Abstract
Objective
Many studies indicate that early life stress leads to negative health outcomes in adulthood, and some suggest that high-quality parenting might buffer these effects. Most prior research, however, has relied on cross-sectional retrospective reports of stress and parenting. Our study tests how coder-rated stress and parenting quality assessed at different life stages predict adult health outcomes in a prospective, longitudinal study.
Methods
Participants were 163 individuals in the Minnesota Longitudinal Study of Risk and Adaptation (MLSRA) studied since birth. Physical health was assessed at age 32 with BMI, self-reports of symptoms and illnesses experienced, and self-ratings overall physical health. Stress was assessed by coder-rated interviews involving participants or their mothers at 16 time-points partitioned into five life stages: early childhood, middle childhood, adolescence, young adulthood, and at age 32 (when health was assessed). Parenting quality was measured by coder-ratings of each mother's provision of sensitive, responsive support at 7 time-points between birth and age 13.
Results
Early childhood, adolescent, and concurrent stress predicted adult health outcomes at age 32. Early childhood and adolescent stress, and adolescent and concurrent stress, both showed a “dual-risk” pattern, such that experiencing higher stress at both of these life stages predicted the worst health outcomes. Higher maternal sensitivity, however, buffered these deleterious effects.
Conclusions
Our prospective data reveals that early childhood and adolescence are important developmental periods during which stress is influential for adult physical health. However, parenting interventions that promote greater sensitivity may help children in high-stress environments avoid negative adult health outcomes.
Keywords: Stress, Parenting, Developmental Antecedents, Longitudinal Study
Experiencing high levels of stress is a major risk factor for poor physical health outcomes (Cohen et al., 2007). However, the timing of life stress may be important in determining its long-term impact. There may be sensitive periods when stress is more likely to generate poor long-term health outcomes (e.g., Lupien et al., 2009; Miller et al., 2011). Little research has examined the effects of stress at different life stages, and very few studies have explored protective factors.
Past research has sought to identify whether there are sensitive periods during development when stress has a stronger impact on adult physical health. Much of this work has focused on early childhood effects (Fagundes & Way, 2014; Miller et al., 2011). Miller and colleagues (2011), for example, propose that early life stress affects adult health by programming the immune system to be hyperactive to potential threats. This process results in chronic inflammation, which in turn predicts several negative health outcomes, such as high blood pressure and poor immune functioning. This early biological embedding model has considerable empirical support. Exposure to greater stress early in life forecasts many deleterious health outcomes, including coronary heart disease (Dong et al., 2004) and earlier mortality (Galobardes et al., 2006).
However, life stages other than early childhood may also affect long-term health. Greater current stress, for example, is also associated with poorer health outcomes (DeLongis et al., 1988; Uchino et al., 1995). During adolescence, individuals gain greater control over health behaviors, establishing habits that often continue into adulthood (Tamelin et al., 2003; Lake et al., 2006). Greater stress during adolescence also predicts pro-inflammatory tendencies (Ehrlich et al., in press), metabolic syndrome (Gustafsson et al., 2012), and headaches (Waldie, 2001). Animal studies show that early life and adolescence are two critical periods for long-term health (e.g., Meaney & Szyf, 2001; McCormick et al., 2010). The long-term effects of stress beyond early childhood on human health, however, have rarely been tested, and never with a prospective longitudinal study.
Another major limitation of prior research is that life stress has typically been measured retrospectively. During adolescence/adulthood (when health measures are collected), individuals are asked to report on the stressfulness of their earlier experiences (e.g., Chen et al., 2011; Miller et al., 2009; Miller et al., 2011). Retrospective reports are problematic because individuals may not accurately remember their earlier experiences (Rubin et al., 1998) or third variables may lead people to exaggerate their earlier life stress and/or the severity of their health problems, artificially inflating these associations. In addition, many studies have treated socioeconomic status (SES) as a proxy of life stress (e.g., Raposa et al., 2014; Taylor et al., 2006). SES, however, may not capture forms of stress that middle and upper-class individuals encounter, and it may not be sensitive to the variability in stress often experienced by low-SES individuals.
Only two prospective studies have examined early life stress and later physical health. Essex and colleagues (2011) followed children from infancy to adolescence and found that greater stress in infancy and preschool (operationalized as having depressed parents and being exposed to anger in the family) resulted in atypical diurnal cortisol patterns in middle childhood and adolescence. Raposa and colleagues (2014) investigated how young adult health outcomes were affected by early adversity (measured by mother's psychopathy, parental discord, harsh discipline, family income, and parental criminal behavior in the first five years of life). Children who experienced greater adversity in early in life had higher levels of inflammation, BMI, and smoking at age 20. Although these two studies did not use retrospective reports of early life adversity, they examined the effects of stress only during early childhood and did not test the effects of stress at other life stages. Furthermore, the stress measures used in these studies are limited in the range of stressors they assessed, and may not comprehensively measure participants’ actual life stress.
Experiencing stress is unavoidable. However, one protective factor may be the quality of care provided by parents (especially mothers, who tend to be children's primary caregivers). Social support promotes better health because it buffers stress (Cohen & Smye, 1985; Uchino et al., 1996). High-quality parenting tends to be a protective factor for various outcomes associated with child adversity because it reduces children's stress responses. This suggests that higher-quality parenting could have buffering effects on the relations between earlier stress and adult health (Cicchetti & Blender, 2006; Gunnar & Quevedo, 2007). Indeed, greater self-reported maternal warmth and nurturance early in life appears to buffer the impact of low SES on inflammation and metabolic syndrome in adulthood (Chen et al., 2011; Miller et al., 2011), and parenting interventions have been found to be effective in improving child health (Miller et al., 2014).
