Abstract
Background
The influence of insulin and insulin resistance (IR) on children’s weight and fat gain is unclear.
Objective
To evaluate insulin and IR as predictors of weight and body fat gain in children at high-risk for adult obesity. We hypothesized that baseline IR would be positively associated with follow-up BMI and fat mass.
Subjects/Methods
249 healthy African American and Caucasian children, age 6–12y, at high-risk for adult obesity because of early-onset childhood overweight and/or parental overweight, were followed for up to 15y with repeated BMI and fat mass measurements. We examined baseline serum insulin and HOMA-IR as predictors of follow-up BMI Z score and fat mass by DEXA in mixed model longitudinal analyses accounting for baseline body composition, pubertal stage, sociodemographic factors, and follow-up interval.
Results
At baseline, 39% were obese (BMI ≥95th percentile for age/sex). Data from 1,335 annual visits were examined. Children were followed for an average of 7.2±4.3y, with a maximum follow up of 15 years. After accounting for covariates, neither baseline insulin nor HOMA-IR was significantly associated with follow up BMI (p’s>.26), BMIz score (p’s>.22), fat mass (p’s>.78), or fat mass percentage (p’s>.71). In all models, baseline BMI (p<.0001), body fat mass (p<.0001), and percentage fat (p<.001) were strong positive predictors for change in BMI and fat mass. In models restricted to children without obesity at baseline, some but not all models had significant interaction terms between body adiposity and insulinemia/HOMA-IR that suggested less gain in mass among those with greater insulin or insulin resistance. The opposite was found in some models restricted to children with obesity at baseline.
Conclusions
In middle childhood, BMI and fat mass, but not insulin or IR, are strong predictors of children’s gains in BMI and fat mass during adolescence.
Keywords: Obesity, overweight, prospective, longitudinal, prediction, hyperinsulinemia, insulin resistance, pediatric, child, maturation, growth
Introduction
It is well-established that excessive weight gain is associated with increases in fasting insulin concentrations and the development of greater insulin resistance.1–7 Some evidence, however, points to a perhaps more intriguing possibility: that hyperinsulinemia and/or insulin resistance may primarily induce undue weight gain. Experimental data demonstrate that insulin may act within the central nervous system to regulate energy homeostasis.8 Central insulin administration affects energy balance in a manner similar to acute injections of leptin within the hypothalamus,9, 10 causing decreased energy intake. The ability of central insulin administration to diminish food intake is, however, impaired in both obese animals11 and obese humans,12 suggesting that both animals and humans with obesity and insulin resistance might be at particularly high risk for weight gain that might be attributable to their insulin resistance. Indeed, there are data suggesting that, among obese children, hyperinsulinemia and insulin resistance are positively associated with energy intake at buffet meals independent of children’s body composition.13
Epidemiological studies remain inconclusive as to whether hyperinsulinemia or insulin resistance may influence weight gain.14, 15 Some,16, 17 but not all,18–26 longitudinal reports in adults have found that higher insulin concentrations or greater insulin resistance predict greater weight and fat gain. Similarly, among pediatric cohorts, some,27–31 but not all32–34 studies have found that those with the highest insulin concentrations or insulin resistance have the greatest increases in weight and adipose tissue over time; some pediatric studies find differences in the insulin resistance-weight gain relationship according to sex7 or age.35 However most childhood studies have reported results from unselected samples that were not representative of children at unusually high risk for development of adult obesity. Thus extant findings may not necessarily identify predictors of excessive fat gain that clinicians might use to select children at the highest risk who should be especially targeted for early intensive interventions. Research that examines baseline insulinemia and insulin resistance as predictors of future adiposity in children believed to be at high-risk for adult obesity is lacking.
We therefore analyzed data from a prospective, longitudinal study of children at high risk for development of adult obesity to determine the influence of serum insulin and insulin resistance as predictors of change in body mass index (BMI) and adiposity during childhood and adolescence. We hypothesized that there would be a positive association between baseline serum insulin/insulin resistance and weight/fat mass over time in children at high risk for adult obesity. We expected that children with the most severe insulin resistance would be relatively insensitive to insulin’s central weight-regulating effects throughout childhood, and would therefore have increased BMI and fat mass over time as compared to children with insulin concentrations more commensurate with their fat mass.
Subjects and Methods
Subjects
African American and Caucasian children, ages 6–12y, from the Washington, DC-Metropolitan region were recruited from 1996–2008 to participate in a longitudinal observational study (www.ClinicalTrials.gov ID: NCT00001522) using mailings sent to Washington, DC metropolitan area schools, advertisements in local newspapers, and referrals from local physicians for participation in non-intervention studies of healthy volunteers. Children were eligible if they were believed to be at high-risk for adult overweight/obesity either because they were obese during childhood, with a BMI for age, race, and sex ≥95th percentile based on the first National Health and Nutrition Examination Survey standards36 or were not obese (BMI between the 5th and 95th percentile) but had two overweight parents with BMI ≥ 25kg/m2. Participants were included only if they reported that all of their grandparents were either all Non-Hispanic Black or all Non-Hispanic White. Exclusion criteria included any serious renal, hepatic, pulmonary, endocrinologic, or psychiatric disorder. A negative pregnancy test was required from all female participants of childbearing age. The protocol was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Written consent was obtained from parents/guardians of all participants under age 18y and from participants age 18y or older; written assent was obtained from all minors.
Methods
Participants were seen at the Clinical Research Center of the National Institutes of Health for a baseline visit and subsequently invited for annual follow-up visits for a maximum of 15 years. Subjects who did not attend a visit were invited to return each subsequent year. At the baseline physical examination, testicular volume (mL) was measured using a set of orchidometer beads as standards according to Prader;37 breast and pubic hair development were assigned according to the five stages of Tanner.38, 39 Height and weight were measured at each visit after an overnight fast and in minimal clothing without shoes. Weight was measured to the nearest 0.1kg (Scale-Tronix, Wheaton, IL). Height was recorded as the average of three measurements to the nearest 1mm using a stadiometer, calibrated prior to each measurement (Holtain Ltd., Crymych, UK). BMI standard deviation (BMIz) scores were calculated for analyses according to the Centers for Disease Control and Prevention 2000 standards.40 Weight status was characterized as non-overweight (BMI 5th to < 85th percentile), overweight (BMI ≥85th to <95th percentile), or obese (BMI≥95th percentile).41 Body fat mass was measured at each visit by dual-energy x-ray absorptiometry (DEXA, QDR 2000 pencil beam or 4500A fan beam densitometer; Hologic, Waltham, MA) as previously described.42 Baseline serum insulin and glucose were measured in the morning after an overnight fast of 10 to 12 hours. Glucose samples were centrifuged rapidly and measured on a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN). Insulin concentrations were determined using an immunochemiluminometric assay (Diagnostic Product Corporation) on an Immulite 2000 machine (Diagnostic Product Corporation, Los Angeles, CA). The cross-reactivity of the insulin assay with proinsulin was < 8% and with C-peptide was < 1%, sensitivity was 2 μU/mL and the mean inter- and intra-assay coefficients of variation were 5.8 and 3.6%, respectively. Homeostasis model of assessment-insulin resistance (HOMA-IR) was calculated43 as the product of fasting glucose concentration (mmol/L) and fasting insulin concentration (μU/L) divided by 22.5. Socioeconomic status was determined at baseline for both parents by the Hollingshead two factor index of social position.44 Educational attainment and type of employment were used to assign participants to 5 strata. For parents with discordant strata, the higher stratum was assigned.
