Abstract
Mg2+ is one of the essential elements for bacterial cell growth. The presence of the magnesium cation (Mg2+) in various concentrations often affects cell growth restoration in plant-associating bacteria. This study attempted to determine whether Mg2+ levels in Sphingomonas yanoikuyae EC-S001 affected cell growth restoration in the host plant and what the threshold level is. S. yanoikuyae EC-S001, isolated from the rhizoplane of spinach seedlings grown from surface-sterilized seeds under aseptic conditions, displayed uniform dispersion and attachment throughout the rhizoplane and phylloplane of the host seedlings. S. yanoikuyae EC-S001 did not grow in potato-dextrose broth medium but grew well in an aqueous extract of spinach leaves. Chemical investigation of the growth factor in the spinach leaf extract led to identification of the active principle as the magnesium cation. A concentration of ca. 0.10 mM Mg2+ or more allowed S. yanoikuyae EC-S001 to grow in potato-dextrose broth medium. Some saprophytic and/or diazotrophic bacteria used in our experiment were found to have diverse threshold levels for their Mg2+ requirements. For example, Burkholderia cepacia EC-K014, originally isolated from the rhizoplane of a Melastoma sp., could grow even in Mg2+-free Hoagland's no. 2 medium with saccharose and glutamine (HSG medium) and requires a trace level of Mg2+ for its growth. In contrast, S. yanoikuyae EC-S001, together with Bacillus subtilis IFO12113, showed the most drastic restoring responses to subsequent addition of 0.98 mM Mg2+ to Mg2+-free HSG medium. Our studies concluded that Mg2+ is more than just the essential trace element needed for cell growth restoration in S. yanoikuyae EC-S001 and that certain nonculturable bacteria may require a higher concentration of Mg2+ or another specific essential element for their growth.
Through evolution, certain plants and fungi have developed unique endosymbiotic relationships. Specifically, arbuscular mycorrhizal (AM) fungi are regarded as important symbionts of herbaceous plants and function to increase nutrition uptake, desiccation tolerance, and disease resistance in their host plants (8, 19, 22). Conversely, plant species that do not associate with AM fungi might have evolved alternative survival mechanisms that compensate for the lack of AM symbionts. For example, white lupin (Lupinus albus L.), known as a non-AM leguminous plant, forms cluster roots which exude organic acids and acid phosphatase, thereby functioning to solubilize soil-bound phosphates (16, 29). In many non-AM herbaceous plants, however, rhizoplane bacteria, including free-living nitrogen fixers and P solubilizers, likely compensate for the absence of AM fungi (11).
In the present study, we utilized spinach (Spinacia oleracea L.) as a model system in which to investigate the role of alternative epiphytes in a non-AM plant. Islam et al. initially noted the presence of bacteria on the rhizoplane of spinach seedlings germinated from surface-sterilized seeds during study of preinfection events of Aphanomyces cochlioides on the seedlings under a scanning electron microscope (SEM) (9). This observation drew our attention toward this epiphytic bacterium. Upon subsequent isolation of this gram-negative, rod-shaped bacterium from the seedlings, we identified it as Sphingomonas yanoikuyae by 16S rRNA gene sequencing and phenotypic characterization (28, 32) and specified it as strain EC-S001. One unique characteristic of EC-S001 was its unilaminar colonization on the phylloplane and/or rhizoplane. Such bacterial-cell dispersion is often due to quorum sensing (also known as autoinduction) mediated by N-acyl-l-homoserine lactones of the endogenous quorum-sensing signal molecules, and Brelles-Marino and Bedmar have shown in their review (3) that many plant-associated bacteria indeed possess quorum-sensing signals and display characteristic responses and behaviors on the rhizoplane or phylloplane.
A more important finding was that the isolate did not grow in potato-dextrose broth (PD) medium but could grow in an aqueous extract of spinach leaves or in nutrient broth (NB) medium. The isolate survived relatively longer in the spinach leaf extract (SLE) than in NB medium; however, it died shortly after it was cultured in the NB medium. This was in agreement with findings for other phyllospherous Sphingomonas spp. (10). Therefore, we were interested in investigating the S. yanoikuyae EC-S001 growth factor(s) in the aqueous extracts of spinach leaves, and we identified it as the magnesium cation (Mg2+). Here, we report Mg2+ as the restoring principle for S. yanoikuyae EC-S001 and discuss its ecological significance in the host plant.
MATERIALS AND METHODS
Culture medium preparation.
Twenty-four grams of PD medium (lot 0192000; Difco) was dissolved in 1 liter of ion-exchanged water, filtered to remove insolubles, and subsequently autoclaved. A second medium was prepared by dissolving 30 g of NB powder (lot 104110; Nissui) in 1 liter of ion-exchanged water without filtration. Hoagland's no. 2 mineral salt mixture (2) was prepared with a minor variation by replacing EDTA-Fe with FeSO4, using the same molar concentration of Fe2+. As a minimal basal medium for the experiments, we prepared 1× Hoagland's no. 2 mineral salt mixture containing 1.0% saccharose and 0.1% l-glutamine (HSG medium). Experiments which utilized HSG medium enabled EC-S001 to grow, but the growth of EC-S001 in HSG medium was comparatively slower than the growth observed in PD medium to which the ingredients of 1× Hoagland's no. 2 salt mixture was added. When Mg2+-free Hoagland's no. 2 mineral salt mixture was used for HSG medium, the growth of EC-S001 was severely inhibited. As a result, experiments were designed to determine the effect of EDTA or citric acid on the restoring activity of the Mg2+-containing HSG medium.
Preparation of aqueous extracts of spinach leaves.
