Abstract
Twelve ruminally cannulated cattle, adapted to forage or grain diet with or without monensin, were used to investigate the effects of diet and monensin on concentration and duration of ruminal persistence and fecal shedding of E. coli O157:H7. Cattle were ruminally inoculated with a strain of E. coli O157:H7 (1010 CFU/animal) made resistant to nalidixic acid (Nalr). Ruminal and fecal samples were collected for 11 weeks, and then cattle were euthanized and necropsied and digesta from different gut locations were collected. Samples were cultured for detection and enumeration of Nalr E. coli O157:H7. Cattle fed forage diets were culture positive for E. coli O157:H7 in the feces for longer duration (P < 0.05) than cattle fed a grain diet. In forage-fed cattle, the duration they remained culture positive for E. coli O157:H7 was shorter (P < 0.05) when the diet included monensin. Generally, ruminal persistence of Nalr E. coli O157:H7 was not affected by diet or monensin. At necropsy, E. coli O157:H7 was detected in cecal and colonic digesta but not from the rumen. Our study showed that cattle fed a forage diet were culture positive longer and with higher numbers than cattle on a grain diet. Monensin supplementation decreased the duration of shedding with forage diet, and the cecum and colon were culture positive for E. coli O157:H7 more often than the rumen of cattle.
Enterohemorrhagic strains of Escherichia coli, especially serotype O157:H7, have been linked in humans with hemorrhagic colitis, hemolytic-uremic syndrome, and thrombocytopenic purpura from eating contaminated foods, such as beef and dairy products, vegetables, cider, etc., contaminated drinking water, or contact with colonized animals or animal environments (2, 3, 17, 18). Significant correlations between bovine fecal and hide prevalence with beef carcass contamination indicate a role for controlling E. coli O157 in live cattle (12). Simulation modeling has shown that reducing the prevalence of E. coli O157 in cattle would substantially reduce contamination of beef (23). Diet and feeding practices are considered to be important factors affecting fecal E. coli O157:H7 (6, 7, 19, 31, 32).
Data on grain versus forage effects on fecal E. coli O157:H7 are limited and are somewhat conflicting. It is reported that grain feeding, as opposed to hay feeding, favored growth of acid-resistant generic E. coli because of lower ruminal and possibly cecal pH (9). However, Hovde et al. (21) indicated that the acid sensitivity of the organism was not affected by diet and that hay-fed cattle shed E. coli O157:H7 longer than grain-fed cattle. Jordan and McEwen (22) found no difference in fecal E. coli between cattle fed a grain- or forage-based diet. Tkalcic et al. (35) reported that fecal levels of E. coli O157:H7 in calves fed diets high in roughage or grain were highly variable. Keene et al. (W. E. Keene, G. A. Uhlich, and R. O. Elder, Abstr. 80th Conf. Res. Work. Anim. Dis., 1999) studied the effect of grain-to-hay shift on the prevalence of E. coli O157:H7 in cattle and reported that 52% of the cattle maintained on grain continued to shed E. coli O157:H7 but only 18% of the cattle that were switched to hay were culture positive for E. coli O157:H7.
Ionophores, particularly monensin, are used extensively in the cattle industry to improve weight gain in cattle on pasture and feed efficiency in feedlot cattle fed grain-based diets (13, 28). In other countries, monensin is approved for use in dairy cows for increased milk production, improved feed efficiency, and control of ketosis and bloat (27). Improved animal performance with ionophore feeding is generally attributed to increased fermentation efficiency associated with altered microbial flora of the rumen (33). Given the temporal relationship between initial ionophore use in the U.S. cattle industry and the increase in E. coli O157:H7 cases and speculation that ionophores could increase the prevalence of E. coli O157:H7 by inhibiting competing gram-positive organisms (11, 31), there is interest in the potential influence of ionophore feed additives on fecal E. coli O157:H7 in cattle. Among herd management factors surveyed for their association with the prevalence of Shiga toxin-positive E. coli O157 in feedlot cattle, a positive association was observed between prevalence and inclusion of ionophores in the feed (20). Short-term feeding (12 days) of ionophores, monensin, or laidlomycin was shown to have no effect on fecal E. coli O157: H7 (11). However, the influence of monensin on ruminal persistence and fecal E. coli O157 in relation to forage versus grain has not been directly investigated. Therefore, the objective of the present study was to compare the influence of diet, grain versus forage, with and without monensin on ruminal persistence and fecal E. coli O157:H7 in cattle ruminally inoculated with the organism.
MATERIALS AND METHODS
Experimental animals.
