Abstract
A number of retrospective studies have suggested that choice of anesthetic drugs during surgical tumor resection might affect tumor recurrence/ metastasis, or outcome of patients. The recent study showed that volatile anesthetics-based general anesthesia was associated with the worse outcomes than intravenous anesthetics-based general anesthesia. However, the underlying mechanism is yet to be determined. Because natural killer (NK) cells are implicated as important immune cells for tumor recurrence/ metastasis in the perioperative period, we examined the effect of different anesthetics on NK cell-mediated tumor cytotoxicity. Because adhesion molecule leukocyte function-associated antigen-1 (LFA-1) is functionally important in NK cells and is inhibited by commonly used volatile anesthetics isoflurane and sevoflurane, we hypothesized that these anesthetics would attenuate NK cell-mediated cytotoxicity. Using human NK cell line NK92-MI cells and tumor cell line K562 cells as a model system, we performed cytotoxicity, proliferation, conjugation and degranulation assays. Lytic granule polarization was also assessed. We showed that isoflurane, sevoflurane and LFA-1 inhibitor BIRT377 attenuated cytotoxicity, and reduced conjugation and polarization, but not degranulation of NK cells. Our data suggest that isoflurane and sevoflurane attenuated NK cell-mediated cytotoxicity at least partly by their LFA-1 inhibition in vitro. Whether or not isoflurane and sevoflurane attenuate NK cell-mediated tumor cytotoxicity in patients needs to be determined in the future.
Keywords: Volatile anesthetic, tumor recurrence/ metastasis, natural killer cell, leukocyte function-associated antigen-1
Graphical abstract
1. Introduction
Local tumor recurrence and/or distal metastasis after surgical resection remain to be the main cause of morbidities and mortalities in solid tumors despite significant advancement in tumor therapies over years (Gottschalk, Sharma et al. 2010). Causes of recurrence and/or metastasis are potentially multifactorial, including the dissemination of tumor cells during surgical resection and the functional suppression of immune cells in the perioperative period (Tavare, Perry et al. 2012). Various retrospective studies have demonstrated the association between anesthetic regimens and tumor recurrence/ metastasis and/or patient survival, suggesting that anesthesia regimens might play an important role in tumor metastasis and/or recurrence after surgery (Schlagenhauff, Ellwanger et al. 2000, Exadaktylos, Buggy et al. 2006, Biki, Mascha et al. 2008, Christopherson, James et al. 2008, Lin, Liu et al. 2011). Overall, these studies reported that the anesthetic regimens sparing general anesthesia by incorporating regional anesthesia or no use of general anesthesia were associated with better survival, or less recurrence/ metastasis. Furthermore, a recent retrospective study showed an association of poorer outcomes with patients who received volatile anesthetics-based general anesthesia for tumor resection surgery than patients who received total intravenous anesthetics-based general anesthesia (Wigmore, Mohammed et al. 2016). The foundation of these associations remains unclear, however.
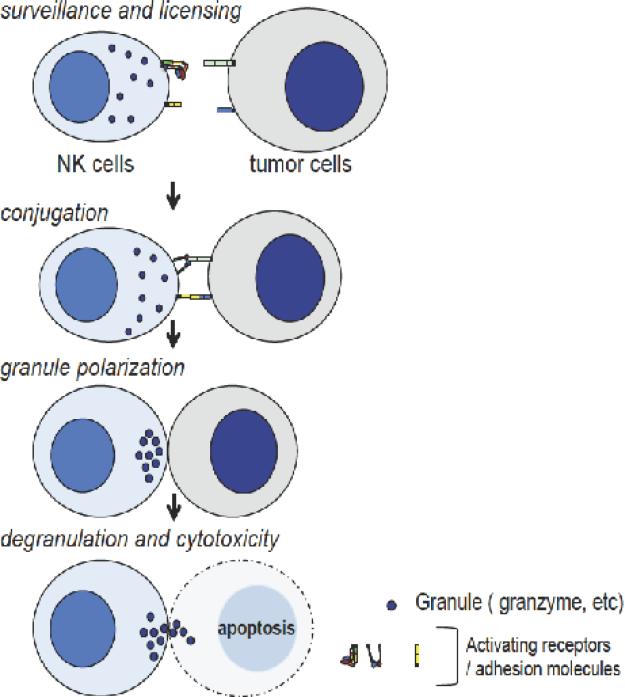
Natural killer (NK) cells are a phenotypically distinct population of lymphocytes (CD56+/CD3−) that lyse tumor cells using constitutively expressed lytic machinery independent of prior immunization. NK cells survey and conjugate with tumor cells devoid of major histocompatibility complex (MHC) class I, and polarize lytic granules toward them. Subsequent degranulation of lytic proteins such as perforin, granzyme and Fas ligands leads tumor cells to apoptosis (Figure 1). The correlation of perioperative NK cell suppression with tumor recurrence and mortality after surgical resection of colorectal and lung tumors suggests that adequate, perioperative NK cell function is critical to minimize post-resection tumor recurrence (Tartter, Steinberg et al. 1987, Fujisawa and Yamaguchi 1997). NK cells express a number of activating and inhibitory receptors on their cell surfaces to recognize stress ligands as well as MHC class I (Pegram, Andrews et al. 2011). Leukocyte function-associated antigen-1 (LFA-1) is one of the activating receptors and a major adhesion molecule on NK cells. It binds to intercellular adhesion molecule 1 (ICAM-1) on tumor cells, assisting the conjugation of NK cells with tumor cells. The binding of LFA-1 to ICAM-1 then reorganizes cytoskeletal structures within NK cells and induces lytic granule polarization (Figure 1). And LFA-1 inhibition or deficiency was found to impair NK cell-mediated cytolysis (Kohl, Springer et al. 1984, Weitz-Schmidt, Chreng et al. 2009).