One kilogram of fresh spinach leaves was washed, chopped, soaked in 2.5 liters of ion-exchanged water, and autoclaved for 10 min. The resulting yellowish brown aqueous layer was filtered through a double layer of cheesecloth and was subsequently centrifuged (at 8,000 ×g for 10 min). The resulting supernatant was collected and defatted by partitioning the aqueous layer and one-half volume of ethyl acetate in a shaking funnel. After evaporation of the remaining ethyl acetate, the aqueous layer was freeze-dried to yield approximately 20 g of a dry, pale yellowish powder (SLE).
SEM analysis.
A JEOL JSM-6301F SEM was used for all SEM observations. The root tissues were fixed with 2% glutaraldehyde and processed as described by Islam et al. (9).
Mg analysis.
Mg2+ contents in SLE, fresh spinach leaves, and the commercial broth media were measured with a Shimadzu AA-6400F atomic absorption flame emission spectroscope. Measurements for individual samples were averaged over triplicates, and data were analyzed as averages ± standard errors.
Isolation of epiphytic bacteria from spinach seedlings.
Seeds of spinach (Spinacia oleracea cv. Lead) purchased from Daigaku Nouen, Tokyo, Japan, were soaked in 5% sodium hypochlorite for 15 min and then rinsed with sterilized water. Seeds were germinated under aseptic conditions on wet filter paper in petri dishes at 23°C in a plant incubator under a photoperiodic condition of 16 h of light and 8 h of darkness. For bacterial isolation, a typical 10-day-old seedling was vortexed in 2 ml of sterile water, and 100-μl aliquots of the washing were plated onto cornmeal agar in 9-cm-diameter petri dishes. Typically, three different types of bacterial colonies were apparent on the plates. The predominant (>80%) type was purified on NB agar plates and appeared as smooth, convex, creamy white, glossy colonies.
Identification of the isolated bacteria.
Identification of the bacterial isolates utilized both phenotypic and physiological characterization (32) as well as partial 16S rRNA gene sequence analysis (30). Bacterial colonies grown on an NB agar slant were scraped with a loop, and cellular lipids were extracted as previously described by Yabuuchi et al. (32). Half of the extracted lipids was redissolved in a chloroform-methanol (2:1) mixture, and an equal volume of 0.5 M KOH was added to effect a mild alkaline hydrolysis at 40°C for 1 h. The reaction mixture was neutralized with acetic acid and checked by thin-layer chromatography to detect glycosylsphingolipids that tolerated the alkaline hydrolysis. Total DNA was purified with Isoplant II (Wako Pure Chemical Industries Ltd.) and used as the template for PCR amplification with either Gene Taq (Nippon Gene) or HotStarTaq DNA polymerase (QIAGEN). The first amplification for the 16S ribosomal DNA (rDNA) region with universal forward (27f) and reverse (1525r) primers (30) was performed with 30 cycles of denaturation at 94°C for 1 min, annealing at 53°C for 1 min, and extension at 72°C for 1 min. PCR products were sequenced by an ABI PRISM 310 Genetic Analyzer with a BigDye Terminator (version 3.0) Cycle Sequencing Ready Reaction kit (Applied Biosystems). Four forward (357f, 536f, 926f, and 1112f) and five reverse (327r, 518r, 803r, 1080r, and 1389r) primers were utilized for PCR direct sequencing. The sequence homology of the 1,457 bases determined (accession no. AB120764) was then searched on the BLASTN database program, provided by the DNA Data Bank of Japan (DDBJ; National Institute of Genetics, Mishima, Japan) on its website (http://www.ddbj.nig.ac.jp/E-mail/homology-j.html).
Measurement of bacterial cell growth.
Bacterial cell growth was monitored by measuring optical density at 660 nm (OD660) with a HITACHI U-3210 photospectrometer. For the OD measurements, the bacterial culture was diluted with a known volume of ion-exchanged water until the OD660 was lower than 0.8. Then the relative cell population was calculated from the dilution ratio. During the OD660 measurement, a noninoculated culture medium was used as a blank. The blank was used without dilution, unless otherwise mentioned.
The bacterial growth test for the SLEs was conducted as follows. A 100-ml volume of a test medium in a 300-ml Erlenmeyer flask with baffles was cultured with shaking (100 rotations per min) at 25°C. S. yanoikuyae colonies that had been precultured on NB agar slants for 2 days were scraped off the agar with a loop and suspended in 10 ml of sterilized water. Each medium of the growth assay was inoculated with 100 μl of the bacterial cell suspension and incubated for specific time periods. A procedure similar to that described above was used to monitor the cell growth of additional bacterial strains.
Investigation of Mg2+ as a restoring principle for other bacteria.
To determine whether Mg2+ acted as a restoring principle for additional bacterial strains, the effects of MgSO4 on Escherichia coli IFO3301, Staphylococcus aureus AHU1142, Bacillus subtilis IFO12113, and some other rhizoplane bacteria were also tested in HSG medium and Mg2+-free HSG medium. The rhizoplane bacterial species used in this experiment were those isolated from host plants and were tentatively identified by partial 16S rRNA gene sequences as follows: Caulobacter sp. strain EC-S044 (DDBJ/EMBL/GenBank accession no. AB086019) from Plantogo lanceolata, Sphingomonas sp. strain EC-K013 (AB121233) and Burkholderia cepacia EC-K014 (AB121232) from a Melastoma sp., and Sphingomonas sp. strain EC-K005 from a South Kalimantan local variety of Oryza sativa. After a 48-h-incubation, bacterial cell growth was monitored by OD660 in order to ascertain the responses of these bacteria to Mg2+.
Effect of EDTA or citric acid on growth of S. yanoikuyae EC-S001.
EDTA and citric acid were utilized to test the effect of an Mg2+-chelating reagent on the ability of Mg2+ to restore the growth of EC-S001 cells. Each chelating reagent was prepared in a series of concentrations from 0.03 to 3.4 mM and was added to HSG medium containing 0.98 mM Mg2+. After a 48-h-incubation, cell growth was monitored by OD660.
