Abstract
Background/Objective
Youth with obesity have an altered HDL subspecies profile characterized by depletion of large apoE rich HDL particles and an enrichment of small HDL particles. The goal of this study was to test the hypothesis that this atherogenic HDL profile would be reversed and that HDL function would improve with metabolic surgery.
Methods
Serum samples from adolescent males with severe obesity mean ± SD age of 17.4 ± 1.6 years were studied at baseline and 1 year following vertical sleeve gastrectomy (VSG). HDL subspecies and HDL function were evaluated pre and post VSG using paired t-tests. A lean group of adolescents was included as a reference group.
Results
After VSG, BMI decreased by 32% and insulin resistance as estimated by HOMA-IR decreased by 75% (both p<0.01). Large apoE rich HDL subspecies increased following VSG (p<0.01) and approached that of lean adolescents despite participants with considerable residual obesity. Additionally, HDL function improved compared to baseline (cholesterol efflux capacity increased by 12%, HDL lipid peroxidation potential decreased by 30%, and HDL anti-oxidative capacity improved by 25%, all p<0.01).
Conclusions
Metabolic surgery results in a significant improvement in the quantity of large HDL subspecies and HDL function. Our data suggest metabolic surgery may improve cardiovascular risk in adolescents and young adults.
Key Terms: high density lipoproteins, cholesterol efflux, adolescents, vertical sleeve gastrectomy
Introduction
Pediatric obesity is a major public health concern. More alarming, however, is the rapid rise in severe obesity among US adolescents. Severe obesity is defined as an absolute body mass index (BMI) >35 or a BMI >120% of the 95th percentile for age and sex, and currently affects about 6% of all youth, or 4 million adolescents (1). Emerging data show that youth with severe obesity have higher numbers of cardiovascular risk factors, more dyslipidemia, and early evidence of cardiac and vascular dysfunction (2) compared to youth with less severe obesity. Despite these immediate and serious cardiovascular consequences, treatments are limited. Lifestyle intervention, the mainstay of therapy, is often ineffective (3, 4).
As a result, metabolic surgery (also known as bariatric or weight loss surgery) has emerged as a potential treatment option in adolescents with severe obesity with studies showing effective weight loss (5, 6) and a favorable benefit to risk profile (7). Specifically, weight loss surgery improves important metabolic parameters such as insulin resistance (8), as well as blood pressure and lipids (5, 6). One important change that occurs following metabolic surgery in both adults and youth is a significant and sustained rise in high density lipoprotein cholesterol (HDL-C) of 40% or greater (9). However, while a remarkable and reproducible finding, it is not clear whether this rise in HDL-C translates into improved cardiovascular disease (CVD) risk. Indeed, in adult populations, increases in HDL-C by as much as 70% (10, 11) either through pharmacologic intervention or in the setting of genetic mutations have not consistently been shown to reduce CVD risk (12).
As a result, attention has shifted from evaluating HDL-C quantity to assessing HDL quality as a CVD risk marker. A landmark study by Khera et al. found that HDL function, as measured by cholesterol efflux capacity or HDL’s ability to promote efflux of cholesterol from macrophages, was independently associated with both subclinical atherosclerosis (measured by carotid artery intima-media thickness) and angiographically confirmed obstructive coronary heart disease (CAD). Furthermore, in their study cholesterol efflux capacity outperformed clinically important measures of total cholesterol, low density lipoprotein cholesterol (LDL-C) and HDL-C with respect to decreasing the odds of CAD (13). This was later confirmed prospectively by Rohatgi et al. (14). Similarly, other HDL functions including HDL anti-oxidative capacity has been shown to be independently associated with acute coronary syndrome (15).
It is often incorrectly assumed that all HDL particles are compositionally homogeneous. However, HDL exists as different subspecies each with unique lipid and protein compositions (see (16) for a review). Furthermore, not all subspecies are equally atheroprotective (17, 18). Thus, it is critically important to evaluate HDL’s subspecies that may be integral to vascular function and susceptibility to CVD.
We have previously shown that youth with obesity and those with combined obesity and type 2 diabetes exhibit an altered HDL subspecies profile characterized by depletion of large apoE rich HDL particles and an enrichment of small HDL particles compared to lean adolescents (17). These adolescents also exhibited increased arterial stiffness measured non-invasively by pulse wave velocity compared to controls (17, 19). Strikingly, a higher number of large HDL particles was inversely associated with arterial stiffness but traditional lipid markers of total cholesterol, LDL-C and HDL-C were not. From these data, we surmised that this altered HDL subspecies profile (reduction in large HDL particles and increased small particles) was disadvantageous-tied to either excess adiposity or insulin resistance, or perhaps both. In this current study, we asked whether reduction in weight with metabolic surgery reverses this unhealthy HDL subspecies profile and improves HDL function. This surgical model was chosen for two reasons: 1) significant weight loss and reversal of insulin resistance can be achieved with metabolic surgery in a consistent manner (6, 8), and importantly 2) studying participants pre- and post-surgery allows each subject to serve as his own control thus reducing the number of confounding variables encountered in cross-sectional studies of individuals with a broad range of BMI values.