Statistical Analysis
Data were analyzed using a series of eight mixed models that differed with respect to the combination of dependent variable (BMIz, BMI, fat mass, or percentage fat) and primary independent variable (log(insulin) or log(HOMA-IR)). Both BMI and BMIz were modeled because there are differences of opinion in the literature regarding which represents the most appropriate metric for longitudinal studies in children,45 largely because of the insufficient data at the extremes of BMI in the samples used to establish BMIz standards. Each model included fixed effects for the primary independent variable and child-specific random intercepts and slopes over time, along with a random quadratic time effect. Fixed subject-specific covariates included sex, race, socioeconomic status, age at baseline and pubertal stage. Height (both baseline and follow-up) was included in models with fat mass as the dependent variable. Each model included all two- and three-way interactions among the primary independent variables (baseline log(insulin) or log(HOMA-IR), baseline value of the dependent variable, follow-up interval (years in study), and follow-up interval squared for quadratic models). Interactions with race were not modeled. Baseline BMI, BMIz, fat mass, fat percent, log(insulin) and log(HOMA-IR) were centered so that interaction coefficients could be interpreted at the mean of the other variables. Initially each model fitted a quadratic growth curve to the available outcome data for each child. However the quadratic term was not significant (all P > .98 based on a likelihood ratio test) for models with fat mass as the dependent variable and was subsequently deleted. Only the linear models are therefore presented for fat mass and fat percentage. The error structure included serial correlation with a power structure allowing the correlation to depend on the time interval between repeated observations on the same child. For models with fat mass or percentage fat as the dependent variable, this error structure yielded an infinite likelihood so a simpler compound symmetric error structure was assumed. Model parameters were estimated by restricted maximum likelihood using the MIXED procedure in SAS 9.2 (SAS Institute, Inc., Cary, NC). The full results of these models are shown in Supplemental Appendix A, Tables 1–8.
Based on the use of the data to fit 8 models, a Bonferroni correction was applied; thus only nominal p-values < .00625 were considered significant. Significance levels in tables are reported as adjusted p-values (value × 8) and as 1.00 when multiplication would produce values exceeding 1. The models were then used to generate fitted values for individuals over time at the 25th (lower) and 75th (higher) percentiles for the observed data for baseline BMI/BMIz, insulin, and HOMA-IR, holding all other variables in the model constant at the mean value. These fitted values along with corresponding standard errors are used for graphical presentation of the data in Figures.
To examine the possibility that children with- and without obesity have a different relationship between insulinemia/insulin resistance and growth in BMI/fat mass, the data were also split into cohorts of children with obesity and children without obesity at baseline, and similar exploratory analyses performed with adjusted p-values (value × 8) reported (Supplementary Appendices B and C).
Results
A total of 283 eligible children completed an initial visit for determination of BMI. During that visit, 263 had baseline insulin and glucose values. 249 returned for at least one follow-up visit and thus met the inclusion criteria for BMI analysis. 218 had both baseline fat mass measurement and cotemporaneous laboratory data, among whom 178 children had follow-up data available for fat mass analysis (Table 1). Children not included in the follow-up analyses were not significantly different from those included with regard to baseline age, socioeconomic status, BMIz, fat mass, sex, or race distribution for either cohort (all p>.10, data not shown). Among children in the BMI-analysis cohort, at baseline, 15% were overweight (BMI 85th to <95th percentile) and 39% obese (BMI ≥95th percentile). Among children in the fat mass-analysis cohort, at baseline 16% were overweight and 36% obese. Longitudinally, a total of 1335 subject visits were included in the final BMI analysis. For BMI, median follow-up time was 6.5 years (mean±SD 7.2±4.3y) with a median of 4 visits. For fat mass, a total of 844 subject visits were included in the analysis; median follow-up time was 7.1 years (mean±SD 7.4±4.0y) with a median of 4 visits. 18% of subjects were overweight and 40% were obese at their last follow-up visit.
Table 1.
Participant characteristics at baseline.
| BMI cohort (n=249) | Body fat mass cohort (n=178) | |
|---|---|---|
|
| ||
| Sex, n (%) | ||
| Female | 130 (52%) | 93 (52%) |
| Male | 119 (48%) | 85 (48%) |
|
| ||
| Race, n (%) | ||
| Caucasian | 153 (61%) | 113 (63%) |
| African American | 96 (39%) | 65 (37%) |
|
| ||
| Age at baseline (y)* | 9.02 ± 2.0 | 9.33 ± 1.9 |
|
| ||
| Socioeconomic Status (median) | 3.0 | 3.0 |
|
| ||
| Girls’ Tanner stage, n (%) | ||
| Tanner I | 52 (40%) | 42 (45%) |
| Tanner II or III | 66 (51%) | 41 (44%) |
| Tanner IV or V | 10 (7.7%) | 10 (11%) |
| Not available | 2 (1.5%) | 0 (0%) |
|
| ||
| Boys’ Tanner stage based on testicular size (mL), n (%) | ||
| 1–3 ml | 98 (82%) | 67 (79%) |
| 4–12 ml | 16 (13%) | 15 (18%) |
| ≥12 ml | 4 (3.4%) | 3(3.5%) |
| Not available | 1 (0.8%) | 0 (0%) |
|
| ||
| BMI (kg/m2)* | 21.8 ± 7.0 | 22.7 ± 8.2 |
|
| ||
| BMI Z-score* | 1.18 ± 1.15 | 1.08 ± 1.31 |
|
| ||
| Weight status, n (%) | ||
| Non-overweight (BMI 5th to <85th percentile) | 115 (46%) | 86 (48%) |
| Overweight (BMI 85th–95th percentile) | 38 (15%) | 29 (16%) |
| Obese (BMI≥95th percentile) | 96 (39%) | 64 (36%) |
|
| ||
| Parent weight status, n (%) | ||
| Two overweight parents | 156 (62.7%) | 105 (59.0%) |
| One overweight parent | 58 (23.3%) | 48 (27.0%) |
| No overweight parents | 35 (14.0%) | 25 (14.0%) |
|
| ||
| Total body fat mass (kg)* | --- | 14.8 ± 12.4 (1.7–69.8) |
|
| ||
| Fasting insulin (uU/mL)* | 10.0 ±10.1 (2.0–57.7) | 10.2 ± 10.5 (2.0–57.7) |
|
| ||
| HOMA-IR index* | 2.2 ± 2.1 (0.4–12.3) | 2.2 ± 2.3 (0.3–12.3) |
Mean ± SD (range)
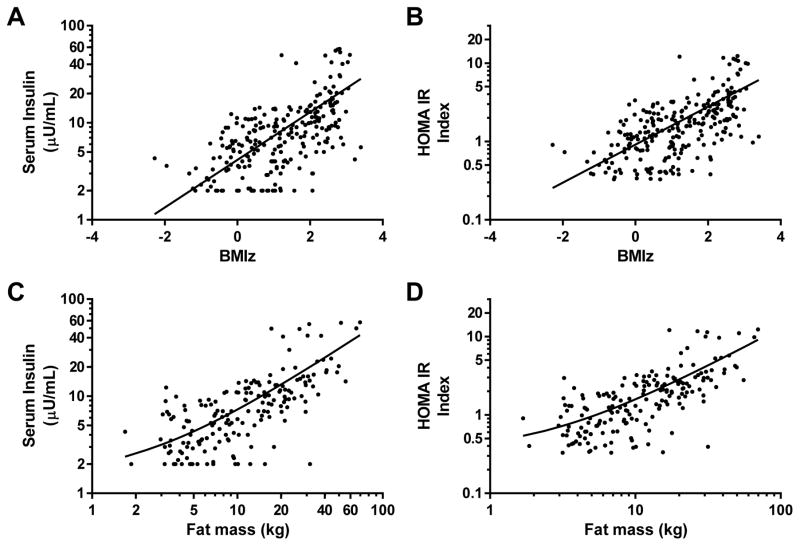
At participants’ baseline visits, fasting insulin and HOMA-IR were significantly related to both BMIz (Figures 1A and 1B) and fat mass (Figures 1C and 1D). However, there was considerable variability in these relationships, such that some children had much higher insulin
Figure 1. Univariate correlations.