Twelve beef and Holstein-beef cross yearling cattle (eight heifers and four steers with average body weight of 223 ± 40 kg) were surgically fitted with ruminal cannulae and used for this experiment. Fecal samples were collected before surgical cannulation and cultured for E. coli O157:H7 by an enrichment technique to assure that cattle were free of E. coli O157:H7. Ruminal cannulation was done 2 weeks before beginning the experiment. The animals were blocked by body weight and randomly assigned to one of four dietary treatments in a 2 by 2 factorial design. The treatments were as follows: (i) forage diet (85% forage, 15% grain) with no monensin, (ii) forage diet (85% forage, 15% grain) with 1.32 g of monensin/kg, (iii) grain diet (15% forage, 85% grain) with no monensin, and (iv) grain diet (15% forage, 85% grain) with 1.32 g of monensin/kg. Each group of three animals was housed in a separate pen, all in a climate-controlled (biosecurity level 2) indoor facility. This arrangement prevented nose-to-nose contact among animals in different pens. A separate water trough and feed manger were provided to each pen.
Diet and feeding.
The grain portion of the diet was composed of cracked corn, soybean meal, corn gluten meal, urea, and vitamin and mineral supplement with or without monensin (Gainmore R1200 or Maxi-Gain R1000; Suther Feeds Inc., Frankfort, Kans.) (Table 1). The forage portion consisted of prairie hay and chopped alfalfa hay. All diet formulations were on a dry matter (DM) basis. Monensin (1.32 g/kg; Elanco Animal Health, Greenfield, Ind.) was included in the vitamin and mineral supplement and premixed with the grain portion of the diet. The grain portions of the diets were mixed and bagged off site, and sufficient grain mixes were prepared for the entire study. The rations were fed ad libitum once daily, and cattle had access to water at all times. Cattle were adapted to the diets for 14 days before inoculating with E. coli O157:H7.
TABLE 1.
Ingredient and nutrient compositions of diets
| Item | Forage diet
|
Grain diet
|
||
|---|---|---|---|---|
| No monensin | Monensin | No monensin | Monensin | |
| Ingredient composition (% DM basis) | ||||
| Soybean meala | 3.8 | 3.8 | 2.5 | 2.5 |
| Alfalfa hay, midbloom | 14.8 | 14.8 | 15.0 | 15.0 |
| Corn grain, cracked | 6.3 | 6.3 | 79.8 | 79.8 |
| Prairie hay | 69.2 | 69.2 | 0.0 | 0.0 |
| Corn gluten mealb | 3.5 | 3.5 | 0.0 | 0.0 |
| Urea | 0.0 | 0.0 | 0.7 | 0.7 |
| Maxi-Gain vitamin and mineral supplement without monensinc | 2.4 | 0.0 | 0.0 | 3.0 |
| Maxi-Gain vitamin and mineral supplement with monensind | 0.0 | 2.4 | 0.0 | 0.0 |
| Gainmore vitamin and mineral supplement without monensine | 0.0 | 0.0 | 3.0 | 0.0 |
| Gainmore vitamin and mineral supplement with monensinf | 0.0 | 0.0 | 0.0 | 3.0 |
| Nutrient composition | ||||
| Dry matter (%) | 90.5 | 90.4 | 85.9 | 85.1 |
| Crude protein (%) | 12.5 | 12.7 | 11.6 | 15.0 |
| Soluble protein (%) | 19.9 | 19.9 | 30.0 | 34.0 |
| Crude fat (%) | 2.3 | 2.3 | 4.0 | 3.4 |
| Neutral detergent fiber (%) | 59.1 | 59.3 | 17.2 | 18.6 |
| Acid detergent fiber (%) | 37.2 | 37.3 | 10.4 | 10.8 |
| NEg (mcal/kg)g | 0.44 | 0.44 | 1.26 | 1.19 |
49% ruminally degradable.
60% ruminally undegradable.
Maxi-Gain R1000 consisting of 18 to 20% Ca, ≥2% P, ≥4.2% K, 9.5 to 11% NaCl, 220,000 IU of vitamin A/kg, 22,000 IU of vitamin D3/kg, 22 IU of vitamin E/kg.
Maxi-Gain R1000 plus 1.32 g of monensin/kg.
Gainmore R1200 consisting of 13 to 15% Ca, ≥4% P, ≥5% K, 9.5 to 11.5% NaCl, 264,000 IU of vitamin A/kg, 22,000 IU of vitamin D3/kg, 22 IU of vitamin E/lb.
Gainmore R1200 plus 1.32 g of monensin/kg.
NEg, net energy for growth.
Animal inoculation.
Animals were inoculated with E. coli O157:H7, strain FRIK 1123, which was made resistant to nalidixic acid (Nalr) in the laboratory (20 μg/ml). The strain, originally isolated from a dairy cow, was obtained from Andrew Benson (University of Nebraska, Lincoln). The organism was grown in GN broth (Difco Laboratories, Detroit, Mich.) for 7 h (approximately 0.8 absorbance at 600 nm), and colony counts of the culture were done by spread plate technique. Each animal was inoculated directly into the rumen via ruminal cannula with 100 ml of the culture containing 108 CFU of Nalr E. coli O157:H7/ml (1010 CFU/animal).