Figure1. Steps of natural killer (NK) cell-mediated cytotoxicity.
NK cells survey and are educated by their surrounding cells (surveillance and licensing), and conjugate with cells devoid of MHC I (conjugation). The lytic granules in NK cells are polarized (granule polarization) and released (degranulation). Tumor cells undergo apoptosis subsequently (cytotoxicity).
Previously, we demonstrated that volatile anesthetics isoflurane and sevoflurane allosterically blocked LFA-1 (Yuki, Astrof et al. 2008, Zhang, Astrof et al. 2009, Yuki, Astrof et al. 2010, Yuki, Bu et al. 2012). LFA-1 is a structurally dynamic protein consisting of a large, extracellular component, and small transmembrane and cytoplasmic domains. It is a heterodimeric molecule with the α and β subunits. The α subunit contains the ligand-binding domain on its top. During conformational changes of LFA-1 as a whole, this ligand-binding domain also undergoes significant changes. And the pocket called ‘lovastatin site’ in the ligand-binding domain allosterically regulates the exposure of the ligand-binding site. A small molecule BIRT377 binds to ‘lovastatin site’ and prevents the exposure of ICAM-1 binding site (Kelly, Jeanfavre et al. 1999, Woska, Shih et al. 2001). Overall, LFA-1 transforms itself to bind to ICAM-1 only when it is fully activated. Isoflurane and sevoflurane also bind to this ‘lovastatin site’ and stabilize LFA-1 in a conformation that is not favorable for ICAM-1 binding. Here, we hypothesized that isoflurane and sevoflurane would impair NK cell function through their LFA-1 inhibition. We tested this hypothesis using NK cell line NK92-MI cells and tumor cell line K562 cells. We also used BIRT377 as a reference of LFA-1 inhibition via occupancy of ‘lovastatin site’.
2. Methods
2.1. Cell cultures
NK92-MI cells, K562 cells and Jurkat cells were purchased from ATCC (Manassas, VA, USA). NK92-MI cells were cultured in alpha Minimum Essential Medium without ribonucleosides and deoxyribonucleosides (Sigma Aldrich, St. Louis, MO, USA), with 0.2 mM inositol (Alfa Aesar, Haverhill, MA, USA), 0.1 mM 2-mercaptoethanol (Gibco, Waltham, MA, USA), 0.02 mM folic acid (Sigma Aldrich), 12.5 % fetal bovine serum (FBS) (Atlantic Biologicals, Miami, FL, USA) and 12.5% horse serum (Gibco). K562 cells and Jurkat cells were grown in RPMI1640 with 10% FBS. All the cells were cultured at 37°C, 5% CO2.
2.2. Tumor cytotoxicity assay
NK92-MI cells and K562 cells were used as effector cells (E) and target cells (T), respectively. After K562 cells were stained with carboxyfluorescein succinimidyl ester (CFSE, Life Technologies, Carlsbad, CA, USA), NK92-MI cells and K562 cells were co-incubated in the NK cell growth medium in 96 well V-bottom plates with the E/T ratio of 5:1 at 37°C for 4 hours with or without anesthetic exposure. Volatile anesthetics isoflurane (Baxter International, Deerfield, IL, USA) and sevoflurane (Abbott laboratories, Chicago, IL, USA) were exposed to cells in an air-tight chamber. Intravenous anesthetics ketamine, midazolam, propofol, fentanyl (all from Sigma Aldrich), dexmedetomidine and etomidate (both from Tocris Bioscience, Bristol, UK) were added into the medium directly. The stocks of propofol, etomidate and BIRT377 (Tocris) were dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the assay medium was 0.1%. Following 4-hour incubation, V-bottom plates were centrifuged at 1,500 rpm × 5 min, then the culture medium was removed and replaced with phosphate buffer saline (PBS). Fluorescence intensity was read using a plate reader at fluorescence of 485 nm and emission of 535 nm (Synergy H4; Bio Tek, Winooski, VT, USA). Percent cytotoxicity was calculated as [fluorescence intensity of input K562 cells –fluorescence intensity of samples + fluorescence intensity of NK cells (background)]/ [fluorescence intensity of input K562 cells] × 100 (%).
