Abstract
Physiological and behavioral evidence supports that dopamine (DA) receptor signaling influences hippocampal function. While several recent studies examined how DA influences CA1 plasticity and learning, there are fewer studies investigating the influence of DA signaling to the dentate gyrus. The dentate gyrus receives convergent cortical input through the perforant path fiber tracts and has been conceptualized to detect novelty in spatial memory tasks. To test whether DA-receptor activity influences novelty-detection, we used a novel object recognition (NOR) task where mice remember previously presented objects as an indication of learning. Although DA innervation arises from other sources and the main DA signaling may be from those sources, our molecular approaches verified that midbrain dopaminergic fibers also sparsely innervate the dentate gyrus. During the NOR task, wild-type mice spent significantly more time investigating novel objects rather than previously observed objects. Dentate granule cells in slices cut from those mice showed an increased AMPA/NMDA-receptor current ratio indicative of potentiated synaptic transmission. Post-training injection of a D1-like receptor antagonist not only effectively blocked the preference for the novel objects, but also prevented the increased AMPA/NMDA ratio. Consistent with that finding, neither NOR learning nor the increase in the AMPA/NMDA ratio were observed in DA-receptor KO mice under the same experimental conditions. The results indicate that DA-receptor signaling contributes to the successful completion of the NOR task and to the associated synaptic plasticity of the dentate gyrus that likely contributes to the learning.
Keywords: dentate gyrus, AMPA/NMDA ratio, memory, learning, CA1
Graphical Abstract
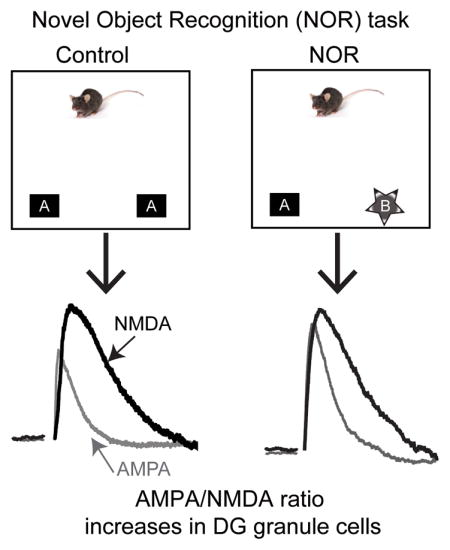
Novel object recognition (NOR) engages the hippocampus as mice remember previously presented objects as an indication of learning. In this study, Yang et al. measured the ratio of AMPA to NMDA currents. When mice were exposed to novel objects (right), the AMPA/NMDA ratio increased in dentate granule neurons. Accurately performing the task and the associated increase of the AMPA/NMDA ratio were dependent upon D1-like receptor activity.
INTRODUCTION
While there is increasing evidence that dopaminergic neurotransmission influences hippocampal learning and memory, many recent behavioral experiments have restricted study to the CA1 region, which is the final node of the trisynaptic loop of the hippocampus (McNamara et al., 2014; Rosen et al., 2015; Broussard et al., 2016). Drugs of abuse, such as nicotine (Tang & Dani, 2009; Zhang et al., 2010) and methylphenidate (Jenson et al., 2015), have been shown to influence synaptic plasticity in the dentate gyrus (DG), the first node in the hippocampal loop. Furthermore, D1 and D5 receptors are prominently expressed in the DG (Mu et al., 2011). Therefore, we set out to test the hypothesis that dopaminergic signaling participates in DG-specific synaptic plasticity associated with the novel object recognition (NOR) task.
The NOR task allows researchers to examine an animal’s behavioral changes in response to a new object introduced into a familiar environment (Bevins & Besheer, 2006; Broadbent et al., 2010; Antunes & Biala, 2012; Cohen & Stackman, 2015). The natural preference for novel objects displayed by an animal suggests that it retains a memory of the familiar objects presented previously in the environment (Ennaceur, 2010). Unlike many other behavioral assays, the NOR task does not require external motivation, reward, or punishment but only requires habituation and training (Antunes & Biala, 2012; Leger et al., 2013). Thus, the NOR task has been routinely applied to evaluate object recognition memory and development of attention processes in rodents and humans (David & Witryol, 1990; Carey et al., 2008; Taglialatela et al., 2009).
The hippocampus is integral for spatial memory (King et al., 2004) and aversive contextual memory (Broussard et al., 2016). Studies specifically designed to test subregions of the hippocampus have indicated that the DG participates in spatial pattern separation (Rolls & Kesner, 2006; Kesner, 2013; Kesner & Rolls, 2015). Some studies report impaired object recognition performance caused by hippocampal damage (Clark et al., 2000; Broadbent et al., 2010; Cohen et al., 2013), and blockade of neurogenesis within the DG was sufficient to block NOR in mice (Jessberger et al., 2009). Altered intracellular zinc signaling spatially restricted to the DG also was sufficient to affect NOR (Tamano et al., 2015). In addition, NOR produces in vivo LTP in mice (Clarke et al., 2010). All of those studies served as a background that inspired us to ask whether successful completion of the NOR task engaged synaptic plasticity that required DA-receptor participation.
Evidence supports that substantial dopaminergic signaling in the hippocampus arises from outside the midbrain ventral tegmental area (VTA) and substantia nigra compacta (SNc) (Smith & Greene, 2012; Walling et al., 2012). Other studies, however, also indicate a sparse direct dopaminergic projection from midbrain DA neurons to the CA1 region of the hippocampus (Gasbarri et al., 1994; Gasbarri et al., 1996; McNamara et al., 2014; Rosen et al., 2015; Broussard et al., 2016). Electrophysiological evidence from freely moving mice and from ex vivo hippocampal slices shows that contextual learning during inhibitory avoidance training directly induces LTP expressed in CA1 pyramidal neurons, but not in DG granule cells. Furthermore, D1-like receptor activation is required for inhibitory avoidance to induce CA1 synaptic plasticity (Broussard et al., 2016). Both D1 and D5 receptors are also important for controlling spike timing-dependent plasticity (STDP) of the medial perforant path synapse onto DG granule cells (Yang & Dani, 2014; Jenson et al., 2015). In addition, D1 and D5 receptors are integral for hippocampal neurogenesis in the DG (Mu et al., 2011). Taken together, DA signaling has broad influence over learning, memory, and synaptic plasticity in the hippocampus, including both the CA1 and the DG. However, it remains unclear whether DA significantly participates during NOR and regulates the associated synaptic plasticity in the hippocampal DG.