Similar to the research on stress, virtually all prior research on parenting as a protective factor has relied on retrospective accounts, typically by asking adults to report on their mother's warmth and nurturance when they were children (e.g., Chen et al., 2011; Miller et al., 2011). As with retrospective reports of life stress, there may be a gap between actual childhood experiences and adult perceptions of those experiences (e.g., Roisman et al., 2002). In addition, past studies have conflated parenting quality and life stress by including parenting or parenting-relevant issues (e.g., maternal depression; Essex et al., 2011) in life stress measures. Although parenting quality and life stress usually correlate negatively, they are independent constructs.
This research utilizes the Minnesota Longitudinal Study of Risk and Adaptation (MLSRA)—a long-term, initially low SES prospective sample—to examine the effects of the timing of life stress on adult physical health and to test a plausible protective factor—the quality of maternal care during childhood. The current study fills several major gaps in the early stress to adult health literature by examining: (a) the long-term effects of stress prospectively assessed at different life stages on adult health, and (b) the protective effect of high-quality parenting. It also measures and tests these constructs in a more rigorous and detailed way than prior studies. The Minnesota Longitudinal Study participants have been followed from birth into adulthood. Life stress was assessed by coder-rated interviews with mothers or participants and then grouped into five life stages: early childhood, middle childhood, adolescence, young adulthood, and at age 32 (concurrent with health assessments). Parenting quality was assessed with observations of maternal sensitivity during childhood. The effects of stress and parenting were examined for three self-reported health outcomes: an overall rating of physical health, BMI, and the number of illnesses/symptoms experienced.
Greater stress in early life, adolescence, and concurrently should exert the strongest negative effects on adult health (Hypothesis 1). The study also explored whether experiencing greater stress at multiple key life stages had additive effects in predicting adult health outcomes, or whether it showed a “dual-risk” pattern, with higher stress at multiple stages resulting in worse health outcomes than main effects alone (Exploratory Question 1). In addition, we explored whether stress at certain life stages predicted adult health outcomes more strongly than the cumulative stress experienced across the lifespan (Exploratory Question 2). Finally, higher-quality maternal care should have a buffering effect on adult health, such that higher-quality parenting would reduce or eliminate the negative effects of stress on adult health (Hypothesis 2).
Methods
Participants
The study involved 163 participants (51% female, 49% male) from the Minnesota Longitudinal Study of Risk and Adaptation (MLSRA) who participated in the 32-year assessment in 2007-2008. 65.9% of participants were White, 10.4% were African American, 18.3% were multiracial, 2.4% were Native American, Hispanic, or Asian American, and the race/ethnicity of 3% could not be determined. The sample did not differ significantly from the initial sample of children recruited in 1975-76 (N = 267), all of whom were born to women living below the poverty line and receiving free prenatal services through the Minneapolis Department of Health (see the supplemental file for more information about the sample and retention). At the child's birth, 48% of the mothers were teenagers, 65% were single, and 42% had not completed high school.
Measures
Descriptive statistics for all measures are reported in Table 1.
Table 1.
Correlations and descriptive statistics for key variables
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Overall Physical Health | -- | ||||||||||
| 2. BMI | −.38*/−.37* | -- | |||||||||
| 3. Symptom/Illness Count | −.41*/−.33* | .23*/.21* | -- | ||||||||
| 4. Early Childhood Stress | −.15†/−.17† | .19*/.23* | .09/.05 | -- | |||||||
| 5. Middle Childhood Stress | −.08 −.03 | .18*/.14 | .09/.09 | .45*/.44* | -- | ||||||
| 6. Adolescent Stress | −.17*/−.05 | .01/.02 | .16†/.05 | .26*/.23* | .24*/.19* | -- | |||||
| 7. Young Adulthood Stress | −.18*/−.02 | .16†/.15† | .05/.08 | .21*/.19* | .09/.02 | .33*/.27* | -- | ||||
| 8. Concurrent Stress | −.19*/−.13 | .09/.10 | .16*/.11 | .17*/.09 | −.01/−.04 | .21*/.15† | .53*/.48* | -- | |||
| 9. Maternal Sensitivity | .19*/.04 | −.23*/−.22 | −.14†/−.06 | −.16*/−.14 | −.18*/−.19* | −.09/−.06 | −.13†/−.07 | −.15†/−.13 | -- | ||
| 10. Sex | −.09/.13 | −.05/−.09 | .16*/.11 | .14*/.11 | .04/−.04 | .12†/.01 | .09/−.04 | .16*/.10 | .05/.02 | -- | |
| 11. Race (white vs. nonwhite) | .11/.03 | −.25*/−.14 | .14†/.13 | −.02/.08 | −.14†/−.13 | .04/.02 | −.12/−.08 | −.18*/−.17† | .31*/.27* | .03/.12 | -- |
| Mean | 3.25 | 28.49 | .39 | .002 | .003 | .009 | .004 | 8.54 | .01 | −.09 | .64 |
| SD | 1.01 | 7.29 | .53 | .76 | .74 | .72 | .82 | 6.80 | .70 | 1.0 | .48 |
Values listed are rwithout partialling/rpartialling neuroticism
p<.10
p<.05
**p<.01
***p<.001
Physical health measures
The physical health measures were collected at age 32. Participants provided an overall Physical Health Rating on a Likert-type scale ranging from 5 (Excellent) to 1 (Poor. To assess body fat, their body mass index (BMI) was calculated from self-reports of their height and weight (Center for Disease Control, 2011). Illness/Symptom Counts were assessed using the Adult Health Survey, a health questionnaire adapted from the Adolescent Health Survey appropriate for use with adults (Blum et al., 1989). Participants indicated how often they had experienced different physical illnesses/symptoms within the past 12 months. Sixty percent reported no physical illness/symptoms at age 32. Given the skewness of this measure, responses were log transformed using the natural log.