Nucleotide sequence accession number.
The S. yanoikuyae EC-S001 sequence determined in this study has been deposited in DDBJ as accession no. AB120764.
RESULTS
Isolation and identification of S. yanoikuyae EC-S001.
In the prescreening for epiphytic bacteria from spinach seedlings, three different strains of bacteria were apparent. The predominant strain (>80%), which formed the smallest colonies, was isolated on NB agar plates and was subsequently named strain EC-S001. The partial 16S rRNA gene sequence from position 8 to 1542 (E. coli numbering) was analyzed, and DNA sequence analysis of the isolate (accession no. AB120764) confirmed the highest score for alignment to Sphingobium (a synonym of Sphingomonas) yanoikuyae X85023, a polycyclic aromatic hydrocarbon-degrading strain (27); 99.4% homology was found between the sequences of EC-S001 (AB120764) and X85023. High homology was also detected for Sphingomonas sp. strain SS3 DSM6432 (accession no. X87165; 99.6%) (13), S. yanoikuyae KF706 (AB109749; 99.6%), GIFU9882 (D16145; 99.4%), B1 (U37524; 99.6%), and Q1 (U37525; 99.5%), and an unnamed S. yanoikuyae strain (AF331661; 99.6%) (25, 28, 31, 33), whereas S. yanoikuyae IFO15102 (D13728; 99.1%) and GIFU9882T (D84526; 98.1%) and another unnamed S. yanoikuyae strain (AY047219; 97.5%) had relatively low homology (23, 33). SEM analysis showed that S. yanoikuyae EC-S001, exhibiting a rod-shaped morphology with a polar flagellum, formed unilaminar colonization on the phylloplane or rhizoplane (Fig. 1). These data are consistent with previous observations for the standard strain of S. yanoikuyae, JCM 7371 (32). Moreover, analysis by silica gel thin-layer chromatography confirmed that the lipid composition of the isolate was also comparable with that of S. yanoikuyae JCM 7371; two spots of glycosylsphingolipids, a phenomenon unique to the genus Sphingomonas (32), were detected by our chromatographic analysis.
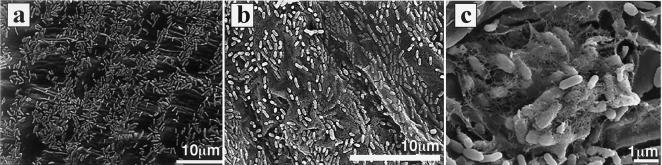
FIG. 1.
SEM photographs of S. yanoikuyae EC-S001 forming a fine layer on the rhizoplane of a spinach seedling. S. yanoikuyae EC-S001 cells that had been cultured in SLE for 2 days at 20°C without shaking were suspended in sterile water, and sterilized seedlings were soaked in the bacterial cell suspension for 10 min. The seedlings were then transferred to a sterilized, wet filter paper in a petri dish and maintained for 2 days in a plant incubation chamber. (a and b) S. yanoikuyae EC-S001 is shown on the cotyledon (a) and on the rhizoplane (b). (c) Adhesive, fibrous polysaccharide polymers on the bacterial cells. The polysaccharides are associated with the bacterial cells on the root surface.
Some of the primary metabolic properties of S. yanoikuyae EC-S001 were tested. As shown in Table 1, discrepancies between several metabolic characteristics of S. yanoikuyae EC-S001 and S. yanoikuyae JCM 7371 were apparent. It is well known that the primary metabolic characteristics of a bacterial species are dependent on its native environment (6). As a result, it is possible that the differences observed between the metabolic characteristics of the two strains are due to their different habitats. Indeed, JCM 7371 originally came from a clinical specimen (32), while EC-S001 was an isolate from the rhizoplane of a spinach seedling. Considering the findings described above, the isolate was identified as S. yanoikuyae despite discrepancies in some primary metabolic properties between JCM 7371 and EC-S001.
TABLE 1.
Comparisons of the phenotypes of S. yanoikuyae JCM 7371 and EC-S001
Cell growth factor in SLE.
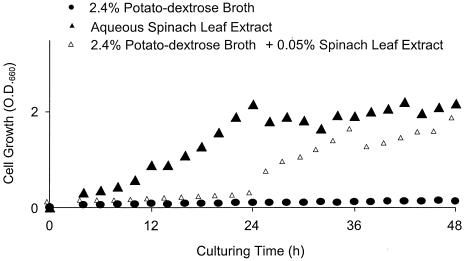
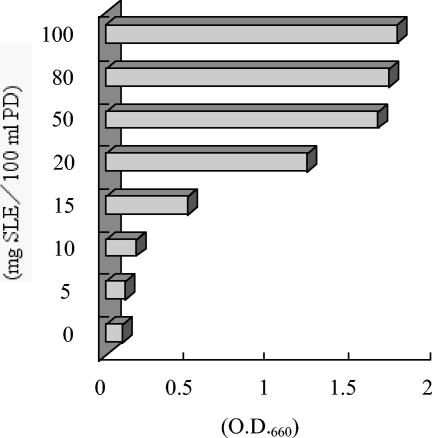
Cell growth of the isolate was completely inhibited when it was cultured in a plain PD medium. However, cell growth increased significantly with time when the isolate was grown in an aqueous SLE (Fig. 2). These data suggested that SLE contains certain growth factors that are absent in PD medium. In a second experiment, we added approximately 2.5 ml of SLE, corresponding to the extract from 1.0 g of fresh leaves (ca. 20 mg of soluble material) to 100 ml of plain PD medium. In the SLE-containing PD medium, S. yanoikuyae EC-S001 cell growth was restored. As a result, we further fractionated SLE and monitored the active matter(s) that passed through porous polystyrene beads (XAD-2 resin) in relation to bacterial cell growth. The SLE was initially partitioned between the aqueous layer and one-half volume of ethyl acetate, and only the resulting aqueous layer (95% recovery) showed growth-stimulating activity for S. yanoikuyae EC-S001. Subsequently, the defatted SLE was freeze-dried after removal of the remaining ethyl acetate by evaporation. In a preliminary dose-response examination using the freeze-dried powder (SLE), the maximal effect on the cell growth of S. yanoikuyae EC-S001 in PD medium was apparent with 20 mg of SLE powder/100 ml of medium, thus indicating a threshold level of required Mg2+ (Fig. 3).