2.3. Cell proliferation assay
The impact of various anesthetics on the proliferation of NK92-MI cells and K562 cells was examined using WST-1 (Sigma Aldrich). Briefly, either NK92-MI cells or K562 cells were incubated for 4 hours with or without 2% isoflurane or 3% sevoflurane at 37°C. In addition, BIRT377 (10 μM) was tested. Absorbance was read at 450 nm.
2.4. Conjugation assay
First, K562 cells were stained with CFSE, and NK92-MI cells were stained with CMS Ros (Life Technologies). Then, NK92-MI cells and K562 cells were co-incubated with the ratio of 2.5: 1 for 15 minutes in the presence or absence of isoflurane, sevoflurane or BIRT377. The samples were subject to flow cytometry analysis using Accuri 6 (BD Biosciences, San Jose, CA, USA). Double positive cells were considered to be positive for conjugation.
2.5. CD2-CD58 binding assay
The binding of CD2 (LFA-2)-CD58 (LFA-3) was performed based on the previously published protocol with minor modifications (Satyanarayanajois, Ronald et al. 2011). Briefly, 96 wells were coated with 5 μg/mL of LFA-3 protein (Sino Biological Inc., Beijing, China) overnight. Following wash, wells were blocked with 1% bovine serum albumin (BSA) for 2 hours at room temperature. Jurkat cells, which expressed CD2 constitutively, were stained with 2’7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM, Life Technologies) and suspended in PBS, then aliquoted to each well. Cells were incubated with or without 2% isoflurane, 3% sevoflurane or 10 μM BIRT377 at 37°C for 30 minutes. After incubation, wells were subject to wash and fluorescence intensity was read using a plate reader at fluorescence of 485 nm and emission of 535 nm. The binding percentage was calculated as [fluorescence intensity of samples/fluorescence intensity of input Jurkat cells] × 100(%).
2.6. CD107a exposure
NK92-MI cells and K562 cells were co-incubated with the ratio of 5:1 with or without isoflurane or sevoflurane along with fluorescein (FITC) labeled anti-human CD107a antibody (Biolegend, San Diego, CA, USA). Following 2-hour incubation at 37°C, cells were washed and stained with APC anti-human CD56 antibody (Biolegend). Cells were then subject to flow cytometry analysis. CD56 positive cells were gated and examined for CD107a exposure.
2.7. Fluorescence microscopy
NK92-MI cells and K562 cells were co-incubated with the ratio of 2.5: 1 with or without isoflurane, sevoflurane or BIRT377 for 30 minutes on lysine-coated slides. Then, cells were fixed with acetone at −20°C. Cells were blocked with PBS/ 1% FBS and stained with alexa fluor 647 conjugated anti-granzyme B antibody (Biolegend) and phalloidin CruzFluor 488 conjugate (Santa Cruz Biotechnology, Inc., Dallas, Texas, USA) and subject to microscope analysis.
2.8. Statistical Analysis
All the statistical analyses were performed using PRISM5 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analyses used were included in the corresponding figure legends. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Volatile anesthetics isoflurane and sevoflurane, not intravenous anesthetics attenuated NK cell-mediated cytotoxicity
The effect of various anesthetics on NK cell-mediated tumor cytotoxicity was studied using NK92-MI cells as effector cells and K562 cells as target cells. Volatile anesthetics isoflurane and sevoflurane attenuated tumor cytotoxicity (Figure 2). However, none of the intravenous anesthetics tested reduced NK cell-mediated cytotoxicity (Figure 2). The intravenous anesthetic fentanyl rather increased the degree of tumor killing, which was in line with the previously published data (Yeager, Procopio et al. 2002). LFA-1 inhibition by BIRT377 reduced NK cell-mediated tumor killing. Because LFA-1 inhibition significantly attenuated cytotoxicity in this model, and both isoflurane and sevoflurane are known LFA-1 inhibitors (Yuki, Astrof et al. 2008, Yuki, Astrof et al. 2010), we speculated that LFA-1 inhibition by isoflurane and sevoflurane was at least partly responsible for the impairment of NK cell-mediated cytotoxicity by both anesthetics. We also tested the effect of isoflurane or sevoflurane on NK cell-mediated cytotoxicity in the presence of 10 μM BIRT377. 10 μM is the saturating concentration of BIRT377 (Kelly, Jeanfavre et al. 1999), and BIRT377 at this concentration presumably fully occupies ‘lovastatin site’. The co-incubation of isoflurane or sevoflurane with 10 μM BIRT377 did not provide additional tumor killing (data not shown), further supporting the idea that isoflurane and sevoflurane attenuated tumor cytotoxicity by interacting with the ‘lovastatin site’ on LFA-1.