Life stress
Life stress was assessed at 16 time-points when participants were between 12 months and 32 years old (see Figure 1). At each time-point, life stress was measured by an in-depth interview, the Life Events Schedule (LES), which contains 39-47 questions (questions were added over time) that assess whether and the extent to which different potentially stressful life events occurred during the past year. The questions tapped a wide variety of stressors, ranging from financial issues, to fighting with romantic partners, to being a victim of crime. Each participant's answers were audio-recorded. Each stressful event was then rated by trained coders according to how disruptive it was on a scale ranging from 0 (no disruption; e.g., minor changes in job responsibilities) to 3 (extreme disruption; e.g., having a parent commit suicide). Each mother completed the LES until her child (the target participant) was 17.5 years old, after which each target participant completed the LES (ICC > .94). At age 16 and 19, each target participant completed an adolescent life stress measure, which contained 67 potentially stressful events (similar to those in the LES), but geared to an adolescent population. For each event, participants reported whether or not a potentially stressful event had occurred (1=yes, 2=no), how frequently it occurred (1=less than once a month, 2=once a month or more), and how much it impacted them (0=very little/no impact to 2= major/extreme negative impact). Occurrence, frequency, and impact were then multiplied to create a stress score for each item.
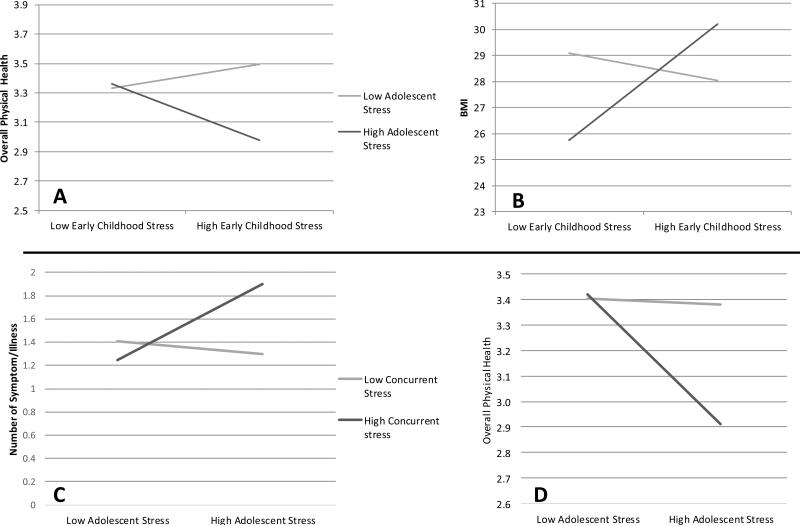
Figure 1.
Interaction effects of stress at multiple life stages
Stress ratings for each event were summed within each assessment period to create a total stress score for each participant. Assessments were then grouped into four life stages: early childhood (5 assessments between birth and age 5), middle childhood (4 assessments between ages 6-12), adolescence (3 assessments between age 13-19), and young adulthood (3 assessments between age 21-31). Total life stress scores were standardized at each assessment to account for varying numbers of items/responses, after which all of the standardized total stress scores within each life stage were averaged to create a Total Stress Composite for that life stage. Life stress at age 32 was also standardized and used to index concurrent life stress (ICC=.98).
Maternal sensitivity
Ratings of developmentally age-appropriate maternal sensitivity were made by a different set of independent trained coders. (Paternal sensitivity was not used because father involvement in the sample was intermittent or absent). These ratings were based on observational assessments of mother-child interactions in the home or lab conducted at seven time-points between 3 months and 13 years, which were coded for the extent to which mothers gave responsive, sensitive support to their child (see the supplemental file for descriptions of tasks, coding, and reliability for each assessment; Raby et al., 2015).
Control measures
Demographics, including the sex and race/ethnicity of each participant, were assessed at birth. Because most participants were white, race/ethnicity was treated as a binary variable: white (1) or non-white (0). Neuroticism was assessed at age 32 using the 35-item scale from the Berkeley Personality Profile (α=.84) (John & Srivastava, 1999).
Procedure
Initial consent to participate was provided by each target participant's mother when the target participant was an infant. Target participants began giving their own consent for each assessment at age 13. The age 32 year assessment involved interviews and questionnaires administered by trained staff. Assessments took place in the lab, in participants’ homes, or over the phone. Each participant received $100 for participating at age 32.