Associations at baseline between BMI standard deviation score (BMIz) and (A) log fasting serum insulin (r2=.32, p<.001) and (B) log homeostasis model assessment for insulin resistance (HOMA-IR) index (r2=.32, p<.001). Associations between log fat mass, assessed by dual-energy x-ray absorptiometry and (C) log fasting serum insulin (r2=.49, p<.001) and (D) log homeostasis model assessment for insulin resistance (HOMA-IR) index (r2=.47, p<.001).
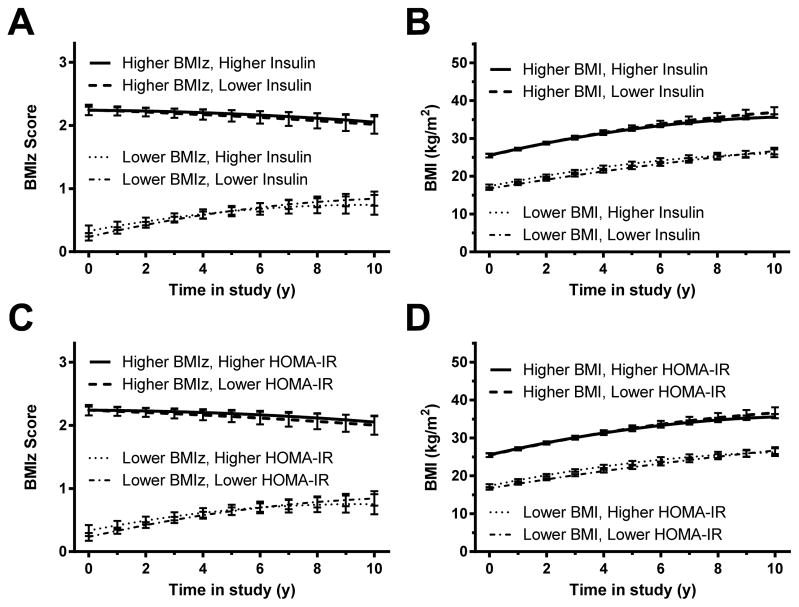
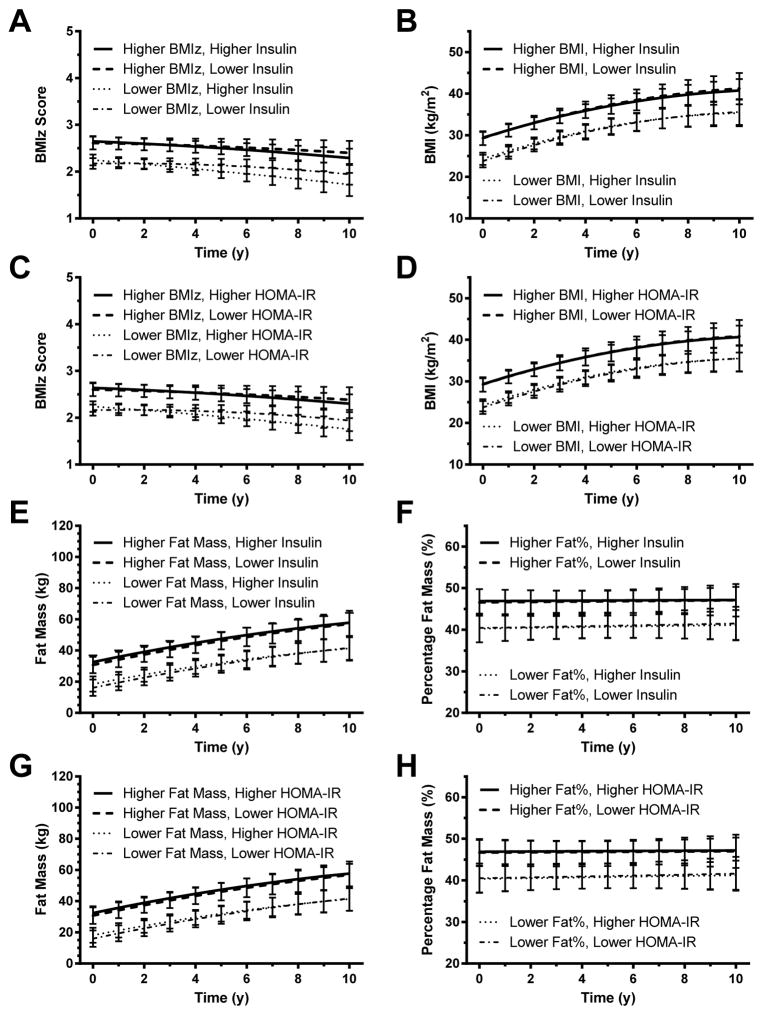
Prediction of change in BMIz and BMI by insulin (Figures 2A and 2B; Supplemental Appendix A, Tables 1 and 2)
Figure 2. Changes in BMI – full cohort.