Figure 2. The effect of various anesthetics on NK cell cytotoxicity.
Cytotoxicity of K562 cells by NK92-MI cells was tested under different anesthetics at various concentrations. In addition, the effect of LFA-1 allosteric antagonist BIRT377 was tested. Cells were co-incubated for 4 hours. Data are shown as mean +/− S.D. of 10 replicates for anesthetic experiments and 4 replicates for BIRT377 experiment. Statistical analyses were performed using one-way analysis of variance with Tukey post hoc analysis. * denotes p < 0.05 versus mock.
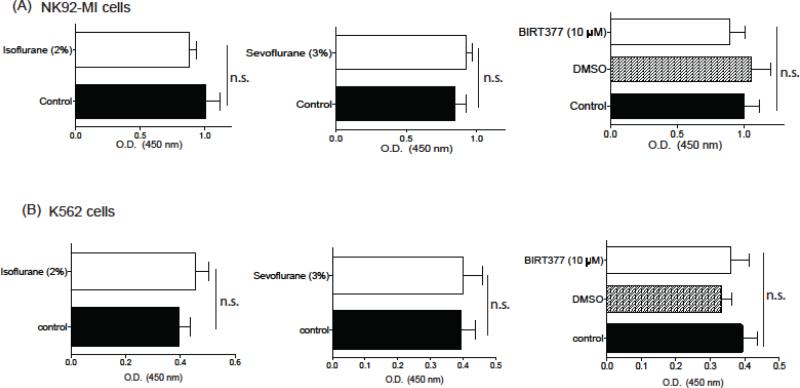
3.2. Isoflurane and sevoflurane did not affect the proliferation of NK92-MI cells and K562 cells
Previously sevoflurane and isoflurane enhanced proliferation of breast tumor cell MDA-MB-231 and kidney tumor cell RCC4 (Benzonana, Perry et al. 2013, Ecimovic, McHugh et al. 2013). If isoflurane or sevoflurane enhances tumor cell or NK cell proliferation in our model system, the interpretation of our cytotoxicity data may be affected. Our data showed that isoflurane, sevoflurane and BIRT377 did not significantly affect the metabolism and proliferation of NK cells and K562 cells (Figure 3).
Figure 3. The effect of isoflurane, sevoflurane and BIRT377 on NK92-MI cell and K562 cell proliferation.
The effect of volatile anesthetics and BIRT377 on NK92-MI cell proliferation (A) and K562 cell proliferation (B) was examined. Data are shown as mean +/− S.D. of 10 replicates. Statistical analyses were performed using student's t test for isoflurane and sevoflurane experiment and one-way analysis of variance with Tukey post hoc analysis for BIRT377. We did not observe any statistical significance. n.s. = not significant.
3.3. Isoflurane and sevoflurane attenuated the conjugation of NK92-MI cells with K562 cells
Because the conjugation of NK cells with tumor cells precedes NK cell-mediated cytotoxicity, we tested the impact of isoflurane and sevoflurane on conjugation. Both isoflurane and sevoflurane attenuated conjugation (Figure 4). BIRT377 also inhibited conjugation. Previously, Zheng et al. showed that the conjugation of NK cells with K562 cells occurred mainly through LFA-1 and CD2 on NK cells (Zheng, Wang et al. 2009). Our data using BIRT377 also showed the involvement of LFA-1 in conjugation
Figure 4. The effect of isoflurane and sevoflurane on NK cell conjugation.
Representative flow cytometry analysis data of NK92-MI cells: K562 cells conjugation assay under isoflurane, sevoflurane and BIRT377 are shown. The percentages in the figure represent conjugation percentage. Four independent experiments were performed with similar results.
