Abstract
Dopamine was first identified as a neurotransmitter localized to the midbrain over 50 years ago. The dopamine transporter (DAT; SLC6A3) and the vesicular monoamine transporter 2 (VMAT2; SLC18A2) are two regulators of dopamine homeostasis in the presynaptic neuron. DAT transports dopamine from the extracellular space into the cytosol of the presynaptic terminal. VMAT2 then packages this cytosolic dopamine into vesicular compartments for subsequent release upon neurotransmission. Thus, DAT and VMAT2 act in concert to move transmitter efficiently throughout the neuron. The accumulation of dopamine in the neuronal cytosol can trigger oxidative stress and neurotoxicity, suggesting that the proper compartmentalization of dopamine is critical for neuron function and risk of disease. For decades, studies have examined the effects of reduced transporter function in mice (e.g. DAT-KO, VMAT2-KO, VMAT2-deficient). However, we have only recently been able to assess the effects of elevated transporter expression using BAC transgenic methods (DAT-tg, VMAT2-HI mice). Complemented with in vitro work and neurochemical techniques to assess dopamine compartmentalization, a new focus on the importance of transporter proteins as both models of human disease and potential drug targets has emerged. Here we review the importance of DAT and VMAT2 function in the delicate balance of neuronal dopamine.
Keywords: dopamine, DAT, VMAT2, Parkinson’s disease
I. DOPAMINE AND NEUROTOXICITY
Dopamine was first identified in 1910, but the compound was thought to be merely an intermediary chemical in the synthesis of norepinephrine for decades (Hornykiewicz, 2002). In 1958, dopamine was identified in the brain (Carlsson et al., 1958), and the massive depletion of dopamine was linked to Parkinson’s disease in the 1960s (Ehringer and Hornykiewicz, 1960; Hornykiewicz, 1966). Dopamine neuron populations and pathways were soon identified as responsible for reward, cognition, and motor function (Haber, 2014; Swerdlow and Koob, 1987). Dopamine receptors are not saturated upon typical neurotransmission, suggesting that the dopamine system is capable of a large range of signaling intensities, the uppermost of which are not tapped at baseline. Thus, the dopamine system is not acting at its maximum function, leaving room for novel manipulations that could augment dopaminergic neurotransmission (Rice and Cragg, 2008). Here we discuss transgenic mouse models of altered dopamine output achieved by modifying dopamine compartmentalization and storage within the neuron terminal (Table 1). These mice provide novel insights into the neurochemical, behavioral, and protective or pathological effects of modified dopamine dynamics at the synapse.
Table 1. Mouse models with varying DAT and VMAT2 transporter levels.
“Transporter protein level” refers to DAT protein for DAT mouse models and VMAT2 protein for VMAT2 mouse models. Similarly, “dopamine uptake” refers to plasma membrane uptake for DAT mouse models and vesicular uptake for VMAT2 mouse models. Percentages reflect increases or decreases compared to measurements from wild type littermate control animals. Extracellular dopamine was measured by microdialysis. Stimulated dopamine release was measured by cyclic voltammetry or fast scan cyclic voltammetry. Tissue dopamine levels were measured by HPLC
| Genetic manipulation |
Transporter protein level (DAT or VMAT2) |
Dopamine uptake (plasma membrane or vesicular) |
Extracellular dopamine |
Dopamine release |
Tissue dopamine level |
Pathophysiological changes |
Behavior | Toxicant vulnerability |
Response to psychostimulants |
References |
|---|---|---|---|---|---|---|---|---|---|---|
| DAT mouse models | ||||||||||
| DAT-knockout | None | None | Increased (400%) | Decreased (75%) | Decreased (95%) in striatum | Dopamine dysregulation (e.g. desensitization of autoreceptors, reduced TH expression, increased dopamine synthesis) | Hyperlocomotion, impaired habituation, cognition, sensorimotor gating | Resistant (MPTP) | Paradoxical calming effect of cocaine and amphetamine on locomotion | Giros et al, 1996; Jaber et al, 1997; Jones et al, 1998; Bezard et al, 1999; Gainetdinov et al, 1997, 1999; Ralph et al, 2001; Barr et al, 2003; Sotnikova et al, 2005; Yamashita et al, 2006; Weiss et al, 2007 |
| DAT-over expressor | Increased (100–200% in dopamine cells only) | Increased (50%) | Decreased (40%) | Decreased (50%) | Decreased (30%) in striatum | Loss of midbrain dopamine neurons, oxidative stress | Fine motor deficits (rescued by L-DOPA) | Enhanced (MPTP) | Increased locomotion with amphetamine but not cocaine | Salahpour et al, 2008; Ghisi et al, 2009; Masoud et al, 2015 |
| VMAT2 mouse models | ||||||||||
| VMAT2-knockout | None | None | Not measured | Not measured | Not measured | Post-natal lethality due to lack of feeding | n/a | n/a | Amphetamine increases movement, promotes feeding, and prolongs survival | Wang et al, 1997; Fon et al, 1997; Takahashi et al 1997 |
| VMAT2-heterozygote | Decreased (50%) | Decreased (30%) | Decreased (40%) | Not measured | Decreased (20–40%)* in striatum | None reported | Increased depressive-like behavior | Enhanced (MPTP, meth-amphetamine) | Increased locomotion with amphetamine and cocaine; reduced amphetamine-conditioned reward | Wang et al, 1997; Takahashi et al 1997; Gainetdinov et al, 1998; Fumagalli et al, 1999; Fukui et al., 2007 |
| VMAT2-deficient | Decreased (95%) | Decreased (>80%) | Not measured | Decreased (>80%) | Decreased (>80%) in striatum | Dopaminergic and noradrenergic neurodegeneration, cellular oxidative stress, alpha-synuclein accumulation | Reduced locomotion and gut motility, increased depressive-like behavior and sleep disturbances | Enhanced (MPTP, meth-amphetamine) | Not measured | Mooslehner et al, 2001; Caudle et al, 2007; Taylor et al, 2009; Guillot et al, 2008; Taylor et al 2014; Lohr et al, 2016 |
| VMAT2-over expressor | Increased (100–200%) | Increased (100%) | Increased (40%) | Increased (80%) | Increased (20%) in striatum | Enlarged vesicles in TH-positive striatal terminals | Enhanced locomotion, reduced anxiety and depressive-like behaviors | Resistant (MPTP, meth-amphetamine) | Not measured | Lohr et al, 2014; Lohr et al, 2015; Lohr et al, 2016 |
n/a = not applicable, TH = tyrosine hydroxylase,
conflicting reports
Neurotoxic mechanisms of cytosolic dopamine
Dopamine is synthesized via the conversion of the amino acid tyrosine into L-dihydroxyphenylalanine (L-DOPA) by the rate-limiting enzyme tyrosine hydroxylase (TH) (Axelrod and Weinshilboum, 1972). L-DOPA is then converted into dopamine by amino acid decarboxylase (AADC) in the neuronal cytosol (Kuhar et al., 1999). Several studies have shown that accumulation of dopamine in the cytosolic space can lead to deleterious consequences (Caudle et al., 2007; Chen et al., 2008; Masoud et al., 2015; Mosharov et al., 2009). In a unique study, Mosharov and colleagues directly measured cytosolic dopamine in cultured neurons using intracellular patch electrochemistry (Mosharov et al., 2009). They demonstrated that manipulations that increased cytosolic dopamine levels (such as inhibiting dopamine metabolism) exacerbate toxicity, whereas reducing cytosolic dopamine levels (by inhibiting conversion of L-DOPA to dopamine or by increasing VMAT2 levels) is protective and improves cell survival. These results suggest that cytosolic dopamine levels can directly determine toxic outcomes in dopaminergic cells.
Cytosolic dopamine is highly reactive and causes toxicity by two mechanisms: deamination by cytosolic enzymes or autooxidation. Dopamine can be deaminated by mitochondrial monoamine oxidase (MAO), which converts cytosolic dopamine to DOPAL, a reactive aldehyde intermediate, and hydrogen peroxide (Eisenhofer et al., 2004; Goldstein et al., 2013; Rees et al., 2009). Luckily, DOPAL is most often converted to DOPAC, a relatively benign metabolite, via aldehyde dehydrogenase. However, our understanding of DOPAL toxicity in disease pathology, like that seen in Parkinson’s disease, is growing. DOPAL itself can be oxidized, creating reactive oxygen species (Eisenhofer et al., 2004). DOPAL has been shown to induce neurotoxicity in numerous culture systems and in a mouse model lacking cytosolic and mitochondrial aldehyde dehydrogenases (Kristal et al., 2001; Mattammal et al., 1995; Rees et al., 2009; Wey et al., 2012). Additionally, DOPAL is increased in the post mortem brains of individuals with Parkinson’s disease, suggesting a dysfunctional compartmentalization or breakdown of dopamine in those affected by the disease (Goldstein et al., 2011).
Cytosolic dopamine can be also autooxidized to form reactive oxygen species including hydroxyl radicals, superoxide, and hydrogen peroxide (Graham, 1978; Jenner, 2003). Oxidized dopamine can then be converted to dopamine-quinones and protein function-altering cysteinyl adducts (LaVoie and Hastings, 1999; Lotharius and O’Malley, 2001; Rabinovic et al., 2000). Additionally, dopamine synthesis proteins have been reported to interact with the vesicular monoamine transporter 2 (VMAT2), helping to accumulate dopamine in synaptic vesicles from the cytosolic space perhaps as a protective mechanism. For example, TH and AADC have been immunoprecipitated with VMAT2, and vesicular filling is reduced following the disruption of these interactions (Cartier et al., 2010). Based on the detrimental effects of cytosolic dopamine, it has been speculated that the relative distribution of dopamine within the neuronal terminal, possibly mediated by vesicular storage and plasma membrane uptake, may be responsible for a neuron’s vulnerability to injury (Guillot and Miller, 2009; Miller et al., 1999b; Uhl, 1998).
II. PLASMA MEMBRANE TRANSPORT
Plasma membrane transport via the dopamine transporter
Transport of dopamine across the plasma membrane is an important process that contributes to overall dopamine neurotransmission and compartmentalization. This process is mediated by the dopamine transporter (DAT, SLC6A3), a membrane protein located on dopaminergic cells. Similar to other monoamine transporters, DAT has 12 transmembrane domains with intracellular N- and C- termini and belongs to the SLC6A family of Na+/Cl−-dependent symporters (Gainetdinov and Caron, 2003). In particular, DAT couples the active transport of dopamine with the movement of one Cl− and two Na+ ions along the concentration gradient. This concentration gradient is created by the plasma membrane Na+/K+ ATPase and serves as the driving force for DAT-mediated dopamine uptake (Gether et al., 2006; Kanner and Schuldiner, 1987). Dopamine translocation across the plasma membrane occurs as a result of conformational changes in DAT. The uptake cycle begins when DAT is open to the extracellular space in an outward facing state (Reith et al., 2015). In this conformation, Na+ and Cl− ions bind to DAT and prepare the transporter for dopamine binding. Upon binding of dopamine, the extracellular gate closes, generating an occluded DAT state. Importantly, dopamine binding induces a conformational change allowing the transporter to open on the cytosolic side. In this inward facing state, dopamine and ions dissociate from DAT. Finally, the cycle is reset once DAT returns to the outward facing conformation (Reith et al., 2015).