Here we wanted to test two hypotheses: (1) that NOR is associated with synaptic plasticity as indicated by the AMPA/NMDA-receptor ratio in the DG, and (2) that DA-receptor activity influences this plasticity and successful completion of the NOR task. First, we verified that DA projections originating in the midbrain contribute to the DG innervation (Broussard et al., 2016). Then, we assessed which subregion of the hippocampus participates in synaptic plasticity associated with the later phases of the NOR task by measuring AMPA/NMDA ratios in both the DG and CA1 at 3 h after NOR testing. The next aim was to determine whether D1-like receptors are important for the NOR task using either a D1-like receptor antagonist, SCH 23390 (SCH, 0.05 mg/kg i.p.), or D1- or D5-receptor knockout (KO) mice.
After finding sparse GFP-labeled DA projections directly innervating the DG, we found that the NOR task is associated with an increased AMPA/NMDA ratio measured in the DG of hippocampal slices only in wild-type mice, but not in D1- or D5-KO mice. D1-like receptor inhibition not only blocked successful completion of the NOR task but also prevented the increase in the AMPA/NMDA ratio. Our findings indicate that dopaminergic signaling has a significant role in regulating NOR behavior and in the associated DG synaptic plasticity.
MATERIALS AND METHODS
Dopamine transporter fluorescent labeling
Dopamine transporters (DAT) have been shown to be located on DA fibers and terminals (Shimada et al., 1991; Shimada et al., 1992; Nirenberg et al., 1996). To examine whether there is a direct midbrain dopaminergic projection to the hippocampus, we injected a recombinant adeno-associated virus (Grimm et al., 2008; Tsai et al., 2009) AAV-EF1a-DIO-synaptophysin:GFP containing a double-floxed inverted open reading frame encoding synaptophysin-GFP into the midbrain DA area of adult DATires-cre/+ knock-in (n = 5) and DAT+/+ (WT, n = 3) mice (Fig. 1A). We bilaterally injected AAV-EF1α-DIO synaptophysin-GFP (1 μl) into the VTA (± 3.64 A/P, ± 0.5 M/L, ± 4.0 D/V) of DATcre/+ or WT C57BL/6J mice (Backman et al., 2006). This AAV vector facilitates the expression of GFP specifically in the synaptic terminals of neurons containing DAT, exclusively in Cre-expressing cells. Because synaptophysin is a synaptic vesicle protein, this procedure concentrated the GFP fluorophore into DA terminals of neurons from the ventral tegmental area (VTA) and the substantia nigra (SN). Control mice were littermates of the mutants. The production of the synaptophysin vector was described previously (Broussard et al., 2016). Briefly, synaptophysin-GFP was excised using NotI and BsrGI and shuttled into a custom chloramphenicol resistant shuttle plasmid that contained the MCS SpeI-NotI-BsrGI-ScaI-XhoI-SpeI. From there, synaptophysin-GFP was excised using SpeI and cloned into the pAAV-flex vector. Clones were verified for flex orientation by restriction digest and transfection (Arenkiel & Ehlers, 2009; Arenkiel, 2011). After two weeks we transcardially perfused the mice, fixed the brains, and cut slices (40 μm). Sections were analyzed in 2-μm steps on a confocal microscope and images from the DG and CA1 were analyzed for green fluorescence using ImageJ (http://rsbweb.nih.gov/ij/).
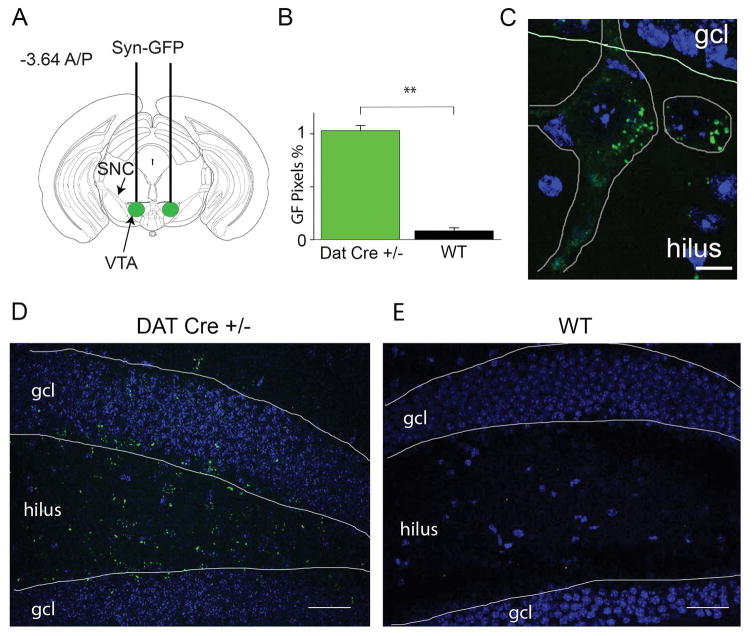
Figure 1. Midbrain DAT-containing neurons project to the DG.
(A) We bilaterally injected AAV-EF1a-DIO-synaptophysin:GFP virus into the midbrain of DAT-cre (i.e., putative DA neurons) and wild-type mice as a control. The coordinates were −3.64 A/P, 0.5 M/L, and 4.0 D/V. (B) Bar graph representing the quantification of the number of green pixels in the DG from DATcre +/− mice (green) compared to WT littermates (black) with p = 0.001. (C) A confocal image (40X) taken from the DG of a DATcre +/− mouse illustrating the synaptic contact from DAT neurons on two hilar neurons outlined in gray. The larger multipolar neuron has about 12 contacts, and the smaller neuron has 6. Blue and green images are from four 2-μm sweeps that were combined at max projection. Scale bar = 10 μm. (D, E) Confocal images (20x) taken from the DG of a DATcre +/− mouse (left, D) or from a wild-type (right, E) mouse injected with synaptophysin-GFP virus. The light blue is DAPI (Vector laboratories, Burlingame CA), and green fluorescence indicates the reporter from synaptophysin-GFP, which targets the putative DA axonal terminals. The white horizontal scale bars represent 50 μm. The green images were combined at max projection and blue images taken from a single confocal plane. gcl = granule cell layer.