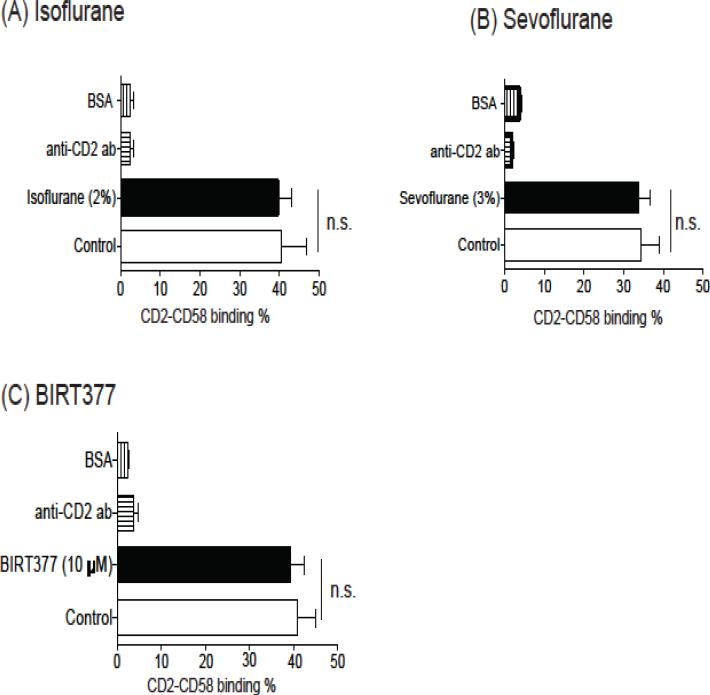
3.4. Isoflurane and sevoflurane did not affect the binding of CD2 to CD58
We previously demonstrated that both isoflurane and sevoflurane blocked LFA-1 (Yuki, Astrof et al. 2008, Yuki, Astrof et al. 2010). Here we tested if they also affected CD2. CD2 bound to CD58 on K562 cells (Zheng, Wang et al. 2009). Isoflurane and sevoflurane did not affect CD2 binding (Figure 5A and 5B). BIRT377 did not affect CD2 binding as well (Figure 5C). These results supported the idea that isoflurane and sevoflurane impaired conjugation by inhibiting LFA-1.
Figure 5. The effect of isoflurane, sevoflurane and BIRT377 on CD2-CD58 binding.
The binding of CD2 to CD58 was tested with or without isoflurane (A), sevoflurane (B) or BIRT377 (C). Data are shown as mean+/− S.D. of 4 replicates. Statistical analysis was performed using one-way analysis of variance with Tukey post hoc analysis. No statistical significance was observed between control and isoflurane (or sevoflurane, BIRT377) (n.s. = not significant).
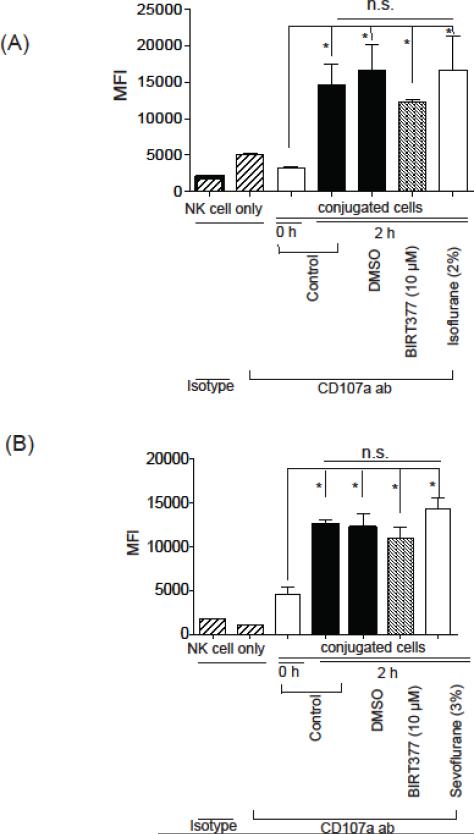
3.5. Isoflurane and LFA-1 did not attenuate degranulation process
Once NK cells conjugate with K562 cells, degranulation occurs. Lytic granules are secretory lysosomes containing various lytic proteins including perforin and granzyme. CD107a is a lysosomal-associated membrane glycoprotein around this lysosome and cellular surface expression of CD107a can be used as a surrogate of exocytosis of these lytic granules (Bryceson, Pende et al. 2012). Our data showed that isoflurane, sevoflurnane and BIRT377 did not affect the degree of degranulation (Figure 6A and 6B). In line with our findings, it is reported that degranulation occurs without LFA-1 involvement (Bryceson, March et al. 2005).
Figure 6. CD107a exposure under isoflurane and sevoflurane.
The degree of degranulation during NK92-MI cells and K562 cells co-incubation for 2 hours was studied using CD107a cell surface exposure with or without isoflurane (A) or sevoflurane (B). Data represent mean +/− S.D. of 4 replicates. Statistical analysis was performed using one-way analysis of variance with Tukey post hoc analysis. * denotes p < 0.05 versus 0 hour incubation. n.s. =not significant.
