||
| Startle Amplitude (μV) | |||||
| No Shock ISI | 33.4 | 29.5 | 5.3 | 148.0 | |
| No Shock CD | 32.7 | 27.5 | 3.6 | 110.1 | |
| Predictable ISI | 32.6 | 28.9 | 4.5 | 158.6 | |
| Predictable CD | 40.7 | 30.3 | 4.1 | 136.3 | |
| Unpredictable ISI | 48.8 | 34.9 | 3.5 | 161.4 | |
| Unpredictable CD | 52.3 | 36.2 | 3.6 | 155.0 | |
| Potentiation Scores | |||||
| Predictable Threat | 7.9 | 13.2 | −29.6 | 48.7 | |
| Unpredictable Threat | 17.5 | 15.7 | −22.7 | 60.2 | |
|
| |||||
| Questionnaires | |||||
|
| |||||
| Trait Anxiety | 41.2 | 10.1 | 23 | 72 | |
| State Anxiety | 37.3 | 12.0 | 20 | 80 | |
| Intolerance of Uncertainty | 28.2 | 8.9 | 14 | 50 | |
Note. ANT-R = Attention Network Test - Revised; NPU = Neutral-predictable-unpredictable.
2.2. Measures and Procedure
After informed consent, participants completed the NPU threat task, the ANT-R, and questionnaires in a randomized counterbalanced order. To minimize effects of fatigue, participants were given a short break after each task.
2.2.1. Attention Network Test - Revised
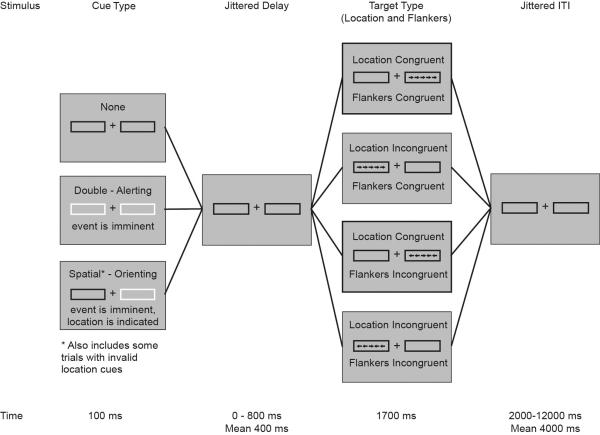
The ANT-R (Fan et al., 2009; Figure 1) is a computerized behavioral task assessing efficiency of alerting, orienting, and executive control of attention. The primary scores generated by the task have shown acceptable retest reliability (Fan et al., 2002). The task was administered using E-Prime 2.0 Professional (Psychology Software Tools, Sharpsburg, PA). Participants were seated approximately 18 inches from a 19-inch computer monitor. Instructions for the ANT-R were presented on the computer, followed by a block of practice trials.
Figure 1.
Schematic of the Attention Network Test - Revised (ANT-R).
The task is described in detail elsewhere (Fan et al., 2009). Briefly, a fixation cross with boxes on each side is displayed continuously throughout each block. During each trial, an arrow pointing right or left appears in either the right or left box for 500 ms, flanked by four additional arrows pointing in the same or opposite direction. Participants are instructed to indicate the direction of the central arrow as quickly and accurately as possible.
The task consists of 288 trials. During 48 trials, the boxes on both sides of the screen flash for 100 ms before the target array appears (double cue), which allows measurement of the degree to which the participant benefits from temporal alerting cues. During another 48 trials, no cues are presented before target onset, which provides a comparison condition for the alerting effect.
During the remaining 192 trials, the box on one side of the screen flashes before the target appears (spatial cue). For 144 of these trials, the box flashes on the same side of the screen as the target it precedes (valid cue), whereas for the other 48 of trials, the box flashes on the opposite side (invalid cue). This allows measurement of (1) the speed with which the participant engages with valid spatial cues and (2) the ease with which the participant is able to disengage from invalid spatial cues.
Finally, in 144 trials, the arrows flanking the central arrow point in the same direction as the central arrow (e.g., > > > > >; congruent), whereas for the other 144 of trials, the flanker arrows point in the opposite direction (e.g., < < > < <; incongruent). This allows measurement of the degree to which the participant can inhibit conflicting information when responding – an indicator of executive control of attention. The task lasts approximately 30 minutes.
Scores for efficiency of alerting, engaging, disengaging, and executive control of attention were computed based on reaction times during each condition as described by Fan and colleagues (2009). Trials for which participants responded inaccurately were excluded. Scores for disengaging and executive control of attention were multiplied by −1 so that higher values indicate more efficient performance for all four aspects of attention.
2.2.2. No Shock-Predictable-Unpredictable (NPU) Threat Task
2.2.2.1. NPU task
All stimuli for the NPU task were administered using PSYLAB (Contact Precision Instruments, London, United Kingdom) and startle data were acquired using NeuroScan 4.4 (Compumedics, Charlotte, NC). The task was modeled after the NPU threat task described by Schmitz and Grillon (2012) with modifications as previously described (Nelson et al., 2014; Sarapas et al., 2014).