Novel object recognition (NOR) task
The Institutional Animal Care and Use Committee at Baylor College of Medicine or University of Pennsylvania approved all of the protocols. Male wild-type C57BL/6J (Jackson Laboratory, Bar Harbor, Maine) or DA D1- or D5-receptor KO mice 2–3 months old had free access to food. Experiments were conducted in the dark, active cycle. A 25-cm cube with no top was covered in black contact paper on the sides and white paper on the bottom. Two 2x2 Lego brackets held the Lego objects for training and testing. Mice were habituated to the empty training box for 20 min/d for 4 d before training (Fig. 2A). Following each habituation to the box, mice were injected with small volumes of saline. In the training phase of this task, a mouse was placed in a box for five minutes with two identical objects stuck to the floor (e.g., two Lego blocks that look like steering wheels, the “A A” condition, Fig. 2B). The training box was cleaned with 70% ethanol between each mouse to dissipate the odors. After a 24 h interval, we tested the mice by placing them back in the same box for 5 min with the familiar object (“A”, e.g., steering wheel) and a new object (“B”, e.g., a Lego block with roses, the “A B” condition, Fig. 2D, top). As a control, a separate group of mice was presented the same objects from the training session (“A A”, Fig. 2C, top). In separate experiments, we injected animals with a low dose of the D1-like receptor antagonist, SCH 23390, after training to test whether DA-receptor signaling contributes to the successful completion of the NOR task (Broussard et al., 2016).
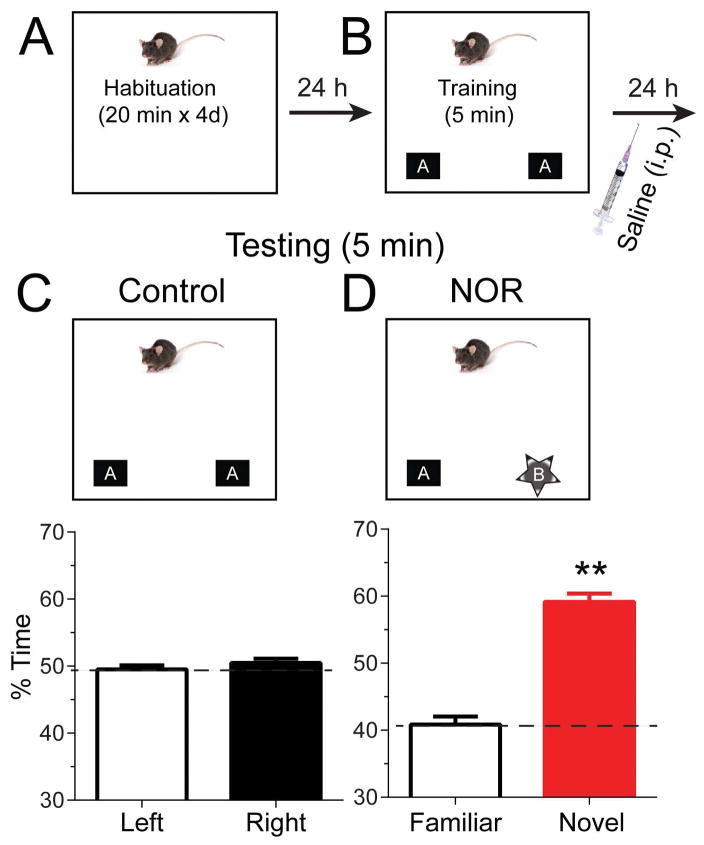
Figure 2. Novel object recognition (NOR) task in wild-type mice.
The experimental protocol is illustrated. (A) Mice were placed in an open field procedural arena for 20 min daily for 4 days (Habituation). (B) 24-hours after habituation, the animals were exposed to two identical objects for 5 min in the familiar arena (Training). Immediately afterward, they were injected (i.p.) with saline. (C, top) The next day, one group of mice was placed in the same familiar field with the same familiar objects (Control). (C, bottom) The percentage of time spent exploring two identical objects was similar and not statistically different. (D, top) The second group of mice was placed in the same familiar field with one familiar object and one novel object. (D, bottom) The percentage of time spent exploring the novel object (red bar) was significantly greater than the time spent exploring the familiar object (white bar).
Simple webcams captured the interactions with the objects, and independent investigators blind to the circumstances scored the videos. For the videos for each animal, a card with the subject number was presented before the mouse was placed to act as a watermark. A mouse was scored as interacting with an object when its nose was in contact with the object or was directed toward the object within 2 cm. Moving past or resting on or over the object was not considered interaction (Bevins & Besheer, 2006). Time spent with objects during training (A A) was recorded to assess any left or right biases of the mice, and during testing a discrimination ratio was calculated (novel object interaction/total interaction with both objects). A discrimination ratio above 0.5 (i.e., 50%) indicates greater preference for the novel object.
For measurement of the AMPA/NMDA ratio associated with NOR, whole cell patch-clamp recordings were made from ex vivo hippocampal slices prepared from either NOR tested or littermate controls. The experimenter was blind to the behavioral condition of the mouse at the time of the electrophysiology recordings.
Hippocampal slice preparation
Hippocampal slices were prepared from mice 1.5 h after testing with the novel object. To obtain slices, all the mice were deeply anesthetized with an over-dose of ketamine and xylazine (4:1) and were transcardially perfused (Yang et al., 2011; Broussard et al., 2016) with ice-cold oxygenated sucrose based low-calcium, high-magnesium artificial cerebrospinal fluid (ACSF, in mM): 250 sucrose, 11 glucose, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2. After decapitation, the brain was rapidly dissected and sliced horizontally at a thickness of 220 μm in the same solution using a vibratome (Leica VT 1000S). Horizontal hippocampal slices were kept in low-calcium, high-magnesium ACSF (in mM): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 7 MgSO4, 0.5 CaCl2, and 25 glucose saturated with 95% O2 and 5% CO2 at 32 °C for 20 min and then at room temperature for at least 1 h until recording.