Results
Hypothesis 1: Associations between Life Stress Stages and Age 32 Health Outcomes
The effects of stress at each life stage on the three health outcomes (Overall Physical Health, BMI, and Illness/Symptom Count) are shown in Table 1. Concurrent stress was significantly correlated with two of the three health outcomes (Overall Physical Health and Illness/Symptom Count), and adolescent stress was significantly associated with Overall Physical Health and marginally associated with Symptom/Illness Count. Early childhood stress was significantly associated with BMI and marginally associated with Overall Physical Health, while young adulthood stress was significantly correlated with Overall Physical Health and marginally correlated with BMI. Middle childhood stress was significantly associated with only BMI. Given the theoretical importance of early childhood stress (Miller et al., 2011), the large time gap between early childhood and age 32, and the high covariation between young adult and concurrent stress (see Table 1), the analyses reported below focus on early childhood stress, adolescent stress, and concurrent stress.
Exploratory Question 1: Interaction Effects between Stress at Different Life Stages
Table 2 shows the tests for interactions between stress at different life stages that are related to adult health. The first model tested the predictive power of early childhood stress, adolescent stress, and their interaction. Early childhood and adolescent stress significantly interacted to predict BMI (see Figure 1, panel A), such that individuals with higher stress at both life stages had the highest BMI at age 32. There also was a marginal interaction between early childhood and adolescent stress predicting Overall Physical Health (see Figure 1, panel B). Specifically, individuals who experienced higher stress at both life stages rated their physical health as marginally worse than those who experienced higher stress at only one or neither life stage.
Table 2.
Regression models predicting health from early childhood, adolescent, and concurrent life stress. Grey boxes represent terms not included in that model.
| Early & Adolescent | Early & Concurrent | Adolescent & Concurrent | Early, Adolescent, & Concurrent | |
|---|---|---|---|---|
| b | b | b | b | |
| Overall Physical Health Rating | ||||
| Early Childhood Stress | −.10/−.08 | −.16/−.16 | -- | −.08/−.08 |
| Adolescent Stress | −.17/−.10 | -- | −.18†/−.14 | −.19†/−.13 |
| Concurrent Stress | -- | −.18*/−.07 | −.11/−.03 | −.16*/−.06 |
| Early * Adolescent Stress | −.25†/−.18 | -- | -- | −.26†/−.21 |
| Early * Concurrent Stress | -- | .03/.07 | -- | .12/.12 |
| Adolescent*Concurrent Stress | -- | -- | −.17†/−.12 | −.28*/−.19 |
| Early*Adolescent*Concurrent Stress | -- | -- | -- | .18/.11 |
| BMI | ||||
| Early Childhood Stress | 1.36†/1.13 | 1.62*/1.64* | -- | 1.32†/1.11 |
| Adolescent Stress | −.42/−.35 | -- | −.08/.07 | −.40/−.29 |
| Concurrent Stress | -- | .34/.35 | .53/.53 | .51/.44 |
| Early * Adolescent Stress | 2.16*/2.16* | -- | -- | 2.46*/2.49* |
| Early * Concurrent Stress | -- | .58/.58 | -- | .59/.62 |
| Adolescent*Concurrent Stress | -- | -- | .06/.03 | −.35/−.33 |
| Early*Adolescent*Concurrent Stress | -- | -- | -- | −.66/−.70 |
| Symptom/Illness Count | ||||
| Early Childhood Stress | .04/.03 | .05/.04 | -- | .04/.03 |
| Adolescent Stress | .11/.06 | -- | .12†/.08 | .11†/.08 |
| Concurrent Stress | -- | .09*/.06 | .06/.05 | .07/.06 |
| Early * Adolescent Stress | −.03/−.05 | -- | -- | −.05/−.06 |
| Early * Concurrent Stress | −.06/−.06 | −.06/−.07 | ||
| Adolescent * Concurrent Stress | .17*/.15* | .18*/.16* | ||
| Early*Adolescent*Concurrent Stress | .01/−.01 | |||
Values listed are bno control/bneuroticism control
p<.10
p<.05
**p<.01
Next, models were run predicting health outcomes from early childhood stress, concurrent stress, and the interaction between the two. No interactions were found (see Table 2).
Additionally, models were run predicting health outcomes from adolescent stress, concurrent stress, and the interaction between the two (see Table 2). There was a significant interaction predicting Symptom/Illness Count, such that individuals who experienced higher stress in adolescence and concurrently reported more symptoms and illnesses than those who experienced higher stress at only one or neither life stage (see Figure 1, panel C). There was also a marginally significant interaction predicting Overall Physical Health, such that individuals who experienced higher stress at both life stages rated their physical health as somewhat worse than those who experienced higher stress at only one or neither life stage (see Figure 1 panel D).
Finally, a model was run that included early childhood stress, adolescent stress, concurrent stress, and all possible two-way and three-way interactions. The previously identified early childhood by adolescent stress interactions remained robust in this larger model, and no significant three-way interactions emerged (see Table 2).
Exploratory Question 2: Comparing Life Stages and Cumulative Stress Models
Stepwise regression models tested for additive predictive effects of life stress at other life stages. The base model, identified in Exploratory Question 1, included the main effects of early childhood, adolescent, and concurrent life stress and the interactions between early and adolescent life stress and between adolescent and concurrent stress. This model explained 9.7%, 7.7%, and 5.1% of the variance in Overall Physical Health, BMI, and Illness/Symptom Count, respectively. Adding middle childhood and/or young adulthood life stress did not produce significant changes in life stress (R2 change < .02, all ps > .056). The regression coefficients for middle childhood and young adult stress were non-significant in all of these models.