Briefly, the task included three conditions – no shock (N), predictable shock (P), and unpredictable shock (U). A 6 s countdown (CD) was displayed five times within each 90 s condition. During the N condition, no shocks were delivered. During the P condition, participants received a 400 ms shock to their left wrist when the countdown reached 1, making the shock completely predictable. During the U condition, shocks could occur at any time, including when the countdown was not on the screen (i.e., during ISIs). During each condition, text was continuously displayed at the bottom of the screen indicating “no shock,” “shock at 1,” or “shock at any time.”
Acoustic startle probes were delivered during countdowns and ISIs across all conditions. Each of the 3 conditions included 16 startle probes (8 each during countdowns and ISIs). Startle response was recorded from two electrodes placed over the orbicularis oculi muscle below the right eye. The NPU task lasted nine minutes. To ensure equality in perceived shock aversiveness, the level of shock intensity was ideographically set for each participant at a level they described as “highly annoying but not painful” (Rollman & Harris, 1987; Grillon et al., 2004). The maximum shock level a participant could achieve was 5 mA. The mean shock level in the present sample was 2.4 mA (SD = 1.5 mA). After the task, participants rated their level of “nervousness/anxiety” during the countdowns and ISIs for each condition on 7-point Likert scales ranging from 1 (Not at all) to 7 (Extremely).
2.2.2.2. Physiological data processing
Startle eyeblink responses were scored according to published guidelines (Blumenthal et al., 2005). After exclusion of missing blinks, each cell included from 3 to 8 blinks (M = 7.3, SD = 0.9). We conducted analyses using both blink magnitude (i.e., including values of 0 for non-response trials) and amplitude (i.e., non-response trials not included). Results presented below are based on amplitude, although results for magnitude were similar. Amplitude- and magnitude-derived potentiation scores were correlated at r = .94 (predictable threat) and r = .97 (unpredictable threat). Predictable threat potentiation was defined as startle amplitude during P countdowns minus amplitude during N countdowns. Unpredictable threat potentiation was defined as average amplitude during U ISIs and countdowns minus average amplitude during N ISIs and countdowns. Analyses examining N and U condition countdowns and ISIs separately, rather than averaging them, yielded comparable results.
2.2.3. Questionnaires
2.2.3.1. State-Trait Anxiety Inventory
The State-Trait Anxiety Inventory (STAI) consists of two 20-item self-report scales assessing the respondent's current level of anxiety (state form) and general propensity to experience anxiety (trait form; Spielberger et al., 1983). Cronbach's alphas for the STAI in the present sample were .94 (state form) and .92 (trait form).
2.2.3.2. Intolerance of Uncertainty Scale-12
The Intolerance of Uncertainty Scale - 12-Item Version (IUS-12) is a self-report measure that assesses the degree to which individuals find uncertainty to be distressing, frustrating, and undesirable (Freeston et al., 1994; Carleton et al., 2007). The 12-item version demonstrates better psychometric properties than the original 27-item scale (Freeston et al., 1994). Intolerance of uncertainty has been associated with a number of anxiety disorders (Carleton, 2012). Cronbach's alpha for intolerance of uncertainty in the present sample was .88.
2.3. Data Analysis
First, we examined whether each task functioned as expected. For the NPU task, we conducted a 3 (Condition: N, P, U) X 2 (Cue: ISI, countdown) repeated measures ANOVA on startle amplitude as well as nervousness/anxiety ratings. For the ANT-R, we conducted separate repeated measures ANOVAs comparing the four pairs of conditions from which scores are derived (i.e., alerting cue vs. no cue; valid cue vs. alerting cue; invalid cue vs. alerting cue; incongruent flankers vs. congruent flankers).
Second, we examined relationships among components of attention and defensive responding by computing correlations of neutral startle, startle potentiation to predictable threat, and startle potentiation during unpredictable threat with alerting, engaging, disengaging, and executive control of attention.
Third, we computed correlations of attention and startle potentiation with self-reported state and trait anxiety and intolerance of uncertainty. We also conducted analyses of indirect effects in 5,000 bootstrap samples to test whether fear or anxiety potentiation mediated effects of attention on these self-report measures. Specific paths tested were based on results of bivariate correlational analyses (i.e., an A to B to C path was tested only if A was correlated with B and B was correlated with C).
Finally, because gender is linked to anxiety (McLean & Anderson, 2009), startle response (Kofler et al., 2001), and attention (Stoet, in press), we tested for gender differences in variables of interest. We also examined whether results differed after covarying for gender and whether the pattern of results was similar within each gender.
3. Results
Descriptive statistics for startle, attention, and questionnaire data are presented in Table 1.