Electrophysiology
The slices were placed in a recording chamber and were continuously bathed (1–2 ml/min) in oxygenated ACSF (in mM): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 25 D-(+)-glucose, maintained at 32–34°C via an inline heater system (TC-324B, Warner Instrument Corp, Hamden, CT). Picrotoxin (100 μM, Sigma-Aldrich), a noncompetitive GABAA receptor antagonist, was routinely included in the ACSF. Recording electrodes (2–3 MΩ) were pulled from borosilicate glass capillaries (TW 150-4, WPI) using a Narishige PC-10 micropipette puller and were filled with a cesium-based intracellular solution (in mM): 130 cesium methanesulfonate, 10 KCl, 10 HEPES, 1 BAPTA, 3 Na2ATP, 10 TEA-Cl, 1 GTP, 2 MgSO4, pH 7.3 with CsOH. After the pipette formed a gigaseal (> 2 GΩ), a gentle suction was briefly applied to achieve a whole-cell patch-clamp recording configuration (access resistance < 10 MΩ). We recorded AMPA and NMDA receptor mediated excitatory postsynaptic currents (EPSCs) under voltage clamp conditions (+40 mV, to enable NMDA-receptor currents) from either DG granule cells or CA1 pyramidal neurons. We stimulated using a bipolar electrode (Stereotrode Tungsten, WPI, Inc., Sarasota, FL) placed 100–150 μm away from the recording electrode within the medial perforant pathway or the Schaffer collateral pathway. Input-output curves were first generated by various intensity of stimulation pulses with a duration of 0.1 ms and approximately 50–60% of maximal peak currents were used for current measurements. Both the NOR tested and control slices and recording were handled in the same way.
To obtain pure AMPA receptor-mediated EPSCs, the competitive NMDA receptor antagonist, DL-APV (100 μM, Sigma-Aldrich), was bath applied to inhibit NMDA receptor-mediated EPSCs right after a stable 10-sweep (at 0.1 Hz) baseline was recorded. Pure NMDA receptor-mediated EPSCs were digitally defined by subtraction with the aid of the Clampfit 10.4 (Molecular Devices, LLC). Electrophysiological signals were acquired using Axopatch 200B (Axon Instruments) via a 1322A digitizer (Axon Instruments). All signals were averaged from 10 EPSCs evoked by repetitive stimuli generated by a stimulus isolator (A365, WPI, Inc, Sarasota, FL) at 0.1 Hz (Broussard et al., 2016). For all recordings made in the CA1 area, connections between CA3 and CA1 were cut (see didactic white “cut”, Fig. 3C) prior to bath application of 100-μM picrotoxin to prevent the spread of recurrent electrical activity into the CA1 (Kehoe et al., 2014). The electrode access resistance (Ra) was monitored before and after recordings. The recordings were not used if the Ra changed by 30% or more. Investigators blind to the behavioral conditions did all the electrophysiological recordings.
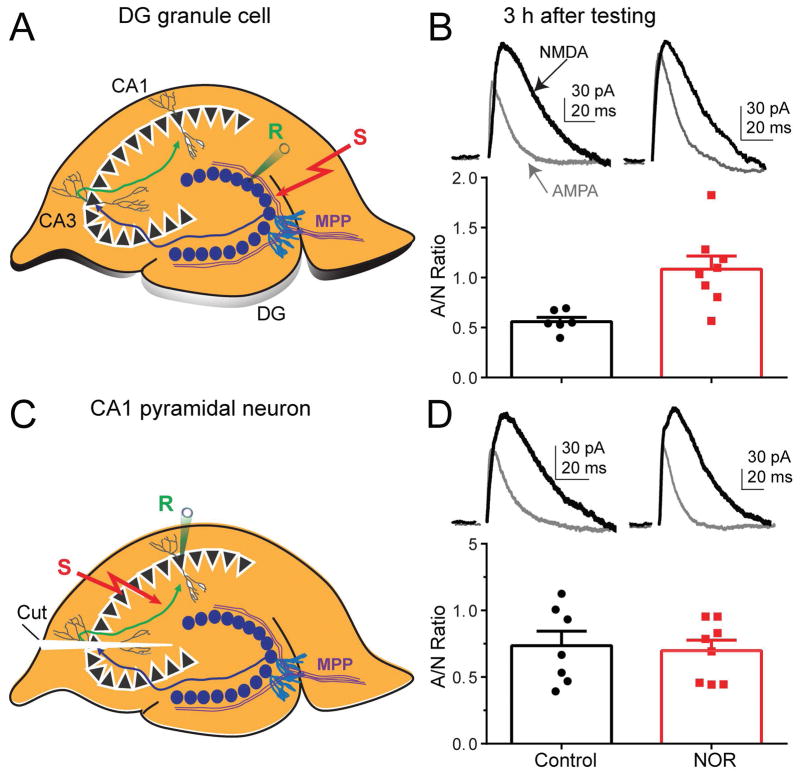
Figure 3. NOR test increased the AMPA/NMDA ratio in DG granule cells.
(A) Diagram illustrating a whole-cell recording from a DG granule cell in the hippocampal slice. We placed the patch-clamp recording electrode (R) onto a granule cell, and the stimulating electrode (S) into the medial perforant path (MPP). (B, top) Representative traces of AMPA-receptor (gray traces) and NMDA-receptor (black traces) mediated whole-cell currents recorded from granule cells obtained from wild-type mice 3 h after NOR testing. (B, bottom) The tabulated individual plasticity values and averaged AMPA/NMDA ratios from recorded granule cells in controls (open black bar) or from mice that explored a novel object (open red bar). (C) Diagram illustrating a whole-cell recording from a CA1 pyramidal neuron in the hippocampal slice. We placed the patch-clamp recording electrode (R) onto a pyramidal neuron and the stimulating electrode (S) into the Schaffer collateral path. The white triangle (labelled, Cut) indicates that connections between CA3 and CA1 were cut to prevent the spread of recurrent electrical activity into the CA1. (D, top) Representative traces of AMPA-receptor (gray traces) and NMDA-receptor (black traces) mediated whole-cell currents recorded from CA1 pyramidal neurons obtained from wild-type mice 3 h after NOR testing. (D, bottom) The tabulated individual plasticity values and averaged AMPA/NMDA ratios from recorded pyramidal neurons in controls (open black bar) or from mice that explored a novel object (open red bar).