FIG. 2.
Growth curves of S. yanoikuyae EC-S001 in PD medium (•), aqueous SLE (▴), and PD medium plus aqueous SLE (▵). Cell growth was monitored by OD660 and plotted without any correction.
FIG. 3.
Effects of addition of SLE powder to plain PD medium on the growth of S. yanoikuyae EC-S001. Values are averages from duplicate experiments after 48 h of incubation.
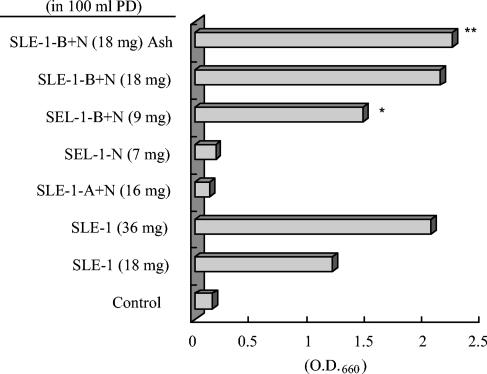
The SLE powder dissolved in water was first chromatographed on an XAD-2 (porous polystyrene beads; Rohm & Haas Co.) column, and the active principle(s) was completely passed through the XAD-2 column by water to recover 90% water-soluble matter. Dry materials from the active fraction (SLE-1, 18 mg), showing activity almost equivalent to that of 20 mg of SLE in 100 ml of PD medium, were further subjected to approximately 1 g of a carboxymethyl (CM)-cellulose column (cation-exchange) and/or approximately 1 g of a DEAE-cellulose column (anion-exchange) (both from Wako Pure Chemical Industries Ltd.). The acidic-plus-neutral fraction (SLE-1-A+N, ca. 16 mg), which passed through an H+-type CM-cellulose column, showed no growth-stimulating activity; on the contrary, it suppressed cell growth relative to that in plain PD medium. On the other hand, the basic-plus-neutral fraction (SLE-1-B+N, ca. 9 mg), which was obtained from an OH−-type DEAE-cellulose column, stimulated the growth of S. yanoikuyae EC-S001 with activity equivalent to that of 20 mg of SLE in 100 ml of PD medium. The neutral fraction (SLE-1-N, ca. 7 mg), obtained by passage through both columns, did not demonstrate any activity (Fig. 4).
FIG. 4.
Effects of an SLE solution passed through anion- or cation-exchange resin or of an SLE ash solution on the growth of S. yanoikuyae EC-S001. SLE-1, substances from SLE passed through XAD-2 resin with water; SLE-1-A+N, acidic and neutral substances from SLE-1 passed through a cation-exchange resin (CM-cellulose) column; SLE-1-B+ N, basic and neutral substances from SLE-1 passed through an anion-exchange resin (DEAE-cellulose) column; SLE-1-N, SLE-1 passed through both CM- and DEAE-cellulose columns; SLE-1-B+N Ash, ash from SLE-1-B+N. Values are averages from duplicate experiments after 48 h of incubation. *, active fraction after ion-exchange chromatography; **, equivalent activity of the ash to the original amount of SLE-1-B+N.
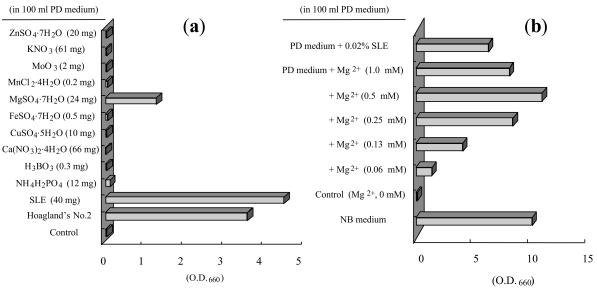
Due to the fact that amino acids cannot pass through the DEAE-cellulose column, it was possible that the active principle(s) in this phenomenon was simple amines or inorganic cations. Based on this assumption, we burned the freeze-dried powder of SLE-1-B+N (18 mg) and added the remaining ash to 100 ml of PD medium. The growth-stimulating activity of the ash from SLE-1-B+N was maintained, adding further evidence that the active principles in SLE were indeed inorganic cations (Fig. 4). Furthermore, we investigated the effects of several inorganic elements on cell growth by using Hoagland's no. 2 salt mixture (normally used for plant hydroponic cultures). When S. yanoikuyae EC-S001 was cultured in Hoagland's no. 2 medium containing 2.4% PD powder, the resulting medium showed an effect on cell growth equivalent to that of 40 mg of SLE in 100 ml of PD medium. This result indicated that Hoagland's no. 2 salt mixture contains an active principle(s) at a level above the threshold required for cell growth (Fig. 5a).
FIG. 5.
Restoring effect of Mg2+ on the growth of S. yanoikuyae EC-S001 in PD medium. (a) Amounts of each ingredient added to plain PD medium were equal to concentrations in the original Hoagland's no. 2 mineral solution. Among the ingredients of Hoagland's no. 2 mineral medium, only 0.98 mM MgSO4 · 7H2O allowed cell growth of EC-S001. (b) To examine the threshold level of Mg2+ for EC-S001 cell growth, a series of concentrations of MgSO4 · 7H2O was added to PD medium. Values are averages from duplicate experiments after 48 h of incubation.