The function of DAT is to rapidly transport dopamine from the extracellular space into the cytosol of the presynaptic neuron. At the plasma membrane, DAT is located peri-synaptically, where it removes extracellular dopamine and provides spatial and temporal control of the dopamine signal (Cragg and Rice, 2004; Hersch et al., 1997; Jones et al., 1998). In dopaminergic brain regions such as the striatum and nucleus accumbens, DAT provides the principal mechanism of clearing extracellular dopamine and terminating neurotransmission (Ciliax et al., 1995). Aside from modulating the dynamics of released dopamine, DAT is also responsible for recycling the neurotransmitter back into the dopaminergic cell, allowing it to be reused (Sotnikova et al., 2006). By loading the presynaptic neuron with dopamine, DAT directly contributes to the buildup of cytosolic dopamine and indirectly influences vesicular dopamine as well. Accumulation of cytosolic dopamine can produce neurotoxicity as previously discussed. Hence, DAT is a key player in dopamine compartmentalization that can have significant consequences for the presynaptic neuron. Collectively, DAT regulates the concentrations of both 1) extracellular dopamine at the synapse and 2) intracellular dopamine within the presynaptic neuron.
Pharmacological manipulation of plasma membrane transport
DAT is the primary target for many compounds including psychostimulants, medications and neurotoxicants (Miller et al., 1999b; Torres et al., 2003). Since uptake of dopamine is dependent on DAT, pharmacological manipulation of DAT can produce profound effects on dopamine neurotransmission. Two classical psychostimulants that operate by altering DAT function are cocaine and amphetamine. Cocaine binds to DAT and blocks the transport of dopamine from the extracellular space to the presynaptic neuron (Ritz et al., 1987). Cocaine is a competitive inhibitor of dopamine transport because its binding site overlaps with dopamine’s site of action, precluding the endogenous substrate from binding (Beuming et al., 2008). As a result, dopamine accumulates in the extracellular space where it can reinforce downstream signaling. Conversely, amphetamines (amphetamine, methamphetamine, MDMA) compete with dopamine to enter dopaminergic cells, acting as a substrate for DAT (Sulzer et al., 2005). Once inside the cell, amphetamine disrupts the proton gradient required for vesicular storage of dopamine (Sulzer et al., 1995). This leads to leakage of dopamine from the vesicles into the cytoplasm of the presynaptic neuron. Ultimately, accumulation of cytosolic dopamine in combination with the actions of amphetamine on DAT cause a reversal of the transporter, resulting in efflux of intracellular dopamine into the extracellular space. This DAT-mediated release of dopamine produces a surge in dopamine signaling in response to amphetamine. While cocaine and amphetamine can also produce other effects in the CNS, it is the manipulation of DAT function that directly enhances dopamine neurotransmission and is thought to underlie the reinforcing properties of these psychostimulants (Donovan et al., 1999; Howell and Kimmel, 2008). In addition to enhancing extracellular dopamine levels, these psychostimulants have also been shown to activate phasic dopamine signaling events causing release of dopamine that contributes to drug reinforcement (Aragona et al., 2008; Daberkow et al., 2013; Wanat et al., 2009).
Drugs that modulate DAT are also used for therapeutic purposes in diseases such as attention deficit hyperactivity disorder (ADHD), depression, narcolepsy, obesity and drug dependence. Indeed, dextroamphetamine and methylphenidate, both act by modifying DAT function and are the main components of the leading medications for ADHD, Adderall and Ritalin, respectively (Gether et al., 2006; Gowrishankar and Hahn, 2014). In particular, dextroamphetamine causes DAT reversal and dopamine release similar to amphetamine, while methylphenidate is a DAT inhibitor. These drugs relieve disease symptoms by increasing dopamine signaling which is disrupted in ADHD patients (Sagvolden et al., 2005; Volkow et al., 2010). In addition to dopamine, these drugs also elevate norepinephrine levels which may contribute to their therapeutic effects (Del Campo et al., 2011). Furthermore, some antidepressants like bupropion also block DAT-mediated dopamine uptake and derive at least part of their therapeutic benefit from this inhibition (Learned-Coughlin et al., 2003). Modafinil, prescribed for the treatment of narcolepsy, is a selective DAT inhibitor that elevates dopamine transmission and promotes wakefulness (Volkow et al., 2009). Since the dopamine system has several physiological roles (cognition, motivation, reward, motor control), inhibiting DAT function provides a unique therapeutic opportunity to enhance dopamine signaling and impact diverse behaviors.
In addition to these examples, several compounds can inhibit DAT function with varying levels of selectivity and potency. Initially, it was postulated that all DAT inhibitors would have cocaine-like stimulant and reinforcing properties (Ritz et al., 1987). However, over the past 10–15 years, accumulating evidence has challenged this notion, showing heterogeneity among DAT inhibitors (Schmitt et al., 2013). In fact, different compounds preferentially bind and stabilize distinct structural states of DAT. Typical DAT inhibitors such as cocaine and methylphenidate have been shown to stabilize the outward facing conformation and produce locomotor stimulation and behavioral reinforcement (Loland et al., 2007). However, atypical DAT inhibitors such as modafinil, buproprion and vanoxerine (GBR12909) tend to promote occluded/inward facing conformations (Schmitt et al., 2013). Interestingly, these compounds also lack cocaine-like behavioral effects and possess limited rewarding properties (Schmitt and Reith, 2011). Very recently, ligands that bind to allosteric sites on DAT have been identified and shown to block dopamine uptake as well (Janowsky et al., 2016). Hence, it seems that the specific pharmacological profile of a drug and its behavioral effects are heavily dependent on how the drug interacts with DAT and which structural conformation is favored. In general, DAT antagonists lock the transporter in a certain structural state, preventing the conformational transitions that are required to shuttle dopamine across the plasma membrane (Reith et al., 2015). Taken together, these data exemplify 1) the diversity of DAT ligands and 2) the responsiveness of DAT to different types of pharmacological manipulation.
As a plasma membrane transporter, DAT also provides a site of entry into dopaminergic cells. The most potent dopaminergic toxicants, 6-hydroxydopamine and MPP+, are substrates of DAT (Gainetdinov et al., 1997; Miller et al., 1999b; Schober, 2004). These compounds are used to mimic symptoms of Parkinson’s disease in animal models because they cause robust degeneration of dopaminergic cells. Since 6-hydroxydopamine is a structural analog of dopamine, it can hijack the DAT-mediated uptake mechanism to access dopamine cells. It should be noted that 6-hydroxydopamine is also a substrate for the norepinephrine transporter and thus, must be administered specifically to dopaminergic regions to exert its toxicity. With regards to MPTP, after crossing the blood brain barrier, this compound is converted to its toxic metabolite MPP+ by monoamine oxidase. MPP+ is specifically translocated into dopaminergic neurons by DAT (Javitch et al., 1985). Once these toxins accumulate in dopaminergic cells, they cause oxidative stress and mitochondrial dysfunction, culminating in neurotoxicity (Abdulwahid Arif and Ahmad Khan, 2010; Miller et al., 1999a; Simola et al., 2007). Hence, DAT provides a molecular gateway for toxicants to selectively access and damage dopaminergic cells.
In summary, various compounds produce significant effects in the brain as a result of their actions on DAT. Given this rich pharmacology, manipulation of DAT function occurs in different ways: 1) by inhibiting DAT and causing buildup of extracellular dopamine, 2) by reversing DAT and causing release of dopamine and 3) by acting as substrate of DAT and using the transporter to access dopamine cells. Interestingly, compounds that increase DAT activity are currently lacking.
DAT-knockout mice
The critical role of DAT in maintaining appropriate dopaminergic function is clearly demonstrated by DAT knockout mice (DAT-KO). Genetic ablation of this plasma membrane transporter produces dramatic changes in extracellular and intracellular dopamine dynamics (Giros et al., 1996; Jaber et al., 1997; Jones et al., 1998). DAT-KO mice display 5-fold elevated extracellular dopamine levels due to lack of uptake. Additionally, dopamine remains in the extracellular space 300 times longer since diffusion is the only mechanism to clear the neurotransmitter in DAT-KO mice. Conversely, intracellular dopamine content is reduced by 95% demonstrating that DAT-mediated recycling of dopamine is chiefly responsible for maintaining presynaptic dopamine levels (Sotnikova et al., 2005). Due to depleted intracellular stores, evoked dopamine release is also diminished by 75% in DAT-KO mice (Jones et al., 1998). These neurochemical changes illustrate the vital role of DAT in balancing dopamine levels across different cellular compartments.
Furthermore, lack of DAT activity also triggers compensatory alterations in other pre- and post-synaptic markers of the dopamine system. Striatal post-synaptic D1 and D2 receptors are downregulated by 60% and 40% respectively, to adapt to high extracellular dopamine (Ghisi et al., 2009). Presynaptic D2 autoreceptors are also desensitized, disrupting regulatory negative feedback mechanisms (Giros et al., 1996; Jones et al., 1999). Levels of dopamine metabolites, HVA and 3-MT, are increased suggesting that dopamine degradation may be altered in these mice (Jones et al., 1998). Without DAT-mediated dopamine recycling, presynaptic dopamine levels in DAT-KO mice are solely dependent on synthesis by TH. Paradoxically, while TH expression is reduced, dopamine synthesis rates are doubled, highlighting major adaptive changes in attempts to stabilize dopamine levels in DAT-KO mice (Jaber et al., 1999; Jones et al., 1998). Behaviorally, these animals show spontaneous hyperlocomotion and impaired habituation as a result of increased extracellular dopamine (Giros et al., 1996). DAT-KO mice also display disturbances in cognition (Weiss et al., 2007) and sensorimotor gating (Barr et al., 2003; Ralph et al., 2001; Yamashita et al., 2006). Pharmacologically, DAT-KO mice are insensitive to the classical stimulant actions of cocaine and amphetamine but show paradoxical calming effects instead (Gainetdinov et al., 1999; Giros et al., 1996). In particular, when treated with cocaine or amphetamine, dopamine release and locomotor activity are not enhanced in DAT-KO mice, validating that transporter function is compulsory for psychostimulant effects (Giros et al., 1996). Also, DAT-KO mice are completely resistant to nigrostriatal damage induced by MPTP (Bezard et al., 1999; Gainetdinov et al., 1997) demonstrating that DAT-mediated uptake of MPP+ is required for neurotoxic effects. Collectively, mice lacking DAT show dramatic neurochemical, adaptive and behavioral changes in the dopamine system as summarized in Table 1. Investigation of DAT-KO mice has contributed essential knowledge on the physiological role of DAT as well as the importance of this transporter as a pharmacological target.