Ruminal fluid and fecal sampling.
Following inoculation, ruminal and fecal samples were collected three times a week for 4 weeks and twice a week for the next 7 weeks, for a total of 11 weeks. Ruminal digesta were collected 3 h after feeding from the midventral sac of the rumen. The digesta in the ventral sac were hand stirred and then scooped with a 100-ml plastic beaker. The pH was recorded immediately after collection with a Fischer AR model 10 pH meter (Fischer Inc., Hanover, Ill.). Approximately 25 g of digesta was transferred into a Whirl-Pak and shipped overnight in a cooler to the laboratory for detection and enumeration of Nalr E. coli O157:H7. The remaining digesta were strained through four layers of cheesecloth, and 10 ml of the strained ruminal fluid was pipetted into a vial containing 2.5 ml of 25% metaphosphoric acid. Vials were immediately stored at −20°C for volatile fatty acid (VFA) analysis.
Feces were collected from the rectum immediately after collecting ruminal samples. Approximately 25 g of feces was placed into a Whirl-Pak for shipment to the laboratory for detection and enumeration of Nalr E. coli O157:H7. About 10 g was placed into a centrifuge tube containing 25 ml of distilled water and was vortexed thoroughly to obtain a suspension to measure pH.
Necropsy samples.
At the end of the experimental period (11 weeks), cattle were euthanized and digesta were collected from the rumen, omasum, abomasum, ileum, cecum, colon (spiral loop), and rectum. The pH of the digesta was recorded. Also, mesenteric, parotid, and pharyngeal lymph nodes, tonsils, and Peyer's patches were collected. Tissue samples were cut into approximately 1-g pieces, suspended in GN broth, and homogenized for 1 min with a tissue homogenizer (Brinkmann Instruments, Westbury, N.Y.). Digesta samples and homogenized tissue samples were used for enumeration and/or detection of Nalr E. coli O157.
Detection and enumeration of Nalr E. coli O157:H7.
One gram of feces, digesta, or homogenized tissue sample was added to 9.0 ml of GN broth containing 50 μl (0.05 mg/liter) of cefixime, 200 μl (10 mg/liter) of cefsulodin, and 100 μl (8 mg/liter) of vancomycin (GNccv). Samples were vortexed for 30 sec, and 100 μl of undiluted (final dilution, 10−1) or serially diluted (10−1 and 10−2 dilutions) portions were spread plated, in triplicate, onto sorbitol MacConkey agar plates containing 20 μg of Nal/ml (SMAC-N). The remaining GN broth was incubated as an enrichment step in the isolation procedure. After 6 h of incubation at 37°C, 1.0 ml was transferred into 9.0 ml of GNccv broth and incubated an additional 18 to 24 h at 37°C. The inoculated SMAC-N plates were incubated for 24 h at 37°C, and typical sorbitol-negative (gray-colored) colonies were counted. A maximum of three colonies per sample per animal were collected, streaked onto blood agar plates, and incubated for 24 h at 37°C. The indole test was done on colonies from the blood agar plates; indole-positive colonies were tested for agglutination specific for O157 (Oxoid Diagnostic Reagents, Basingstoke, Hampshire, England).
If E. coli O157:H7 colonies were not detected by direct plating (detection limit, ≥102 CFU/g), GNccv broth incubated for 18 to 24 h was plated, in duplicate, on SMAC-N plates and incubated for 24 h at 37°C. Following incubation, three colonies per sample with typical colony morphology (from the enriched samples) were streaked on blood agar plates and incubated for 24 h at 37°C. The indole test was done on colonies from the blood agar plates, and indole-positive colonies were tested for agglutination specific for O157.
VFA analysis.
Thawed ruminal fluid samples were clarified by centrifugation at 18,457 × g at 4°C for 20 min before analyzing by gas chromatography. Concentrations of VFA (acetic, propionic, isobutyric, butyric, isovaleric, and valeric acids) were determined using a Shimadzu GC-14A gas chromatograph (Shimadzu, Kyoto, Japan) with an AOC-20I auto-injector and an AOC-20S auto-sampler. The gas chromatograph was equipped with a flame ionization detector and a Supelco column (15-m by 0.53-mm internal diameter, 0.5-um film) (Nukol; Sigma Aldrich Co., St. Louis, Mo.). The carrier gas was helium flowing at a rate of 20 ml/min. The analysis was isothermal for 7 min at 100°C, with an injector temperature of 150°C and detector temperature of 150°C. VFAs were identified and quantified from chromatograph peak areas using calibration with external standards.