Material and Methods
Ten male participants 14–20 years of age undergoing metabolic surgery at Cincinnati Children’s Hospital between 2009 and 2011 were offered enrollment in a prospective biospecimen repository protocol (Pediatric Obesity Tissue Repository). The decision to undergo metabolic surgery and the type of surgery was made collaboratively by the patient, caregiver(s), and clinical staff, independent of this research protocol. For this pilot study of HDL, only males were selected to eliminate known differences in lipoproteins by sex (20). All participants were assessed at baseline and 1 year post-operatively. Height was measured to the closest 0.1 cm on a wall mounted stadiometer and weight was measured on an electronic scale. Blood pressure was obtained manually using a sphygmomanometer (with appropriate cuff size) and auscultation. Major comorbid conditions and laboratory data were abstracted from medical records. Written informed consent was obtained from participants ≥ 18 years old or from the parent or guardian if age <18 years. The study was approved by the Institutional Review Board at Cincinnati Children’s Hospital.
Clinical Measurements
Measurements of total cholesterol, HDL-C, and triglycerides (TGs) were performed as previously described (17). LDL-C was calculated using the Friedewald equation. Glucose was measured using a Hitachi model 704 glucose analyzer (Roche Hitachi, Indianapolis, IN) and insulin was measured by radioimmunoassay (RIA) with an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St Louis, MO) and a double antibody method to separate bound from free tracer.
HDL Functional Assays
Serum stored at −80°C was thawed. HDL was isolated by removing apoB containing lipoproteins from serum using polyethylene glycol (PEG, MW 6000, Sigma Aldrich; St. Louis, MO). 400 μl PEG (20% in water) was added to 1 ml serum, incubated for 20 min at RT, and then centrifuged at 10,000 RPM for 30 min.
A. Cholesterol efflux capacity
HDL most well recognized function is the ability to move (efflux) cholesterol from the plasma membrane of macrophages (13). This assay measures the net movement (aqueous diffusion, ATP-binding cassette sub-family A1 (ABCA1) and G member 1 (ABCG1) mediated) of 3H-labeled cholesterol from J774 cells, a mouse macrophage cell line (ATCC; Manassas, VA) to an acceptor (apoB depleted plasma) where a higher cholesterol efflux capacity is indicative of improved HDL function. J774 cells were plated in 48-well plates at 200,000 cells per well and radiolabeled with 1 μCi/ml of 3H-cholesterol (PerkinElmer; Boston, MA). The next day 150 μl treatments containing 2.8% v/v of apoB depleted serum were made in basal media (DMEM containing 0.2% BSA) with 2 μg/ml ACAT inhibitor and 0.3 mM 8-Br-cAMP were added and incubated for 6 h.
Liquid scintillation counting was used to detect the 3H-cholesterol effluxed to an acceptor via scintillation counting. Percent cholesterol efflux is expressed using the following formula: 3H count in efflux media/(3H count in efflux media + mean 3H count extracted from untreated cells) × 100. All samples were assayed in triplicate. All data was normalized to a plasma control and is expressed as cholesterol efflux capacity. Positive controls included plasma at 2% v/v, lipid-free apoA-I and HDL (each at 10 μg/ml protein) containing the ACAT inhibitor with and without 8-Br-cAMP. Supplement Figure 1 shows reproducibility. Interassay coefficient of variation (CV) is 5.3%
HDL lipid peroxidation
HDL lipid peroxidation (or the measured amount of hydrogen peroxide in HDL) was measured using an established protocol published by Kelesidis et al (21). This assay utilizes Amplex Red in the presence of horseradish peroxidase (HRP) to assess the rate of formation of endogenous lipid hydroperoxides in a specific amount of HDL. 50 μl of apoB depleted serum with reaction buffer (0.5 M potassium phosphate, pH 7.4, 0.25 M NaCl, 25 mM cholic acid, 0.5% Triton X-100) were added to a 96-well black plate. Controls included ultracentrifugally isolated HDL (20 μg cholesterol) in 50 μl reaction buffer and a 50 μl reaction buffer blank. 50 μl of a solution containing 1–10 U/ml HRP in reaction buffer was then added to each well and incubated at 37°C for 1 h. Following incubation, 50 μl of a reaction buffer (300 μM Amplex Red and 0.2 U/ml cholesterol esterase) was added. Fluorescence was measured at one-minute intervals for 1 h using a Biotek Synergy HT plate reader at absorption 530nm and emission 590 nm. Kinetic curve data was analyzed using Gen5 2.01 (Biotek; Winooski, VT). A buffer blank control was subtracted from each experimental sample. HDL lipid peroxidation was calculated as the rate of oxidation (relative fluorescent units per minute) divided by the cholesterol concentration of each sample as measured using a colorimetric kit (Pointe Scientific; Canton, MI).
A lower amount of lipid peroxidation in HDL indicates improved HDL function. Reproducibility is shown in ref (21). Interassay CV is 4.0%.