DAT-overexpressing mice
On the other end of the spectrum are mice that over-express DAT. DAT was selectively over-expressed in dopaminergic neurons of DAT-transgenic (DAT-tg) mice using bacterial artificial transgenesis (Salahpour et al., 2008). DAT-tg mice display approximately a 3-fold increase in total striatal DAT protein and a 30% increase specifically in the synaptic plasma membrane fraction. Functionally, this translates to a 40–50% increase in dopamine uptake. Due to increased dopamine clearance, DAT-tg mice also have about a 40% reduction in extracellular dopamine levels (Salahpour et al., 2008). While increased DAT expression is expected to enhance dopamine accumulation in the presynaptic neuron, both striatal dopamine tissue content and evoked dopamine release were reduced in DAT-tg mice (Masoud et al., 2015). These results reflect compromised integrity of dopamine neurons in DAT-tg mice as evidenced by 30–40% loss of midbrain dopamine cells (Masoud et al., 2015). Neuronal loss is accompanied by increases in dopaminergic markers of oxidative stress, cysteinyl-dopamine and cysteinyl-DOPAC. These detrimental consequences in DAT-tg mice are likely due to the neurotoxic effects of high cytosolic dopamine. Although it is difficult to assess cytosolic dopamine concentrations in an intact animal, increased dopamine-to-metabolite ratios in these mice indicate buildup of cytosolic dopamine. Behaviorally, DAT-tg mice display fine motor deficits that are reversed by L-DOPA treatment, suggesting that dopamine neuronal loss negatively impacts motor behavior that can be rescued by restoring dopaminergic tone (Masoud et al., 2015). Pharmacologically, DAT-tg mice are particularly vulnerable to MPTP toxicity and show enhanced response to amphetamine, as expected (Masoud et al., 2015; Salahpour et al., 2008). These outcomes reinforce the physiological importance of proper dopamine compartmentalization in the vulnerable nigrostriatal pathway. Characteristics of these mice are summarized in Table 1. Since DAT-tg mice display phenotypes related to Parkinson’s disease (loss of dopamine cells, reduced dopaminergic tone, oxidative stress, L-DOPA responsive motor deficits), characterization of these mice could inform potential disease mechanisms.
DAT in disease
Although the physiological actions of DAT are well-elucidated, deciphering the role of this transporter in disease pathogenesis has been complex. In fact, the DAT protein sequence appears to be highly conserved, possibly as an evolutionary mechanism to preserve appropriate function of the dopamine system (Vandenbergh et al., 2000). Evidence surrounding the involvement of DAT in neurological and psychiatric diseases has only recently been uncovered and is still developing. The first genetic condition directly caused by DAT mutations was discovered in 2009 (Kurian et al., 2009). DAT deficiency syndrome (DTDS) is caused by autosomal recessive loss-of-function mutations in the DAT gene (SLC6A3) (Kurian et al., 2011, 2009). In vitro analyses indicate that the mutations associated with this disease obstruct maturation of the DAT protein, leading to reduced transporter expression at the plasma membrane. As a result, overall dopamine uptake is impaired triggering a complex motor disorder characterized by progressive parkinsonism-dystonia. Typically, the syndrome manifests in infancy and severely reduces life expectancy (Ng et al., 2014). The striking phenotypes in DTDS patients convincingly demonstrate the significance of DAT genetics in controlling motor behavior.
The most common neurodegenerative movement disorder in humans is Parkinson’s disease. Motor symptoms of Parkinson’s disease are characterized by muscle rigidity, postural instability, resting tremor and bradykinesia (Jankovic, 2008). These motor deficits are caused by degeneration of nigrostriatal dopamine neurons that lead to reduced dopaminergic tone in the basal ganglia (Dauer and Przedborski, 2003). Unlike DTDS, concrete evidence of a causal link between genetic DAT mutations and Parkinson’s disease is lacking. This is probably because the etiology of Parkinson’s disease is multifactorial and genetic mutations account for only a small proportion of cases (5–10%) (Dauer and Przedborski, 2003; Sulzer, 2007). However, neuroanatomical analyses indicate that regions of the human brain containing the highest levels of DAT protein – the caudate and putamen – are most sensitive to damage in PD (Miller et al., 1997), suggesting that DAT may act as risk factor. The potential role of DAT in enhancing vulnerability of dopamine neurons in PD is two-fold: first, it functions to increase the pool of cytosolic dopamine, which is highly reactive and second, it allows toxicants such as MPTP selective access to dopaminergic cells. Hence, DAT activity could sensitize dopamine neurons to both intrinsic oxidative stress as well as extrinsic environmental insult. In fact, a study by Ritz et al demonstrates that DAT genetic variants in combination with pesticide exposure can increase risk of PD by several fold (Ritz et al., 2009). These DAT variants include single nucleotide polymorphisms in the 5′ region as well as variable number tandem repeats (VNTR) at the 3′ region of the gene. Although the functional consequences of these DAT variants are unclear, these results highlight the importance of considering genetic and environmental interactions in PD (Kelada et al., 2006; Sulzer, 2007). In summary, while DAT mutations produce drastic childhood-onset motor syndromes like DTDS, in a progressive age-related disorder like PD, DAT is more likely to play a modulatory role in combination with other risk factors.
Another disorder that has received considerable attention with regards to DAT dysfunction is ADHD (Gowrishankar and Hahn, 2014). There are two reasons that spurred particular interest in ADHD: 1) DAT is fundamental in dopamine neurotransmission and a large body of work indicates that dopamine signaling is dysregulated in ADHD and 2) the most common pharmacological therapies for this disorder, dextroamphetamine and methylphenidate, directly target and antagonize DAT. Hence, genetic links between DAT and ADHD have been investigated. Initially, Cook et al. reported that the VNTR polymorphism in the 3′ untranslated region of the DAT-1 allele conferred significant risk for developing ADHD (Cook et al., 1995). Other independent studies including a meta-analysis confirmed this result (Chen et al., 2003; Gill et al., 1997; Yang et al., 2007). While the precise molecular basis for this association is unclear, it has been shown that the VNTR polymorphism can impact expression of the DAT gene (Michelhaugh et al., 2001; Mill et al., 2002). Aside from polymorphisms in the non-coding region, rare mutations in the coding sequence of the DAT gene have also been revealed in ADHD. In particular, a point substitution, Ala559Val was discovered in two male siblings diagnosed with ADHD (Mazei-Robison et al., 2008). When tested in vitro, this variant caused abnormal DAT-mediated efflux of dopamine (Mazei-Robison et al., 2008). In vivo analysis of DAT Val 559 knock-in mice confirmed in vitro findings by demonstrating elevated extracellular dopamine levels consistent with DAT-mediated leakage of cytosolic dopamine (Mergy et al., 2014). Basally, these mice demonstrate context-dependent hyperactivity when handled, which may represent phenotypes similar to ADHD patients. In response to amphetamine, these mice show diminished dopamine release and blunted locomotor activation (Mergy et al., 2014), suggesting reduced sensitivity to psychostimulant effects. Hence, the Ala559Val DAT mutation produces noteworthy changes in dopamine dynamics and behavior that may relate to ADHD pathogenesis. In another subject with ADHD, Sakrikar and colleagues described an additional DAT coding variant, Arg615Cys (Sakrikar et al., 2012). This mutation affects the C-terminus of the DAT protein and gives rise to a transporter that is constitutively recycled. Hence, DAT localization and trafficking are largely impacted by this mutation. Taken together, these findings suggest that genetic alterations in DAT structure, expression or function can impose an increased risk for the development of ADHD, a disorder that is linked to aberrant dopamine signaling (Sagvolden et al., 2005; Volkow et al., 2010).
In addition to ADHD, point mutations in the DAT gene have been associated with other neuropsychiatric diseases as well. The Ala559Val DAT variant that was detected in 2 ADHD patients, was also identified in subjects with bipolar disorder and autism spectrum disorder, suggesting that altered DAT function and consequently, modified dopamine signaling may represent a common pathological pathway for these diseases (Bowton et al., 2014; Grünhage et al., 2000; Mergy et al., 2014). Furthermore, a whole-exome sequencing study in families with autism spectrum disorder uncovered another missense DAT mutation, Thr356Met. Similar to the Ala559Val DAT variant, the Thr356Met DAT mutation also produced persistent efflux of dopamine, although the two variants differed in other characteristics (Gowrishankar and Hahn, 2014; Hamilton et al., 2013). Interestingly, transgenic expression of the mutated human Thr356Met DAT in drosophila produced hyperactivity (Hamilton et al., 2013), implying possible overlap in the mechanisms of ADHD and autism spectrum disorder. Indeed, a significant proportion of individuals with autism spectrum disorder concurrently display ADHD symptoms (Goldstein and Schwebach, 2004). Another systematic screening approach of the DAT coding sequence revealed a missense substitution, Glu602Gly in an individual with bipolar disease that was inherited from an affected parent (Grünhage et al., 2000). The role of this mutation in disease mechanisms is currently unknown since the Glu602Gly mutant shows similar surface DAT expression and dopamine transport as wild-type DAT in vitro (Mazei-Robison and Blakely, 2005). This raises the possibility that there may be other properties of DAT dynamics and regulation that have not yet been identified in this mutant. Finally, when DAT coding exons were sequenced in a cohort of patients with atypical movement disorders, it revealed 2 variants Ile312Phe and Asp421Asn (Hansen et al., 2014). Remarkably, both mutations were detected in an individual with comorbid adult-onset neurodegenerative parkinsonism and ADHD. DAT-Ile312Phe was inherited from one of the parents while DAT-Asp421Asn appeared to be a de novo mutation. In vitro, both mutations result in reduced dopamine uptake capacity and the Asp421Asn DAT variant also shows anomalous efflux of dopamine (Hansen et al., 2014). The comorbidity in this individual suggests that DAT variants could provide a unifying mechanism between movement and psychiatric disorders.