Effect of Mg2+ on S. yanoikuyae EC-S001 cell growth and dose-response.
All inorganic ingredients of Hoagland's no. 2 solution were tested in order to assess their effects on S. yanoikuyae cell growth. Individual solutions of 2.4% PD medium containing N, B, Mg, K, Ca, Cu, Fe, Zn, Mo, or Mn at concentrations similar to those in the original Hoagland's medium were examined for their effects on S. yanoikuyae growth. After 48 h of incubation, significant cell growth was observed only in the PD medium supplemented with MgSO4 · 7H2O (1 mM). These results strongly suggested that Mg2+ was the only active principle ion in spinach leaves, required for S. yanoikuyae to grow in PD medium (Fig. 5a). In addition, we tested two other Mg2+ salts [MgCl2 · 6H2O and Mg(NO3)2 · 6H2O] at 1 mM and observed similar effects on the bacterium after 48 h of incubation. Taking these results together, we confirmed that the growth-stimulating activity of the Mg2+ occurred independently of its corresponding anion.
The dose response relationship of Mg2+ with EC-S001 was concurrently examined by using MgSO4 · 7H2O. When the concentration of Mg2+ was higher than 0.5 mM, the stimulating effect of Mg2+ on cell growth seemed to reach its maximal level, equivalent to 500 mg of SLE/liter or an extract from 25 g of fresh spinach leaves/liter. The minimal dose of Mg2+ that showed a significant, positive effect on bacterial growth was 0.13 mM (Fig. 5b). Based on our measurement by the atomic absorption spectrophotometer, the magnesium content of SLE was 0.98 mg/100 mg of dry powder (Table 2). PD medium containing Mg2+-free Hoagland's no. 2 medium or a minimal basal medium (HSG medium) without Mg2+ failed to promote any significant cell growth of S. yanoikuyae EC-S001. These results confirmed that the sole growth-stimulating component of SLE for S. yanoikuyae EC-S001 is Mg2+.
TABLE 2.
Mg2+ contents in the medium used in the study and minimal effective dose of Mg2+ for S. yanoikuyae EC-S001
| Sample or medium | Concn or amt of Mg2+ |
|---|---|
| SLE (from 1 kg of leaves/2.5 liters) | 7.39 mM |
| SLE freeze-dried powder | 9.8 mg/g |
| Minimal SLE powder in PD medium (200 mg/liter) | 0.05 mM (1.3 mg/liter) |
| Plain PD medium | 0.05 mM |
| Plain NB medium | 0.70 mM |
| Hoagland's no. 2 mineral mixture in PD medium | 0.97 mM |
| Minimally effective PD medium with SLE added | 0.10 mM |
| Minimally effective PD medium with Mg2+ addeda | 0.11 mM |
As MgSO4·7H2O.
Effects of Mg2+-chelating reagents on cell growth of S. yanoikuyae EC-S001.
S. yanoikuyae EC-S001 cultured in Mg2+-free HSG medium failed to grow throughout a 2-week incubation period. However, when 0.98 mM MgSO4 · 7H2O was added to the culture medium after a 1-day incubation period, bacterial cell growth recovered dramatically. In Mg2+-containing HSG medium, however, EC-S001 started to show slight growth suppression when grown in the presence of 0.03 mM EDTA, and its cell growth was thoroughly inhibited at concentrations of 0.27 mM EDTA or more. When the Mg2+ concentration in HSG medium was increased to 9.8 mM, growth inhibition by 0.27 mM EDTA did not diminish. Therefore, we conclude that growth inhibition of EC-S001 by EDTA is not due to chelation of Mg2+ but may be due to some other cationic trace elements.
Restoring effects of Mg2+ on cell growth of some reference and rhizoplane bacteria.
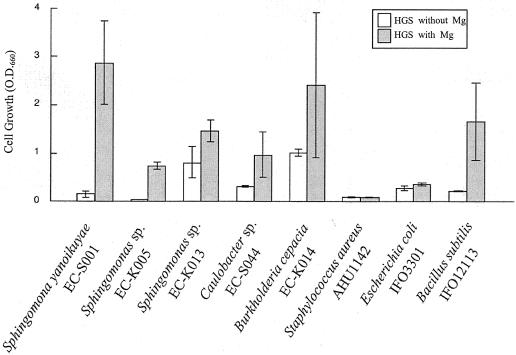
To investigate whether the restoring effect of Mg2+ on bacterial growth is a widely observed phenomenon, additional saprophytes were used as references. Stored cultures of S. aureus AHU1142, B. subtilis IFO12113, E. coli IFO3301, and a few other isolated rhizoplane bacteria were used in our analyses. In addition to S. yanoikuyae EC-S001 and B. subtilis IFO12113, some other rhizoplane bacteria demonstrated restoration of cell growth in response to Mg2+ in HSG medium (Fig. 6). All of the rhizoplane bacteria tested here showed better cell growth in Mg2+-containing than in Mg2+-free HSG medium. However, B. subtilis IFO12113 was the only bacterium to demonstrate a threshold level of Mg2+ similar to that of EC-S001. Both B. cepacia EC-K014 and Sphingomonas sp. strain EC-K013 grew comparatively well in Mg2+-free HSG medium. The levels of cell growth of Caulobacter sp. strain EC-S044, Sphingomonas sp. strain EC-K013, and Sphingomonas sp. strain EC-K005 in Mg2+-containing HSG medium were only ca. 35 to 60% that of S. yanoikuyae EC-S001. Interestingly, neither S. aureus AHU1142 nor E. coli IFO3301 (both originally isolated from mammals, not plants) grew well in HSG medium, irrespective of the presence or the absence of Mg2+. In fact, the B. subtilis strain IFO12113, which shows a clear restoring response to Mg2+, is of plant origin.