3.1. Task Effects
3.1.1. NPU task
3.1.1.1. Startle amplitude
Main effects for both Condition, F(2, 160) = 63.46, p < .001, , and Cue, F(1, 80) = 17.32, p < .001, , emerged, as well as a Condition by Cue interaction, F(2, 160) = 7.37, p < .001, . As expected, follow-up analyses indicated that startle amplitude during the UISI was higher than during the PISI, F(1, 80) = 59.86, p < .001, , which did not differ from amplitude during the NISI, F < 1. In contrast, amplitude during the UCD was higher than that during the PCD, F(1, 80) = 35.26, p < .001, , and amplitude during the PCD was higher than during the NCD, F(1, 80) = 28.96, p < .001, . In sum, the threat-of-shock task manipulated startle responding as expected: startle was potentiated during the countdown, but not during the ISI during the P condition (when the countdown reliably signaled shock); whereas startle was potentiated during both the countdown and the ISI during the U condition (when shocks could be delivered at any time).
3.1.1.2. Nervousness ratings
A parallel analysis of self-reported nervousness/anxiety during the task also revealed main effects for Condition, F(2, 160) = 207.49, p < .001, , and Cue, F(1, 80) = 9.77, p < .01, , as well as a Condition by Cue interaction, F(2, 160) = 8.99, p < .001, . Follow-up analyses at each level of Cue indicated a pattern of results similar to that found for startle amplitude, with the exception that participants reported more nervousness during the PISI than during the NISI.
3.1.2. ANT-R
Analyses revealed that participants responded more quickly to targets preceded by double cues than targets without cues, F(1, 80) = 287.78, p < .001, . They responded more quickly when targets were preceded by valid spatial cues, F(1, 80) = 252.29, p < .001, , and more slowly when targets were preceded by invalid spatial cues, F(1, 80) = 221.51, p < .001, , compared to trials preceded by double cues. Participants also responded more slowly to targets with incongruent flankers than those with congruent flankers, F(1, 80) = 757.06, p < .001, . The ANT-R therefore manipulated responding as expected, producing alerting, engaging, disengaging, and executive conflict effects in predicted directions.
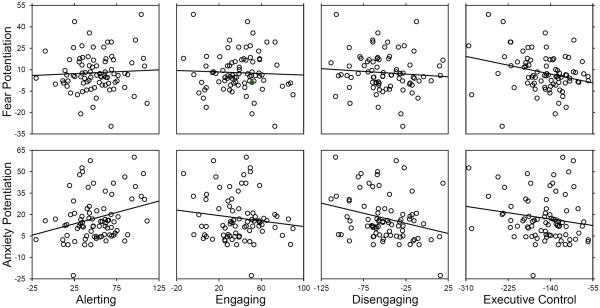
3.2. Relationships Among Attention Networks and Defensive Responding
Relationships among components of attention and threat responding are presented in Table 2 and Figure 2. No aspect of attention was related to startle amplitude during the no shock condition (all ps > .11). Individuals with poorer executive control showed greater startle potentiation during the predictable threat condition, but not the unpredictable threat condition. In contrast, individuals with better alerting and poorer disengaging ability showed greater startle potentiation during the unpredictable, but not predictable, threat condition. Neither fear nor anxiety potentiation were related to attentional engaging.
Table 2.
Correlations of Attention and Questionnaires with Startle Potentiation
| Potentiation to Predictable Threat |
Potentiation to Unpredictable Threat |
|||
|---|---|---|---|---|
| r | 95% CI | r | 95% CI | |
| Attention Score | ||||
| Alerting | .05 | [−.17, .27] | .27 | [.06, .46] |
| Engaging | −.04 | [−.26, .18] | −.13 | [−.34, .09] |
| Disengaging | −.09 | [−.30, .13] | −.28 | [−.47, −.07] |
| Executive control | −.28 | [−.47, −.07] | −.17 | [−.37, .05] |
| Questionnaires | ||||
| Trait Anxiety | .27 | [.06, .46] | .24 | [.02, .43] |
| State Anxiety | .12 | [−.10, .33] | .12 | [−.10, .33] |
| Intolerance of Uncertainty | .15 | [−.07, .36] | .22 | [.005, .42] |
Note: Boldfaced values significant at p < .05.
Figure 2.
Relationships among components of attention and startle potentiation to predictable and unpredictable threat.
3.3. Relationships with Self-Reported Anxiety and Intolerance of Uncertainty
Individuals reporting higher trait anxiety demonstrated greater startle potentiation during both predictable and unpredictable threat (Table 2). Higher intolerance of uncertainty was associated with startle potentiation during unpredictable, but not predictable, threat. State anxiety was not related to startle potentiation.