Data analysis
We analyzed the data using Microsoft Excel and GraphPad Prism 6. All the data were expressed as mean ± SEM. Behavioral data were expressed as % of total exploration time for each object and analyzed by using a one-sample Student t test (α = 0.05) by comparing the group’s means with the fixed value of 50%, which represents no change in exploration between objects. Comparisons between differences in means were assessed by paired/unpaired Student’s t test with the criterion significance set at α = 0.05.
RESULTS
Evidence for sparse innervation of the dentate gyrus by midbrain dopamine neurons
The AAV-EF1a-DIO-synaptophysin:GFP vector facilitates the expression of GFP specifically in the synaptic terminals of neurons containing DAT, exclusively in Cre-expressing cells. Thus, when injected into the midbrain of DATires-cre/+ mice, this procedure labeled with GFP DA terminals from neurons of the ventral tegmental area (VTA) and the substantia nigra (SN), as shown previously (Broussard et al., 2016). Sparse GFP positive terminals were found throughout the DG region of DATires-cre/+ mice in significantly greater proportion than WT (Fig. 1B): 1.06% ± 0.03, n = 5 for DATires-cre/+; 0.08% ± 0.004, n = 3 for WT; p = 0.001, with the greatest representation in the hilus region (green, Fig. 1C, D, compared to Fig. 1E).
Novel object recognition (NOR) task
For behavior studies, wild-type mice were habituated for 20 min/d for 4 d to the training box (Fig. 2A). Then we trained the mice with 2 identical objects under the “A A” condition (Fig. 2B). The littermate mice were then randomly separated into 2 groups. One group was tested the next day under the “A A” condition with the same identical objects (Fig. 2C Control, top). These mice showed no significant difference for the percent of time spent exploring the two identical objects (Fig. 2C, bottom): right object, 50.5 ± 0.6%; left object, 49.5 ± 0.6%; p = 0.4, right vs left, n = 221. The other group of mice were tested under the “A B” condition, where one original object was replaced by a novel object (Fig. 2D NOR, top). The mice spent more time exploring the novel object (Fig. 2D, bottom): familiar object, 40.8 ± 1.2%; novel object, 59.2 ± 1.2%; with one-sample t test = t(116) = 7.6, p < 0.0001, novel vs familiar, n = 117. Consistent with previously published reports (Clark et al., 2000; Bevins et al., 2002; Mumby et al., 2002; Bevins & Besheer, 2006; Fernandez et al., 2008), the results indicate that wild-type mice exhibit a preference for the novel object.
NOR increased the AMPA/NMDA ratio in the dentate gyrus
Because the lateral entorhinal cortex projects directly to the DG and this circuit is critical for NOR memory (Kinnavane et al., 2015), we tested whether synaptic potentiation, which increases the AMPA/NMDA-receptor current ratio (Ungless et al., 2001), occurred in ex vivo hippocampal slices following NOR testing with the novel object. In order to determine which subregion of the hippocampus contributes to the synaptic plasticity underlying the final NOR task, both DG granule cells (Fig. 3A) and CA1 pyramidal neurons (Fig. 3C) were recorded 3 h after testing either with the novel object (test group) or with the same, familiar objects in littermates (control group). We found that after NOR testing (3 h) the AMPA/NMDA ratio increased in DG granule cells (Fig. 3B, red data): 0.56 ± 0.04 in control vs 1.08 ± 0.13 after NOR, p = 0.003, n = 6, 8. After NOR testing (3 h) the ratio did not change in CA1 pyramidal cells (Fig. 3D): 0.74 ± 0.11 in littermate control vs 0.69 ± 0.08 after NOR, p = 0.78, n = 7, 8. These findings support an association between successful NOR testing and increases in the AMPA/NMDA ratio in the DG (3 h after testing).
D1-like DA receptors required for NOR performance and synaptic plasticity
It was previously demonstrated that D1-like DA receptors facilitate synaptic plasticity at the medial perforant path to DG granule cell synapses owing to an addictive drug (Tang & Dani, 2009; Zhang et al., 2010; Jenson et al., 2015), and also enhance aversive contextual memory and plasticity in the CA3-CA1 synapses (Broussard et al., 2016). We asked whether D1/D5 receptors may have a role in regulating successful NOR performance and the associated increase in the AMPA/NMDA ratio. First, we injected wild-type mice with a low dose of the D1-like receptor inhibitor, SCH (0.05 mg/kg, i.p.), immediately after training to examine DA’s effect on memory of the original objects. Mice injected with SCH did not visit the novel object more (Fig 4A): 50.4 ± 3.6% with the novel object, 49.6 ± 3.6% with the familiar object; t(9) = 0.09, p = 0.46, n = 10. Littermates trained in NOR but receiving saline, a subset of the control group, interacted with the novel object 62 ± 3% of the time, t(10) = 4.02, p <0.05, n = 11.
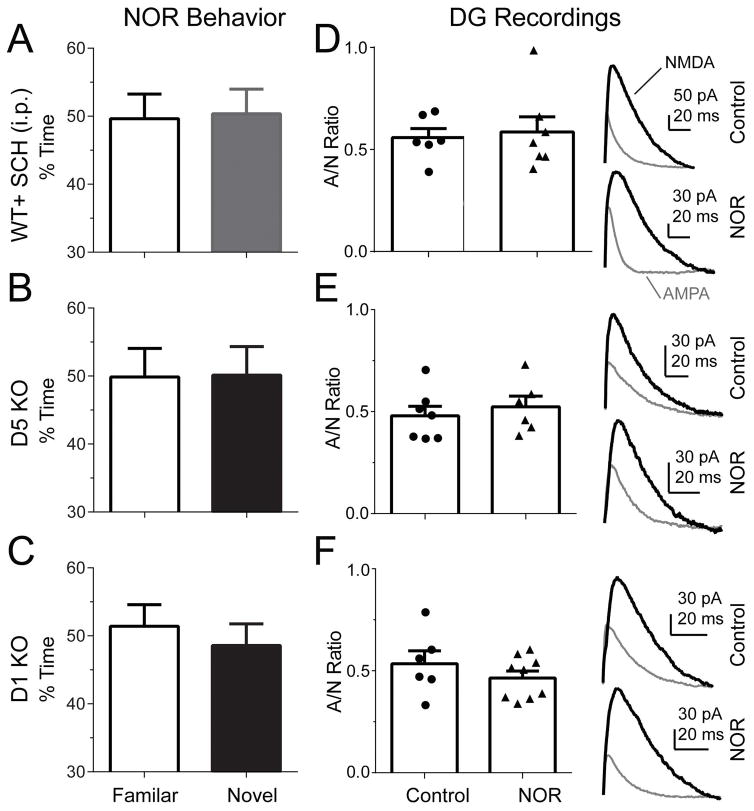
Figure 4. D1-like receptor inhibition or D1 and D5 KO on performance in the NOR test and AMPA/NMDA ratio in the dentate gyrus.