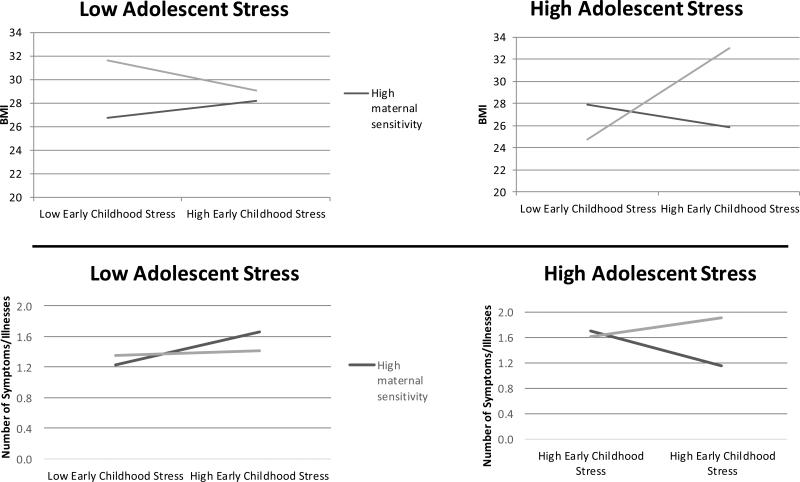
Hypothesis 2: Buffering Effects of Maternal Sensitivity
The tests of buffering effects of maternal sensitivity on life stress predicting health outcomes are shown in Table 3. For maternal sensitivity, early life stress, and adolescent stress, there was a significant three-way interaction predicting BMI and a marginal three-way interaction predicting Symptom/Illness Count (see Figure 2). In both cases, experiencing higher stress at both life stages in combination with lower maternal sensitivity predicted poor adult health outcomes, whereas experiencing lower life stress at both life stages in combination with higher maternal sensitivity predicted lower BMI and fewer Symptoms/Illnesses. Importantly, however, individuals who experienced higher life stress during both early childhood and adolescence and also experienced higher maternal sensitivity had equally good health outcomes as those with no risk factors (i.e., lower life stress at both life stages and higher maternal sensitivity; >56% overlap in marginal mean confidence intervals).
Table 3.
Regression models showing the buffering role of maternal supportive care on stress and health outcomes
| Overall Physical Health Rating | BMI | Symptom/Illness Count | |
|---|---|---|---|
| Early by Adolescent Model | b | b | b |
| Early Childhood Stress | −.04/−.06 | .97/.79 | .03/.03 |
| Adolescent Stress | −.11/−.08 | −.74/−.60 | .07/.03 |
| Concurrent Stress | −.12/−.03 | .24/.25 | .06/.04 |
| Maternal Sensitivity | .21/.06 | −1.90*/−1.67 | −.07/−.04 |
| Early Childhood Stress * Adolescent Stress | −.18/−.14 | 1.52/1.54 | −.12/−.13 |
| Early Childhood Stress * Maternal Sensitivity | .16/.18 | −1.31/−1.53 | −.06/−.05 |
| Adolescent Stress * Maternal Sensitivity | .02/−.07 | .38/.84 | −.13/−.16 |
| Early Childhood Stress * Adolescent Stress * Maternal Sensitivity | .29/.23 | −4.56*/−4.82* | −.25†/−.23 |
| Adolescent by Concurrent Model | |||
| Early Childhood Stress | −.03/−.06 | 1.09/1.05 | −.01/.00 |
| Adolescent Stress | −.12/−.09 | −.45/−.31 | .10/.06 |
| Concurrent Stress | −.13/−.06 | .39/.50 | .06/.05 |
| Maternal Sensitivity | .20/.08 | −2.09*/−2.16 | −.09/−.07 |
| Adolescent Stress * Concurrent Stress | −.23*/−.17 | .40/.50 | .16*/.14† |
| Adolescent Stress * Maternal Sensitivity | .23/.14 | −1.46/−1.31 | −.13/−.17 |
| Concurrent Stress * Maternal Sensitivity | .11/.11 | −.88/−1.21 | −.04/−.03 |
| Adolescent Stress * Concurrent Stress * Maternal Sensitivity | .90*/.77* | −4.79*/−5.47* | −.16/−.13 |
Values listed are bno control/bneuroticism control
p<10
p<.05
**p<.01
***p<.001
Figure 2.