Components of attention were not directly related to self-report questionnaires (all ps > .10). However, analyses revealed several indirect effects of attention scores on trait anxiety. These included an indirect effect of executive control on trait anxiety via predictable condition startle potentiation, β = .07, 95% CI [.001, .26], and indirect effects of alerting, β = .07, 95% CI [.01, .16], and disengaging, β = .07, 95% CI [.004, .18], on trait anxiety via unpredictable condition startle potentiation. Marginal indirect effects of attention on IU were observed. Specifically, alerting, β = .06, 95% CI [−.003, .15], and disengaging, β = .07, 95% CI [−.0009, .21] had marginal indirect effects on IU via unpredictable condition startle potentiation.
3.4. Gender Differences
Compared to male participants, female participants demonstrated faster engaging, t(79) = 3.26, p < .01, and reported higher levels of trait anxiety, t(78) = 2.65, p < .01. Female participants trended towards greater startle potentiation to unpredictable threat, t(79) = 1.80, p < .10. Nonetheless, relationships among components of attention and startle potentiation were similar within each gender (Supplementary Table 1). Specifically, correlations remained significant or trend-significant, with the single exception of the correlation between disengaging and potentiation to unpredictable threat in the underpowered male subgroup (n = 20). The magnitude of this correlation (r = −.27) was nonetheless identical to that observed in the female subgroup and similar to that observed in the combined sample. Results were also unchanged when gender, years of education, or age were included as covariates.
4. Discussion
Humans are continuously confronted by an array of external and internal stimuli, which are triaged for access to processing streams by a set of brain networks labeled “attention.” The present study investigated how the functioning of these attention networks is related to individual differences in physiological responding to predictable and unpredictable threat. We found that efficiency of the relatively automatic processes of alerting and orienting was related to responding during unpredictable, contextual threat. Specifically, faster responding to alerting cues and slower disengaging from previously attended stimuli were each associated with startle potentiation during unpredictable but not predictable threat. Conversely, poorer executive control of attention was related to startle potentiation during predictable, imminent threat, but not during unpredictable threat. In support of the clinical relevance of these findings, we found that broad trait anxiety was related to responding to both predictable and unpredictable threat, whereas IU (a somewhat more specific construct) was related only to responding to unpredictable threat. We also observed small but significant indirect effects of attention variables on trait anxiety, statistically mediated by either unpredictable threat responding (alerting and disengaging) or predictable threat responding (executive control of attention).
4.1 Supporting and Refining Theories of Attention and Anxiety
Our findings are broadly consistent with extant theories of anxiety and non-emotional attention (Bishop, 2009; Eysenck & Derakshan, 2011; Sylvester et al., 2012). First, each of these theories predicts an inverse relationship between executive control of attention and trait anxiety; we found that attentional control was inversely correlated with responding to predictable threat. Second, our finding that poor disengaging was associated with unpredictable threat responding is consistent with Eysenck and Derakshan's (2011) prediction that trait anxiety is related to difficulty shifting attention. Third, the positive association between alerting and unpredictable threat responding is in line with Sylvester and colleagues' (2012) model, which posits that anxiety is associated with enhanced stimulus-driven attention due to increased functioning of the ventral attention network. Although it should be noted that there are important differences among the three models, the present study was not designed to test these theories against one another.
Each of these theories treats “anxiety” as a single, monothetic construct with static or uniform relations to various aspects of attention. In contrast, we examined relationships of attentional abilities with two separable psychophysiological processes thought to underlie trait anxiety, based on evidence that important differences exist between defensive responding to predictable versus unpredictable aversive events. This distinction may help explain why prior studies have conflicted regarding whether trait anxiety is related to alerting, orienting, or executive control of attention (e.g., Dennis et al., 2008; Pacheco-Unguetti et al., 2011; Tull et al., 2012). Specifically, the present results suggest that all three processes are important, but pertain to different aspects of defensive responding. Because prior studies have relied on self-report measures of broad trait anxiety or negative affect, they have been unable to differentiate these aspects of defensive responding and detect the more fine-grained relationships apparent in the present results. Consistent with these fine-grained relationships, we observed that indirect relationships of several aspects of attention with broad trait anxiety were statistically mediated by different types of threat responding.
Finally, the relationship between faster alerting and sensitivity to unpredictable threat complements a behavioral genetics literature suggesting that some individuals possess a general sensitivity to environmental cues that has both adaptive (e.g., facilitated alerting) and maladaptive (e.g., heightened anxiety) consequences. For instance, Homberg and Lesch (2011) argued that the short allele of the serotonin transporter-linked polymorphic region (5HTTLPR) contributes to a phenotype characterized by heightened sensitivity to environmental cues, resulting in both enhanced attentional vigilance and vulnerability to anxiety. Similarly, the “warrior/worrier” hypothesis (Stein et al., 2006) posits that catechol-O-methyltransferase (COMT) val158met methionine carriers demonstrate superior attention and memory as well as greater risk for anxiety disorders. The present findings provide further evidence for a phenotype characterized by both heightened anxiety and superior attentional vigilance.