HDL anti-oxidative capacity
HDL anti-oxidative capacity measures HDL’s ability to mitigate oxidation of LDL. Using a modified assay from Patel et al.(15), we tested the ability of HDL to react with oxidized LDL in the presence of a dihydrorhodamine 123 (DHR, Life Technologies). DHR is a non-fluorescent substrate that reacts with reactive oxygen species (ROS) in oxidized LDL to form the fluorescent compound rhodamine 123. HDL prevents conversion to rhodamine 123 thus a lower anti-oxidative index indicates improved HDL anti-oxidative capacity. LDL was isolated by ultracentrifugation from a single donor (Hoxworth Blood Center, Cincinnati OH) and oxidized with 20 nmol CuSO4 (per 1 mg LDL protein). LDL was then dialyzed into PBS and diluted to a final concentration of 80.4 μg/ml prior to use in this assay. DHR was prepared as a 50 mM stock solution in anhydrous DMSO. 5 μl apoB depleted serum was combined with oxidized LDL protein at a final concentration of 1.4 μg/ml and brought up to a volume of 173 μl with phosphate buffered saline (PBS). DHR was diluted with PBS to a 500 μM concentration and 2 μl was added to each well for a final DHR concentration of 5.95 μM. All samples were assayed in triplicate. Fluorescence of each well was measured for 1 h at 37 °C using a BioTek Synergy HT plate reader at absorption 485nm and emission 528 nm. HDL anti-oxidative capacity was calculated as the endpoint fluorescence of DHR incubated with apoB depleted serum minus the optical density of DHR alone. Data was normalized to the LDL blank on the plate. Supplement Figure 2 shows reproducibility. Interassay CV is 3.5%
HDL Subspecies
370 μl of frozen serum (−80°C) was applied to three Superdex 200 Increase columns (10/300, GE Healthcare) arranged in series (22). To relate gel filtration fractions to traditional density-centric lipoprotein definitions (LDL, VLDL and HDL) we use the presence of apoB, the core constituent of LDL, as the key distinguisher. Therefore, the VLDL/LDL range is defined as fractions 15−19 due to the presence of apoB. Fractions 20–29 are considered HDL because their diameters are consistent with measurements for density-isolated HDL and because of their abundance of the major HDL protein, apoA-I (details have been previously published (22) and see Supplement Figure 3). Choline-containing phospholipid and total cholesterol were measured in each fraction with kits from Wako (Richmond, VA) and Pointe Scientific (Canton, MI), respectively. Supplement Figure 4 shows the subspecies profile is not altered by freezing.
Cholesterol efflux in HDL Subspecies
Cholesterol efflux was also measured in the aforementioned HDL-associated gel filtration fractions. Given we have previously shown that the majority of cholesterol efflux occurs between fractions 20–25 (23), only these fractions were evaluated. Efflux across HDL fractions and subsequent mass spectrometry (MS) analysis was performed on a subset of n=6 individuals due to unavailable sample on n=4. Characteristics of the participants studied were no different from the entire group. As above, efflux was assessed as net movement of 3H cholesterol from J774 macrophage cells. Instead of apoB depleted serum, we used 26.7% v/v of each serum fraction in freshly made in basal media (consisting of DMEM containing 0.2% BSA) with 2 μg/ml ACAT inhibitor and 0.3 mM 8-Br-cAMP. It should be noted that each serum fraction is approximately 50-fold diluted compared with serum. HDL lipid peroxidation and HDL inflammatory index assays were attempted on HDL subspecies but data were not dose dependent nor reproducible. Supplement Figure 5 demonstrates that the increase in cholesterol efflux capacity represents an increase in the number of particles contained in that fraction.
HDL Proteomics
We applied shotgun proteomics to determine if there were changes in the HDL proteome following VSG (17). HDL fractions were prepared as previously described (22). Further details on sample preparation and mass spectrometry data analysis are provided in the supplement. Raw spectral counts are presented and no data normalization was performed. Correction for multiple comparisons for the proteomic data was not performed given the exploratory nature of this work.
Results
Clinical Characteristics
Characteristics of the participant’s pre-surgery and 1 year post VSG are presented in Table 1. At the baseline visit prior to surgery, participants were a mean age of 17.4 ± 1.6 years (age range 15.5–19.8 years). 90% of the participants were Caucasian and all were male. All participants were severely obese defined as body mass index ≥35 or a BMI ≥120% of the 95th percentile for age and sex. None were on lipid lowering medication or metformin. One participant reported taking an ACE inhibitor for hypertension.
Table 1.
Study participants at pre and post sleeve gastrectomy.
| Variable | Baseline | 1 year post VSG | Percent Change |
|---|---|---|---|
| Age (years) | 17.4±1.6 | 18.4±1.5 | – |
| Race (Caucasian) | 9 (90%) | – | – |
| Height (cm) | 126±86 | 126±86 | 0% |
| Weight (kg)* | 167 ±36 | 111± 25 | ↓32% |
| Body Mass Index (kg/m2)* | 52.1± 9.8 | 35.3± 9.0 | ↓32% |
| Total cholesterol (mg/dL) | 167± 56 | 157± 29 | ↓1.4% |
| LDL cholesterol (mg/dL) | 102 ±41 | 96± 27 | ↓1.4% |
| HDL cholesterol (mg/dL) | 34± 7 | 42±11 | ↑ 23% |
| Triglycerides (mg/dL) | 154± 99 | 98±49 | ↓25% |
| Fasting insulin (mcIU/mL)* | 43.1±19.1 | 13.0±10.0 | ↓71% |
| Fasting glucose (mg/dL)* | 98 ± 14 | 78±8.2 | ↓19% |
| HOMA-IR* | 10.0 ± 5.3 | 2.5 ± 2.0 | ↓75% |
| Systolic Blood Pressure (mmHg)* | 136±9 | 124±10 | ↓8.7% |
| Diastolic Blood Pressure (mmHg) | 70±11 | 69±10 | ↓0.8% |
VSG= vertical sleeve gastrectomy. HOMA-IR= Homeostatic Model Assessment of Insulin Resistance. Data from n=10 participants. Mean ± SD or number (percent).
indicates p <0.05 between baseline and post VSG tested by paired t-test.