The percentage of time spent exploring one familiar object (open bars) and a novel object (solid bars) in wild-type mice injected with the D1-like receptor antagonist, SCH, (A); or D5-receptor KO mice (B); or D1-receptor KO mice (C). The tabulated individual plasticity values and averaged AMPA/NMDA ratios (left panel) and representative traces of AMPA-receptor (gray traces) and NMDA-receptor (black traces) mediated whole-cell currents (right panel) recorded in granule neurons from mice injected with the D1-like receptor antagonist, SCH, (D); or from D5-receptor KO mice (E); or from D1-receptor KO mice (F). The hippocampal slices were obtained from mice 1.5 h after NOR testing, and the currents were measured about 3 h after testing. The number of mice or slices and the statistics are given in the text. The baselines for the currents (right panel) were left off to conserve space.
Using D1- or D5-receptor KO mice, we examined the participation of these individual DA receptor subtypes. Consistent with the results from systematic administration of SCH, both D5 KO (Fig. 4B) and D1 KO (Fig. 4C) prevented novel objection recognition. The mice spent nearly equal times with both the familiar and the novel objects: D5 KO, 49.9 ± 4.2% vs 50.1 ± 4.1%, t(8) = −0.44, p = 0.66, n = 9 (Fig. 4B); D1 KO, 51.4 ± 3.2% vs 48.6 ± 3.2%, t(8) = −0.26, p = 0.60, n = 9 (Fig. 4C). These data indicate that both D1 and D5 DA receptors influence NOR performance in mice. Like the WT mice, the DA-receptor heterozygous littermates explored novel objects more commonly: D1 heterozygous, 58 ± 3%, t(9) = 3.56, p < 0.05, n = 10; and D5 heterozygous, 60 ± 2%, t(10) = 3.78, p < 0.05, n = 11.
Preventing D1-like receptor activity throughout the brain with DA-receptor KO or systemic DA-receptor inhibition, prevents normal NOR behavior. Therefore, we examined whether the NOR associated DG synaptic plasticity also required D1-like receptor activity. When wild-type mice were injected with SCH (0.05 mg/kg, i.p.), then there was no significant change in the AMPA/NMDA ratio 3 h after the NOR test compared to the control condition with both familiar objects (Fig 4D): 0.52 ± 0.02 in control vs 0.59 ± 0.08 after SCH; p = 0.49, n = 6, 7. Also using the D5- or D1-receptor KO mice, we measured the AMPA/NMDA ratios 3 h after the NOR test: 0.48 ± 0.04 in control vs 0.52 ± 0.05 in D5 KO; p = 0.53, n = 7, 6 (Fig. 4E); and 0.53 ± 0.06 in control vs 0.46 ± 0.03 in D1 KO; p = 0.31, n = 6, 9 (Fig. 4F). These electrophysiological data support that D1 and D5 DA receptor activity is necessary for the synaptic plasticity (as indicated by the AMPA/NMDA ratio) associated with the NOR memory task.
DISCUSSION
The dopamine transporter (DAT) is a marker for most DA neurons (Shimada et al., 1991; Shimada et al., 1992; Nirenberg et al., 1996). Fluorescent markers associated with DAT indicated sparse dopaminergic terminals arising from the midbrain ventral tegmental area and substantia nigra innervate the dorsal DG (Fig. 1). After establishing a consistent, stable protocol for NOR (Fig. 2), the presentation of novel objects in the testing phase was associated with an increased AMPA/NMDA ratio in the DG, but not in the CA1, 3 h after NOR testing (Fig. 3). A low systemic dose of a D1-like receptor antagonist post-training decreased the exploration time of novel objects and prevented the subsequent hippocampal plasticity, an effect that was replicated in both D1 and D5 KO mice (Fig. 4). In all 3 of these cases, the DA receptors were affected throughout the brain. Taken together, these results suggest that D1-like receptor activity in the brain contributes to DG synaptic plasticity associated with the NOR task. While the synaptic plasticity was recorded specifically in the DG, the treatments with SCH or with D1- and D5-receptor KO mice acted throughout the brain, and it should be anticipated DA has related roles beyond the hippocampus (Kudolo et al., 2010).
Anatomical evidence for DA innervation in the dentate gyrus
DA signaling to the hippocampus can arise from sources outside the midbrain VTA/SNc. There is known noradrenergic innervation to the hippocampus, and much of the DA receptor activity may arise from neurons originating in the locus coerulus (Smith & Greene, 2012; Walling et al., 2012). Our results, however, also support a direct projection from midbrain VTA/SNc DA neurons to the dentate gyrus as indicated by synaptophysin-GFP staining of DAT-expressing neurons (Fig. 1). Direct DA projections were particularly prominent in the hilus. The hilus is comprised of many cell types, but the most common ones are excitatory mossy cells and HIPP interneurons (Andersen et al., 2006), the latter providing feed-forward and feedback inhibition onto dentate granule cells (Zhang et al., 2010). Nicotinic enhancement of LTP in the perforant path to DG circuitry was dependent upon D1-like receptor activity (Tang & Dani, 2009; Zhang et al., 2010). However, D1-like receptor activation in the nucleus accumbens has also been shown to influence plasticity in the dentate (Kudolo et al., 2010), and likely D1-like receptor activation in many locations contributes to hippocampal-dependent memory.