4.2 Implications for Understanding Biased Attention to Threat
Together with previous studies of the ANT in anxiety, the present results suggest that anxious individuals may show biased attention to threat not only because threat cues are more salient for them, but also because these individuals detect all salient cues more quickly, and disengage from them more slowly, regardless of their emotional content. The results also complement findings that self-reported attentional control moderates the relationship between anxiety and biased attention to threat (Bardeen & Orcutt, 2011; Derryberry & Reed, 2002).
There is an ongoing debate whether attentional bias in anxiety reflects faster engaging with threat or slower disengaging from threat among anxious individuals (e.g., Armstrong & Olatunji, 2012; Yiend et al., 2010). However, although some dot probe tasks can distinguish effects due to engaging versus disengaging (both aspects of spatial orienting), they generally do not assess effects due to temporal alerting, and this component of attention has rarely been considered in the biased attention literature. The present results support impaired disengaging from salient stimuli among individuals who are more sensitive to potential threat. Results do not indicate facilitated spatial engaging with stimuli, but do support more efficient temporal alerting to salient cues in threat-sensitive individuals. It is possible that apparent findings of facilitated engaging in some dot probe studies may actually reflect facilitated alerting. This possibility may be directly investigated using modifications of traditional dot probe tasks (e.g., Osborne et al., 2013).
4.3 Cognitive and Neural Mechanisms for Attention-Anxiety Relationships
The present pattern of results may reflect the differing cognitive demands of responding to unpredictable, contextual versus predictable, cued threat. Unpredictably threatening contexts require sustained vigilance and the ability to quickly detect and evaluate threat when it unexpectedly appears. Consistent with this, participants with greater trait vigilance for non-emotional stimuli (i.e., faster alerting; longer dwelling on attended stimuli) also showed more reactivity to unpredictable threat. These findings may also reflect greater connectivity of alerting and orienting networks with lateral CeA and BNST than with medial CeA, possibly mediated by the noradrenergic system (Stamatakis et al., 2014; Cecchi et al., 2002) or by structures such as IFG (Cha et al., 2016) or vmPFC (Motzkin et al., 2015).
In contrast, temporally predictable threat does not demand tonic attentional vigilance, but does facilitate effortful preparatory strategies. That is, cues signaling that danger is imminent give individuals greater opportunity to prepare for danger (e.g., by engaging in distraction or emotion regulation; D'Amato & Safarjan, 1979; Imada & Nageishi, 1982; Perkins, 1968; Sheppes et al., 2015). The relationship observed between executive control of attention and predictable threat responding may therefore indicate that individuals with greater capacity for executive control were better able to down-regulate emotional responding (e.g., through effortful attentional redeployment; Gross & Jazaieri, 2014), but only when the predictability of threat stimuli afforded sufficient opportunity to do so.
This hypothesis merits examination in a study including direct measures of emotion regulatory ability, which the present study lacked. Nonetheless, attentional control and emotion regulatory abilities are closely related (Posner & Rothbart, 2013; Zelazo & Cunningham, 2007) and share underlying brain networks (in particular, the ACC and dlPFC; Beauregard et al., 2001; Bush et al., 2000; Ochsner et al., 2002). These regions both show structural (Eden et al., 2015; Johansen-Berg et al., 2008) and functional (Quaedflieg et al., 2015) connectivity with the amygdala. The specificity of the present findings to predictable threat responding may suggest stronger connectivity of ACC and dlPFC with medial CeA than with lateral CeA and BNST. More fine-grained investigation of connectivity among ACC, dlPFC, and limbic system structures, perhaps using animal models or high-resolution fMRI, would provide evidence for or against this hypothesis, and would further elucidate the complex set of neural network interactions underlying emotion experience and regulation.
The present study's correlational design cannot establish the direction of relationships between attention and defensive responding, and effects may operate in either, or both, directions. Of note, functional connectivity among attention networks and the extended amygdala does not imply unidirectional top-down cortical control of fear and anxiety. Indeed, afferent fibers originating in the amygdala and BNST project to ACC and dlPFC (Bracht et al., 2009), locus coeruleus (Dong & Swanson, 2003), and other brainstem neurotransmitter hubs (Bechara, 2005). Thus, extended amygdala activation may directly influence activity of executive control networks, and may indirectly affect cortical regions important for alerting and orienting by modulating noradrenergic tone and other neurotransmitter systems (Bechara, 2005). Likewise, some studies suggest that induced anxiety or worry reduces capacity for executive control of attention (Garner et al., 2012; Philippot & Brutoux, 2008; but see Finucane and Power, 2010). More studies that experimentally manipulate either state anxiety or attention (via training or by manipulating cognitive load) are needed to clarify the causal relationships among these constructs.