Follow-up data were obtained at a mean age of 18.4 ±1.5 years, approximately 1 year after VSG. At follow-up, the mean BMI decreased by 32%, p<0.01, Figure 1. Total cholesterol and LDL-C minimally changed at 1 year post-surgery, while HDL-C increased by 23% percent and triglycerides decreased by 25%. Fasting insulin, glucose, and HOMA-IR were significantly lower at follow-up (p<0.05). Systolic blood pressure was also lower (p<0.05). None of the participants were on lipid or blood pressure lowering medication or metformin 1 year post VSG.
Figure 1.
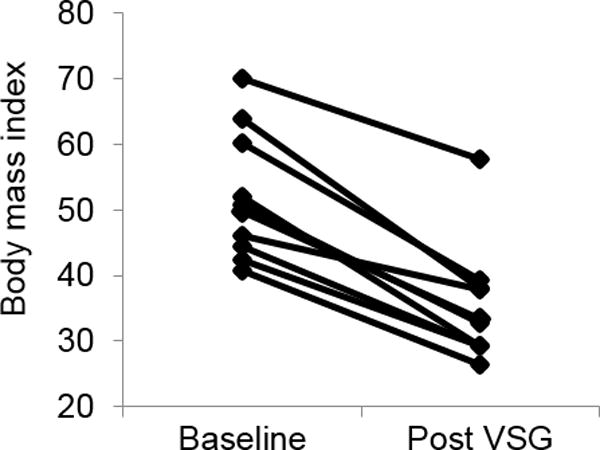
Body mass index pre-surgery and post VSG. Individual data points are plotted for n=10 participants. p value from paired t-test was 0.003.
HDL Function
We tested HDL function both pre and post-surgery using three independent assays. The function most commonly associated with HDL is the ability to move (efflux) cholesterol from the plasma membrane of macrophages. Using J774 mouse macrophages (ATCC, Manassas, VA) stimulated with cyclic AMP, we measured the capacity of plasma samples to accept radiolabeled cholesterol. Since apoB containing lipoproteins (VLDL and LDL) are depleted in these samples, the primary lipoprotein acceptor is HDL. Figure 2a shows that overall cholesterol efflux capacity improved post VSG. Mean cholesterol efflux capacity was 12% higher in participants post VSG (baseline mean ± SE 0.94 ± 0.05 vs. post VSG 1.04 ± 0.06, p=0.0056). We next measured HDL lipid peroxidation. Figure 2b shows mean HDL lipid peroxidation decreased by 30% post VSG (baseline mean ± SE 16.4 ± 1.1 vs. 11.4 ± 0.9, p=0.0047). Finally, we measured the ability of HDL in apoB depleted plasma to mitigate oxidation of LDL. Figure 2c shows that mean HDL anti-oxidative capacity improved by 25% post VSG (baseline mean ± SE 4.8 ± 0.3 vs. 3.6 ± 0.3, p=0.0029).
Figure 2.
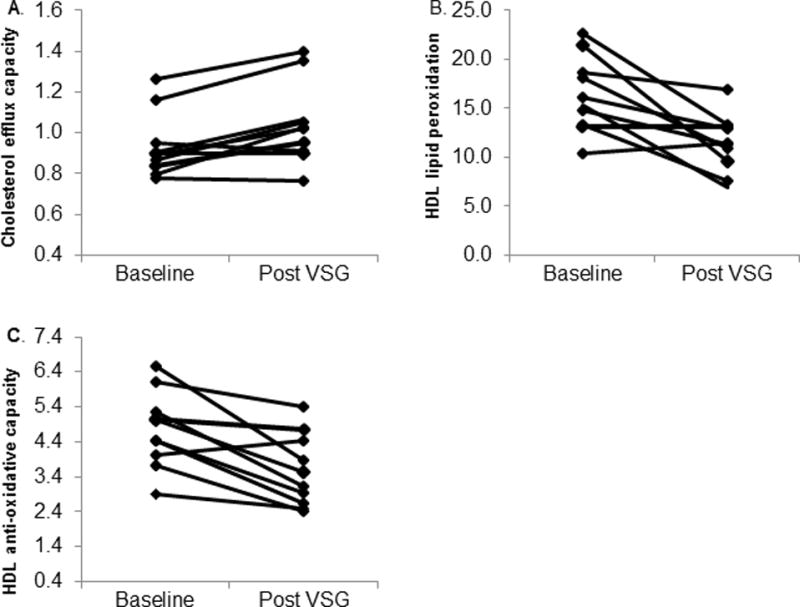
HDL Function pre and post VSG. Individual data points are plotted for n=10 participants. p value obtained via paired t-test. A) Cholesterol efflux capacity, p=0.0056. B) HDL oxidation, p-value =0.0047. C). HDL anti-oxidative index, p = 0.0029.