Modulatory influence of D1 and D5 receptors on NOR-related memory and synaptic plasticity in the hippocampus
In the present study, testing in the NOR task increased the AMPA/NMDA ratio in the primary excitatory cells in the DG, but not the CA1 when examined 3 h after testing. Other forms of spatial novelty have enhanced tetanus-induced plasticity in the DG (Straube et al., 2003). The lack of NOR-associated plasticity in the CA1 (at > 1.5 h) seems to contrast with a previous study that measured in vivo fEPSP responses from the CA1 (Clarke et al., 2010). In that study, NOR testing immediately increased the slope of the fEPSP, then decreased the fEPSP at 1.5 h, followed by no significant change at 3 h. In our studies, we sacrificed mice at 1.5 h, and began recording around 3 h after NOR testing. Thus, the lack of NOR-associated plasticity in the CA1 in our study very likely reflects the time window we used for the measurement. Given our time window, our results are consistent with the earlier in vivo study that indicated no NOR-associated CA1 plasticity at the 3h time point (Clarke et al., 2010).
DA has a role in learning, memory, and synaptic plasticity via activation of D1/D5 receptors expressed in the hippocampus (Tang & Dani, 2009; Sarinana et al., 2014; Jenson et al., 2015; Broussard et al., 2016). Systemic administration of the D1-like receptor antagonist, SCH, effectively inhibited the preference for the novel object in the NOR task in wild-type mice (Fig. 4A). This finding is consistent with a previous study showing SCH, but not the D2/D3 receptor antagonist, eticlopride, blocks novelty-induced place preference in rats (Besheer et al., 1999). The systemic SCH injection given after training prevented increases in the AMPA/NMDA ratio associated with NOR 24 h later, indicating a role for D1-like receptors. (Fig. 4D). To better distinguish the roles of D1-receptor and D5-receptor subunits in novel object recognition and the associated synaptic plasticity, D1 KO and D5 KO mice were used in this study. Both D1 KO and D5 KO mice failed to show preference for the novel object (Fig. 4B, C). Furthermore, no significant changes in the hippocampal DG AMPA/NMDA ratios were detected following NOR testing (Fig. 4E, F). Therefore, both D1- and D5-receptor activation in the brain was required for novelty detection. Both D1 and D5 receptors can be found in the DG (Mu et al., 2011; Sarinana et al., 2014). We recently demonstrated that D1 and D5 KO mice had similar fEPSP responses in the DG to stimulation of the perforant path, and that spike timing-dependent protocols that produce hippocampal LTP required both D1 and D5 receptors (Yang & Dani, 2014). In previous experiments, D1 KO mice have normal basal synaptic transmission in the hippocampus, but have impaired E-LTP (Granado et al., 2008).
A key limitation to our studies is that our manipulations (systemic injections, whole brain KO mice) fail to target the function of D1 or D5 receptor neurotransmission specifically in the DG. An approach that could be used to solve this problem is to use the POMC-Cre mouse line (McHugh et al., 2007; Sarinana et al., 2014) that selectively targets the DG. Then, DA signaling could be optogenetically (Liu et al., 2014) or chemogenetically (Zhao et al., 2016) manipulated at precise times to determine whether DA influences consolidation of learning pairs or influences the recognition of novel objects. Recent experiments with D1/D5 receptors selectively deleted in DG granule cells demonstrate that basal synaptic transmission is intact in the DG, but late phase LTP was not (Sarinana et al., 2014).
There is ample evidence in the literature that D1-like receptors throughout the brain respond to novelty. Using in vivo microdialysis, previous studies reported that novel stimulation increases DA efflux in the medial prefrontal cortex (Feenstra & Botterblom, 1996), the nucleus accumbus shell (De Leonibus et al., 2006), and the insular cortex (Guzman-Ramos et al., 2012), suggesting broad DA influence. It also was found that rats receiving an injection (i.p.) of the specific D1-like receptor agonist, SKF38393, immediately after training dose–dependently produced a long-term enhancement of NOR memory measured at 24 and 72 h after training (de Lima et al., 2011). Post-training administration of the nonselective DA receptor agonist, apomorphine, in combination with the D2 receptor antagonist, raclopride, also increased novel object exploration time, suggesting that selective activation of D1-like receptors enhances NOR memory (de Lima et al., 2011). Thus, the activation of D1-like receptors (possibly at numerous locations) either by novelty-induced increases in extracellular DA levels or by systematic administration of D1-like receptor agonists can be proposed as a contributor to NOR memory consolidation. Associated with the NOR task is the plastic neuronal changes in the hippocampal DG.
In conclusion, D1-like receptor activity is necessary for successful completion of the NOR task and is necessary for the associated DG plasticity in mice. Synaptic plasticity, as indicated by an increased AMPA/NMDA ratio, is associated with NOR performance. These findings support the hypothesis that the processing of the overall NOR task engages the DG to remember the original objects in the presence of the novel objects (Antunes & Biala, 2012), and D1-like receptors participate in this process.
Acknowledgments
This work was supported by grants from the National Institutes of Health: NS21229 and DA09411 to JAD. We thank Dr. David R. Sibley for providing D1/D5 knockout mice.
Footnotes
Authors Contributions: Kechun Yang did the ex vivo electrophysiology, and he wrote the first draft and edited the manuscript. John I. Broussard did the anatomy experiments, and he wrote the first draft and edited the manuscript. Amber T. Levine did the behavioral experiments. Daniel Jenson assisted and cooperated with the experiments. Benjamin Arenkiel provided probes and advised for the anatomy experiments. John A. Dani conceived the experiments, directed the work, and wrote the final manuscript.
Competing Interests: The authors do not have conflicts of interest.