Associations between baseline fasting insulin and gain in (A) BMI standard deviation score (BMIz) and (B) BMI. Associations between homeostasis model assessment for insulin resistance (HOMA-IR) index and gain in (C) BMIz and (D) BMI. In each analysis, those with higher baseline insulin or HOMA-IR (represented graphically using the 75th percentile for the cohort) did not gain significantly more body mass over time, independent of baseline fat mass and other covariates, than those with lower baseline insulin or HOMA-IR (represented graphically using the 25th percentile; see Subjects and Methods). However, baseline BMIz and BMI were significant positive predictors of later body mass (p<.001 in each analysis).
For the model testing baseline insulin as a predictor of BMIz, children with higher baseline BMIz had significantly higher follow-up BMIz (Figure 2A); baseline BMIz coefficient = 1.0012, adjusted p< .001). Baseline log(insulin) concentration was not, however, significantly associated with follow-up BMIz (adjusted p=1.00). Sex, race, socioeconomic status, baseline age, and pubertal status were similarly not significant predictors of follow-up BMIz.
For the model testing baseline insulin as a predictor of BMI, BMI increased over time (follow-up interval coefficient = 1.4514, p<.001) but at a decreasing rate ([follow-up interval]2 coefficient = −.06022, adjusted p<.001; Figure 2B). Children with higher baseline BMI values had higher BMI at follow-up (baseline BMI main effect = 1.0502, adjusted p<.001); baseline age (coefficient = −.2344, adjusted p=.01) also significantly predicted follow-up BMI. However, baseline log(insulin) was not a significant predictor for BMI (adjusted p=1.00).
Prediction of change in BMIz and BMI by HOMA-IR (Figures 2C and D; Supplemental Appendix A, Tables 3 and 4)
For the model testing baseline HOMA-IR as a predictor of BMIz, baseline BMIz (coefficient = .9993, adjusted p<.001) and the interaction between baseline BMIz and follow-up interval (coefficient = −.05209, adjusted p<.002) were the most important predictors of follow-up BMIz, indicating that while participants with higher baseline BMIz tended to have higher BMI at all time points, BMIz in those with low baseline BMIz tended to increase over time and BMIz in those with high baseline BMIz tended to decrease over time (Figure 2C). This effect may represent regression to the mean. Baseline log(HOMA-IR) was not significantly associated with follow-up BMIz (adjusted p=1.00).
Findings were similar for the model predicting follow-up BMI with baseline HOMA-IR. Baseline BMI was a significant predictor of follow-up BMI (coefficient = 1.0507, adjusted p<.001), along with length of follow-up interval (coefficient 1.4446, adjusted p<.001), baseline age (coefficient −.2399, adjusted p=.008), and [follow-up interval]2 (coefficient −.05951, adjusted p<.001). Baseline log(HOMA-IR), however, was not significantly associated with follow-up BMI (adjusted p=1.00). These results indicate that participants’ BMI tended to increase and then level off over time; those with higher baseline BMI had higher follow-up BMI at all time points, and BMI was lower in those who were older at baseline (Figure 2D).
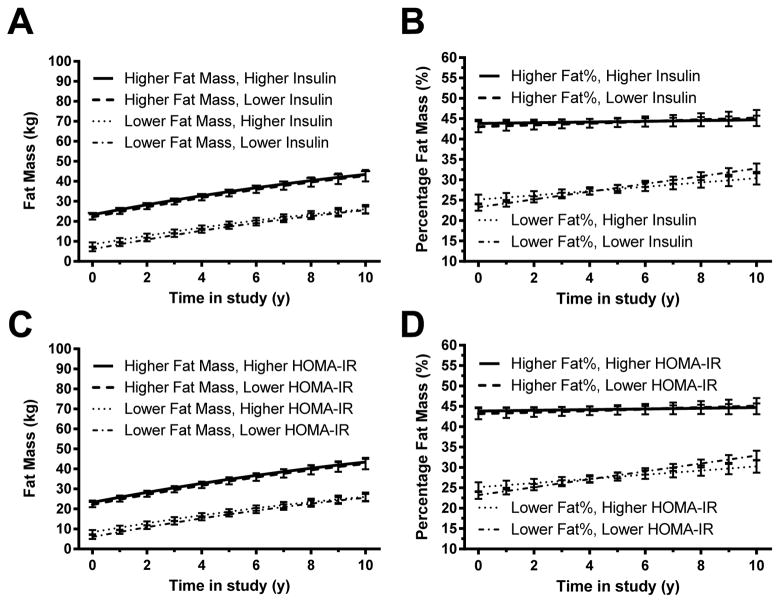
Prediction of change in fat mass and percentage fat by insulin (Figures 3A and B; Supplemental Appendix A, Tables 5 and 6)
Figure 3. Changes in Fat Mass – full cohort.
Associations between baseline fasting insulin and gain in (A) fat mass and (B) percentage body fat mass. Associations between homeostasis model assessment for insulin resistance (HOMA-IR) index and gain in (C) fat mass, and (D) percentage body fat mass. In each analysis, those with higher baseline insulin or HOMA-IR (represented graphically using the 75th percentile) did not gain significantly more body fatness over time, independent of baseline fat and other covariates, than those with lower baseline insulin or HOMA-IR (represented graphically using the 25th percentile; see Subjects and Methods). However, baseline body fat mass and percentage body fat mass were significant positive predictors of later body fatness (p<.001 in each analysis).
For the model predicting change in fat mass with insulin , fat mass was predicted by baseline fat mass (coefficient = 1.1086, adjusted p<.001; Figure 3A), sex (coefficient −2.7937 for females, adjusted p=.018), baseline height (coefficient −.1772, adjusted p=.023), follow-up height (coefficient .2169, adjusted p<.001), and the interaction between length of follow-up interval and sex (coefficient +1.4107 for females, p<.001). Baseline log(insulin), however, did not predict follow-up fat mass (adjusted p=1.00). In general, fat mass was lower in females at first but then increased more quickly over time, was higher in those with higher baseline fat mass and shorter baseline height, and increased with increasing height over time.
For the model predicting change in percentage fat with insulin, percentage fat decreased significantly over time in males at the average value of insulin (follow-up interval coefficient = − 1.0104, adjusted p<.001) and increased over time in females at the average value of baseline insulin (sex x time-in-study interaction coefficient 1.4709 for in females, adjusted p<.001). Participants with higher baseline percent fat had higher values at all time points (coefficient = .9135, adjusted p<.001; Figure 3B), and participants who were younger at the baseline visit had higher percentage fat (coefficient −1.021, adjusted p<.001). Baseline log(insulin), however, did not predict follow-up percentage fat (adjusted p=1.00).