Buffering effects of maternal sensitivity on early childhood stress and adolescent stress
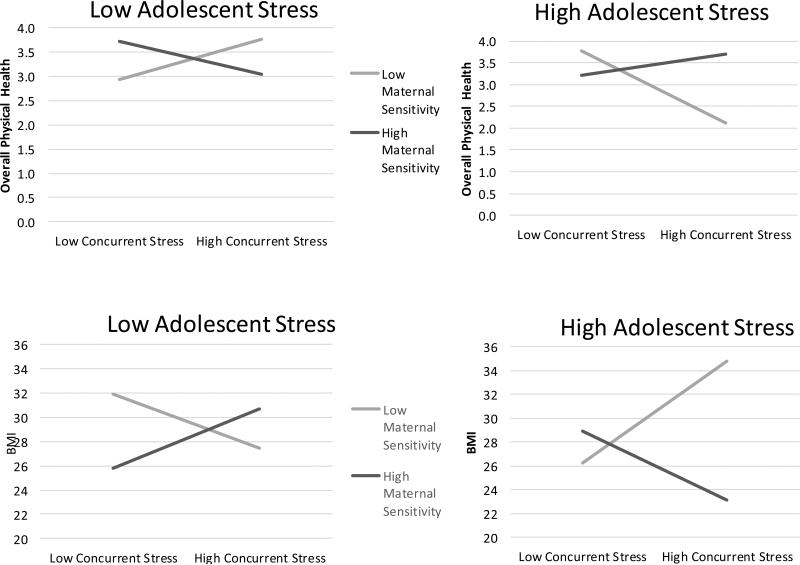
Similar patterns were found for the interaction between maternal sensitivity, adolescent stress, and concurrent stress. The three-way interaction was significant for both BMI and Overall Physical Health. As in previous analyses, experiencing higher stress at both life stages and lower maternal sensitivity predicted poorer adult health outcomes, whereas experiencing lower life stress at both life stages in combination with higher maternal sensitivity predicted lower BMI and fewer Symptoms/Illnesses. Once again, individuals who experienced higher life stress during both adolescence and concurrently and also received higher maternal sensitivity had equally good health outcomes as those with no risk factors (i.e., lower life stress at both life stages and higher maternal sensitivity; >58% overlap in marginal mean confidence intervals).
Control Variables
All of the models reported above were also run controlling for participants’ sex and race (white/nonwhite). Neither demographic variable changed the pattern of results—all significant effects remained significant or marginal. Controlling for neuroticism, however, resulted in the correlations between stress beyond early childhood and health no longer being significant (see Table 1). Controlling for neuroticism also rendered the early childhood stress by adolescent stress interaction for Overall Physical Health (see Table 2) and the three-way interaction with maternal sensitivity for Symptom/Illness Count (see Table 3) no longer significant. All effects for BMI, however, remained statistically significant controlling for neuroticism.
Discussion
This study is the first prospective longitudinal research to test for the effects of stress at different life stages on physical health outcomes in adulthood. As expected, exposure to greater stress in early childhood (0-5 years old), adolescence (16-21 years), and concurrently (at age 32) was associated with adult health outcomes, with higher levels of stress at each period predicting poorer health outcomes. These findings are consistent with previous research emphasizing the importance of early life experiences for later health outcomes (see Miller et al., 2011). However, they also suggest that focusing on only the first few years of life may lead researchers to underappreciate the impact that stress at other life stages exerts on health outcomes.
These effects were not perfectly consistent across the three health outcomes. However, the outcomes we measured were diverse and only moderately correlated, so some variation across measures would be expected. The effects were also fairly small in magnitude, with adolescent and concurrent stress having larger (but not significantly larger) correlations with adult health outcomes than early life stress did. This may be due to the shorter time-lags between these more contemporary assessments and our age 32 health measures. In general, the impact of earlier developmental experiences—even those that have lasting direct effects—typically decline dramatically before asymptoting at non-zero levels (Fraley et al., 2013). Early life stress may have a similar developmental pattern in terms of forecasting adult health problems. In addition, there are many other predictors of health that may be unrelated to life stress, such as engaging in healthier behaviors, which might explain a greater portion of the variance in health outcomes.
The current study also provides evidence that experiences at different life stages should be studied in concert. Early and adolescent stress, and adolescent and concurrent stress, combined to reveal a dual-risk pattern for two of the health outcomes, such that individuals who experienced greater stress at both of these life stages had the worst health outcomes at age 32. Prior studies have not tested for these types of effects because they have focused on stress at a single life stage. These findings should encourage future researchers to examine interaction effects more systematically by determining whether and how separate “sensitive periods” may be linked or codependent with regard to predicting different types of adult health outcomes.
Our findings also reveal that measuring stress at all life stages does not necessarily increase predictive power. Focusing on stress during early childhood, adolescence, and concurrently (rather than using a cumulative model) provided the best fit to the MLSRA data. When middle childhood and young adult stress were added to the model, neither had a significant or marginally significant effect, and they added little predictive power. Similar patterns of “early childhood risk” results have been found to predict other outcomes, such as negative adolescent behaviors across development (e.g., Appleyard et al., 2005). Viewed together, these results lend further support for the importance of early childhood, adolescent, and current stress experiences as key life stages impacting adult health outcomes. It also suggests that these life stages may play a more important role in affecting later health outcomes, and they may be fruitful periods to target interventions.
Finally, receiving more supportive, non-obtrusive maternal care during childhood had important effects on adult health outcomes in combination with life stress. Specifically, experiencing higher stress and lower maternal sensitivity resulted in poor health outcomes at age 32. However, individuals who experienced higher stress yet also received more maternal sensitivity in childhood had equally good health outcomes compared to those who experienced lower stress and received higher maternal sensitivity. This shows the potential importance and relevance of close relationships for long-term health outcomes. Higher quality relationships fully buffered the most at-risk individuals (i.e., those who encountered higher levels of early childhood and adolescent stress, or adolescent and concurrent stress) from experiencing poor health outcomes. This mirrors the findings regarding romantic relationship quality and health outcomes in adulthood (Robles et al., 2014). Similar caregiving “resilience” effects have also been found in other domains: for example, although exposure to greater stress early in life predicts more behavior problems later in development, this connection does not exist when children are securely attached to their primary caregivers (Pianta et al., 1990).