4.4 Clinical Implications
There has been growing interest in attention bias modification treatments for mood and anxiety disorders (see review by MacLeod & Mathews, 2012, and meta-analysis by Hallion & Ruscio, 2011), which target relatively automatic processes such as orienting to threat. A smaller literature has instead focused on training effortful cognitive abilities such as cognitive flexibility (Brockmeyer et al., 2014; Elgamal et al., 2007; Meusel et al., 2013; Porter et al., 2013). However, these literatures have so far remained independent. Given the present and previous findings that aspects of threat sensitivity are related to both effortful and automatic attentional processes (e.g., Bishop, et al., 2004; Bishop, 2009), treatments that include training of both types of processes may have synergistic effects and prove more efficacious than interventions focused on either process alone.
4.5 Limitations & Strengths
Several limitations should be considered in interpreting the present results. First, our sample consisted of unselected undergraduates. This sample did yield a sufficient range of individual differences in threat responding and self-reported anxiety to detect significant intercorrelations. Nonetheless, replication in clinical samples is needed to examine whether our findings hold among individuals with higher levels of threat sensitivity. Second, we employed a correlational design and therefore could not establish how components of attention and threat responding are causally related. The present findings may be useful in guiding the design of future experimental studies. Third, our sample was underpowered for analysis of indirect effects (Fritz & MacKinnon, 2007) or to detect significant differences among correlations. Results of both correlational and path analyses require replication in larger samples. The study also had several strengths, including examination of well-validated subcomponents of both threat responding and attention and use of psychophysiological (rather than exclusively self-reported) indicators of threat responding.
5. Conclusions
The present study demonstrated a double dissociation among components of attention and sensitivity to threat. Specifically, better alerting and poorer attentional disengaging were related to responding to unpredictable threat (often labeled “anxiety”), whereas poorer executive control of attention was related to responding to predictable threat (often labeled “fear”). The findings help to integrate models of attention and anxiety, and may be useful in guiding basic and applied research on cognition in anxiety disorders.
Supplementary Material
Highlights.
We examined relationships between non-emotional attention and sensitivity to threat.
Faster temporal alerting and slower spatial disengaging were related to reactivity to unpredictable threat.
Poorer executive control of attention was related to reactivity.
These relationships mediated modest indirect effects of attention on self-reported anxiety.
Acknowledgements
This research was supported by National Institute of Mental Health grants F31 MH100823 to Casey Sarapas and R01MH098093 to Stewart A. Shankman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: 5HTTLPR, serotonin transporter-linked polymorphic region; ACC, anterior cingulate cortex; ANT-R, Attention Network Test – Revised; BNST, bed nucleus of the stria terminalis; CD, countdown; CeA, central amygdala; COMT, catechol-O-methyltransferase; dlPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; ISI, interstimulus interval; IUS, Intolerance of Uncertainty Scale; NPU task, No Shock-Predictable-Unpredictable task; vmPFC, ventromedial prefrontal cortex.
Of note, vmPFC may have stronger functional connectivity with the BNST than with fear-related structures such as the amygdala, hypothalamus, or periaqueductal gray (Motzkin et al., 2015; also see primate study by Fox et al., 2010).
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Olatunj BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clin. Psychol. Rev. 2012;32:704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Zald DH, Olatunji BO. Attentional control in OCD and GAD: Specificity and associations with core cognitive symptoms. Behav. Res. Ther. 2011;49:756–762. doi: 10.1016/j.brat.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Seymour B, Dolan RJ. Neural activity associated with the passive prediction of ambiguity and risk for aversive events. J. Neurosci. 2009;29:1648–1656. doi: 10.1523/JNEUROSCI.4578-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeen JR, Orcutt HK. Attentional control as a moderator of the relationship between posttraumatic stress symptoms and attentional threat bias. J. Anx. Dis. 2011;25:1008–1018. doi: 10.1016/j.janxdis.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Ionescu DF, Vande Voort JL, Slonena EE, Franco-Chaves JA, Zarate CA, Jr, Grillon C. Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J. Affect. Dis. 2014;162:34–38. doi: 10.1016/j.jad.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am. Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bracht T, Tüscher O, Schnell S, Kreher B, Rüsch N, Glauche V, Lieb K, Ebert D, Il'yasov KA, Hennig J, Weiller C, van Elst LT, Saur D. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res. 2009;174:217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Brockmeyer T, Ingenerf K, Walther S, Wild B, Hartmann M, Herzog W, Bents H, Friederich HC. Training cognitive flexibility in patients with anorexia nervosa: A pilot randomized controlled trial of cognitive remediation therapy. Int. J. Eat. Disorder. 2014;47:24–31. doi: 10.1002/eat.22206. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Norton MA, Asmundson GJ. Fearing the unknown: A short version of the Intolerance of Uncertainty Scale. J. Anxiety Disord. 2007;21:105–117. doi: 10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Carleton RN. The intolerance of uncertainty construct in the context of anxiety disorders: Theoretical and practical perspectives. Expert Rev. Neurother. 2012;12:937–947. doi: 10.1586/ern.12.82. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Cha J, DeDora D, Nedic S, Ide J, Greenberg T, Hajcak G, Mujica-Parodi LR. Clinically anxious individuals show disrupted feedback between inferior frontal gyrus and prefrontal-limbic control circuit. J. Neurosci. 2016;36:4708–4718. doi: 10.1523/JNEUROSCI.1092-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson's method. Tutorials Quant. Methods Psychol. 2005;1:42–45. [Google Scholar]