Prediction of change in fat mass and percentage fat by HOMA-IR (Figures 3C and D; Supplemental Appendix A, Tables 7 and 8)
Findings were similar when baseline log(HOMA-IR) was used in the models for fat mass and percentage fat instead of baseline log(insulin). Changes in fat mass over time were not associated with baseline log(HOMA-IR) (adjusted p=1.00). The only significant predictors of fat mass change were baseline fat mass (coefficient = 1.1108, adjusted p<.001; Figure 3C), height at baseline (coefficient = −.1785, adjusted p=.022), follow-up height (coefficient = .2165, adjusted p<.001), sex (coefficient −2.765 for females, adjusted p=.019), and the interaction between follow-up interval and sex (coefficient 1.4154 for females, adjusted p<.001). In this model, fat mass was higher in those with higher baseline fat mass and shorter baseline height and increased with increasing height. Females tended to have lower initial fat mass and a greater increase in fat mass over time.
Changes in percentage fat over time were not associated with baseline log(HOMA-IR) (adjusted p=1.00). Significant predictors of percentage fat were baseline percentage fat (coefficient = .9192, adjusted p<.001; Figure 3D), baseline age (coefficient = −1.0118, adjusted p<.001), length of follow-up interval (coefficient −1.0172, adjusted p<.001), and the interactions of length of follow-up interval with baseline percentage fat (coefficient −.02764, adjusted p=.027) and sex (coefficient −2.2423, adjusted p<.001). Percentage fat was lower in females than males at baseline and tended to decrease over time in males but increase slightly in females. Participants with higher baseline percentage fat and lower baseline age tended to have higher percentage fat.
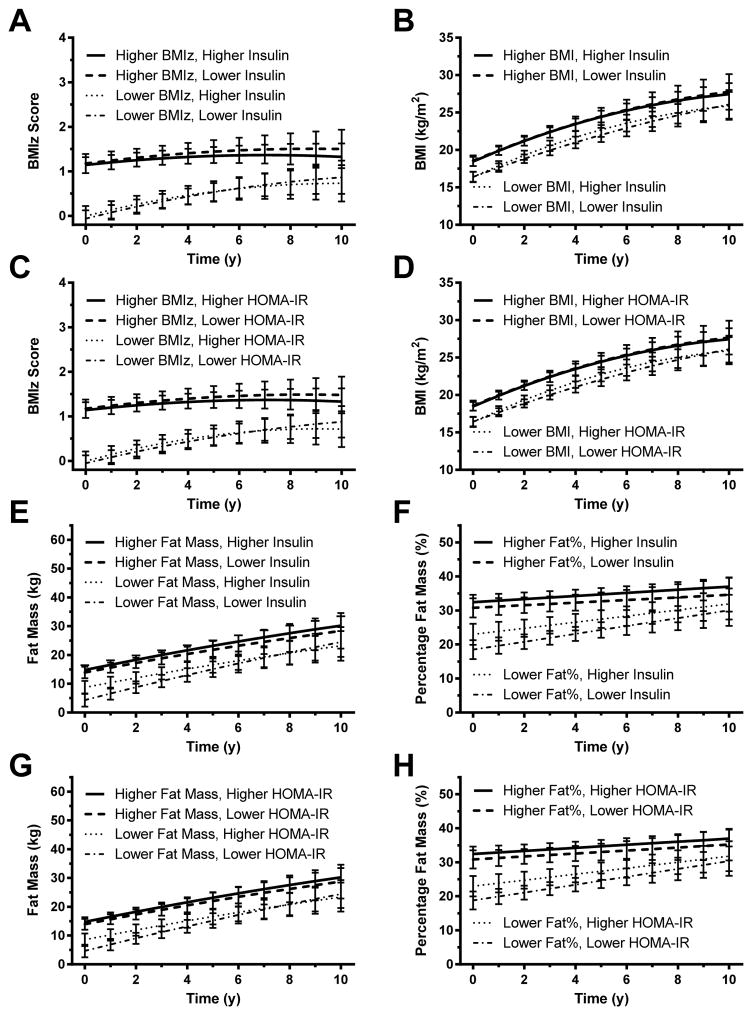
Exploratory analyses of changes in BMI/fat in children without obesity at baseline (Figure 4; Supplemental Appendix B) and in children with obesity at baseline (Figure 5; Supplemental Appendix C)
Figure 4. Children without obesity.
Analysis restricted to children without obesity. Associations between baseline fasting insulin and gain in (A) BMI standard deviation score (BMIz), (B) BMI, (E) fat mass, and (F) percentage fat mass. Associations between homeostasis model assessment for insulin resistance (HOMA-IR) index and gain in (C) BMIz, (D) BMI, (G) fat mass, and (H) percentage fat mass. In each analysis, those with higher baseline insulin or HOMA-IR are represented graphically using the 75th percentile for the cohort and those with lower baseline insulin or HOMA-IR are represented graphically using the 25th percentile; see Subjects and Methods).
Figure 5. Children with obesity.
Analysis restricted to children with obesity. Associations between baseline fasting insulin and gain in (A) BMI standard deviation score (BMIz), (B) BMI, (E) fat mass, and (F) percentage fat mass. Associations between homeostasis model assessment for insulin resistance (HOMA-IR) index and gain in (C) BMIz, (D) BMI, (G) fat mass, and (H) percentage fat mass. In each analysis, those with higher baseline insulin or HOMA-IR are represented graphically using the 75th percentile for the cohort and those with lower baseline insulin or HOMA-IR are represented graphically using the 25th percentile; see Subjects and Methods).
Among children without obesity at baseline (61% of the cohort), initial body composition was a strong, significant predictor of later adiposity (Figure 4). Insulinemia and insulin resistance were not significantly predictive of any of the outcomes examined as main factors (Supplemental Tables 9–16). However, for prediction of BMI (but not BMIZ) by serum insulin, the follow-up interval x baseline BMI x baseline log(insulin) interaction was significant with a small negative coefficient (Supplemental Table 10, p=0.036). This finding suggests that while the increase in BMI over time was greatest in those with low baseline BMI and high baseline insulin, BMI may have decreased over time in those with both low baseline BMI and low baseline insulin. Similarly, for prediction of BMI (but not BMIZ) by baseline log(HOMA-IR), the follow-up interval x baseline BMI x baseline log(HOMA-IR) interaction was significant (Supplemental Table 12, p=0.016) with a small negative coefficient; the baseline log(HOMA-IR) x baseline BMI x (Time in study)2 interaction was also significant (Table 12, p=0.027), but with a small positive coefficient. These findings show a similar pattern to the associations among BMI, time in study, baseline BMI and baseline log(insulin) with the added finding that that the degree of leveling off observed in Figure 4D (shown at the average value of log(insulin) may have been less for participants with both high baseline BMI and high baseline log(HOMA-IR). For prediction of fat mass (but not percentage fat mass) by serum insulin, the baseline fat mass x baseline log(insulin) interaction was significant (Table 13, p=0.012) with a small negative coefficient. Similarly, for prediction of fat mass (but not percentage fat mass) by HOMA-IR, the baseline fat mass x baseline log(HOMA-IR) interaction was significant (Table 15, p=0.018) with a small negative coefficient, suggesting that the positive association between baseline fat mass and follow-up fat mass is slightly diminished in participants with higher baseline log(insulin) or higher baseline log(HOMA-IR).