Statistical analysis.
Analysis of variance was performed using a PROC mixed procedure of SAS (34) to analyze ruminal and fecal E. coli O157:H7 concentrations, pH, and ruminal VFA concentrations. Data were analyzed as a 2 by 2 factorial repeated measures design. Bacterial counts were log transformed before analysis. Two animals in the grain diet without monensin group died (on day 10 and day 36) from conditions unrelated to the experiment. Therefore, all four treatments were included for analysis of data from days 2 to 35, and for the remaining time (days 40 to 77), the grain without monensin group was eliminated. The least-squared means test was used to separate means when significant (P < 0.05) treatment effect or treatment by day interaction was observed.
The binomial data (presence or absence of E. coli O157:H7 by direct plating or enrichment) was analyzed using PROC GENMOD in SAS. The prevalence data in gut digesta collected at necropsy (rumen versus cecum or rumen versus colon) were analyzed by the chi-square test. The effect of diet or monensin on duration of fecal shedding was assessed by performing a survival analysis using PROC LIFETEST in SAS. The outcome was the final day in the sampling period that an animal shed the organism in the feces.
RESULTS
Diet and monensin effects on fecal E. coli O157:H7.
Cattle on all four diets shed at least 104 CFU of Nalr E. coli O157:H7/g of DM (range 104 to 107) in the feces during the first week after inoculation. After that there was a general decrease in magnitude of shedding, and the numbers of Nalr E. coli O157:H7 recovered ranged from 105 to undetectable (Table 2). Cattle fed the forage diet had higher concentrations of Nalr E. coli O157:H7 (P < 0.05) in the feces and a longer duration than the cattle fed the grain diet. No quantifiable numbers of Nalr E. coli O157:H7 were recovered after day 63 postinoculation in any group (Table 2).
TABLE 2.
Fecal shedding of E. coli O157:H7 in cattle fed high-forage or high-grain diet with or without monensina
| Days postinoculation | Shedding (CFU/g of DM) withb:
|
|||
|---|---|---|---|---|
| High-forage diet
|
High-grain diet
|
|||
| No monensin | Monensin | No monensin | Monensin | |
| 2 | 1.3 × 105a | 5.3 × 104a | 8.9 × 107b | 4.9 × 104a |
| 5 | 1.1 × 106ab | 7.7 × 104a | 1.2 × 107b | 1.4 × 105a |
| 7 | 1.4 × 105 | 1.0 × 104 | 6.3 × 105 | 8.0 × 104 |
| 9 | 4.6 × 103 | 7.5 × 103 | 3.6 × 104 | 4.3 × 103 |
| 12 | 6.2 × 102 | 8.7 × 104 | 2.1 × 106 | 1.6 × 103 |
| 14 | 4.3 × 104a | 3.8 × 104a | 1.2 × 104ab | 2.2 × 102b |
| 16 | 4.9 × 104a | 2.6 × 104a | 4.2 × 103ab | 4.1 × 102b |
| 19 | 1.9 × 104a | 1.1 × 103b | 0c (2) | 0c (3) |
| 21 | 2.5 × 103 | 3.2 × 103 | 0 | 0 |
| 23 | 2.5 × 103 | 3.3 × 102 | 0 (1) | 0 (3) |
| 26 | 3.0 × 103a | 7.4 × 102a | 0b (1) | 0b (2) |
| 28 | 7.2 × 103a | 8.1 × 102a | 0b (2) | 4.6 × 101b |
| 33 | 1.3 × 103a | 9.4 × 103a | 0b | 5.8 × 102a |
| 35 | 1.5 × 102 | 8.7 × 101 | 0 (2) | 0 |
| 40 | 5.0 × 102a | 0b (2) | 0 | 0b (1) |
| 42 | 3.9 × 102a | 0b (3) | 0 (1) | 0b (2) |
| 47 | 2.5 × 102 | 1.9 × 103 | 0 | 0 |
| 49 | 6.8 × 103a | 2.8 × 105a | 0 (1) | 0b |
| 54 | 0 (1) | 1.9 × 102 | 0 | 0 |
| 56 | 4.2 × 101 | 4.6 × 101 | 0 | 0 |
| 61 | 2.0 × 103 | 1.8 × 102 | 0 | 0 |
| 63 | 3.1 × 103 | 0 (1) | 0 | 0 |
| 68 | 0 (2) | 0 | 0 (1) | 0 |
| 70 | 0 (2) | 0 | 0 (1) | 0 |
| 75 | 0 (2) | 0 | 0 | 0 |
| 77 | 0 (1) | 0 | 0 | 0 |
n = 3 in all groups, except in the grain with no monensin group in which n was 2 from day 12 and 1 from day 40. Numbers in parentheses represent the number of animals positive based on the enrichment technique.