In summary, mutations in the coding and non-coding regions of the DAT gene have been associated with various motor and neuropsychiatric disorders. While the mechanisms of these mutations in disease pathogenesis are yet to be fully elucidated, they have been shown to cause changes in dopamine signaling that is important for multiple brain functions. Identification of discrete DAT mutations in rare individuals is a significant finding given the genetic conservation of DAT and multifactorial nature of complex brain disorders.
III. VESICULAR MEMBRANE TRANSPORT
Synaptic vesicle filling via the vesicular monoamine transporter 2
Neurotransmission relies on the proper storage and release of neurotransmitters from synaptic vesicles. Without precise control of the quantal release of these molecules, the fundamental processes of effective signaling would be disrupted. Synaptic vesicles are small spherical lipid bilayers formed in the Golgi apparatus and transported to the synaptic terminal (Südhof, 2004; Zimmermann et al., 1993). In the terminal, vesicles are responsible for packaging and concentrating neurotransmitters for signaling events. The majority of the neurotransmitters are sequestered into synaptic vesicles at any given moment, making vesicular storage the primary determinant of transmitter capacity of the brain. Through active transport mechanisms, synaptic vesicles package high concentrations of neurotransmitters within their lumen. A vacuolar proton pump creates the electrochemical gradient in the vesicle (Maycox et al., 1988) It is estimated that the proton pump takes up about 10% of the vesicle’s volume (Stadler and Tsukita, 1984). Each type of neuron is defined by the vesicular transmitter transporter expressed on vesicles within the cell. For example, GABA, glutamate, and monoaminergic cells can be defined by their expression of VGAT, VGLUT1 and 2, or VMAT1 and 2, respectively.
The vesicular monoamine transporter 2 (VMAT2, SLC18A2) is responsible for the packaging of cytosolic monoamines (dopamine, serotonin, norepinephrine, histamine) into small synaptic and dense core vesicles in monoaminergic neurons throughout the brain (Eiden and Weihe, 2011; Erickson and Eiden, 1993; Erickson et al., 1992; Liu et al., 1992a). VMAT2 is a member of the SLC18 family of transporter proteins, where both VMAT1 and the vesicular acetylcholine transporter (VAChT) are members (Lawal and Krantz, 2013). VMAT1 is preferentially expressed in large dense core vesicles of neuroendocrine cells, including chromaffin and enterochromaffin cells; whereas VMAT2 is primarily expressed in monoaminergic neurons in the CNS, sympathetic nervous system, mast cells, and histamine containing cells in the gut (Peter et al., 1995; Schuldiner et al., 1995). Though its crystal structure is unknown, VMAT2 is characterized by 12 transmembrane helices (Peter et al., 1994). Both the N- and C-termini of VMAT2 are localized in the cytosol, and there is a large glycosylated intralumenal loop between the first and second transmembrane domains of the transporter (Wimalasena, 2011). VMAT2 is an H+-ATPase antiporter, which uses the vesicular electrochemical gradient to drive transport (Parsons et al., 1993; Reimer et al., 1998). The pH gradient across the vesicular membrane is established by the vacuolar H+-ATPase, which uses ATP hydrolysis to generate the energy required to move H+ ions into the vesicle (Kanner and Schuldiner, 1987; Moriyama and Futai, 1990). It is this movement of H+ ions that creates the vesicular proton gradient and establishes an acidic environment inside the vesicle (pH of ~5.5). This electrochemical gradient drives VMAT2-mediated transport (Rudnick et al., 1990). For every single monoamine molecule transported into the vesicular lumen via VMAT2, two protons are extruded into the cytosol. By driving the sequestration of neurotransmitter into the vesicle, VMAT2 is a critical mediator of dopamine dynamics in the neuronal terminal.
It had previously been postulated that a variety of factors may limit the packaging of neurotransmitters into monoaminergic vesicles (Edwards, 2007). These models include the set point, equilibrium, leak, and swelling models of vesicular storage. The set point model suggests that vesicles have a set maximum independent of the number of vesicular transporters on the membrane. This could be dictated by equilibrium across the membrane or even physical limitations of the biomechanics of the vesicular membrane. In the equilibrium model, the vesicular ATPase creates the electrochemical H+ gradient that is the driving force for filling via VMAT2. Once the H+ gradient is eliminated and equilibrium is reached between the cytosol and the vesicular lumen, monoamine packaging would stop. Finally, it is possible that biomechanics of the vesicular membrane may act as the limiting factors for filling. In vitro evidence has shown that L-DOPA application and VMAT2 inhibition has the potential to alter vesicle size (Bruns et al., 2000; Colliver et al., 2000; Gong et al., 2003; Pothos, 2002). This suggests that the concentration of transmitter inside of a vesicle does not increase; rather it is merely the volume of the vesicle increasing. These previous results also showed that limitations to the elasticity of membranes may be less stringent than previously thought. Finally, it was assumed that an increase in vesicular filling would result in compensatory changes to the rest of the dopamine system (e.g. increased extracellular dopamine clearance, decreased dopamine synthesis, altered receptor sensitivity) that would eventually downregulate dopamine output.
VMAT2 as a neuroprotective mechanism
Vesicular dopamine is protected in the synaptic vesicle. However, dopamine can be left in the cytosol following its synthesis, uptake via the DAT, or after leak out of the synaptic vesicle. The correlation between vesicular function and neuronal vulnerability is well established. VMAT2 is phylogenetically related to a family of toxin extruding antiporters (TEXANs) in prokaryotes and eukaryotes and is also a member of the major facilitator superfamily (MFS) which remove toxins from the cytosol and sequester them into other intracellular compartments (Guillot and Miller, 2009; Schuldiner et al., 1995; Vardy et al., 2004). In fact, the cloning of VMAT2 and its initial characterization was based on the protein’s ability to confer protection from MPP+ toxicity in Chinese hamster ovary fibroblasts (CHO cells) (Liu et al., 1992a). In addition to its ability to protect from exogenous toxic insult, VMAT2 has also been shown to protect against native threats to neuronal health, including unpackaged cytosolic dopamine that can be neurotoxic (Guillot and Miller, 2009).
Pharmacological manipulations of vesicular filling
VMAT2 has two well-characterized inhibitors, reserpine and tetrabenazine. Reserpine, a compound isolated from Indian snakeroot Rauwolfia serpentine, was originally used in the treatment of hypertension (Freis, 1954). However, in addition to reserpine’s ability to inhibit dopamine uptake (Kirshner, 1962; Kirshner et al., 1963), it was also noted that reserpine treatment caused a variety of side effects including gastrointestinal dysfunction, movement deficits, and negative mood (Carlsson, 1976, 1972). It was these mood-altering effects of reserpine that supported the idea that monoamine neurotransmitters may be key in affective behaviors. While the pharmacological target of reserpine was not identified for many years (Erickson et al., 1992; Liu et al., 1992b), reserpine treatment in rats recapitulated many of the behavioral effects seen in parkinsonian humans, including profound akinesia (Colpaert, 1987). In this way, reserpine provided a Parkinson’s disease-like set of behavioral characteristics. VMAT2 inhibition via reserpine is also reversible by the application of a dopamine replacement therapy like L-DOPA (Carlsson, 1972). However, reserpine is not selective between VMAT1 and VMAT2 (Peter et al., 1994). Due to the differing distributions between VMAT1 and VMAT2 in the body, reserpine-mediated VMAT2 inhibition makes CNS-specific effects more difficult to assess. Tetrabenazine is a VMAT2-selective inhibitor (IC50 = 0.3 μM), since it has less of an inhibitory effect on VMAT1 (IC50 = 3 μM) (Chaudhry et al., 2008; Lawal and Krantz, 2013; Pothos, 2002; Wimalasena, 2011). Unlike reserpine, tetrabenazine acts at a binding site on VMAT2 distinct from the substrate binding site and allows for reversible VMAT2-specific inhibition of vesicular filling (Peter et al., 1994). Tetrabenazine and other derivate compounds are currently used clinically in the treatment of hyperkinetic disorders such as chorea associated with Huntington’s disease in Canada, Australia, the United Kingdom, and, more recently, in the United States (Wimalasena, 2011; Yero and Rey, 2008). Recent work has suggested that VMAT2 inhibition by lobeline may be therapeutic in the treatment of psychostimulant abuse (Dwoskin and Crooks, 2002; Nickell et al., 2010). Lobeline acts at the tetrabenazine binding site to decrease amphetamine-evoked dopamine release, suggesting that lobeline selectively inhibits amphetamine effects. It has been suggested that this reduction in dopamine signal occurs via increased metabolism and less dopamine available for reverse transport through the DAT (Miller et al., 2001). Thus, by taking a VMAT2 inhibitor, dopamine-releasing drugs such as amphetamine have less of a rewarding or euphoric effect.
Based on the importance of vesicular storage in neurotransmission and neuroprotection, an increase in VMAT2 function could be a novel clinical target for diseases characterized by loss of dopamine neurons and, subsequently, dopamine release. Despite an extensive history of studying the effects of pharmacological VMAT2 inhibition, there are no known positive modulators of VMAT2 function (Osherovich, 2014).
VMAT2-deficient mice
Previous data clearly show that disruption of VMAT2 function produces adverse effects (Caudle et al., 2008; Taylor et al., 2011). Pharmacological VMAT2 inhibition by reserpine or tetrabenazine results in monoamine depletion and negative behavioral consequences, including akinesia and depressive behaviors (Kirshner, 1962; Kirshner et al., 1963; Pettibone et al., 1984). Genetic knockout of VMAT2 is lethal, with animals dying a few days after birth (Mooslehner et al., 2001; Wang et al., 1997). Through serendipitous recombination events, mice with only 5% of VMAT2 levels were also created (Caudle et al., 2007; Mooslehner et al., 2001). These mice survive into adulthood, allowing for assessments of long-term vesicular deficits. The large reduction in VMAT2 levels in the VMAT2-deficient mice dramatically reduces vesicular filling (approximately 80% reduction) and also causes depletion of dopamine, norepinephrine, and serotonin levels throughout the brain (Caudle et al., 2007; Taylor et al., 2014). VMAT2-deficient mice also show progressive neurodegeneration in multiple monoaminergic regions, including the substantia nigra pars compacta and the locus coeruleus (Caudle et al., 2007; Fon et al., 1997; Taylor et al., 2014). Additionally, these animals show accumulation of α-synuclein, the signature protein component in Lewy body pathology (Caudle et al., 2007).