3.6. The impact of isoflurane, sevoflurane and BIRT377 on granzyme localization
Although degranulation is not dependent on LFA-1 signal, polarization of lytic granules is LFA-1- dependent (Chen, Trivedi et al. 2007). Once LFA-1 on NK cells bind to its ligand, granzyme accumulates toward the microtubule-organizing center (MTOC) via LFA-1- dependent mechanism (Figure 7A). While the polarization of granzyme was seen in the control sample, isoflurane, sevoflurane and BIRT377-treated samples showed global distribution, not polarization of granzyme (Figure 7B).
Figure 7. The effect of isoflurane, sevoflurane and BIRT377 on granzyme polarization.
(A) LFA-1 signal and lytic granule polarization along with the microtubule-organizing center (MTOC). (B) The effect of isoflurane, sevoflurane and BIRT377 on granzyme polarization. Cyan; granzyme, green; F-actin.
4. Discussion
Here we demonstrate that commonly used volatile anesthetics isoflurane and sevoflurane attenuated tumor cytotoxicity by NK cells, while intravenous anesthetics did not. Isoflurane and sevoflurane attenuated conjugation and granzyme polarization, not degranulation of NK cells as LFA-1 inhibitor BIRT377 did. We concluded that the inhibition of LFA-1 by isoflurane and sevoflurane was at least in part responsible for their effects on NK cells.
The association between anesthetics and postoperative metastasis has been suggested in animal models more than three decades ago. Shapiro et al. demonstrated that halothane and nitrous oxide anesthesia exposure was associated with enhanced metastasis of lung carcinoma and melanoma after surgical resection in mice (Shapiro, Jersky et al. 1981). Since then, a number of retrospective studies have reported the association between the anesthetics used for surgical resection of various tumors and tumor recurrence and/or metastasis. Accordingly, the underlying mechanism behind these observations became the scope of further investigations. The clinical trial led by Buggy et al. (clinical trial registration NCT041857) was to study the impact of anesthetic regimens in women undergoing primary breast cancer surgery. In the trial, patients were randomized to propofolparavertebral block anesthesia (PPA) or sevoflurane-opioid anesthesia (GA). The serum from patients in the PPA group facilitated NK cell-mediated cytotoxicity more than the serum from patients in the GA group (Buckley, McQuaid et al. 2014). Although the PPA subjects also had lower serum interleukin (IL)-1β (Deegan, Murray et al. 2010), it was unlikely that this was responsible for higher NK cell-mediated cytotoxicity. The attenuation of NK cell-mediated cytotoxicity was also reported in the postoperative period by another group (Pollock, Lotzova et al. 1991, Kutza, Gratz et al. 1997). Whether certain anesthetics could contribute to NK cell dysfunction directly or only indirectly via mediators in the serum as described by Buckley et al. is an important question to be answered. Buggy group also reported that the serum collected from GA subjects reduced apoptosis of MDA-MB-231 breast tumor cells more than the serum from PPA subjects (Jaura, Flood et al. 2014). Lastly, serum vascular endothelial growth factor (VEGF) C concentration was significantly higher in the GA group than in the PPA group (Looney, Doran et al. 2010). VEGF C promotes angiogenesis and aids tumor cell dissemination into the systemic circulation. Although the type of anesthetic drugs is often the scope of interest, consideration should be also given how long and how much they are used. A brief and small anesthetic exposure may not have a significant impact on immune function (Procopio, Rassias et al. 2001). These results suggest that multiple factors may be taken into consideration when we study the impact of anesthetics in tumor immunology.
The effects of anesthetic drugs should be studied on both immune cells and tumor cells. The direct effect of anesthetics on tumor cells has been well studied in vitro. Ecimovic et al studied the effect of sevoflurane on MCF7 breast tumor cells (Ecimovic, McHugh et al. 2013). A 6-hour of sevoflurane exposure enhanced MCF7 proliferation. However, the dose of sevoflurane tested in the study was rather high (> 3.3%). Benzonana et al. demonstrated that a 2- hour of 2% isoflurane exposure increased the proliferation of renal tumor RCC4 cells at 6 hours after the exposure (Benzonana, Perry et al. 2013). Here we examined the effect of isoflurane and sevoflurane on K562 cells. We did not observe their significant effect on K562 cell proliferation. The difference among these studies might be explained by different observation timeframe or cell type used. We studied the direct effect of anesthetics on NK cell functions and found that volatile anesthetics reduced NK cell functions, but intravenous anesthetics did not. Melamed et al. examined the effect of various anesthetics on lung retention of intravenously injected breast adenocarcinoma cell line MADB106 cells in rats (Melamed, Bar-Yosef et al. 2003). They found that ketamine and halothane enhanced lung retention of tumor cells along with the reduction of NK cell activity, but propofol did not. Ketamine injection was associated with reduced NK cell number in their study, and the reduction in NK cell activity by ketamine might be simply explained by reduction in NK cell number.