Among children with obesity at baseline (39% of the cohort), initial body composition, was again a strong, significant predictor of later adiposity (Figure 5). Insulinemia and insulin resistance were not significantly predictive of any of the outcomes when examined as main factors (Supplemental Tables 17–24). However, for prediction of BMIz (but not BMI) by insulin, the follow-up interval x baseline BMIz x baseline log(insulin) interaction was significant (Table 17, p=0.044) with a small positive coefficient. Similarly for prediction of BMIz (but not BMI) by HOMA-IR, the follow-up interval x baseline BMIz x baseline log(HOMA-IR) interaction was significant (Table 19, p=0.030) with a small positive coefficient, indicating that the effect of baseline BMIz on change over time is somewhat more positive in participants with higher baseline log(HOMA-IR). For predicting fat mass and percentage fat mass in the obese cohort, no such interactions with insulin or HOMA-IR were significant.
Discussion
The aims of the present study were to evaluate serum insulin and insulin resistance, estimated by the HOMA-IR index, as predictors of follow-up weight and fat gain in children at high risk for adult obesity. We found little evidence for hyperinsulinemia or high insulin resistance as strong predictors of gain in body mass. Although a minority of exploratory models analyzing children with obesity separately had p-values for interaction terms between 0.01 and 0.05 that suggested insulin resistance might predispose to greater weight gain, the opposite was found for models in children without obesity. Regardless of the model, those with higher baseline BMI or fat mass demonstrated greater gains in body mass throughout the study with p-values <0.001 for the main effect of baseline body adiposity.
Many longitudinal pediatric studies have observed that baseline adiposity and BMI are strong prospective predictors of weight and fat gain.7, 31, 35 Since genetic/physiological factors contribute to the tendency to develop obesity even during early childhood, it is possible that controlling for initial body composition in longitudinal studies could already account for at least some of the potential effect of variables that, like insulin resistance, are cross-sectionally well correlated with adiposity. Controlling for baseline body size might thus make it difficult to detect the roles of variables like insulin resistance that, in analyses uncontrolled for initial body composition, would likely make a significant contribution to the prediction of change in adiposity. If insulin resistance were able to be more reliably and inexpensively determined than BMI, there might conceivably be a rationale for its measurement. However, given the expense and difficulty of accurately measuring insulin resistance,46, 47 the data of the current study offer little justification for measuring insulin or insulin resistance in clinical practice as a predictor of later adiposity in children believed to be at high-risk for adult obesity.
Some investigations have suggested there may be sex-related disparities in the impact of insulin resistance on adipose tissue gain. For example, Hosking et al7 studied HOMA-2 IR as a predictor of gain in body fat among 238 mostly non-overweight children who were followed for 6y between ages 7 to 13y, finding that baseline insulin resistance was positively associated with long term rate of adiposity (but not BMIz) gain in boys, while insulin resistance was negatively associated with long-term adiposity (but not BMIz) gain in girls, perhaps due to sex disparities in humoral factors. In their short term (1y) analyses, no significant effects of insulin resistance, or sex x insulin resistance interactions, on gain in adiposity or BMIz were observed; even the significant long-term associations had small effect sizes.7 In our cohort of children at particularly high-risk for adult obesity, among whom over 50% were overweight or obese at baseline, we found no interaction between insulin resistance and sex in predicting gain in adipose tissue or BMI. In addition to the differences in obesity prevalence, racial differences in study samples might also potentially contribute to these differing results, as the Hosking et al cohort included only five non-white participants.7 Similarly, the findings of Sinaiko et al31 in a cohort of Black and White adolescents finding high baseline glucose infusion rate adjusted for lean body mass from clamp studies (Mlbm) predicted lower BMIz in adolescents had a relatively small effect size. We believe the results of the studies suggest at most a small independent effect of insulin resistance on gain in adiposity. These results are also consistent with the relatively small impact of insulin sensitizers like metformin on body weight in children and adolescents with obesity.48–58
The strengths of the present study lie in its subject selection, its longitudinal nature and the size of its cohort. Most previous studies examining the relationship between insulin resistance and weight gain have either been cross-sectional, or when longitudinal, spanned only a few years or focused more on adults or adolescents than children. The present study included a large cohort of children followed, on average, for more than 6 years, providing one of the larger pools of pediatric longitudinal data reported on this topic across puberty. There are some limitations to our study that should be noted. We used HOMA-IR from fasting samples as our measure of insulin resistance, rather than a clamp-derived index. Although HOMA-IR has been reported to have a high correlation with insulin sensitivity measured by euglycemic-hyperinsulinemic clamps in children,59 it may reflect hepatic insulin sensitivity more than the insulin sensitivity of the whole body or muscle60 and other investigators have not found as high correlations with clamp data.61, 62 Indeed, it is not clear that HOMA-IR offers marked advantages over measuring fasting insulin.47, 62 Our results cannot be considered generalizable to all children because our participants were selected to be at particularly high-risk for developing obesity and were limited to non-Hispanic White and Black American children living in a limited geographical area. Studies conducted using cohorts that represent the normal distribution of weight status in children who lived in a broader region conceivably could reach different conclusions. There were also variations in the duration and frequency of follow-up of study subjects that might have affected results. Thus, it is possible that a larger cohort or a similarly sized starting cohort31 with even more observations might find a contribution of insulinemia or insulin resistance to gain in body mass that could not be detected in the current study. This study is limited by the absence of reliable measures of lifestyle factors such as duration of breast feeding, habitual diet quality, sleep quality, and level of usual physical activity that might be expected to impact growth of adipose tissue. Future research in this area is also needed to examine how baseline insulin resistance and other factors such as adiponectin63 or other adipokines or cytokines may predict development of total fat mass and visceral abdominal, hepatic, or intramyocellular fat, as these fat depots are particularly well correlated with insulin sensitivity/glucose metabolism.64–66
In sum, we found little evidence that hyperinsulinemia or insulin resistance is a strong independent predictor of excessive weight or fat gain among children at high risk for later obesity. Further research is required to identify useful physiological predictors for obesity beyond anthropometric variables.