VMAT2-deficient mice and VMAT2 heterozygote mice also have exacerbated dopaminergic responses to toxicant exposure, including treatment with both MPTP and methamphetamine (Gainetdinov et al., 1998; Guillot et al., 2008; Lohr et al., 2016; Takahashi et al., 1997). Reduced VMAT2 modifies a variety of monoamine-mediated behaviors, including reduced locomotor activity (Caudle et al., 2007) as well as non-motor behaviors reminiscent of pre-clinical Parkinson’s disease, including depressive-like and anxiety-like behaviors, olfactory deficits, altered sleep latency, and gastrointestinal disturbances (Fukui et al., 2007; Taylor et al., 2009). Taken together, evidence from VMAT2-deficient mice has pointed out the numerous deficits arising from reduced vesicular function, while also highlighting the potential benefits of upregulating vesicular storage. Characteristics of these mice are summarized in Table 1.
VMAT2-overexpressing mice
Although the detrimental effects of reduced VMAT2 function are recognized, our understanding of the potential benefits of increased VMAT2 function in vivo had been limited to a Drosophila model (Chang et al., 2006; Lawal et al., 2010; Sang et al., 2007). Recently, BAC-mediated VMAT2 overexpression in mice (VMAT2-HI) was shown to enhance the capacity of the vesicle to store dopamine (Lohr et al., 2014). Due to this enhanced storage, VMAT2-overexpressing mice have increased total dopamine content and synaptic vesicle size. The elevated vesicular filling translates to elevated dopamine release and changes in monoamine-related behaviors, including locomotor activity, forced swim test, and the marble burying assay. As shown in Table 1, VMAT2-overexpressing mice are also resistant to neurotoxic insults, including MPTP and methamphetamine intoxication, based on VMAT2’s neuroprotective abilities (Lohr et al., 2016, 2015, 2014). Together, these findings suggest that elevated VMAT2 would be beneficial to enhance neuronal monoamine output, protect vulnerable dopamine neuronal populations, and improve monoamine handling at the synapse.
Presynaptic machinery and Parkinson’s disease
Genetic mutations linked to Parkinson’s disease often affect synaptic vesicle machinery, leading to deficits in trafficking, transmitter storage, and release. α-Synuclein, the first PARK locus identified, has long been known to bind to phospholipids on the vesicle membrane (Lotharius et al., 2002; Nemani et al., 2010; Volles and Lansbury, 2002). Protofibrillar α-synuclein can permeabilize the vesicular membrane, spilling dopamine into the cytosol (Volles and Lansbury, 2002). Oxidized dopamine stabilizes protofibrillar α-synuclein, creating a potentially vicious cycle of pathology (Conway, 2001; Mosharov et al., 2009; Ulusoy et al., 2012). While the biological function of α-synuclein is unknown, genetic ablation of the synuclein genes (α, β, γ) increases dopamine release, suggesting that the protein may act as a brake on transmitter release (Anwar et al., 2011).
However, α-synuclein, perhaps the best characterized Parkinson’s disease-related protein, is not the only link between Parkinson’s disease and vesicular function. Leucine-rich repeat kinase 2 (LRRK2) mutations, responsible for the majority of familial Parkinson’s disease, modify vesicle trafficking, localization of vesicular proteins, and release of dopamine (Belluzzi et al., 2012; Cirnaru et al., 2014; Piccoli et al., 2011). Reduced PTEN-induced putative kinase 1 (PINK1) function also decreases synaptic efficiency by immobilizing synaptic vesicles of the reserve pool (Morais et al., 2009). Protein deglycase DJ-1 interacts with synaptic vesicle proteins such as synaptophysin and Rab3A and modifies the expression of VMAT2 (Osherovich, 2014; Usami et al., 2011). DJ-1, PINK1 and parkin knock-out mouse models all show substantial presynaptic deficits in dopamine release (Cookson, 2012; Goldberg, 2005; Kitada et al., 2007). Most recently, synaptojanin, a vesicular protein integral for endocytotic traffic at synapses, was identified as a disease-causing gene of parkinsonism (Krebs et al., 2013). These results lend further support to the convergence of disrupted synaptic vesicle function and the pathogenesis of Parkinson’s disease.
VMAT2 function as a disease modifier in Parkinson’s disease
There is also a growing body of evidence that genetic control of vesicular function, perhaps in the form of VMAT2 function or expression, is a modifier of Parkinson’s disease risk in human populations. Post-mortem Parkinson’s disease brains show dramatically reduced VMAT2-mediated vesicular filling, and this loss is greater than what could be explained by terminal loss alone (Pifl et al., 2014). These results suggest that impaired dopamine storage in vesicles influences the disease process. The Parkinson’s disease brain has also been shown to have higher dopamine turnover, suggesting a higher level of cytosolic dopamine (Goldstein et al., 2011, 2013). These findings could be due to an impaired storage of dopamine or non-specific dopamine leak. Alternatively, it is possible that the increased turnover is due to degradation of dopamine from other monoaminergic neurons or even glial cells. Single nucleotide polymorphisms (SNPs) or mutations within the coding regions of the VMAT2 gene (SLC18A2) are rare, likely due to the fundamental requirement of the protein for neurotransmission. A Saudi Arabian family with multiple members suffering from an infantile-onset parkinsonism with profound motor and cognitive impairments was the first known report of a mutation within the VMAT2 gene (Rilstone et al., 2013). When the mutation was introduced in vitro, it was shown to cause drastic reductions in vesicular filling. Although this familial mutation represents a pediatric neurotransmitter disease, the VMAT2-deficient mice recapitulated many of the neurochemical and behavioral deficits of the affected individuals (Caudle et al., 2007; Taylor et al., 2009).
Genetic evidence from human populations also suggests that increased VMAT2 level or function protects against the development of Parkinson’s disease. Gain-of-function VMAT2 promoter haplotypes protect against the development of Parkinson’s disease in women (Glatt et al., 2006). Two other SNPs in the promoter region of the VMAT2 gene have also been linked with reduced Parkinson’s disease risk, suggesting that increases in VMAT2 level protect against the disease (Brighina et al., 2013). Based on this growing evidence in humans, a better understanding of the neurochemical and toxicological effects of elevated vesicular function in a mammalian system may be of interest for future therapeutic pursuits.
Despite the familial and spontaneous mutations linked to Parkinson’s disease, it is estimated that nearly 90–95% of disease cases are of unknown causes (Dauer and Przedborski, 2003; Hatcher et al., 2008). Parkinson’s disease incidence rates between monozygotic and dizygotic twins are similar and the concordance rate between twins sets is extremely low (Tanner et al., 1999; Wirdefeldt et al., 2011a, 2011b). These findings in human populations suggest that additional factors, beyond genetics, contribute largely to the development of Parkinson’s disease. There has been growing evidence that environmental toxicants, including organochlorine pesticides and polychlorinated biphenyl compounds, are capable of inhibiting VMAT2 activity and altering aspects of the nigrostriatal pathway (Bemis and Seegal, 2004; Guillot and Miller, 2009; Richardson and Miller, 2004). Additionally, it has been shown that chronic exposure of these toxicants and structurally-related compounds (polybrominated diphenyl ethers) modify transporter levels, such as DAT and VMAT2, and other dopamine-related proteins (Bradner et al., 2013; Caudle et al., 2012; Wilson et al., 2014). This may be particularly relevant in the pathophysiology of Parkinson’s disease since these compounds have been identified in postmortem Parkinson’s disease brains (Hatcher-Martin et al., 2012) and correlated with disease incidence (Cannon and Greenamyre, 2011; Hatcher et al., 2008).
VMAT2 and other diseases
Beyond Parkinson’s disease, alterations to VMAT2 function or level has been attributed to a variety of other disorders. For decades, monoamine neurotransmission has been strongly associated with affective disorders. The monoamine hypothesis of depression was largely based on the dramatic mood changes observed in patients taking the VMAT2 inhibitor, reserpine (Krishnan and Nestler, 2008). In humans, changes in VMAT2 level have been correlated with depression, bipolar disorder, and schizophrenia (Laufer et al., 2005; Simons and van Winkel, 2013; Zubieta et al., 2001, 2000; Zucker, 2002; Zucker et al., 2002). Single nucleotide polymorphisms upstream of VMAT2 have also been correlated to the severity of post-traumatic stress disorder (Solovieff et al., 2014). Additional studies have linked changes in VMAT2 level to the risk of Tourette’s syndrome (Ben-Dor et al., 2007), alcohol dependence (Fehr et al., 2013), ADHD symptoms in children (Toren et al., 2005), and even cognitive outcome following traumatic brain injury in adults (Markos et al., 2016). While other monoamines besides dopamine undoubtedly play an important role in these VMAT2-related disorders, all of these disorders have been connected to dopamine neurotransmission. In summary, while VMAT2 does not appear to be causative in disease, its interaction with the dopamine system is likely a contributing factor to complex disease pathogenesis.
IV. DAT AND VMAT2 AT THE SYNAPSE
DAT to VMAT2 ratio
DAT and VMAT2 act in concert to control the compartmentalization and movement of dopamine at the synapse. The interplay between DAT and VMAT2 activity may be best described by their importance in vulnerability to the neurotoxicant, MPTP. As mentioned, MPTP is metabolized into MPP+ by MAO outside of neurons (Ransom et al., 1987). After MPP+ enters dopaminergic neurons via the DAT, it then can be sequestered into the vesicle via VMAT2, which is neuroprotective. Based on the critical actions of both of these transporter mechanisms, the ratio of DAT to VMAT2 function has previously been suspected as a mediator of MPTP vulnerability (Masoud et al., 2015; Miller et al., 1999b; Uhl, 1998). Further, it has been speculated that differences in the ratio between DAT and VMAT2 in midbrain dopamine pathways may explain the preferential loss of nigrostriatal projections in Parkinson’s disease models. As seen in the immunohistochemical staining of DAT and VMAT2, dorsal striatum expresses greater levels of the DAT relative to VMAT2 compared to the ventral striatum (nucleus accumbens), which sees considerable sparing following MPTP administration and in Parkinson’s disease (Miller et al., 1999a, 1999b, 1997; Uhl, 1998). It has also been speculated that vesicular uptake of MPP+ in rats is approximately twice that of a mouse, presumably due to VMAT2 expression differences, suggesting a mechanism for the neuroprotection in these animals (Staal et al., 2000). Indeed, inhibition of VMAT2 in these rats increased the toxicity of MPTP.
In the absence of MPTP, DAT and VMAT2 continue to work to maintain the proper compartmentalization of dopamine within the synapse. The various mouse models of transporter function described by our laboratories and others (DAT-KO, DAT-tg, VMAT2-KO, VMAT2-deficient, VMAT2-HI; Table 1) allow for a neurochemical analysis of new combinations of transporter levels. We hope that genetic crosses of these animals will result in studies examining the contributions of these manipulations to dopamine release, presynaptic dopamine homeostasis, pharmacological responses, and behavioral paradigms ranging from locomotor activity to psychostimulant response.