These interaction effects also yielded some unexpected patterns. For example, individuals who experienced lower early life stress and higher maternal sensitivity had fairly high illness/symptom counts, regardless of their level of adolescent stress. Individuals who experienced lower stress in both early childhood and adolescence and lower maternal sensitivity had particularly high BMI in adulthood. The explanation for these effects is unclear. Future research should replicate these results and test other variables that may be responsible for them.
However, the effects of life stress on overall physical health ratings and self-reported symptoms were reduced when controlling for neuroticism. This may be attributable to the tendency of highly neurotic people to over-report their health difficulties (Watson & Pennebaker, 2009). However, the effects for our most objective health outcome, BMI, remained robust when neuroticism was controlled, suggesting that early stress and parenting may, in fact, exert a biological toll on health outcomes. Furthermore, neuroticism may be a result of experiencing stress earlier in life, so controlling for neuroticism might also camouflage some of the stress effects at different life stages. A better understanding of the mediators linking life stress and later health could help to distinguish mechanistic variables from control variables.
This study addresses several weaknesses in past research. First, it uses a prospective design: Stress at each life stage and parenting across childhood were assessed as they occurred, and physical health was assessed years later in adulthood. Additionally, parenting was measured using coded observations of parent-child interactions (rather than relying on potentially unreliable retrospective reports). Furthermore, our life stress interviews were much more comprehensive than measures of stress in previous studies. Many different types of stress were assessed, which allowed for the partitioning of the effects of stress versus parenting quality. Maternal sensitivity was correlated .14 with stress in early childhood and .16 with middle childhood stress, confirming that these are distinct constructs with additive effects.
However, the current study has some limitations. First, our sample consisted of individuals born to low income (i.e., living below the poverty line), first-time mothers. This sample, therefore, was at risk for experiencing greater life stress. Our ability to find effects even in this relatively at-risk group indicates the importance of not treating SES as a proxy for stress in general. However, the degree to which our results will generalize to other populations remains unclear. Second, we did not correct for family-wise error in our analyses, so that readers could use a standard benchmark to evaluate our unique data and exploratory results and because our pattern of results was reasonably consistent, but future replications of these results will be important for validation. Furthermore, at age 32, this sample was still relatively young and generally healthy—only 40% of the participants reported having any health symptoms or chronic illnesses by age 32. The effects reported could change if health outcomes were assessed later in life. In addition, we assessed a limited range of health outcomes in adulthood—overall rated physical health, BMI, and reports of health symptoms/illnesses. All of these measures were self-reported and assessed at only a single time-point, meaning that changes in health across time could not be examined. To further validate these findings and test their generalizability, future research should explore whether these findings replicate when physiological health measures or health behaviors are assessed. Measures such as inflammation or telomere length might be better indicators of the toll that life stress has on health, especially in younger adult sample. This could explain the somewhat different interaction patterns for different health measures; certain health outcomes (e.g., more vs. less objectively reported ones) could be affected by stress in unique ways. Another concern with self-reported health measures is that they may not necessarily be accurate and can be susceptible to self-report biases. Indeed, when we controlled for neuroticism, the effects of life stress and maternal sensitivity were reduced for our less objective health measures.
Finally, this study did not examine the mechanisms underlying the maternal sensitivity effects. There are multiple possible pathways through which maternal sensitivity could influence long-term health outcomes. As Chen and colleagues (2011) propose, maternal sensitivity might have biological programming effects early in life that set up individuals to have pro-inflammatory phenotypes, eventually leading to health problems. Alternatively, poor maternal sensitivity could cause children to develop insecure attachment orientations, which are associated with perceiving potentially threatening events as being more frequent and/or more intense (Simpson & Rholes, 2012) and with having poorer emotion regulation skills (Mikulincer & Shaver, 2007) in adulthood. Both of these tendencies could, in turn, lead to heightened stress responses, particularly in interpersonal situations, and perhaps the development of pro-inflammatory phenotypes. Similarly, the effects of timing of maternal sensitivity were not explored because most of our maternal sensitivity assessments were conducted early in childhood, but parenting may also have sensitive periods of impact similar to life stress.
In conclusion, this study is the first prospective study to examine the effects of stress and parenting quality at different life stages predicting physical health outcomes in adulthood. Exposure to higher levels of stress in early childhood (0-5 years old), adolescence (16-21 years), and concurrently (at age 32) had the most deleterious effects on adult health outcomes at age 32. These findings, which are based on some of the best measures of life stress and maternal care ever collected as part of a prospective longitudinal study, are consistent with previous cross-sectional/retrospective research suggesting the importance of early life experiences in forecasting adult health outcomes (see Miller et al., 2011). Our results, however, also indicate that the amount of stress experienced at different life stages should be studied in relation to each other and in relation to protective influences. Exposure to greater early and adolescent stress, or greater adolescent and concurrent stress, for instance, showed a dual-risk pattern, with individuals who encountered greater stress at both of these life stages having poor health outcomes at age 32. Importantly, however, receiving more supportive maternal care during childhood buffered the impact of exposure to life stress. Specifically, individuals who experienced higher stress but also received more maternal sensitivity during childhood had equally good health outcomes as did those who experienced lower stress at both stages and received higher maternal sensitivity, revealing the significance of early relationships on adult health outcomes.