FIG. 6.
Effects of Mg2+ on several reference bacteria and rhizoplane bacteria. Test bacteria were inoculated into Mg2+-free HSG medium (open bars) or HSG medium containing 0.98 mM Mg2+ (shaded bars) and were incubated for 2 days. Among the test bacteria, those originally isolated from plant surfaces responded to Mg2+, but the others, from mammals, showed no growth in HSG medium containing 0.98 mM Mg2+. When the cell growth is nearly negative in the Mg2+-free medium while clearly positive in the Mg2+-containing medium, the threshold level of required Mg2+ for the bacterium is thought to be comparatively high (e.g., S. yanoikuyae EC-S001 or B. subtilis IFO12113). On the other hand, bacteria which grow well in the Mg2+-free medium are regarded as possessing a low threshold level of Mg2+ (e.g., B. cepacia EC-K014). Values are averages from triplicate experiments after 48 h of incubation. Error bars, standard deviations. All experiments were performed in triplicate (n = 3), except for S. yanoikuyae EC-S001 (n = 9) and B. subtilis IFO12113 (n = 6).
DISCUSSION
In this study, we demonstrated the role of Mg2+ as a stimulator and also a restoring factor for cell growth of S. yanoikuyae EC-S001. Mg2+ is one of the essential elements for living bacterial cells due to its function as a cofactor of DNA polymerase and ATPase, among others (20). In relation to Mg2+, some additional requirements for certain physiological behaviors of microorganisms are also known. As Nakagawa et al. recently reported, Mg2+ activates the degradation of carbazole by the resting cells of Sphingomonas sp. strain CDH-7 (15). This restoring effect of Mg2+ on certain enzymes associated with xenobiotic metabolism shows that Mg2+ behaves as an activator for Sphingomonas sp. strain CDH-7 in its environmental response and resource utilization. Indeed, Mg2+ is also known as a growth-stimulating factor of submerged mycelia of Streptomyces azureus (18), a role similar to its effect on EC-S001.
In our study, the Mg2+ contents of standard PD medium and NB medium were 1.2 mg/liter (0.05 mM) and 16.6 mg/liter (0.70 mM), respectively. Since the minimal effective concentration of SLE was more than 200 mg per liter of PD medium (Fig. 3), the concentration of Mg2+ in the minimal SLE powder was calculated to be approximately 0.05 mM, and the total estimated concentration of Mg2+ in the SLE-containing PD medium was 0.10 mM. When 0.06 mM supplemental Mg2+ was added to the PD medium as MgSO4 · 7H2O, S. yanoikuyae EC-S001 cell growth was observed (Fig. 5b), so the minimal effective Mg2+ concentration in the Mg2+-enriched PD medium was approximately 0.11 mM. This is almost agreeable with the threshold concentration of Mg2+ in SLE-containing PD medium. The results shown in Table 2 strongly imply that S. yanoikuyae EC-S001 demands more than 0.10 mM Mg2+ as its threshold level for cell growth.
Considering the essential role of Mg2+ mentioned above, it is plausible that Mg2+ is required for bacterial growth; however, many heterotrophic bacteria of phyto-epiphytes are capable of growing in plain PD medium. Therefore, the rationale for the inability of EC-S001 to grow in plain PD medium should be discussed. There are two possible reasons for this phenomenon. EC-S001 may have a high threshold level of Mg2+ for growth, or it may not be able to utilize or decompose the Mg2+ complex formed in PD medium, or both. The latter possibility could be eliminated by the observation that S. yanoikuyae EC-S001 grew well in HSG medium containing 1.7 mM citrate. The dose-response experiments for Mg2+ on some other bacteria and for Mg2+-chelating reagents on EC-S001 itself suggested that the requirement of a comparatively high concentration of Mg2+ for restoring the growth of EC-S001 is simply due to a higher threshold level of Mg2+ for the bacterium. Some of the rhizoplane bacteria tested (e.g., B. cepacia EC-K014) were able to grow in Mg2+-free HSG medium, in which neither S. yanoikuyae EC-S001 nor B. subtilis IFO12113 demonstrated significant growth (Fig. 6). Such Mg2+-free HSG medium-tolerant bacteria had a remarkably low Mg2+ threshold level, since the Mg2+ contents, present only as an impurity, are estimated to be less than 0.01 μM. Hence, threshold levels of Mg2+ for cell growth of bacteria are certainly diverse and are specific characteristic of species and strains. Thus, it seems reasonable that the threshold level of Mg2+ for EC-S001 is higher than others.
Sphingomonas is a genus belonging to the α-4 subclass of the Proteobacteria, and its members often show unique ecological and physiological behaviors relating to biodegradation of artificial chemicals (4, 15, 31), heteropolymer assimilation (7), and biofilm formation (12). In agreement with the biofilm-forming nature of sphingomonads, S. yanoikuyae EC-S001 shows unilaminar cell attachment on the phylloplane or rhizoplane (Fig. 1) as one of its conspicuous features. This is likely a reason why Sphingomonas spp. are often found as plant-associating bacteria, particularly on the surfaces of roots (24), leaves (10), flowers (10), and seeds (10, 17), and even in inner tissues as endophytes (1, 5). Another unique property of Sphingomonas spp. is the presence of an outer membrane outside the cell wall (7, 24, 32). To determine whether the characteristic Mg2+ requirement of S. yanoikuyae EC-S001 is related to the outer membrane, Mg2+ requirements for two more Sphingomonas spp. were investigated. Both showed lower threshold levels of Mg2+ for their cell growth, so any relationship between the Mg2+ requirement and the outer membrane of sphingomonads is excluded.