Previously we demonstrated that isoflurane and sevoflurane inhibited LFA-1. In line with this observation, our data supported that the attenuation of NK cell function by isoflurane and sevoflurane was at least partially explained by their LFA-1 inhibition. LFA-1 exists in different conformations on the cell surface and needs to be in an activated conformation to bind to its ligand. Activation of LFA-1 is triggered via intracellular signals called inside-out signal. Whether or not isoflurane and sevoflurane also affected the process of inside-out signal in addition to their direct interaction with LFA-1 remains to be determined. The involvement of two-pore domain K2P channel TASK-2 on NK cells in LFA-1 dependent adhesion is reported (Schulte-Mecklenbeck, Bittner et al. 2015). In the study, TASK-2 inhibitor inhibited LFA-1 dependent NK cell adhesion (Gray, Zhao et al. 2000). However, isoflurane potentiates TASK-2, and our data are unlikely to be explained by isoflurane's effect on TASK-2, further supporting the idea that isoflurane and sevoflurane directly interacted with LFA-1 on NK cells to attenuate NK cell-mediated tumor cytotoxicity in our study.
Our study has some limitations. First, we used only K562 cells as tumor cells. We chose them because the interaction of K562 cells with NK cells has been well studied, particularly in the context of LFA-1. But there are many different tumor cells with various gene expressional profiles. ICAM-1 expression profiles differ among tumors, and the impact of LFA-1 in NK cell-mediated tumor cytotoxicity likely differs. Further studies are necessary to test the effect of anesthetics on the interaction of NK cells with various tumor types. Second, we used a well-established NK cell line here. However, primary NK cells are heterogenous, and it is also important to study the effect of anesthetics using primary NK cells in the future. Third, we studied the effect of anesthetics only on NK cells. However, it is possible that other type of leukocytes also play a role in tumor recurrence/ metastasis after surgical resection. Lastly, our study is limited to in vitro experiments. In vivo experiments are required in the future.
In conclusion, we demonstrated that isoflurane and sevoflurane attenuated tumor cytotoxicity by NK cells at least partially via their LFA-1 inhibition.
Highlights.
LFA-1 is involved in NK cell-mediated conjugation, polarization and tumor cytotoxicity.
Volatile anesthetics isoflurane and sevoflurane are LFA-1 inhibitors.
Volatile anesthetics attenuated NK-cell mediated conjugation, polarization and cytotoxicity.
Intravenous anesthetics did not affect these NK cell functions.
Acknowledgments
Financial Support
This study was supported by NIH GM101345 (K.Y.) and CHMC Anesthesia Foundation (K.Y. and K.T.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Kazumasa Tazawa
Conflict of Interest: none
Contribution: Performed and analyzed data, prepared manuscript
Sophia Koutsogiannaki
Conflict of Interest: none
Contribution: Designed, performed and analyzed data, prepared manuscript
Matthew Chamberlain
Conflict of Interest: none
Contribution: Designed, performed and analyzed data, prepared manuscript
Koichi Yuki
Conflict of Interest: none
Contribution: Designed, performed and analyzed data, prepared manuscript
References
- Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119(3):593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202(7):1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Pende D, Maul-Pavicic A, Gilmour KC, Ufheil H, Vraetz T, Chiang SC, Marcenaro S, Meazza R, Bondzio I, Walshe D, Janka G, Lehmberg K, Beutel K, zur Stadt U, Binder N, Arico M, Moretta L, Henter JI, Ehl S. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood. 2012;119(12):2754–2763. doi: 10.1182/blood-2011-08-374199. [DOI] [PubMed] [Google Scholar]
- Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth 113 Suppl. 2014;1:i56–62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci U S A. 2007;104(15):6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107(1):325–332. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, Kavanagh BP, Buggy DJ. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;35(6):490–495. doi: 10.1097/AAP.0b013e3181ef4d05. [DOI] [PubMed] [Google Scholar]
- Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013;33(10):4255–4260. [PubMed] [Google Scholar]
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Yamaguchi Y. Autologous tumor killing activity as a prognostic factor in primary resected nonsmall cell carcinoma of the lung. Cancer. 1997;79(3):474–481. [PubMed] [Google Scholar]
- Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110(6):1636–1643. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- Gray AT, Zhao BB, Kindler CH, Winegar BD, Mazurek MJ, Xu J, Chavez RA, Forsayeth JR, Yost CS. Volatile anesthetics activate the human tandem pore domain baseline K+ channel KCNK5. Anesthesiology. 2000;92(6):1722–1730. doi: 10.1097/00000542-200006000-00032. [DOI] [PubMed] [Google Scholar]
- Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth 113 Suppl. 2014;1:i63–67. doi: 10.1093/bja/aet581. [DOI] [PubMed] [Google Scholar]
- Kelly TA, Jeanfavre DD, McNeil DW, Woska JR, Jr., Reilly PL, Mainolfi EA, Kishimoto KM, Nabozny GH, Zinter R, Bormann BJ, Rothlein R. Cutting edge: a small molecule antagonist of LFA-1-mediated cell adhesion. J Immunol. 1999;163(10):5173–5177. [PubMed] [Google Scholar]
- Kohl S, Springer TA, Schmalstieg FC, Loo LS, Anderson DC. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J Immunol. 1984;133(6):2972–2978. [PubMed] [Google Scholar]
- Kutza J, Gratz I, Afshar M, Murasko DM. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg. 1997;85(4):918–923. doi: 10.1097/00000539-199710000-00037. [DOI] [PubMed] [Google Scholar]
- Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011;106(6):814–822. doi: 10.1093/bja/aer055. [DOI] [PubMed] [Google Scholar]
- Looney M, Doran P, Buggy DJ. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor beta in women undergoing anesthesia and surgery for breast cancer. Anesthesiology. 2010;113(5):1118–1125. doi: 10.1097/ALN.0b013e3181f79a69. [DOI] [PubMed] [Google Scholar]
- Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89(2):216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126(3):338–342. doi: 10.1001/archsurg.1991.01410270082013. [DOI] [PubMed] [Google Scholar]
- Procopio MA, Rassias AJ, DeLeo JA, Pahl J, Hildebrandt L, Yeager MP. The in vivo effects of general and epidural anesthesia on human immune function. Anesth Analg. 2001;93(2):460–465. doi: 10.1097/00000539-200108000-00044. 464th contents page. [DOI] [PubMed] [Google Scholar]
- Satyanarayanajois SD, Ronald S, Liu J. Heterotypic cell adhesion assay for the study of cell adhesion inhibition. Methods Mol Biol. 2011;716:225–243. doi: 10.1007/978-1-61779-012-6_14. [DOI] [PubMed] [Google Scholar]
- Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res. 2000;10(2):165–169. [PubMed] [Google Scholar]
- Schulte-Mecklenbeck A, Bittner S, Ehling P, Doring F, Wischmeyer E, Breuer J, Herrmann AM, Wiendl H, Meuth SG, Gross CC. The two-pore domain K2 P channel TASK2 drives human NK-cell proliferation and cytolytic function. Eur J Immunol. 2015;45(9):2602–2614. doi: 10.1002/eji.201445208. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Jersky J, Katzav S, Feldman M, Segal S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68(3):678–685. doi: 10.1172/JCI110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartter PI, Steinberg B, Barron DM, Martinelli G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg. 1987;122(11):1264–1268. doi: 10.1001/archsurg.1987.01400230050009. [DOI] [PubMed] [Google Scholar]
- Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Chreng S, Riek S. Allosteric LFA-1 inhibitors modulate natural killer cell function. Mol Pharmacol. 2009;75(2):355–362. doi: 10.1124/mol.108.051169. [DOI] [PubMed] [Google Scholar]
- Wigmore TJ, Mohammed K, Jhanji S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology. 2016;124(1):69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- Woska JR, Jr., Shih D, Taqueti VR, Hogg N, Kelly TA, Kishimoto TK. A small-molecule antagonist of LFA-1 blocks a conformational change important for LFA-1 function. J Leukoc Biol. 2001;70(2):329–334. [PubMed] [Google Scholar]
- Yeager MP, Procopio MA, DeLeo JA, Arruda JL, Hildebrandt L, Howell AL. Intravenous fentanyl increases natural killer cell cytotoxicity and circulating CD16(+) lymphocytes in humans. Anesth Analg. 2002;94(1):94–99. doi: 10.1097/00000539-200201000-00018. table of contents. [DOI] [PubMed] [Google Scholar]
- Yuki K, Astrof NS, Bracken C, Soriano SG, Shimaoka M. Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology. 2010;113(3):600–609. doi: 10.1097/ALN.0b013e3181e89a77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, Shimaoka M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22(12):4109–4116. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Bu W, Xi J, Sen M, Shimaoka M, Eckenhoff RG. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J. 2012;26(11):4408–4417. doi: 10.1096/fj.12-212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Astrof NS, Liu JH, Wang JH, Shimaoka M. Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. FASEB J. 2009;23(8):2735–2740. doi: 10.1096/fj.09-129908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang Y, Wei H, Sun R, Tian Z. LFA-1 and CD2 synergize for the Erk1/2 activation in the Natural Killer (NK) cell immunological synapse. J Biol Chem. 2009;284(32):21280–21287. doi: 10.1074/jbc.M807053200. [DOI] [PMC free article] [PubMed] [Google Scholar]