Supplementary Material
Figure 3.
Buffering effects of maternal sensitivity on adolescent stress and concurrent stress
Acknowledgements
We thank Manfred van Dulmen and Ohad Szepsenwol for their statistical advice. This research was supported by National Institute of Aging grant # AG039453 (Jeffry A. Simpson, PI).
References
- Appleyard K, Egeland B, van Dulmen MHM, Sroufe LA. When more is not better: The role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry. 2005;46(3):235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Blum RW, Resnick MD, Bergeisen LG. The state of adolescent health in Minnesota. University of Minnesota Adolescent Health Program. Minneapolis, MN.: 1989. [Google Scholar]
- Center for Disease Control Body Mass Index (BMI) 2015 Retrieved from http://www.cdc.gov/healthyweight/assessing/bmi/
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Associate. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen SE, Syme S. Social support and health. Academic Press; San Francisco: 1985. [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Blender JA. A Multiple-Levels-of-Analysis Perspective on Resilience. Annals of the New York Academy of Sciences. 2006;1094:248–258. doi: 10.1196/annals.1376.029. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: Psychological and social resources as mediators. Journal of Personality and Social Psychology. 1988;54:486–495. doi: 10.1037//0022-3514.54.3.486. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, Rohleder N, Adam EK. Trajectories of relationship stress and inflammatory processes in adolescence. Development and Psychopathology. doi: 10.1017/S0954579415000334. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Armstrong JM. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Way B. Early-life stress and adult inflammation. Current Directions in Psychological Science. 2014;23:277–283. [Google Scholar]
- Fraley RC, Roisman GI, Haltigan JD. The legacy of early experiences in development: Formalizing alternative models of how early experiences are carried forward over time. Developmental Psychology. 2013;49:109–126. doi: 10.1037/a0027852. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Janlert U, Theorell T, Westerlund H, Hammarstrom A. Do peer relations in adolescence influence health in adulthood? Peer problems in the school setting and the metabolic syndrome in middle age. PLOS ONE. 2012;7:39385. doi: 10.1371/journal.pone.0039385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, Srivastava S. The Big Five trait taxonomy: History, measurement, and theoretical perspectives. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and research. 2nd ed. Guilford Press; New York: 1999. pp. 102–138. [Google Scholar]
- Lake AA, Mathers JC, Rugg-Gunn AJ, Adamson AJ. Longitudinal change in food habits between adolescence (11–12 years) and adulthood (32–33 years): The ASH30 Study. Journal of Public Health. 2006;28:10–16. doi: 10.1093/pubmed/fdi082. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nature Reviews: Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition. 2010;72:73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in adulthood: Structure, dynamics, and change. Guilford Press; New York: 2007. [Google Scholar]
- Miller GE, Brody GH, Yu T, Chen E. A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences. 2014;111:11287–11292. doi: 10.1073/pnas.1406578111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianta R, Egeland B, Sroufe LA. Maternal stress and children's development: Prediction of school outcomes and identification of protective factors. In: Rolf JE, Masten A, Cicchetti D, Nuechterlein K, Weintraub S, editors. Risk and protective factors in the development of psychopathology. Cambridge University Press; Cambridge, MA: 1990. pp. 215–235. [Google Scholar]
- Raby KL, Roisman GI, Fraley RC, Simpson JA. The enduring predictive significance of early maternal sensitivity: Social and academic competence through age 32 years. Child Development. 2015;86:695–708. doi: 10.1111/cdev.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa EB, Bower JE, Hammen CL, Najman JM, Brennan PA. A developmental pathway from early life stress to inflammation: The role of negative health behaviors. Psychological Science. 2014;25:1268–1274. doi: 10.1177/0956797614530570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychological Bulletin. 2014;140:140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Padrón E, Sroufe LA, Egeland B. Earned-secure attachment status in retrospect and prospect. Child Development. 2002;73(4):1204–1219. doi: 10.1111/1467-8624.00467. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Rahhal TA, Poon LW. Things learned in early adulthood are remembered best. Memory & Cognition. 1998;26:3–19. doi: 10.3758/bf03211366. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Rholes WS. Devine PG, Plant A, Olson J, Zanna M, editors. Adult attachment orientations, stress, and romantic relationships. Advances in Experimental Social Psychology. 2012;45:279–328. [Google Scholar]
- Tammelin T, Näyhä S, Laitinen J, Rintamäki H, Järvelin MR. Physical activity and social status in adolescence as predictors of physical inactivity in adulthood. Preventive Medicine. 2003;37:375–381. doi: 10.1016/s0091-7435(03)00162-2. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Malarkey W, Glaser R. Individual differences in cardiac sympathetic control predict endocrine and immune responses to acute psychological stress. Journal of Personality and Social Psychology. 1995;69:736–743. doi: 10.1037//0022-3514.69.4.736. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. 1996 doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Waldie KE. Childhood headache, stress in adolescence, and primary headache in young adulthood: A longitudinal cohort study. Headache: The Journal of Head and Face Pain. 2001;41:1–10. doi: 10.1046/j.1526-4610.2001.111006001.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.