The role of the critical level of Mg2+ in S. yanoikuyae EC-S001 cell growth is not yet known; however, Mg2+ certainly seems to be more than just an essential element for the general maintenance of the cells of S. yanoikuyae EC-S001 and some other plant-associating bacteria. Although Rovira mentioned in his review that most of the rhizoplane bacteria originate from soil (21), it is likely that S. yanoikuyae EC-S001 originates from the seed coat or the inner space of the seed, considering the source from which this bacterium was isolated and its uniform dispersion. In the interrelationship between spinach seedlings and S. yanoikuyae EC-S001, Mg2+ is probably involved in their cross talk as a communicator. Thus, Mg2+ is a strong candidate for an epiphyte regulator of host plants, considering the fluctuating concentrations of Mg2+ in plants, which is generally high in plant leaves (e.g., 6.4 mg/g [dry weight] in spinach leaves) but relatively low in the rhizosphere (14).
Our conclusion in this research is that more than the threshold concentration of Mg2+ (0.10 mM), which is unusually high, is necessary for the growth of S. yanoikuyae EC-S001. Because Mg2+ is usually one of the major ingredients in many artificial media for bacterial culture, bacteriologists might overlook the necessity of Mg2+ for some slow-growing bacteria, particularly those that are plant associated. Indeed, B. subtilis IFO12113 and some rhizoplane bacteria showed significant responses to Mg2+ in the HSG medium in our investigation. It is well known that great numbers of nonculturable bacteria exist in the soil and/or other natural environments, including the phylloplane and rhizoplane (26). Considering the responses to Mg2+ of the bacteria tested in this study, deficiencies of Mg2+ or some other trace elements in the general bacterial media may partially explain why nonculturable bacteria cannot be cultured.
Acknowledgments
We thank T. Ito of the Graduate School of Agriculture, Hokkaido University, for help with SEM. We also thank M. Yanbuaban, T. Yamamoto, and M. Osaki of the Graduate School of Agriculture, Hokkaido University, for measuring the magnesium contents in SLE and broth media with an atomic absorption flame emission spectrophotometer.
The financial support of the Ministry of Education, Science, Sports and Culture of Japan via grants-in-aid for scientific research (grant 13460143 to Y.H. and grant 14206013 to S.T.) is also gratefully acknowledged.
REFERENCES
- 1.Adhikari, T. B., C. M. Joseph, G. Yang, D. A. Phillips, and L. M. Nelson. 2001. Evaluation of bacteria isolated from rice for plant growth promotion and biological control of seedling disease of rice. Can. J. Microbiol. 47:916-924. [DOI] [PubMed] [Google Scholar]
- 2.Arnon, D. J., and D. R. Hoagland. 1940. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 50:463-485. [Google Scholar]
- 3.Brelles-Marino, G., and E. J. Bedmar. 2001. Detection, purification and characterisation of quorum-sensing signal molecules in plant-associated bacteria. J. Biotechnol. 91:197-209. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin, M. F., B. K. Kinkle, and P. L. Bishop. 2002. Degradation of acid orange 7 in an aerobic biofilm. Chemosphere 46:11-19. [DOI] [PubMed] [Google Scholar]
- 5.Garbeva, P., L. S. van Overbeek, J. W. L. van Vuurde, and J. D. van Elsas. 2001. Analysis of endophytic bacterial communities of potato by planting and denaturing gradient gel electrophoresis (DGGE) of 16S rDNA based PCR fragments. Microb. Ecol. 41:369-383. [DOI] [PubMed] [Google Scholar]
- 6.Hashidoko, Y., E. Itoh, K. Yokota, T. Yoshida, and S. Tahara. 2002. Characterization of five phyllosphere bacteria isolated from Rosa rugosa leaves, and their phenotypic and metabolic properties. Biosci. Biotechnol. Biochem. 66:2474-2478. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto, W., K. Momma, H. Miki, Y. Mishima, E. Kobayashi, O. Miyake, S. Kawai, H. Nankai, B. Mikami, and K. Murata. 1999. Enzymatic and genetic bases of assimilation, depolymerization, and transport of heteropolysaccharides in bacteria. J. Biosci. Bioeng. 87:123-136. [DOI] [PubMed] [Google Scholar]
- 8.Hwang, S. F., K. F. Chang, and P. Chakravarty. 1992. Effects of vesicular-arbuscular mycorrhizal fungi on the development of Verticillium and Fusarium wilts of alfalfa. Plant Dis. 76:239-243. [Google Scholar]
- 9.Islam, M. T., T. Ito, and S. Tahara. 2001. Morphological studies on zoospores of Aphanomyces cochlioides and changes during interaction with host materials. J. Gen. Plant Pathol. 67:255-261. [Google Scholar]
- 10.Kim, H., T. Kunito, K. Senoo, K. Kawahara, K. Murakami, and H. Oyaizu. 1998. High population of Sphingomonas species on plant surface. J. Appl. Microbiol. 85:731-736. [Google Scholar]
- 11.Kim, K.-Y., D. Jordan, and H. B. Krishnan. 1998. Expression of genes from Rahnella aquatilis that are necessary for mineral phosphate solubilization in Escherichia coli. FEMS Microbiol. Lett. 159:121-127. [DOI] [PubMed] [Google Scholar]