- Daly JA, Vangelisti AL, Lawrence SG. Self-focused attention and public speaking anxiety. Pers. Indiv. Differ. 1989;10:903–913. [Google Scholar]
- D'Amato MR, Safarjan WR. Preference for information about shock duration in rats. Anim. Learn. Behav. 1979;7:89–94. [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs. sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Chen CC, McCandliss BD. Threat-related attentional biases: An analysis of three attention systems. Depress. Anxiety. 2008;25:E1–E10. doi: 10.1002/da.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J. Abnorm. Psychol. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: Implications for cerebral hemisphere regulation of ingestive behaviors. J. Comp. Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Eden AS, Schreiber J, Anwander A, Keuper K, Laeger I, Zwanzger P, Zwitserlood P, Kugel H, Dobel C. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci. 2015;35:6020–6027. doi: 10.1523/JNEUROSCI.3659-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: A proof of principle study. Psychol. Med. 2007;37:1229–1238. doi: 10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N. New perspectives in attentional control theory. Pers. Individ. Dif. 2011;50:955–960. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fan J, Gu X, Guise KG, Liu X, Fossella J, Wang H, Posner MI. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009;70:209–220. doi: 10.1016/j.bandc.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz M, Posner MI. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Finucane AM, Power MJ. The effect of fear on attentional processing in a sample of healthy females. J. Anxiety Dis. 2010;24:42–48. doi: 10.1016/j.janxdis.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeston MH, Rhéaume J, Letarte H, Dugas MJ, Ladouceur R. Why do people worry? Pers. Individ. Dif. 1994;17:791–802. [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol. Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M, Attwood A, Baldwin DS, Munafò MR. Inhalation of 7.5% carbon dioxide increases alerting and orienting attention network function. Psychopharmacology (Berl.) 2012;223:67–73. doi: 10.1007/s00213-012-2690-4. [DOI] [PubMed] [Google Scholar]
- Gentes EL, Ruscio AM. A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive-compulsive disorder. Clin. Psychol. Rev. 2011;31:923–933. doi: 10.1016/j.cpr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Neural anxiety systems: Relevant fault-times to trace and treat disorders. Eur. J. Neurosci. 2000;12:311–311. [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biol. Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol. Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol. Bull. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Imada H, Nageishi Y. The concept of uncertainty in animal experiments using aversive stimulation. Psychol. Bull. 1982;91:573–588. [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb. Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Toward a philosophical structure for psychiatry. Am. J. Psychiatry. 2005;162:433–440. doi: 10.1176/appi.ajp.162.3.433. [DOI] [PubMed] [Google Scholar]
- Kofler M, Müller J, Reggiani L, Valls-Solé J. Influence of gender on auditory startle responses. Brain Res. 2001;921:206–210. doi: 10.1016/s0006-8993(01)03120-1. [DOI] [PubMed] [Google Scholar]
- Lavie N. Selective attention and cognitive control: Dissociating attentional functions through different types of load. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention & Performance (Vol. 18) MIT Press; Cambridge, MA: 2000. pp. 175–194. [Google Scholar]
- Lavie N. Distracted and confused? Selective attention under load. Trends Cogn. Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO. Cognitive neuroscience and depression: Legitimate versus illegitimate reductionism and five challenges. Cogn. Ther. Res. 2007;31:263–272. [Google Scholar]
- MacLeod C, Mathews A. Cognitive bias modification approaches to anxiety. Ann. Rev. Clin. Psychol. 2012;8:189–217. doi: 10.1146/annurev-clinpsy-032511-143052. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, Witte EA, Davidson MC. Arousal systems. Curr. Opin. Neurobiol. 1994;4:166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Masters WH, Johnson VE. Human Sexual Inadequacy. Bantam Books; New York: 1970. [Google Scholar]
- Matsumoto K, Tanaka K. Conflict and cognitive control. Science. 2004;303:969–970. doi: 10.1126/science.1094733. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. NEO inventories for the NEO Personality Inventory-3 (NEO-PI-3), NEO Five-Factor Inventory-3 (NEO-FFI-3), NEO Personality Inventory-Revised (NEO-PI-R): Professional manual. PAR; Lutz, FL: 2010. [Google Scholar]
- McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin. Psychol. Rev. 2009;29:496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: From circumscribed fear to broad distress. Depress. Anxiety. 2012;29:264–281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Wangelin BC, Laplante MC, Bradley MM. Defensive mobilization in specific phobia: Fear specificity, negative affectivity, and diagnostic prominence. Biol. Psychiatry. 2012;72:8–18. doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusel LA, Hall GB, Fougere P, McKinnon MC, MacQueen GM. Neural correlates of cognitive remediation in patients with mood disorders. Psychiatry Res. 2013;214:142–152. doi: 10.1016/j.pscychresns.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable vs. predictable threat. J. Abnorm. Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2015;77:276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J, Tanno Y. Dysfunction of attentional networks for non-emotional processing in negative affect. Cogn. Emot. 2009;23:1090–1105. [Google Scholar]
- Nelson BD, Bishop JR, Sarapas C, Kittles RA, Shankman SA. Asians demonstrate reduced sensitivity to unpredictable threat: A preliminary startle investigation using genetic ancestry in a multiethnic sample. Emotion. 2014;14:615–623. doi: 10.1037/a0035776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? Int. J. Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Gorka SM, Katz AC, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J. Abnorm. Psychol. 2013;122:662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Osborne KJ, Tully LM, Pozuelos B, Lincoln SH, Arnold A, Best C, Brady M, Hook A, Yung M, Rosenkrantz S, Hooker CI. The ANT-Emotion: A novel task for the assessment of attentional control in the context of emotional information. Poster presented at 27th annual meeting of the Society for Research in Psychopathology; Oakland, CA. 2013. [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupianez J. Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychol. Sci. 2010;21:298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Marqués E, Lupiáñez J. Alterations of the attentional networks in patients with anxiety disorders. J. Anxiety Disord. 2011;25:888–895. doi: 10.1016/j.janxdis.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Perkins CC., Jr. The stimulus conditions which follow learned responses. Psychol. Rev. 1955;62:341–348. doi: 10.1037/h0040520. [DOI] [PubMed] [Google Scholar]
- Perkins CC., Jr. An analysis of the concept of reinforcement. Psychol. Rev. 1968;75:155–172. doi: 10.1037/h0025509. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Ann. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot P, Brutoux F. Induced rumination dampens executive processes in dysphoric young adults. J. Behav. Ther. Exp. Psychiatry. 2008;39:219–227. doi: 10.1016/j.jbtep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Bowie CR, Jordan J, Malhi GS. Cognitive remediation as a treatment for major depression: A rationale, review of evidence and recommendations for future research. Aust. N. Z. J. Psychiatry. 2013;47:1165–1175. doi: 10.1177/0004867413502090. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Ann. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Development of attention networks. In: Kar BR, editor. Cognition and Brain Development: Converging Evidence from Various Methodologies. American Psychological Association; Washington: 2013. pp. 61–83. [Google Scholar]
- Quaedflieg CW, van de Ven V, Meyer T, Siep N, Merckelbach H, Smeets T. Temporal dynamics of stress-induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PLoS One. 2015;10:e0124141. doi: 10.1371/journal.pone.0124141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Bradley BP. Attention control: Relationships between self-report and behavioural measures, and symptoms of anxiety and depression. Cogn. Emot. 2013;27:430–440. doi: 10.1080/02699931.2012.715081. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discriminability, and perceived magnitude of painful electrical shock. Percept. Psychophys. 1987;42:257–268. doi: 10.3758/bf03203077. [DOI] [PubMed] [Google Scholar]
- Sarapas C, Bishop JR, Patel SR, Nelson BD, Campbell ML, Shankman SA. Variation in serotonin transporter gene predicts startle response to cued but not contextual threat. Psychophysiology. 2012;49:S95. [Google Scholar]
- Sarapas C, Katz AC, Nelson BD, Campbell ML, Bishop JR, Robison-Andrew EJ, Altman SE, Gorka SM, Shankman SA. Are individual differences in appetitive and defensive motivation related? A psychophysiological examination in two samples. Cogn. Emot. 2014;28:636–655. doi: 10.1080/02699931.2013.848787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat. Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, McGowan SK, Katz AC, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J. Abnorm. Psychol. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Ann. Rev. Clin. Psychol. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb. Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology. 2014;76(Pt B):320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Stoet G. Sex differences in the Simon task help to interpret sex differences in selective attention. Psychol Res. doi: 10.1007/s00426-016-0763-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Maack DJ, Viana AG, Gratz KL. Behavioral inhibition and attentional network functioning. Cogn. Behav. Ther. 2012;41:1–4. doi: 10.1080/16506073.2011.614273. [DOI] [PubMed] [Google Scholar]
- Wine J. Test anxiety and direction of attention. Psychol. Bull. 1971;76:92–104. doi: 10.1037/h0031332. [DOI] [PubMed] [Google Scholar]
- Yiend J. The effects of emotion on attention: A review of attentional processing of emotional information. Cogn. Emotion. 2010;24:3–47. [Google Scholar]
- Zelazo PD, Cunningham W. Executive function: Mechanisms underlying emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. pp. 135–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.