Conclusions
We have summarized ways in which changes to transport at the vesicular and plasma membranes influence the synaptic compartmentalization of neuronal dopamine (Figure 1). Disruptions of dopamine storage that have the overall effect of increasing cytosolic dopamine, such as elevated DAT or decreased VMAT2 function, are of particular interest in hypodopaminergic disease states such as Parkinson’s disease. Conversely, manipulations meant to dampen excessive dopaminergic tone may be of use in disorders such as dyskinesias and ADHD. We have described the genetic, epigenetic, and environmental factors that may alter functional levels of DAT and VMAT2 and how these processes influence neuronal viability and susceptibility to neurodegeneration. The recent development of transgenic mouse models of varying levels of DAT and VMAT2 (Table 1) now allows the field to investigate dopamine neurochemistry on a continuum of transporter function and to carefully titrate the handling of dopamine content within the rodent brain. Finally, these mouse models provide insight into both the promise of therapies that modify the function of these transporters, and the pursuit of other potential players to manipulate the delicate balance of dopamine at the synapse.
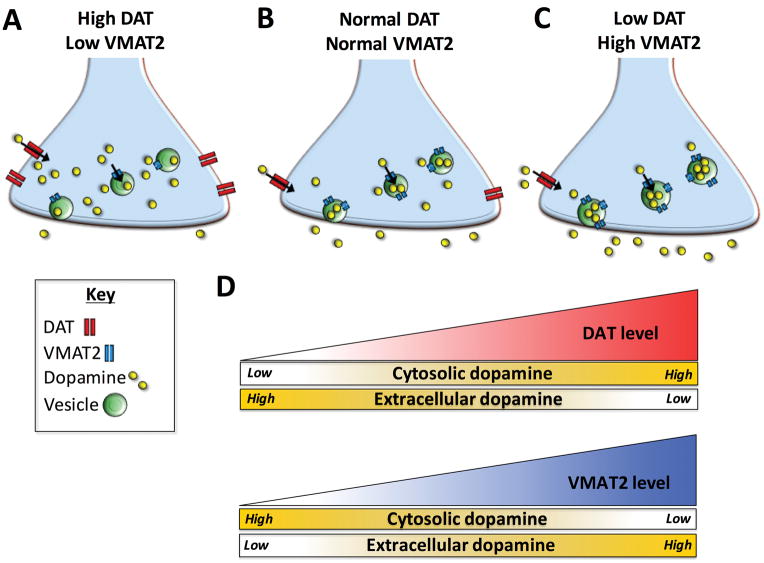
Figure 1. Predicted model of dopamine compartmentalization following manipulation of DAT and VMAT2.
(A) High DAT and low VMAT2 levels would theoretically result in an accumulation of cytosolic dopamine and minimal dopamine release. (B) Normal transporter levels would provide intermediate dopamine content. (C) Low DAT and high VMAT2 levels would theoretically result in low cytosolic dopamine content and elevated extracellular dopamine. (D) A summary of the interaction between DAT and VMAT2 function and resulting dopamine output. Increasing DAT function leads to greater uptake of dopamine from the extracellular space into the cytosol of the presynaptic neuron. This results in higher cytosolic and lower extracellular dopamine levels. Conversely, increasing VMAT2 function enhances packaging of cytosolic dopamine into vesicles, thus reducing cytosolic dopamine levels. Larger vesicular stores also translate into greater amounts of dopamine being released when the neuron in activated, thus increasing extracellular dopamine levels. This is a simplistic model of DAT and VMAT2 function that does not account for other contributors or adaptive changes in the dopamine system that can also influence overall dopamine levels.
Acknowledgments
This work was supported by The National Institutes of Health Grants R01ES023839 (G.W.M.), P30ES019776 (G.W.M.), F31NS084739 (K.M.L.), The Lewis Dickey Memorial Fund (G.W.M.) and by the Canadian Institutes of Health Research Grant 210296 (A.S).
References
- Abdulwahid Arif I, Ahmad Khan H. Environmental toxins and Parkinson’s disease: putative roles of impaired electron transport chain and oxidative stress. Toxicol Ind Health. 2010;26:121–8. doi: 10.1177/0748233710362382. [DOI] [PubMed] [Google Scholar]
- Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, Bolam JP, Chandra SS, Cragg SJ, Wade-Martins R, Buchman VL. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–74. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J, Weinshilboum R. Catecholamines. N Engl J Med. 1972:237–242. doi: 10.1056/NEJM197208032870508. [DOI] [PubMed] [Google Scholar]
- Barr AM, Lehmann-Masten V, Paulus M, Gainetdinov RR, Caron MG, Geyer MA. The Selective Serotonin-2A Receptor Antagonist M100907 Reverses Behavioral Deficits in Dopamine Transporter Knockout Mice. Neuropsychopharmacology. 2003;29:221–228. doi: 10.1038/sj.npp.1300343. [DOI] [PubMed] [Google Scholar]
- Belluzzi E, Greggio E, Piccoli G. Presynaptic dysfunction in Parkinson’s disease: a focus on LRRK2. Biochem Soc Trans. 2012;40:1111–6. doi: 10.1042/BST20120124. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80:288–95. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Ben-Dor DH, Zimmerman S, Sever J, Roz N, Apter A, Rehavi M, Weizman A. Reduced platelet vesicular monoamine transporter density in Tourette’s syndrome pediatric male patients. Eur Neuropsychopharm. 2007;17:523–6. doi: 10.1016/j.euroneuro.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–9. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Fournier MC, Dovero S, Bloch B, Jaber M. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp Neurol. 1999;155:268–73. doi: 10.1006/exnr.1998.6995. [DOI] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Henry LK, Coon H, Sakrikar D, Veenstra-VanderWeele JM, Blakely RD, Sutcliffe J, Matthies HJG, Erreger K, Galli A. SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Transl Psychiatry. 2014;4:e464. doi: 10.1038/tp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Wilson WW, Lazo CR, Stout KA, Kim HM, Wang MZ, Walker DI, Pennell KD, Richardson JR, Miller GW, Caudle WM. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system: role of dopamine handling in neurotoxicity. Exp Neurol. 2013;241:138–47. doi: 10.1016/j.expneurol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina L, Riva C, Bertola F, Saracchi E, Fermi S, Goldwurm S, Ferrarese C. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson’s disease. Neurobiol Aging. 2013;34:1712.e9–13. doi: 10.1016/j.neurobiolaging.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns D, Riedel D, Klingauf J, Jahn R. Quantal release of serotonin. Neuron. 2000;28:205–20. doi: 10.1016/S0896-6273(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci. 2011;124:225–50. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A. The contribution of drug research to investigating the nature of endogenous depression. Pharmakopsychiatr Neuropsychopharmakol. 1976;9:2–10. [PubMed] [Google Scholar]
- Carlsson A. Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol Scand Suppl. 1972;51:11–42. [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T, Waldeck B. On the presence of 3-hydroxytyramine in brain. Science. 1958;127:471. doi: 10.1126/science.127.3296.471. [DOI] [PubMed] [Google Scholar]
- Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, Egaña L, Torres GE. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem. 2010;285:1957–66. doi: 10.1074/jbc.M109.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle W, Guillot T, Lazo C, Miller G. Industrial toxicants and Parkinson’s disease. Neurotoxicology. 2012;33:178–188. doi: 10.1016/j.neuro.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Colebrooke RE, Emson PC, Miller GW. Altered vesicular dopamine storage in Parkinson’s disease: a premature demise. Trends Neurosci. 2008;31:303–308. doi: 10.1016/j.tins.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte Da, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–48. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Boulland J-L, Jenstad M, Bredahl MKL, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb Exp Pharmacol. 2008:77–106. doi: 10.1007/978-3-540-74805-2_4. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen S, Mill J, Huang Y, Lin S, Curran S, Purcell S, Sham P, Asherson P. The dopamine transporter gene is associated with attention deficit hyperactivity disorder in a Taiwanese sample. Mol Psychiatry. 2003;8:393–6. doi: 10.1038/sj.mp.4001238. [DOI] [PubMed] [Google Scholar]
- Chen M, Kuwabara H, Zhou Y, Adams RJ, Bras JR, Mcglothan JL, Verina T, Burton NC, Alexander M, Kumar A, Wong DF, Guilarte R. VMAT2 and dopamine neuron loss in a primate model of Parkinson’s disease. J Neurochem. 2008;105:78–90. doi: 10.1111/j.1471-4159.2007.05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–23. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirnaru MD, Marte A, Belluzzi E, Russo I, Gabrielli M, Longo F, Arcuri L, Murru L, Bubacco L, Matteoli M, Fedele E, Sala C, Passafaro M, Morari M, Greggio E, Onofri F, Piccoli G. LRRK2 kinase activity regulates synaptic vesicle trafficking and neurotransmitter release through modulation of LRRK2 macro-molecular complex. Front Mol Neurosci. 2014;7:49. doi: 10.3389/fnmol.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-mediated changes in quantal size and vesicular volume. J Neurosci. 2000;20:5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert F. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–1440. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Conway KAR. Kinetic Stabilization of the Alpha-Synuclein Protofibril by a Dopamine-Alpha-Synuclein Adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–8. [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb Perspect Med. 2012;2:a009415. doi: 10.1101/cshperspect.a009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–7. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis Sa, Doellman Ma, Ragozzino ME, Garris Pa, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–63. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain S, Sahakian B, Robbins T. The Roles of Dopamine and Noradrenaline in the Pathophysiology and Treatment of Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2011;69:e145–e157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, Schindler CW, Uhl GR. Cocaine reward and MPTP toxicity: Alteration by regional variant dopamine transporter overexpression. Mol Brain Res. 1999;73:37–49. doi: 10.1016/S0169-328X(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/S0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55:835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr. 1960;38:1236–9. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann NY Acad Sci. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–49. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993;61:2314–7. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci. 1992;89:10993–7. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Sommerlad D, Sander T, Anghelescu I, Dahmen N, Szegedi A, Mueller C, Zill P, Soyka M, Preuss UW. Association of VMAT2 gene polymorphisms with alcohol dependence. J Neural Transm. 2013;120:1161–9. doi: 10.1007/s00702-013-0996-y. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–83. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N Engl J Med. 1954;251:1006–8. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–9. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–84. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang Y, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP Neurotoxicity in Vesicular Monoamine Transporter 2 Heterozygote Knockout Mice. J Neurochem. 1998;70:1973–1978. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–83. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Ghisi V, Ramsey AJ, Masri B, Gainetdinov RR, Caron MG, Salahpour A. Reduced D2-mediated signaling activity and trans-synaptic upregulation of D1 and D2 dopamine receptors in mice overexpressing the dopamine transporter. Cell Signal. 2009;21:87–94. doi: 10.1016/j.cellsig.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Mol Psychiatry. 1997;2:311–3. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Wahner AD, White DJ, Ruiz-linares A. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Gen. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. Nigrostriatal Dopaminergic Deficits and Hypokinesia Caused by Inactivation of the Familial Parkinsonism-Linked Gene DJ-1. Neuron. 2005;35:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Goldstein, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18:703–10. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126:591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Schwebach AJ. The comorbidity of Pervasive Developmental Disorder and Attention Deficit Hyperactivity Disorder: results of a retrospective chart review. J Autism Dev Disord. 2004;34:329–39. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- Gong LW, Hafez I, Alvarez de Toledo G, Lindau M. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J Neurosci. 2003;23:7917–21. doi: 10.1523/JNEUROSCI.23-21-07917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar R, Hahn MK. Good riddance to dopamine: Roles for the dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochem Int. 2014;73:42–48. doi: 10.1016/j.neuint.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Molec Pharm. 1978;14:633–43. [PubMed] [Google Scholar]