- 12.Koskinen, R., T. Ali-Vehmas, P. Kaempfer, M. Laurikkala, I. Tsitko, E. Kostyal, F. Atroshi, and M. Salkinoja-Salonen. 2000. Characterization of Sphingomonas isolates from Finnish and Swedish drinking water distribution systems. J. Appl. Microbiol. 89:687-696. [DOI] [PubMed] [Google Scholar]
- 13.Moore, E. 1995. Genetic and serological evidence for the recognition of four pentachlorophenol-degrading bacterial strains as a species of the genus Sphingomonas. Syst. Appl. Microbiol. 18:539-548. [Google Scholar]
- 14.Moritsuka, N., J. Yanai, and T. Kosaki. 2000. Effect of plant growth on the distribution and forms of soil nutrients in the rhizosphere. Soil Sci. Plant Nutr. 46:439-447. [Google Scholar]
- 15.Nakagawa, H., K. Kirimura, T. Nitta, K. Kino, R. Kurane, and S. Usami. 2002. Recycle use of Sphingomonas sp. CDH-7 cells for continuous degradation of carbazole in the presence of MgCl2. Curr. Microbiol. 44:251-256. [DOI] [PubMed] [Google Scholar]
- 16.Neumann, G., A. Massonneau, N. Langlade, B. Dinkelaker, C. Hengeler, V. Romheld, and E. Martinoia. 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann. Bot. 85:909-919. [Google Scholar]
- 17.Oehrle, N. W., D. B. Karr, R. J. Kremer, and D. W. Emerich. 2000. Enhanced attachment of Bradyrhizobium japonicum to soybean through reduced root colonization of internally seedborne microorganisms. Can. J. Microbiol. 46:600-606. [DOI] [PubMed] [Google Scholar]
- 18.Okba, A. K., T. Ogata, H. Matsubara, S. Matsuo, K. Doi, and S. Ogata. 1998. Effects of bacitracin and excess Mg2+ on submerged mycelial growth of Streptomyces azureus. J. Ferment. Bioeng. 86:28-33. [Google Scholar]
- 19.Redman, R. S., D. D. Dunigan, and R. J. Rodriguez. 2001. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol. 151:705-716. [DOI] [PubMed] [Google Scholar]
- 20.Romani, A. M. P., and A. Scarpa. 2000. Regulation of cellular magnesium. Front. Biosci. 5:720-734. [Online.] [DOI] [PubMed] [Google Scholar]
- 21.Rovira, A. D. 1965. Interactions between plant roots and soil microorganisms. Annu. Rev. Microbiol. 19:241-266. [DOI] [PubMed] [Google Scholar]
- 22.Smith, S., and D. J. Read. 1997. Mycorrhizal symbiosis. Academic Press, London, United Kingdom.
- 23.Takeuchi, M., F. Kawai, Y. Shimada, and A. Yokota. 1993. Taxonomic study of polyethylene glycol-utilizing bacteria: emended description of the genus Sphingomonas and new descriptions of Sphingomonas macrogoltabidus sp. nov., Sphingomonas sanguis sp. nov. and Sphingomonas terrae sp. nov. Syst. Appl. Microbiol. 16:227-238. [Google Scholar]
- 24.Takeuchi, M., T. Sakane, M. Yanagi, K. Yamasato, K. Hamana, and A. Yokota. 1995. Taxonomic study of bacteria isolated from plants: proposal of Sphingomonas rosa sp. nov., Sphingomonas pruni sp. nov., Sphingomonas asaccharolytica sp. nov., and Sphingomonas mali sp. nov. Int. J. Syst. Bacteriol. 45:334-341. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi, M., H. Sawada, H. Oyaizu, and A. Yokota. 1994. Phylogenetic evidence for Sphingomonas and Rhizomonas as nonphotosynthetic members of the alpha-4 subclass of the Proteobacteria. Int. J. Syst. Bacteriol. 44:308-314. [DOI] [PubMed] [Google Scholar]
- 26.Torsvik, V., R. Sorheim, and J. Goksoyr. 1996. Total bacterial diversity in soil and sediment communities: a review. J. Ind. Microbiol. 17:170-178. [Google Scholar]
- 27.Wang, R. F., W. W. Cao, and C. E. Cerniglia. 1996. Phylogenetic analysis of Fusobacterium prausnikzii based upon the 16S rRNA gene sequence and PCR. Int. J. Syst. Bacteriol. 46:341-343. [DOI] [PubMed] [Google Scholar]
- 28.Wang, Y., and P. C. Lau. 1996. Sequence and expression of an isocitrate dehydrogenase-encoding gene from a polycyclic aromatic hydrocarbon oxidizer, Sphingomonas yanoikuyae B1. Gene 168:15-21. [DOI] [PubMed] [Google Scholar]
- 29.Wasaki, J., M. Omura, M. Ando, T. Shinano, M. Osaki, and T. Tadano. 1999. Secreting portion of acid phosphatase in roots of lupin (Lupinus albus L.) and a key signal for the secretion from the roots. Soil Sci. Plant Nutr. 45:937-945. [Google Scholar]
- 30.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittmann, C., A. P. Zeng, and W. D. Deckwer. 1998. Physiological characterization and cultivation strategies of the pentachlorophenol-degrading bacteria Sphingomonas chlorophenolica RA2 and Mycobacterium chlorophenolicum PCP-1. J. Ind. Microbiol. Biotechnol. 21:315-321. [Google Scholar]
- 32.Yabuuchi, E., I. Yano, H. Oyaizu, Y. Hashimoto, T. Ezaki, and H. Yamamoto. 1990. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol. Immunol. 34:99-119. [DOI] [PubMed] [Google Scholar]
- 33.Yun, N. R., Y. K. Shin, S. Y. Hwang, H. Kuraishi, J. Sugiyama, and K. Kawahara. 2000. Chemotaxonomic and phylogenetic analyses of Sphingomonas strains isolated from ears of plants in the family Gramineae and a proposal of Sphingomonas roseoflava sp. nov. J. Gen. Appl. Microbiol. 46:9-18. [DOI] [PubMed] [Google Scholar]