Means within a row without a common superscript a or b differ at a P of <0.05.
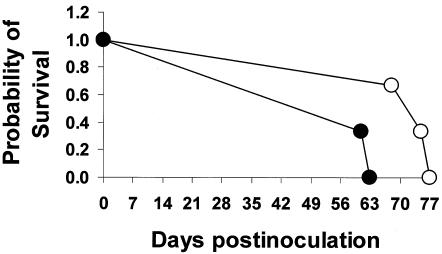
During the first 5 days postinoculation, cattle fed the grain diet without monensin had higher (P < 0.05) concentrations of E. coli O157:H7 in their feces than cattle fed the grain diet with monensin. However, after day 5 postinoculation, there was no difference (P > 0.05) between the two grain diets. In forage-fed cattle, no difference was detected in fecal concentration between monensin and no monensin groups (Table 2). There was no monensin effect observed among diets when data obtained by the enrichment technique (presence or absence) was analyzed for the first 35 days postinoculation; however, feces from cattle fed the forage diet without monensin were culture positive for E. coli O157:H7 more often (P < 0.05) than the monensin-fed cattle between days 40 through 77 postinoculation. A survival analysis was performed to determine if there were differences in the duration of shedding of Nalr E. coli O157:H7 in the feces between monensin and no monensin groups fed forage diets. The duration of shedding decreased (P < 0.05) for cattle fed the forage diet with monensin compared to cattle receiving forage without monensin (Fig. 1). Similar analysis could not be performed for grain-fed groups because of the deaths of two animals in the no monensin group.
FIG. 1.
Duration of E. coli O157:H7 shedding in the feces (survival analysis in which the outcome was the final day in the sampling period that an animal shed the organism in the feces) of cattle fed the forage diet with (closed symbols) or without (open symbols) monensin.
Diet and monensin effects on ruminal persistence of E. coli O157:H7.
Ruminal concentrations of Nalr E. coli O157:H7 in all cattle were in the range of 102 to 104 CFU/g of DM, generally lower than concentrations shed in the feces (Table 3). There were no statistical differences in ruminal Nalr E. coli O157:H7 concentrations between forage and grain diets during the first 9 days postinoculation. Between days 12 and 19, there were minor differences in ruminal concentration of Nalr E. coli O157:H7 in groups fed the two diets with or without monensin, but there was no consistent pattern. No significant differences in the persistence of E. coli O157:H7 in the rumen were observed among the four dietary treatments from days 19 to 35 postinoculation (Table 3).
TABLE 3.
Ruminal concentration of E. coli O157:H7 in cattle fed high-forage or high-grain diet with or without monensin
| Days postinoculation | Ruminal concentration (CFU/g of DM) witha:
|
|||
|---|---|---|---|---|
| High-forage diet
|
High-grain diet
|
|||
| No monensin | Monensin | No monensin | Monensin | |
| 2 | 6.7 × 103 | 7.8 × 103 | 2.0 × 104 | 9.8 × 102 |
| 5 | 1.4 × 102 | 0 (3) | 1.9 × 103 | 2.3 × 103 |
| 7 | 1.4 × 102 | 5.2 × 103 | 1.9 × 103 | 3.5 × 102 |
| 9 | 0 (2) | 3.9 × 102 | 0 (1) | 0 |
| 12 | 0a (3) | 3.0 × 103b | 1.2 × 103bc | 4.3 × 101ac |
| 14 | 3.1 × 103 | 2.9 × 103 | 1.6 × 103 | 0 |
| 16 | 4.0 × 104 | 1.6 × 103 | 0 (2) | 0 (2) |
| 19 | 1.1 × 104a | 0b (2) | 3.2 × 102b | 0b |
| 21 | 1.0 × 103 | 0 | 0 | 0 |
| 23 | 1.6 × 103 | 0 | 0 (1) | 0 |
| 26 | 2.1 × 103 | 0 (2) | 0 | 0 |
| 28 | 2.2 × 102 | 0 (3) | 0 (2) | 0 (2) |
| 33 | 3.6 × 102 | 1.2 × 102 | 0 | 1.5 × 103 |
| 35 | 1.2 × 102 | 3.1 × 102 | 0 (2) | 0 (2) |
| 40 | 1.2 × 102 | 0 | 0 | 0 |
| 42 | 0 (1) | 0 (1) | 0 (1) | 0 (3) |
| 47 | 6.6 × 102 | 7.2 × 101 | 0 (1) | 0 |
| 49 | 0 | 0 | 0 | 0 |
| 54 | 0 | 7.2 × 101 | 0 | 0 |
| 56 | 0 | 0 | 0 | 0 |
| 61 | 0 | 0 | 0 | 0 |
| 63 | 0 | 0 | 0 | 0 |
| 68 | 0 (1) | 0 | 0 | 0 |
| 70 | 0 (1) | 0 | 0 | 0 |
| 75 | 0 (1) | 0 | 0 (1) | 0 (1) |
| 77 | 0 | 0 | 0 | 0 |
n = 3 in all groups, except in the grain with no monensin group in which n was 2 from day 12 and 1 from day 40. Numbers in parentheses represent the number of animals positive based on the enrichment technique. Means within a row without a common superscript a, b, or c differ at a P of <0.05.