Lipoprotein Subspecies
To determine effects of VSG on HDL subspecies, we isolated subspecies by size using gel filtration chromatography. Plasma from n=14 lean adolescent males (dotted line in Figure 3), mean age 16.3 ± 2.7 years, show three distinct peaks when analyzed for phospholipid. Fractions 15–19 contains VLDL/LDL sized particles while fractions 20–30 encompasses HDL sized particles with fractions 27–30 representing relatively small, lipid-poor HDL species. Prior to surgery, adolescents with severe obesity (in black) exhibited a strikingly reduced level of large HDL sized particles (reduced phospholipid and cholesterol concentrations in fractions 20–23, p<0.05) compared to lean adolescents and an increase in small HDL sized particles (higher phospholipid content in fractions 25–26, p<0.05), consistent with our previous findings in youth with obesity and type 2 diabetes (17).
Figure 3.
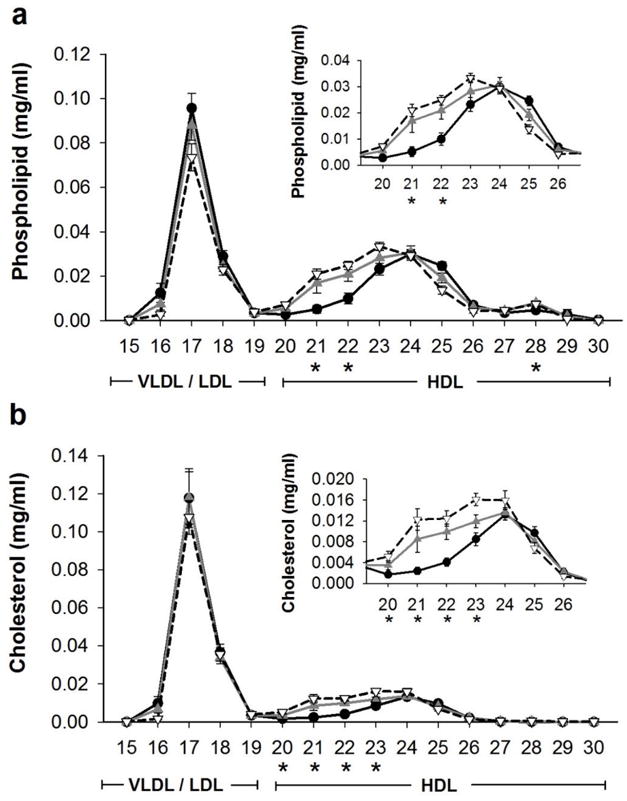
Phospholipid and cholesterol across lipoprotein fractions. A) Phospholipid and B) cholesterol concentration of serum fractionated by gel filtration chromatography pre (—●—black) and post (—▲—gray) vertical sleeve gastrectomy (VSG). Data are mean and SE for pre and post VSG for n=10 participants. Dotted line (–▽–) shows a lean control group (n=14) from a separate study for reference. * indicates significant differences pre and post VSG, p<0.01.
After VSG, there was a remarkable rescue of the large HDL subspecies (phospholipid content fraction 21, p= 0.0055 and fraction 22, p= 0.0002 and cholesterol content in fractions 20–23, all p<0.05) compared to baseline (Figure 3a & b). Indeed there was no difference between individuals who had undergone VSG and lean adolescents. Notably, there was also no change in VLDL/LDL fractions post VSG, a finding consistent with the clinical lipid measurements. To determine if these changes in large HDL particles were associated with a change in HDL function, we assessed cholesterol efflux capacity in equal volumes of these fractions. After VSG, we noted a significant improvement in cholesterol efflux capacity (Figure 4) in HDL fractions 21 (p=0.0211) and 22 (p=0.0170). Taken together, these data show that metabolic surgery in severely obese participants with VSG, increases the concentration of large HDL particles to levels comparable to those observed in lean adolescents and improved HDL function.
Figure 4.
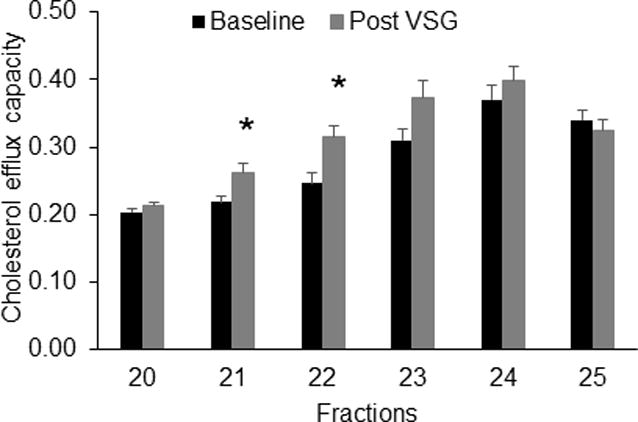
Cholesterol efflux capacity across HDL subspecies. Data are mean and SE for n=6 participants. * indicates p<0.05 by paired t-test. Each sample was assayed in triplicate.
Proteomics
Finally, we also sought to determine if proteins associated with large HDL subspecies in fractions 21–22 (or the HDL proteome) change after VSG. Forty-eight HDL associated proteins (defined as HDL consensus proteins that have been found in 3 or more independent studies of HDL proteomics (16)) were identified in our samples. All proteins were detected both pre- and post-VSG. Twelve proteins showed higher (but not statistically different) peptide counts after VSG, 22 proteins were lower and 14 proteins showed no difference post VSG (see Table S1 in supplement for a complete list of all proteins). In fraction 21, all major HDL apolipoproteins including apoA-I, A-II, apoC-I, apoC-III, apoJ, PON-1 trended higher after VSG (Figure 5, however, only apoE was significantly higher (p<0.05). Proteins that were significantly lower in fraction 21 after VSG included complement factor H and plasma kallikrein. In fraction 22, apoA-I was significantly higher post VSG (Figure 5), while plasma kallikrein was lower, both p<0.05.