Supplementary Material
Acknowledgments
Research support and Acknowledgements: Intramural Research Program, NIH, grant 1ZIAHD000641 (to JAY) from NICHD with supplemental funding from the National Institute for Minority Health and Health Disparities (NIMHD) and the Division of Nutrition Research Coordination (DNRC), NIH. NMS and AJK were supported by the Division of Nutrition Research Coordination and the National Institute of Diabetes and Digestive and Kidney Diseases. JAY is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services. The funding organizations played no role in the design or conduct of the study; the collection, management, analysis, or interpretation of data; or the preparation of the manuscript. JAY, SZY, and VSH conceived the experiment, NMS, LY, WS, AJK, RSK, TC, SMB, APD, APB, JCR, and JAY carried out experiments, NMS, CO, JCR, and JAY analysed data. All authors were involved in writing the paper and had final approval of the submitted and published versions. The first draft of the manuscript was written by NMS and JAY.
Footnotes
Clinical Trial Registration: www.ClinicalTrials.gov ID: NCT00001522
Author Disclosure Summary: All of the authors (NMS, CO, LY, WS, AJK, RSK, TC, SMB, AD, JR, SZY, VSH, JAY) have nothing to declare.
References
- 1.Everson SA, Goldberg DE, Helmrich SP, Lakka TA, Lynch JW, Kaplan GA, et al. Weight gain and the risk of developing insulin resistance syndrome. Diabetes Care. 1998;21(10):1637–43. doi: 10.2337/diacare.21.10.1637. [DOI] [PubMed] [Google Scholar]
- 2.Steinberger J, Moran A, Hong CP, Jacobs DR, Jr, Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J Pediatr. 2001;138(4):469–73. doi: 10.1067/mpd.2001.112658. [DOI] [PubMed] [Google Scholar]
- 3.Lakka HM, Salonen JT, Tuomilehto J, Kaplan GA, Lakka TA. Obesity and weight gain are associated with increased incidence of hyperinsulinemia in non-diabetic men. Horm Metab Res. 2002;34(9):492–8. doi: 10.1055/s-2002-34788. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y, Sung E, Yun KE, Jung HS, Kim CW, Kwon MJ, et al. Weight change as a predictor of incidence and remission of insulin resistance. PLoS ONE. 2013;8(5):e63690. doi: 10.1371/journal.pone.0063690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ondrak KS, McMurray RG, Battaglini CL, Evenson KR, Harrell JS. The Relationship between Changes in Weight Status and Insulin Resistance in Youth. International journal of pediatric endocrinology. 2009;2009:862061. doi: 10.1155/2009/862061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein DJ, Aronson Friedman L, Harlan WR, Barton BA, Schreiber GB, Cohen RM, et al. Obesity and the development of insulin resistance and impaired fasting glucose in black and white adolescent girls: a longitudinal study. Diabetes care. 2004;27(2):378–83. doi: 10.2337/diacare.27.2.378. [DOI] [PubMed] [Google Scholar]
- 7.Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Direction of causality between body fat and insulin resistance in children--a longitudinal study (EarlyBird 51) Int J Pediatr Obes. 2011;6(5–6):428–33. doi: 10.3109/17477166.2011.608800. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13(3):387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 9.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, et al. Crosstalk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13(1):48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]
- 10.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–5. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr, et al. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7(4):381–6. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 12.Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav. 2004;83(1):47–54. doi: 10.1016/j.physbeh.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Han JC, Rutledge MS, Kozlosky M, Salaita CG, Gustafson JK, Keil MF, et al. Insulin resistance, hyperinsulinemia, and energy intake in overweight children. J Pediatr. 2008;152(5):612–7. 617 e1. doi: 10.1016/j.jpeds.2007.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravussin E, Swinburn BA. Metabolic predictors of obesity: cross-sectional versus longitudinal data. Int J Obes Relat Metab Disord. 1993;17(Suppl 3):S28–31. discussion S41–2. [PubMed] [Google Scholar]
- 15.Lazarus R, Sparrow D, Weiss S. Temporal relations between obesity and insulin: longitudinal data from the Normative Aging Study. Am J Epidemiol. 1998;147(2):173–9. doi: 10.1093/oxfordjournals.aje.a009431. [DOI] [PubMed] [Google Scholar]
- 16.Sigal RJ, El-Hashimy M, Martin BC, Soeldner JS, Krolewski AS, Warram JH. Acute postchallenge hyperinsulinemia predicts weight gain: a prospective study. Diabetes. 1997;46(6):1025–9. doi: 10.2337/diab.46.6.1025. [DOI] [PubMed] [Google Scholar]
- 17.Tong J, Fujimoto WY, Kahn SE, Weigle DS, McNeely MJ, Leonetti DL, et al. Insulin, C-peptide, and leptin concentrations predict increased visceral adiposity at 5- and 10-year follow-ups in nondiabetic Japanese Americans. Diabetes. 2005;54(4):985–90. doi: 10.2337/diabetes.54.4.985. [DOI] [PubMed] [Google Scholar]
- 18.Valdez R, Mitchell BD, Haffner SM, Hazuda HP, Morales PA, Monterrosa A, et al. Predictors of weight change in a bi-ethnic population. The San Antonio Heart Study. Int J Obes Relat Metab Disord. 1994;18(2):85–91. [PubMed] [Google Scholar]
- 19.Zavaroni I, Zuccarelli A, Gasparini P, Massironi P, Barilli A, Reaven GM. Can weight gain in healthy, nonobese volunteers be predicted by differences in baseline plasma insulin concentration? The Journal of clinical endocrinology and metabolism. 1998;83(10):3498–500. doi: 10.1210/jcem.83.10.5178. [DOI] [PubMed] [Google Scholar]
- 20.Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88(1):168–73. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoag S, Marshall JA, Jones RH, Hamman RF. High fasting insulin levels associated with lower rates of weight gain in persons with normal glucose tolerance: the San Luis Valley Diabetes Study. Int J Obes Relat Metab Disord. 1995;19(3):175–80. [PubMed] [Google Scholar]
- 22.Wedick NM, Mayer-Davis EJ, Wingard DL, Addy CL, Barrett-Connor E. Insulin resistance precedes weight loss in adults without diabetes : the Rancho Bernardo Study. American Journal of Epidemiology. 2001;153(12):1199–205. doi: 10.1093/aje/153.12.1199. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MW, Boyko EJ, Kahn SE, Ravussin E, Bogardus C. Reduced insulin secretion: an independent predictor of body weight gain. J Clin Endocrinol Metab. 1995;80(5):1571–6. doi: 10.1210/jcem.80.5.7745002. [DOI] [PubMed] [Google Scholar]
- 24.Gould AJ, Williams DE, Byrne CD, Hales CN, Wareham NJ. Prospective cohort study of the relationship of markers of insulin resistance and secretion with weight gain and changes in regional adiposity. Int J Obes Relat Metab Disord. 1999;23(12):1256–61. doi: 10.1038/sj.ijo.0801060. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Vitelli LL, Lewis CE, Schreiner PJ, Watson RL, Wagenknecht LE. Is fasting insulin concentration inversely associated with rate of weight gain? Contrasting findings from the CARDIA and ARIC study cohorts. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1998;22(1):48–54. doi: 10.1038/sj.ijo.0800542. [DOI] [PubMed] [Google Scholar]