Fecal pH, ruminal fluid pH, ruminal VFA concentrations, and acetate-to-propionate (AP) ratio.
Fecal pH was higher (P < 0.05) in cattle fed the forage diet than in cattle fed the grain diet throughout the experiment. Monensin had no effect on fecal pH in cattle that were fed either of the two diets (data not shown). Cattle fed forage diets had higher ruminal pH (P < 0.05) than the cattle fed grain diets throughout the experiment. Ruminal pH in forage-fed cattle without monensin tended (P = 0.08) to be higher (6.29) than in cattle fed the forage with monensin diet (6.23). The cattle fed the grain without monensin diet had a mean pH of 5.39, and cattle fed the grain with monensin diet had a mean pH of 5.49, but the difference was not significant (P > 0.05; data not shown).
Regardless of monensin, cattle fed grain diets had higher (P < 0.05) VFA concentrations than cattle fed forage diets (data not shown). There was a significant interaction (P < 0.05) between treatment and sampling days. The concentration of propionic acid was higher (P < 0.05) in the grain-fed cattle than the forage-fed cattle, and cattle fed the grain diet with monensin had higher (P < 0.05) propionic acid concentrations than cattle fed the grain diet without monensin. The group fed the forage diet without monensin had a mean AP ratio of 4.01, and the group fed the forage diet with monensin had a mean AP ratio of 3.62, but the difference was not significant. The group fed grain without monensin had a mean AP ratio of 2.94, which was higher than the group fed grain with monensin with a mean AP ratio of 1.54 (data not shown).
E. coli O157:H7 in gut digesta and tissue samples collected at necropsy.
There were no quantifiable concentrations of Nalr E. coli O157:H7 in any of the gut digesta or tissues collected at necropsy. None of the tissues and ruminal and omasal digesta was culture positive for E. coli O157:H7. However, Nalr E. coli O157:H7 was recovered from the cecum or spiral colon more often (P = 0.05) than from the rumen or other regions of the gut (Table 2). All three animals in the group fed forage without monensin were culture positive for Nalr E. coli O157:H7 in samples taken from the spiral colon, and only two animals were culture positive in samples from the rectum and cecum. Two out of three animals fed the forage diet with monensin had E. coli O157:H7 in the rectal and spiral colonic samples. One animal was culture positive for E. coli O157:H7 in the ileal sample and another in abomasal digesta. None of the cattle fed the grain diet with monensin had E. coli O157:H7 in any of the gut digesta, and the single animal in the grain without monensin group was culture positive in cecal digesta (Table 4).
TABLE 4.
Recovery of E. coli O157:H7 in gut digesta and tissue samples at necropsy from cattle fed forage or grain diet with or without monensina
| Contents or tissues | Results in cattle feda:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Forage diet
|
Grain diet
|
|||||||||
| No monensin
|
Monensin
|
No monensin | Monensin
|
|||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Rumen | − | − | − | − | − | − | − | − | − | − |
| Omasum | − | − | − | − | − | − | − | − | − | − |
| Abomasum | − | − | − | − | − | − | − | − | − | − |
| Ileum | + | − | − | − | − | + | − | − | − | − |
| Cecum | − | + | + | − | − | + | + | − | − | − |
| Colon | + | + | + | + | + | + | − | − | − | − |
| Rectum | + | + | − | − | + | + | − | − | − | − |
| Mesenteric lymph node | − | − | − | − | − | − | − | − | − | − |
| Parotid lymph node | − | − | − | − | − | − | − | − | − | − |
| Pharyngeal lymph node | − | − | − | − | − | − | − | − | − | − |
| Tonsils | − | − | − | − | − | − | − | − | − | − |
| Peyer's patches | − | − | − | − | − | − | − | − | − | − |
Each group consisted of three cattle, except the grain with no monensin group, in which there was one.