Figure 5.
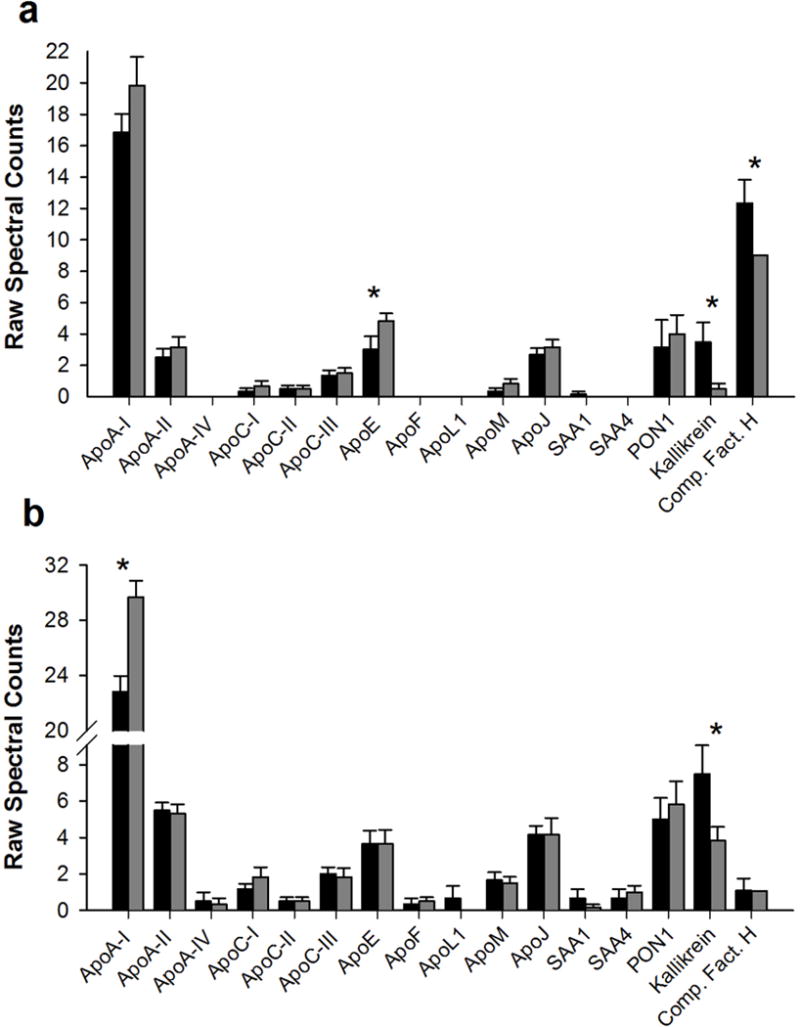
HDL associated proteins in fractions 21 (panel a) and 22 (panel b). Data are mean and SE of raw spectral counts pre (black bars) and post VSG (gray bars) for n=6 participants.
Discussion
We show that one year following VSG there is substantial improvement of atherogenic HDL subspecies profile in youth with severe obesity that approaches lean adolescents, despite incomplete resolution of obesity. Importantly, we also documented there is also significant improvement in HDL function as measured by increased cholesterol efflux capacity, decreased lipid peroxidation, and improved anti-oxidative capacity. These data suggest that metabolic weight loss surgery either via improved weight or insulin resistance or an independent mechanism may be associated with improved cardiovascular health.
To date there has only been a single study conducted in adolescents evaluating HDL function. In that study HDL function measured by the phosphorylation of eNOS did not improve one year after Roux-en-Y gastric bypass. In fact, the authors postulated that HDL function may not improve with surgery in adolescents (24). However, our data show clear improvements in cholesterol efflux capacity, anti-oxidative capacity and lipid peroxidation after one year following VSG. The discrepant results between the former study in adolescents and here may be explained by the larger sample size, alternative HDL functions studied, differences by surgery type, or other factors. Our findings are in agreement with adult studies that show HDL function (apoB depleted plasma or HDL isolated by ultracentrifugation) measured by cholesterol efflux (25), oxidation potential (26) and endothelial function (27) improve following Roux-en-Y gastric bypass.
Not all participants had improved HDL function post VSG as seen in the Figure 2. In fact, some individuals changed very little from baseline to follow-up. However, it was not the same individuals that lacked improvement by all three functional assays. In addition, the degree of improvement did not correlate with BMI loss or HDL-C levels. Thus, improvements in HDL function measured by these assays are likely dedicated by combination of metabolic risk factors.
In addition to evaluating HDL function we show large HDL subspecies increase after metabolic surgery. Prior work has shown that HDL exists as a family of subpopulations that is highly heterogeneous in structure, composition, metabolism, function and likely atherogenic potential (16, 28). Furthermore, large HDL particles have traditionally been inversely associated with cardiovascular risk while high concentrations of small HDL particles are typically positively correlated with cardiovascular risk demonstrating that size of HDL particles is important (29–32). After Roux-en-Y gastric bypass, studies in adults have shown the appearance of large, lipid-rich particles (either alpha-1 particles by 2D gel electrophoresis (26, 33) or HDL2 isolated by ultracentrifugation (25)). We chose to study HDL subpopulations using gel filtration chromatography over the former methods because in addition to allowing the recovery of the particles for functional analysis (23), it is performed at physiological pH and ionic strength without high g-forces resulting in significantly less proteome rearrangement compared to ultracentrifugation (34). We show that large atheroprotective HDL particles increase after VSG. However, whether these particles are directly responsible for the improved HDL function observed post VSG remains to be determined.