References
- Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book 2006 [Google Scholar]
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR. Genetic approaches to reveal the connectivity of adult-born neurons. Frontiers in neuroscience. 2011;5:48. doi: 10.3389/fnins.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Ehlers MD. Molecular genetics and imaging technologies for circuit-based neuroanatomy. Nature. 2009;461:900–907. doi: 10.1038/nature08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Besheer J, Jensen HC, Bevins RA. Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behav Brain Res. 1999;103:35–44. doi: 10.1016/s0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129:41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI, Yang K, Levine AT, Tsetsenis T, Jenson D, Cao F, Garcia I, Arenkiel BR, Zhou FM, De Biasi M, Dani JA. Dopamine Regulates Aversive Contextual Learning and Associated In Vivo Synaptic Plasticity in the Hippocampus. Cell Rep. 2016;14:1930–1939. doi: 10.1016/j.celrep.2016.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN, Shanahan AB. Cocaine effects on behavioral responding to a novel object placed in a familiar environment. Pharmacol Biochem Behav. 2008;88:265–271. doi: 10.1016/j.pbb.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DB, Witryol SL. Gender as a moderator variable in the relationship between an intrinsic motivation scale and short-term novelty in children. J Genet Psychol. 1990;151:153–167. doi: 10.1080/00221325.1990.9914651. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Verheij MM, Mele A, Cools A. Distinct kinds of novelty processing differentially increase extracellular dopamine in different brain regions. Eur J Neurosci. 2006;23:1332–1340. doi: 10.1111/j.1460-9568.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Presti-Torres J, Dornelles A, Scalco FS, Roesler R, Garcia VA, Schroder N. Modulatory influence of dopamine receptors on consolidation of object recognition memory. Neurobiol Learn Mem. 2011;95:305–310. doi: 10.1016/j.nlm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996;742:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33:445–452. doi: 10.1016/0361-9230(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Packard MG, Sulli A, Pacitti C, Innocenzi R, Perciavalle V. The projections of the retrorubral field A8 to the hippocampal formation in the rat. Exp Brain Res. 1996;112:244–252. doi: 10.1007/BF00227643. [DOI] [PubMed] [Google Scholar]
- Granado N, Ortiz O, Suarez LM, Martin ED, Cena V, Solis JM, Moratalla R. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cerebral cortex. 2008;18:1–12. doi: 10.1093/cercor/bhm026. [DOI] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. Journal of virology. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Ramos K, Moreno-Castilla P, Castro-Cruz M, McGaugh JL, Martinez-Coria H, LaFerla FM, Bermudez-Rattoni F. Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn Mem. 2012;19:453–460. doi: 10.1101/lm.026070.112. [DOI] [PubMed] [Google Scholar]
- Jenson D, Yang K, Acevedo-Rodriguez A, Levine A, Broussard JI, Tang J, Dani JA. Dopamine and norepinephrine receptors participate in methylphenidate enhancement of in vivo hippocampal synaptic plasticity. Neuropharmacology. 2015;90:23–32. doi: 10.1016/j.neuropharm.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe LA, Bellone C, De Roo M, Zandueta A, Dey PN, Perez-Otano I, Muller D. GluN3A promotes dendritic spine pruning and destabilization during postnatal development. J Neurosci. 2014;34:9213–9221. doi: 10.1523/JNEUROSCI.5183-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. An analysis of the dentate gyrus function. Behav Brain Res. 2013;254:1–7. doi: 10.1016/j.bbr.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neuroscience and biobehavioral reviews. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- King JA, Trinkler I, Hartley T, Vargha-Khadem F, Burgess N. The hippocampal role in spatial memory and the familiarity--recollection distinction: a case study. Neuropsychology. 2004;18:405–417. doi: 10.1037/0894-4105.18.3.405. [DOI] [PubMed] [Google Scholar]
- Kinnavane L, Albasser MM, Aggleton JP. Advances in the behavioural testing and network imaging of rodent recognition memory. Behav Brain Res. 2015;285:67–78. doi: 10.1016/j.bbr.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudolo J, Tabassum H, Frey S, Lopez J, Hassan H, Frey JU, Bergado JA. Electrical and pharmacological manipulations of the nucleus accumbens core impair synaptic plasticity in the dentate gyrus of the rat. Neuroscience. 2010;168:723–731. doi: 10.1016/j.neuroscience.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Tonegawa S. Inception of a false memory by optogenetic manipulation of a hippocampal memory engram. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369:20130142. doi: 10.1098/rstb.2013.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero A, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–1660. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31:4113–4123. doi: 10.1523/JNEUROSCI.4913-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rosen ZB, Cheung S, Siegelbaum SA. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci. 2015;18:1763–1771. doi: 10.1038/nn.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinana J, Kitamura T, Kunzler P, Sultzman L, Tonegawa S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc Natl Acad Sci U S A. 2014;111:8245–8250. doi: 10.1073/pnas.1407395111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Lin CL, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Walther D, Uhl G. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Brain Res Mol Brain Res. 1992;13:359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 2012;32:6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. The Journal of physiology. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamano H, Minamino T, Fujii H, Takada S, Nakamura M, Ando M, Takeda A. Blockade of intracellular Zn2+ signaling in the dentate gyrus erases recognition memory via impairment of maintained LTP. Hippocampus. 2015;25:952–962. doi: 10.1002/hipo.22418. [DOI] [PubMed] [Google Scholar]
- Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63:673–682. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Walling SG, Brown RA, Miyasaka N, Yoshihara Y, Harley CW. Selective wheat germ agglutinin (WGA) uptake in the hippocampus from the locus coeruleus of dopamine-beta-hydroxylase-WGA transgenic mice. Front Behav Neurosci. 2012;6:23. doi: 10.3389/fnbeh.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, Whiteaker P, Lukas RJ, Wu J. Functional Nicotinic Acetylcholine Receptors Containing {alpha}6 Subunits Are on GABAergic Neuronal Boutons Adherent to Ventral Tegmental Area Dopamine Neurons. J Neurosci. 2011;31:2537–2548. doi: 10.1523/JNEUROSCI.3003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Dani JA. Dopamine d1 and d5 receptors modulate spike timing-dependent plasticity at medial perforant path to dentate granule cell synapses. J Neurosci. 2014;34:15888–15897. doi: 10.1523/JNEUROSCI.2400-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci. 2010;30:6443–6453. doi: 10.1523/JNEUROSCI.0458-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Grunke SD, Keralapurath MM, Yetman MJ, Lam A, Lee TC, Sousounis K, Jiang Y, Swing DA, Tessarollo L, Ji D, Jankowsky JL. Impaired Recall of Positional Memory following Chemogenetic Disruption of Place Field Stability. Cell Rep. 2016;16:793–804. doi: 10.1016/j.celrep.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]