DISCUSSION
Cattle fed forage diets had ruminal persistence and fecal E. coli O157:H7 at quantifiable concentrations for twice as long as cattle fed grain diets. The influence of diet on fecal E. coli O157 observed in our study is in agreement with studies (21, 24, 25) that have reported feces that were culture positive for E. coli O157:H7 for a longer duration in cattle or sheep consuming forage diets than those fed grain diets. Increases in ruminal persistence and fecal shedding duration may be related to ruminal and/or postruminal changes associated with fermentation of feedstuffs (forage versus grain). Diets high in grain generate high VFA concentrations and low pH, creating a less conducive environment for E. coli O157:H7, whereas lower VFA concentrations and higher pH in forage-fed cattle may be more hospitable for the growth and survival of the organism (31, 32). It is known that E. coli O157:H7 is acid resistant and grows better under acidic conditions than other strains of E. coli (10). However, Tkalcic et al. (35) reported no difference in ruminal persistence or fecal E. coli O157:H7 in calves fed the high-roughage or high-concentrate diet, although ruminal pH and VFA concentrations differed between the diets. Diez-Gonzales et al. (9) observed that grain feeding promoted growth of acid-resistant generic E. coli and speculated that hay feeding may be a way to reduce shedding of acid-resistant E. coli. The hypothesis was discounted in a study by Hovde et al. (21) that showed acid resistance of E. coli O157:H7 shed in the feces was similar between grain-fed and hay-fed animals. This is in contrast to the observation by Tkalcic et al. (35) that ruminal fluid from steers fed a high-concentrate diet rapidly induced acid resistance in E. coli O157:H7. Therefore, it appears that change in acidic environment as the possible mechanism to explain the influence of diet on fecal shedding is at best inconclusive. The Nalr E. coli O157:H7 strain used in this study is relatively resistant to acidity. We have determined that exposing the cells to pH of 2.5 for 1 h resulted in less than a 1 log decrease in cell number compared to a strain of E. coli O157:H7 (ATCC 43890) that is acid sensitive and resulted in a 4 to 6 log reduction in colony counts (15). The reasons for the influence of diet on ruminal persistence and fecal E. coli O157:H7 are complex and may involve changes in gut dynamics (motility, rate of passage of digesta, etc.) and changes in chemical and microbiological milieu of the rumen and perhaps the hindgut.
There are other published studies that indicate either no difference or reduced duration or level of shedding in cattle fed a forage-based diet compared to grain-based diet. Jordan and McEwen (22) showed no difference in fecal generic E. coli between cattle fed a grain- or forage-based diet but observed a trend for a decrease in generic E. coli when cattle were switched from grain to hay. Diez-Gonzalez et al. (9) reported that fecal generic E. coli populations declined 1,000-fold when cattle were abruptly switched from a 90% corn-based grain diet to 100% hay diet. Gregory et al. (16) reported that switching cattle from pasture to hay for 2 days before slaughter was able to reduce the fecal E. coli population throughout the gut. Keene et al. (Abstr. 80th Conf. Res. Work. Anim. Dis.) studied the effect of a grain-to-hay shift on the prevalence of E. coli O157:H7 in cattle naturally carrying the organism and reported that the percentage of cattle maintained on grain that continued to shed E. coli O157 was higher than with cattle that were switched to hay (52 versus 18%). In calves experimentally inoculated with E. coli O157:H7, Tkalcic et al. (35) reported that fecal E. coli O157:H7 in calves fed diets high in either roughage or grain was highly variable; however, two calves in the grain-fed group shed approximately 10-fold higher concentrations of E. coli O157:H7 in the feces than did other calves in both groups. In an in vitro study (35), ruminal fluid from cattle fed a forage diet allowed higher growth of E. coli O157:H7 than did ruminal fluid from grain-fed cattle.
Interestingly, studies that have shown forage feeding increased or had no effect on the concentration and or duration of fecal shedding compared to grain feeding, including our study, have involved experimental challenge studies with sheep or cattle inoculated with one or more strains of antibiotic-resistant E. coli O157:H7. Obviously, the advantage with such an approach is that it permits quantification of the shedding. But the limitation is that the conclusion is based on the behavior of laboratory-adapted strains. On the other hand, studies that have shown that forage feeding decreased fecal E. coli O157:H7 involved animals that were naturally shedding or were based on generic E. coli. Although naturally shedding animals would be preferred, it requires sampling large number of animals to obtain sufficient statistical power to observe treatment effects, and more importantly, the results are qualitative and not quantitative. The advantage of monitoring generic E. coli is that it permits quantification, but the limitation is that it is not E. coli O157:H7. The reasons for the conflicting data on diet effects between experimental challenge studies and studies involving natural prevalence or generic E. coli are not known. Further research is needed to better understand the mechanisms explaining the dietary influence on gut persistence and fecal E. coli O157:H7 in cattle.