We noted relatively few changes in the HDL proteome following VSG, similar to prior work (24). While these results could suggest that the HDL proteome does not change with surgery as postulated by Matsou et al (24), it is possible that we were underpowered to detect differences. Indeed, 34 proteins trended toward changes after VSG (up or down). One protein that did significantly change was apoE. This finding parallels our previous study that found obese youth with diabetes have decreased apoE compared to lean individuals (17). Prior work suggests that apoE associated with large HDL particles may confer vascular protection by competing with LDL for heparin-sulfate proteoglycans in the vessel wall, the process that initiates atherosclerosis (35). In fact, lower amount of large apoE HDL particles in patients with type 2 diabetes is thought to be one reason for their accelerated CVD (35). If apoE-rich large HDL competes with LDL, the benefits might not only include the displacement of potentially oxidizable LDL in the vessel wall but our observation of improved HDL anti-oxidative capacity suggest a potential double benefit. ApoE rich large HDL may be an important mechanism by which metabolic surgery improves cardiovascular health. Confirmation of this hypothesis requires further detailed studies.
Other proteins that changed post VSG include ApoA-I and plasma kallikrein. ApoA-I is HDL’s major structural and functional apolipoprotein and is thought to be critical for many of HDL’s functions including cholesterol efflux and generating large HDL particles by lecithin-cholesterol acyltransferase (LCAT). Therefore, it is not surprising apoA-I increased. Plasma kallikrein significantly decreased in both fraction 21 and 22. Plasma kallikrein is a serine protease thought to play a role in the pathogenesis of thrombosis, inflammation, and blood pressure regulation. When plasma concentrations become high, kallikrein is thought to perpetuate CVD (36). In fact, kallikrein inhibitors are being proposed as a novel class of drugs for the treatment of CVD. The fact that following VSG, HDL associated kallikrein decreases suggests there may be additional atheroprotective benefits post VSG. Of note, systolic blood pressure was lower post VSG in these adolescents.
There are some limitations. First, while HDL lipid peroxidation and HDL anti-oxidative capacity are both well-established assays that correlate with HDL functions they are not direct HDL functional assays, per say. However, each was chosen because it has been shown to be a reliable and reproducible surrogate measure that correlates with CVD outcomes (15, 37, 38). Second, we focused on cAMP mediated efflux not cAMP-independent cholesterol efflux or net mass cholesterol flux therefore we cannot determine if these pathways are altered with surgery. Finally, since this was an observational study we cannot address underlying mechanisms. We do not know whether the observed improvements in HDL subspecies and function are due to weight loss, improved insulin resistance, altered gut physiology (27) or another mechanism. Future studies that include larger sample sizes, alternative surgery types (i.e. Roux-en-Y) and multiple time points are needed.
In conclusion, we show large atheroprotective HDL subspecies emerged by 1 year after VSG surgery to levels similar to that observed in lean adolescents. Furthermore, we show improved HDL atheroprotective function. At this time it is unclear whether improved function is a reflection of more particles or improved function of existing particles but at least for efflux our data shows the former is more likely. As metabolic surgery grows in popularity as a weight loss option in adolescents, it is e important to gain a full understanding of its risks and benefits. Our data suggests that weight loss and improvement in the lipid profile may not be the only benefit of weight loss surgery; indeed improved HDL function may alter the risk of future cardiovascular events in this population, an issue of major public health concern (39).
Supplementary Material
Acknowledgments
We would like to acknowledge Aaron Kelly PhD for his helpful discussions and input during preparation of the manuscript, the Pediatric Obesity Tissue Repository co-investigators Todd Jenkins PhD and Stavra Xanthakos MD and the coordinators who helped with the study including Lindsey Shaw, Rosemary Miller, and Jennifer Black.
Funding: K23HL118132 (ASS), R01HL67093 (WSD), R01HL104136 (WSD) from NHLBI, The Central Society for Clinical and Translational Research (ASS) and the Cincinnati Pediatric Diabetes and Obesity Center at Cincinnati Children’s Hospital Medical Center.
Footnotes
Conflict Statement: The authors have no conflicts of interest
Supplementary information is available at International Journal of Obesity’s website
Authors Contributions:
A.S designed the study, analyzed the data and wrote the manuscript, TH recruited the participants and edited the manuscript, HS and AH performed the experiments, analyzed the data and edited the manuscript, DE recruited participants from preliminary experiments, DH edited the manuscript, JM performed the experiments and edited the manuscript, WSD designed the study and wrote the manuscript.