- 26.Silver RJ, Mehta S, Soeldner JS, Martin BC, Warram JH, Goldfine AB. Acute insulin secretion as a predictor of weight gain in healthy humans. Obesity (Silver Spring) 2006;14(1):67–72. doi: 10.1038/oby.2006.9. [DOI] [PubMed] [Google Scholar]
- 27.Odeleye OE, de Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes. 1997;46(8):1341–5. doi: 10.2337/diab.46.8.1341. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MS, Figueroa-Colon R, Huang TT, Dwyer JH, Goran MI. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab. 2001;86(7):3182–7. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 29.Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr. 2007;85(6):1478–85. doi: 10.1093/ajcn/85.6.1478. [DOI] [PubMed] [Google Scholar]
- 30.Morrison JA, Glueck CJ, Horn PS, Schreiber GB, Wang P. Pre-teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18–19 y: a 10-y prospective study of black and white girls. Am J Clin Nutr. 2008;88(3):778–88. doi: 10.1093/ajcn/88.3.778. [DOI] [PubMed] [Google Scholar]
- 31.Sinaiko AR, Steinberger J, Moran A, Hong CP, Prineas RJ, Jacobs DR., Jr Influence of insulin resistance and body mass index at age 13 on systolic blood pressure, triglycerides, and high-density lipoprotein cholesterol at age 19. Hypertension. 2006;48(4):730–6. doi: 10.1161/01.HYP.0000237863.24000.50. [DOI] [PubMed] [Google Scholar]
- 32.Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. J Clin Endocrinol Metab. 2002;87(8):3814–8. doi: 10.1210/jcem.87.8.8765. [DOI] [PubMed] [Google Scholar]
- 33.Maffeis C, Moghetti P, Grezzani A, Clementi M, Gaudino R, Tato L. Insulin resistance and the persistence of obesity from childhood into adulthood. J Clin Endocrinol Metab. 2002;87(1):71–6. doi: 10.1210/jcem.87.1.8130. [DOI] [PubMed] [Google Scholar]
- 34.Salbe AD, Weyer C, Lindsay RS, Ravussin E, Tataranni PA. Assessing risk factors for obesity between childhood and adolescence: I. Birth weight, childhood adiposity, parental obesity, insulin, and leptin. Pediatrics. 2002;110(2 Pt 1):299–306. doi: 10.1542/peds.110.2.299. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan SR, Myers L, Berenson GS. Temporal association between obesity and hyperinsulinemia in children, adolescents, and young adults: the Bogalusa Heart Study. Metabolism. 1999;48(7):928–34. doi: 10.1016/s0026-0495(99)90231-7. [DOI] [PubMed] [Google Scholar]
- 36.Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index (wt/ht2) and triceps skinfold thickness. The American journal of clinical nutrition. 1991;53(4):839–46. doi: 10.1093/ajcn/53.4.839. [DOI] [PubMed] [Google Scholar]
- 37.Prader A. Testicular size: assessment and clinical importance. Triangle. 1966;7(6):240–3. [PubMed] [Google Scholar]
- 38.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 41.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. US Department of Health and Human Services, Centers for Disease Conrol and Prevention; 2010. [PubMed] [Google Scholar]
- 42.Fleisch AF, Agarwal N, Roberts MD, Han JC, Theim KR, Vexler A, et al. Influence of Serum Leptin on Weight and Body Fat Growth in Children at High Risk for Adult Obesity. J Clin Endocrinol Metab. 2007;92(3):948–954. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 44.Hollingshead AB. Hollingshead two factor index of social position (1957) In: Miller DC, editor. Handbook of research design and social measurement. 5. Sage Publications; Newbury Park, CA: 1991. pp. 351–359. [Google Scholar]
- 45.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59(3):419–25. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 46.Levy-Marchal C, Arslanian S, Cutfield W, Sinaiko A, Druet C, Marcovecchio ML, et al. Insulin resistance in children: consensus, perspective, and future directions. The Journal of clinical endocrinology and metabolism. 2010;95(12):5189–98. doi: 10.1210/jc.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown RJ, Yanovski JA. Estimation of insulin sensitivity in children: methods, measures and controversies. Pediatric diabetes. 2014;15(3):151–61. doi: 10.1111/pedi.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutjens A, Smit JL. Effect of biguanide treatment in obese children. Helv Paediatr Acta. 1977;31(6):473–80. [PubMed] [Google Scholar]
- 49.Lustig RH, Mietus-Snyder ML, Bacchetti P, Lazar AA, Velasquez-Mieyer PA, Christensen ML. Insulin dynamics predict body mass index and z-score response to insulin suppression or sensitization pharmacotherapy in obese children. J Pediatr. 2006;148(1):23–9. doi: 10.1016/j.jpeds.2005.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu JF, Liang L, Zou CC, Hong F, Wang CL, Wang XM, et al. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle intervention. Int J Obes (Lond) 2007;31(1):15–22. doi: 10.1038/sj.ijo.0803453. [DOI] [PubMed] [Google Scholar]
- 51.Kay JP, Alemzadeh R, Langley G, D’Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50(12):1457–61. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 52.Freemark M, Bursey D. The Effects of Metformin on Body Mass Index and Glucose Tolerance in Obese Adolescents with Fasting Hyperinsulinemia and a family history of Type 2 Diabetes. Pediatrics. 2001;107(4):e55, 1–7. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91(6):2074–80. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 54.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21(4):339–48. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 55.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152(6):817–22. doi: 10.1016/j.jpeds.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgert TS, Duran EJ, Goldberg-Gell R, Dziura J, Yeckel CW, Katz S, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatric diabetes. 2008 doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 57.Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, et al. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164(2):116–23. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanovski JA, Krakoff J, Salaita CG, McDuffie JR, Kozlosky M, Sebring NG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60(2):477–85. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 60.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 61.Uwaifo GI, Fallon EM, Chin J, Elberg J, Parikh SJ, Yanovski JA. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–7. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31(4):783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 63.Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, Jr, Steinberger J, Moran A, Sinaiko AR. Development of associations among central adiposity, adiponectin and insulin sensitivity from adolescence to young adulthood. Diabetic medicine : a journal of the British Diabetic Association. 2012;29(9):1153–8. doi: 10.1111/j.1464-5491.2012.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caprio S, Hyman LD, Limb C, McCarthy S, Lange R, Sherwin RS, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269(1 Pt 1):E118–26. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 65.D’Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes care. 2010;33(8):1817–22. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bennett B, Larson-Meyer DE, Ravussin E, Volaufova J, Soros A, Cefalu WT, et al. Impaired Insulin Sensitivity and Elevated Ectopic Fat in Healthy Obese vs. Nonobese Prepubertal Children. Obesity (Silver Spring) 2012;20(2):371–5. doi: 10.1038/oby.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.