- Grünhage F, Schulze TG, Müller DJ, Lanczik M, Franzek E, Albus M, Borrmann-Hassenbach M, Knapp M, Cichon S, Maier W, Rietschel M, Propping P, Nöthen MM. Systematic screening for DNA sequence variation in the coding region of the human dopamine transporter gene (DAT1) Mol Psychiatry. 2000;5:275–282. doi: 10.1038/sj.mp.4000711. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;39:149–70. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Shepherd KR, Richardson JR, Wang MZ, Li Y, Emson PC, Miller GW. Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J Neurochem. 2008;106:2205–17. doi: 10.1111/j.1471-4159.2008.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, Belovich AN, Sahai MA, Cook EH, Gether U, McHaourab HS, Matthies HJG, Sutcliffe JS, Galli A. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry. 2013;18:1315–23. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen FH, Skjørringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Andreassen TF, Holy M, Hamilton PJ, Neergheen V, Karlsborg M, Newman AH, Pope S, Heales SJR, Friberg L, Law I, Pinborg LH, Sitte HH, Loland C, Shi L, Weinstein H, Galli A, Hjermind LE, Møller LB, Gether U. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest. 2014;124:3107–20. doi: 10.1172/JCI73778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW, Pennell KD. Association between polychlorinated biphenyls and Parkinson’s disease neuropathology. Neurotoxicology. 2012;33:1298–304. doi: 10.1016/j.neuro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–9. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol. 1997;388:211–27. [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine miracle: from brain homogenate to dopamine replacement. Mov Disord. 2002;17:501–8. doi: 10.1002/mds.10115. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–64. [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Jaber M, Dumartin B, Sagné C, Haycock JW, Roubert C, Giros B, Bloch B, Caron MG. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur J Neurosci. 1999;11:3499–3511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- Jaber M, Jones S, Giros B, Caron MG. The dopamine transporter: a crucial component regulating dopamine transmission. Mov Disord. 1997;12:629–33. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Janowsky A, Tosh DK, Eshleman AJ, Jacobson KA. Rigid Adenine Nucleoside Derivatives as Novel Modulators of the Human Sodium Symporters for Dopamine and Norepinephrine. J Pharmacol Exp Ther. 2016;357:24–35. doi: 10.1124/jpet.115.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci. 1985;82:2173–7. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. Oxidative Stress in Parkinson’s Disease. Ann Neurol. 2003:26–38. doi: 10.1002/ana.10483.uitination. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–55. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci. 1998;95:4029–34. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Kelada SNP, Checkoway H, Kardia SLR, Carlson CS, Costa-Mallen P, Eaton DL, Firestone J, Powers KM, Swanson PD, Franklin GM, Longstreth WT, Weller TS, Afsharinejad Z, Costa LG. 5′ and 3′ region variability in the dopamine transporter gene (SLC6A3), pesticide exposure and Parkinson’s disease risk: A hypothesis-generating study. Hum Mol Genet. 2006;15:3055–3062. doi: 10.1093/hmg/ddl247. [DOI] [PubMed] [Google Scholar]
- Kirshner N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. Science. 1962;135:107–8. doi: 10.1126/science.135.3498.107. [DOI] [PubMed] [Google Scholar]
- Kirshner N, Rorie M, Kamin Inhibition of dopamine uptake in vitro by reserpine administered in vivo. J Pharmacol Exp Ther. 1963;141:285–9. [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci. 2007;104:11441–6. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, Di Paolo G, Walker RH, Shahidi GA, Buxbaum JD, De Camilli P, Yue Z, Paisán-Ruiz C. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat. 2013;34:1200–7. doi: 10.1002/humu.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Biol Med. 2001;30:924–31. doi: 10.1016/s0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Couceyro PR, Lambert PD. Biosynthesis of Catecholamines, Basic Neur. Lippincott-Raven; 1999. [Google Scholar]
- Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Hoffmann GF, Jardine P, von Moers A, Mordekar SR, O’Callaghan F, Wassmer E, Wraige E, Dietrich C, Lewis T, Hyland K, Heales S, Sanger T, Gissen P, Assmann BE, Reith MEA, Maher ER. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 2011;10:54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, Wassmer E, Heales SJR, Gissen P, Reith MEA, Maher ER. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J Clin Invest. 2009;119:1595–603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer N, Zucker M, Hermesh H, Marom S, Gilad R, Nir V, Weizman A, Rehavi M. Platelet vesicular monoamine transporter density in untreated patients diagnosed with social phobia. Psych Res. 2005;136:247–50. doi: 10.1016/j.psychres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: Evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, Krantz DE. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40:102–12. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal HO, Krantz DE. SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med. 2013;34:360–72. doi: 10.1016/j.mam.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry. 2003;54:800–5. doi: 10.1016/s0006-3223(02)01834-6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Privé GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992a;70:539–51. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roghani A, Edwards RH. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci. 1992b;89:9074–8. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, Wang M, Li Y, Fan X, Hess EJ, Yi H, Vecchio LM, Goldstein DS, Guillot TS, Salahpour A, Miller GW. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci. 2014;111:9977–82. doi: 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Chen M, Hoffman CA, McDaniel MJ, Stout KA, Dunn AR, Wang M, Bernstein A, Miller GW. Vesicular monoamine transporter 2 (VMAT2) level regulates MPTP vulnerability and clearance of excess dopamine in mouse striatal terminals. Toxicol Sci. 2016 doi: 10.1093/toxsci/kfw106. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Stout KA, Dunn AR, Wang M, Salahpour A, Guillot TS, Miller GW. Increased Vesicular Monoamine Transporter 2 (VMAT2; Slc18a2) Protects against Methamphetamine Toxicity. ACS Chem Neurosci. 2015;6:790–9. doi: 10.1021/acschemneuro.5b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between Conformational Changes in the Dopamine Transporter and Cocaine-Like Subjective Effects of Uptake Inhibitors. Mol Pharmacol. 2007;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Lotharius, O’Malley KL. Role of mitochondrial dysfunction and dopamine-dependent oxidative stress in amphetamine-induced toxicity. Ann Neurol. 2001;49:79–89. doi: 10.1002/1531-8249(200101)49:1<79::aid-ana11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–94. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- Markos SM, Failla MD, Ritter AC, Dixon CE, Conley YP, Ricker JH, Arenth PM, Juengst SB, Wagner AK. Genetic Variation in the Vesicular Monoamine Transporter: Preliminary Associations With Cognitive Outcomes After Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2016 doi: 10.1097/HTR.0000000000000224. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoud ST, Vecchio LM, Bergeron Y, Hossain MM, Nguyen LT, Bermejo MK, Kile B, Sotnikova TD, Siesser WB, Gainetdinov RR, Wightman RM, Caron MG, Richardson JR, Miller GW, Ramsey AJ, Cyr M, Salahpour A. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and l-DOPA reversible motor deficits. Neurobiol Dis. 2015;74:66–75. doi: 10.1016/j.nbd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration. 1995;4:271–81. doi: 10.1016/1055-8330(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Deckwerth T, Hell JW, Jahn R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport and functional reconstitution in proteoliposomes. J Biol Chem. 1988;263:15423–8. [PubMed] [Google Scholar]
- Mazei-Robison MS, Blakely RD. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacology. 2005;49:737–749. doi: 10.1016/j.neuropharm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–6. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergy MA, Gowrishankar R, Gresch PJ, Gantz SC, Williams J, Davis GL, Wheeler CA, Stanwood GD, Hahn MK, Blakely RD. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc Natl Acad Sci. 2014;111:E4779–88. doi: 10.1073/pnas.1417294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelhaugh SK, Fiskerstrand C, Lovejoy E, Bannon MJ, Quinn JP. The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J Neurochem. 2001;79:1033–8. doi: 10.1046/j.1471-4159.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–9. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP. Lobeline Inhibits the Neurochemical and Behavioral Effects of Amphetamine. J Pharmacol Exp Ther. 2001;296:1023–1034. [PubMed] [Google Scholar]
- Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, Levey AI. Immunochemical analysis of vesicular monoamine transporter (VMAT2) protein in Parkinson’s disease. Exp Neurol. 1999a;156:138–48. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999b;20:424–9. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–9. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21:5321–31. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Futai M. H(+)-ATPase, a primary pump for accumulation of neurotransmitters, is a major constituent of brain synaptic vesicles. Biochem Biophys Res Commun. 1990;173:443–8. doi: 10.1016/s0006-291x(05)81078-2. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips Ka, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–29. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, Ahmad J, Thiele H, Kubisch C, Rider NL, Morton DH, Strauss KA, Puffenberger EG, D’Agnano D, Anikster Y, Carducci C, Hyland K, Rotstein M, Leuzzi V, Borck G, Reith MEA, Kurian MA. Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain. 2014;137:1107–19. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell JR, Krishnamurthy S, Norrholm S, Deaciuc G, Siripurapu KB, Zheng G, Crooks PA, Dwoskin LP. Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2. J Pharmacol Exp Ther. 2010;332:612–21. doi: 10.1124/jpet.109.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherovich L. Priming the PD pump. Sci Exch. 2014:7. doi: 10.1038/scibx.2014.755. [DOI] [Google Scholar]
- Parsons SM, Prior C, Marshall IG. Acetylcholine transport, storage, and release. Int Rev Neurobiol. 1993;35:279–390. doi: 10.1016/s0074-7742(08)60572-3. [DOI] [PubMed] [Google Scholar]
- Peter D, Jimenez J, Liu Y, Kim J, Edwards RH. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J Biol Chem. 1994;269:7231–7. [PubMed] [Google Scholar]
- Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995;15:6179–88. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettibone DJ, Totaro JA, Pflueger AB. Tetrabenazine-induced depletion of brain monoamines: Characterization and interaction with selected antidepressants. Eur J Pharmacol. 1984;102:425–430. doi: 10.1016/0014-2999(84)90562-4. [DOI] [PubMed] [Google Scholar]
- Piccoli G, Condliffe SB, Bauer M, Giesert F, Boldt K, De Astis S, Meixner A, Sarioglu H, Vogt-Weisenhorn DM, Wurst W, Gloeckner CJ, Matteoli M, Sala C, Ueffing M. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J Neurosci. 2011;31:2225–37. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Rajput A, Reither H, Blesa J, Cavada C, Obeso Ja, Rajput AH, Hornykiewicz O. Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J Neurosci. 2014;34:8210–8. doi: 10.1523/JNEUROSCI.5456-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN. Regulation of dopamine quantal size in midbrain and hippocampal neurons. Brain Behav Res. 2002;130:203–207. doi: 10.1016/s0166-4328(01)00419-3. [DOI] [PubMed] [Google Scholar]
- Rabinovic AD, Lewis DA, Hastings TG. Role of oxidative changes in the degeneration of dopamine terminals after injection of neurotoxic levels of dopamine. Neuroscience. 2000;101:67–76. doi: 10.1016/s0306-4522(00)00293-1. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–13. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom BR, Kunis DM, Irwin I, Langston JW. Astrocytes convert the parkinsonism inducing neurotoxin, MPTP, to its active metabolite, MPP+ Neurosci Lett. 1987;75:323–8. doi: 10.1016/0304-3940(87)90543-x. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem Res Toxicol. 2009;22:1256–63. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer RJ, Fon EA, Edwards RH. Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr Opin Neurobiol. 1998;8:405–12. doi: 10.1016/s0959-4388(98)80068-8. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, Partilla JS, Rothman RB, Katz JL. Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend. 2015;147:1–19. doi: 10.1016/j.drugalcdep.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–13. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Rilstone J, Alkhater R, Minassian B. Brain dopamine-serotonin vesicular transport disease and its treatment. New Engl J Med. 2013;368:543–550. doi: 10.1056/NEJMoa1207281. [DOI] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Steiner-Mordoch SS, Fishkes H, Stern-Bach Y, Schuldiner S. Energetics of reserpine binding and occlusion by the chromaffin granule biogenic amine transporter. Biochemistry. 1990;29:603–8. doi: 10.1021/bi00455a002. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-VanderWeele J, Gill M, Blakely RD. Attention Deficit/Hyperactivity Disorder-Derived Coding Variation in the Dopamine Transporter Disrupts Microdomain Targeting and Trafficking Regulation. J Neurosci. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci. 2008;105:4405–10. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, Maidment NT, Krantz DE, Jackson GR. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27:981–92. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith MEA. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PLoS One. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Rothman RB, Reith MEA. Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates. J Pharmacol Exp Ther. 2013;346:2–10. doi: 10.1124/jpet.111.191056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–92. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res. 2007;11:151–67. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- Simons CJP, van Winkel R. Intermediate phenotype analysis of patients, unaffected siblings, and healthy controls identifies VMAT2 as a candidate gene for psychotic disorder and neurocognition. Schiz Bull. 2013;39:848–56. doi: 10.1093/schbul/sbs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N, Roberts AL, Ratanatharathorn A, Haloosim M, De Vivo I, King AP, Liberzon I, Aiello A, Uddin M, Wildman DE, Galea S, Smoller JW, Purcell SM, Koenen KC. Genetic Association Analysis of 300 Genes Identifies a Risk Haplotype in SLC18A2 for Posttraumatic Stress Disorder in two Independent Samples. Neuropsychopharmacology. 2014;39:1872–9. doi: 10.1038/npp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikova TD, Beaulieu JM, Barak LS, Wetsel WC, Caron MG, Gainetdinov RR. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3:e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikova TD, Beaulieu JM, Gainetdinov RR, Caron MG. Molecular biology, pharmacology and functional role of the plasma membrane dopamine transporter. CNS Neurol Disord Drug Targets. 2006;5:45–56. doi: 10.2174/187152706784111579. [DOI] [PubMed] [Google Scholar]
- Staal RG, Hogan KA, Liang CL, German DC, Sonsalla PK. In vitro studies of striatal vesicles containing the vesicular monoamine transporter (VMAT2): rat versus mouse differences in sequestration of 1-methyl-4-phenylpyridinium. J Pharmacol Exp Ther. 2000;293:329–35. [PubMed] [Google Scholar]
- Stadler H, Tsukita S. Synaptic vesicles contain an ATP-dependent proton pump and show “knob-like” protrusions on their surface. EMBO J. 1984;3:3333–7. doi: 10.1002/j.1460-2075.1984.tb02300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 2007;30:244–50. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau Yau Yi, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: Toward a unified hypothesis of cortico-striatopallido-thalamic function. Behav Brain Sci. 1987;10:197–208. [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci. 1997;94:9938–43. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–6. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW. Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology. 2014;76:97–105. doi: 10.1016/j.neuropharm.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Miller GW. VMAT2-Deficient Mice Display Nigral and Extranigral Pathology and Motor and Nonmotor Symptoms of Parkinson’s Disease. Parkinsons Dis. 2011;2011:124165. doi: 10.4061/2011/124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Shepherd KR, Norrian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW, Noorian A. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;29:8103–13. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toren P, Rehavi M, Luski A, Roz N, Laor N, Lask M, Weizman A. Decreased platelet vesicular monoamine transporter density in children and adolescents with attention deficit/hyperactivity disorder. Eur Neuropsychopharm. 2005;15:159–62. doi: 10.1016/j.euroneuro.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Hypothesis: the role of dopaminergic transporters in selective vulnerability of cells in Parkinson’s disease. Ann Neurol. 1998;43:555–60. doi: 10.1002/ana.410430503. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Björklund T, Buck K, Kirik D. Dysregulated dopamine storage increases the vulnerability to α-synuclein in nigral neurons. Neurobiol Dis. 2012;47:367–77. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Usami Y, Hatano T, Imai S, Kubo S, Sato S, Saiki S, Fujioka Y, Ohba Y, Sato F, Funayama M, Eguchi H, Shiba K, Ariga H, Shen J, Hattori N. DJ-1 associates with synaptic membranes. Neurobiol Dis. 2011;43:651–62. doi: 10.1016/j.nbd.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Thompson MD, Cook EH, Bendahhou E, Nguyen T, Krasowski MD, Zarrabian D, Comings D, Sellers EM, Tyndale RF, George SR, O’Dowd BF, Uhl GR. Human dopamine transporter gene: coding region conservation among normal, Tourette’s disorder, alcohol dependence and attention-deficit hyperactivity disorder populations. Mol Psychiatry. 2000;5:283–292. doi: 10.1038/sj.mp.4000701. [DOI] [PubMed] [Google Scholar]
- Vardy E, Arkin IT, Gottschalk KE, Kaback HR, Schuldiner S. Structural conservation in the major facilitator superfamily as revealed by comparative modeling. Protein Sci. 2004;13:1832–1840. doi: 10.1110/ps.04657704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Newcorn J, Kollins S, Wigal T, Telang F, Fowler J, Goldstein R, Klein N, Logan J, Wong C, Swanson J. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2010;16:1147–54. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT. Vesicle Permeabilization by Protofibrillar alpha-Synuclein Is Sensitive to Parkinson’s Disease-Linked Mutations and Occurs by a Pore-like Mechanism. Biochemistry. 2002:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Willuhn I, Clark JJ, Phillips PEM. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2:195–213. doi: 10.2174/1874473710902020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–96. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Weiss S, Nosten-Bertrand M, McIntosh JM, Giros B, Martres MP. Nicotine improves cognitive deficits of dopamine transporter knockout mice without long-term tolerance. Neuropsychopharmacology. 2007;32:2465–78. doi: 10.1038/sj.npp.1301385. [DOI] [PubMed] [Google Scholar]
- Wey MCY, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PLoS One. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WW, Shapiro LP, Bradner JM, Caudle WM. Developmental exposure to the organochlorine insecticide endosulfan damages the nigrostriatal dopamine system in male offspring. Neurotoxicology. 2014;44:279–87. doi: 10.1016/j.neuro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31:483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011a;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Reynolds CA, Prescott CA, Pedersen NL. Heritability of Parkinson disease in Swedish twins: a longitudinal study. Neurobiol Aging. 2011b;32:1923.e1–8. doi: 10.1016/j.neurobiolaging.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Fukushima S, Shen H, Hall FS, Uhl GR, Numachi Y, Kobayashi H, Sora I. Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacology. 2006;31:2132–9. doi: 10.1038/sj.npp.1301009. [DOI] [PubMed] [Google Scholar]
- Yang B, Chan RCK, Jing J, Li T, Sham P, Chen RYL. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3′-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:541–50. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- Yero T, Rey JA. Tetrabenazine (Xenazine), An FDA-Approved Treatment Option For Huntington’s Disease–Related Chorea. Drug Forecast. 2008;33:690–694. [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Volknandt W, Wittich B, Hausinger A. Synaptic vesicle life cycle and synaptic turnover. J Physiol Paris. 1993;87:159–70. doi: 10.1016/0928-4257(93)90027-q. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Taylor SF, Huguelet P, Koeppe RA, Kilbourn MR, Frey KA. Vesicular Monoamine Transporter Concentrations in Bipolar Disorder Type I, Schizophrenia, and Healthy Subjects. Biol Psych. 2001;49:110–116. doi: 10.1016/s0006-3223(00)00981-1. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Huguelet P, Ohl LE, Koeppe RA, Kilbourn MR, Carr JM, Giordani BJ, Frey KA. High vesicular monoamine transporter binding in asymptomatic bipolar I disorder: sex differences and cognitive correlates. Am J Psychiatry. 2000;157:1619–28. doi: 10.1176/appi.ajp.157.10.1619. [DOI] [PubMed] [Google Scholar]
- Zucker M. Increased platelet vesicular monoamine transporter density in adult schizophrenia patients. Eur Neuropsychopharm. 2002;12:343–347. doi: 10.1016/S0924-977X(02)00041-X. [DOI] [PubMed] [Google Scholar]
- Zucker M, Aviv A, Shelef A, Weizman A, Rehavi M. Elevated platelet vesicular monoamine transporter density in untreated patients diagnosed with major depression. Psych Res. 2002;112:251–256. doi: 10.1016/S0165-1781(02)00223-8. [DOI] [PubMed] [Google Scholar]