References
- 1.Claire Wang Y, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe 391 obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2011;6:12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 2.Shah AS, Dolan LM, Khoury PR, Gao Z, Kimball TR, Urbina EM. Severe Obesity in Adolescents and Young Adults Is Associated With Subclinical Cardiac and Vascular Changes. J Clin Endocrinol Metab. 2015;100:2751–2757. doi: 10.1210/jc.2014-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166:1103–1108. doi: 10.1001/2013.jamapediatrics.319. [DOI] [PubMed] [Google Scholar]
- 4.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 5.Olbers T, Gronowitz E, Werling M, Marlid S, Flodmark CE, Peltonen M, et al. Two-year outcome of laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS) Int J Obes (Lond) 2012;36:1388–1395. doi: 10.1038/ijo.2012.160. [DOI] [PubMed] [Google Scholar]
- 6.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374:113–123. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168:47–53. doi: 10.1001/jamapediatrics.2013.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inge TH, Prigeon RL, Elder DA, Jenkins TM, Cohen RM, Xanthakos SA, et al. Insulin Sensitivity and beta-Cell Function Improve after Gastric Bypass in Severely Obese Adolescents. J Pediatr. 2015;167:1042–1048. e1041. doi: 10.1016/j.jpeds.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen NT, Varela E, Sabio A, Tran CL, Stamos M, Wilson SE. Resolution of hyperlipidemia after laparoscopic Roux-en-Y gastric bypass. J Am Coll Surg. 2006;203:24–29. doi: 10.1016/j.jamcollsurg.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 12.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012 doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol. 2011;58:2068–2075. doi: 10.1016/j.jacc.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Shah AS, Tan L, Lu Long J, Davidson WS. The proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013 doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon SM, Davidson WS, Urbina EM, Dolan LM, Heink A, Zang H, et al. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62:2958–2967. doi: 10.2337/db12-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobecourt E, Jacqueminet S, Hansel B, Chantepie S, Grimaldi A, Chapman MJ, et al. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 2005;48:529–538. doi: 10.1007/s00125-004-1655-5. [DOI] [PubMed] [Google Scholar]
- 19.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 28:1692–1698. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA, et al. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–377. doi: 10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Kelesidis T, Roberts CK, Huynh D, Martinez-Maza O, Currier JS, Reddy ST, et al. A high throughput biochemical fluorometric method for measuring lipid peroxidation in HDL. PLoS One. 2014;9:e111716. doi: 10.1371/journal.pone.0111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9:5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson WS, Heink A, Sexmith H, Melchior JT, Gordon SM, Kuklenyik Z, et al. The Effects of Apolipoprotein B Depletion on HDL Subspecies Composition and Function. J Lipid Res. 2016 doi: 10.1194/jlr.M066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo Y, Oberbach A, Till H, Inge TH, Wabitsch M, Moss A, et al. Impaired HDL function in obese adolescents: impact of lifestyle intervention and bariatric surgery. Obesity (Silver Spring) 2013;21:E687–695. doi: 10.1002/oby.20538. [DOI] [PubMed] [Google Scholar]
- 25.Aron-Wisnewsky J, Julia Z, Poitou C, Bouillot JL, Basdevant A, Chapman MJ, et al. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J Clin Endocrinol Metab. 2011;96:1151–1159. doi: 10.1210/jc.2010-2378. [DOI] [PubMed] [Google Scholar]
- 26.Zvintzou E, Skroubis G, Chroni A, Petropoulou PI, Gkolfinopoulou C, Sakellaropoulos G, et al. Effects of bariatric surgery on HDL structure and functionality: results from a prospective trial. J Clin Lipidol. 2014;8:408–417. doi: 10.1016/j.jacl.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Osto E, Doytcheva P, Corteville C, Bueter M, Dorig C, Stivala S, et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation. 2015;131:871–881. doi: 10.1161/CIRCULATIONAHA.114.011791. [DOI] [PubMed] [Google Scholar]
- 28.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 30.Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 32.Salonen JT, Salonen R, Seppanen K, Rauramaa R, Tuomilehto J. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation. 1991;84:129–139. doi: 10.1161/01.cir.84.1.129. [DOI] [PubMed] [Google Scholar]
- 33.Asztalos BF, Swarbrick MM, Schaefer EJ, Dallal GE, Horvath KV, Ai M, et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2010;51:2405–2412. doi: 10.1194/jlr.P900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunitake ST, Kane JP. Factors affecting the integrity of high density lipoproteins in the ultracentrifuge. J Lipid Res. 1982;23:936–940. [PubMed] [Google Scholar]
- 35.Umaerus M, Rosengren B, Fagerberg B, Hurt-Camejo E, Camejo G. HDL2 interferes with LDL association with arterial proteoglycans: a possible athero-protective effect. Atherosclerosis. 2012;225:115–120. doi: 10.1016/j.atherosclerosis.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 36.Feener EP, Zhou Q, Fickweiler W. Role of plasma kallikrein in diabetes and metabolism. Thromb Haemost. 2013;110:434–441. doi: 10.1160/TH13-02-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel PJ, Khera AV, Wilensky RL, Rader DJ. Anti-oxidative and cholesterol efflux capacities of high-density lipoprotein are reduced in ischaemic cardiomyopathy. Eur J Heart Fail. 2013;15:1215–1219. doi: 10.1093/eurjhf/hft084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Distelmaier K, Wiesbauer F, Blessberger H, Oravec S, Schrutka L, Binder C, et al. Impaired antioxidant HDL function is associated with premature myocardial infarction. Eur J Clin Invest. 2015;45:731–738. doi: 10.1111/eci.12466. [DOI] [PubMed] [Google Scholar]
- 39.Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N Engl J Med. 2016 doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