Monensin, a widely used antimicrobial feed additive in beef cattle, improves digestive efficiency in ruminants by modifying ruminal microbial populations and altering fermentation products (28, 33). In feedlot cattle, monensin reduces feed intake while the rate of body weight gain is unaffected, resulting in improved efficiency of feed utilization (33). In contrast to feedlot cattle, monensin supplementation increases body weight gain in forage-fed beef cattle and dairy heifers (13). Although monensin is not approved for use in lactating dairy cows in the United States, it is widely used in dairy cows in many other countries (27). Monensin's effect on ruminal fermentation in our study is evidenced by a reduced ruminal AP ratio. Herriott et al. (20) evaluated a number of herd management factors for their association with the prevalence of Shiga toxin-positive E. coli O157 in dairy cattle and observed a higher prevalence in herds that used feed additives, such as monensin, lasalocid, and/or decoquinate, in heifer rations than in herds not fed these additives. The association appeared to be strongest for monensin with median prevalence of 1.75% in herds fed monensin compared with 0.69% in herds not fed monensin (P = 0.10). Recently, Edrington et al. (11) reported no effects of short-term (12 days) feeding of monensin or laidlomycin propionate on fecal E. coli O157:H7 in growing lambs fed a grain and hay (50:50) diet. The reduced shedding observed in the present study with monensin inclusion in the diet (at least in forage-fed cattle) is in contrast to the epidemiological association reported by Herriott et al. (20). Monensin and other ionophores are inhibitory to gram-positive bacteria, and gram-negative bacteria are generally resistant (30, 33). Because E. coli O157:H7 is gram negative and resistant (1), it was somewhat surprising that monensin reduced the duration of fecal shedding of E. coli O157:H7. However, it has been shown that monensin can reduce growth rates in certain gram-negative bacteria (30). Recently, Bach et al. (1) reported that monensin at concentrations (5 to 10 μg/ml) expected in the rumens of cattle fed at recommended levels (5.5 to 33 mg/kg of diet DM) had no effect on the growth rate of a strain of E. coli O157:H7. It has been determined (M. J. Van Baale and T. G. Nagaraja, unpublished data) that even at a very high concentration (100 μg/ml), monensin had no effect on growth rate and doubling times of Nalr E. coli O157:H7, the FRIK 1123 strain used in this study and the parent strain, FRIK 1123, and four other strains including ATCC 43890. Therefore, it is unlikely that reduced duration of fecal shedding of E. coli O157:H7 is a direct effect of monensin on the organism. However, any indirect effect(s) of monensin mediated by inhibition of gram-positive bacteria, changes in fermentation products, modulation of feed intake, or gut environment cannot be ruled out.
Fecal E. coli O157:H7 is reflective of the ability of the organism to persist or colonize the gastrointestinal tract. However, the site of persistence or colonization in the gastrointestinal tract has not been conclusively determined. Brown et al. (4) reported that rumen and omasum appear to be the primary sites of E. coli O157:H7 localization and proliferation. In contrast, Dean-Nystrom et al. (8) and Buchko et al. (5) reported that E. coli O157:H7 was rapidly eliminated from the rumen of the animals but persisted in the feces of some animals, suggesting that the hindgut may be the site of E. coli O157 persistence. Also, Buchko et al. (5) cultured E. coli O157:H7 from the ruminal digesta once, and it was present in the feces and the saliva on many occasions, suggesting that the growth and maintenance of the organism was most likely to occur in the hindgut as opposed to the rumen. Grauke et al. (14) reported that E. coli O157:H7 persisted in the lower gastrointestinal tract (cecum and colon) and was not harbored in the forestomach (rumen, reticulum, and omasum), abomasum, or duodenum of ruminants. Recently, Laven et al. (26) have reported that E. coli O157:H7 was detected more frequently in the colon than in the rumen. In another study of steers inoculated with E. coli O157:H7, reported by Grauke et al. (15), the organism colonized the most distal region of the gastrointestinal tract and was not consistently cultured from the rumen or the duodenum. A recent study (29) provides evidence that the primary site of E. coli O157:H7 colonization in cattle was the rectoanal junction, specifically the lymphoid follicles. In our study, fecal shedding of E. coli O157 continued long after it was undetectable in the rumen. Also, data collected from necropsied cattle in the present study indicated that none of the ruminal digesta sampled had any E. coli O157:H7; however, E. coli O157:H7 was detected more often in the cecum and spiral colon than other locations in the gut. This provides further evidence for the observation that cecum and or colon are the likely sites of persistence of E. coli O157:H7 in cattle. The reason for the persistence of E. coli O157:H7 in the cecum and colon as opposed to the rumen is not known. Possibly, the conditions in the cecum and colon (higher pH, lower VFA concentrations, absence of ciliated protozoa, slower rate of passage of digesta, etc., compared to the rumen) are more favorable to the survival and growth of E. coli O157:H7.
Acknowledgments
The research was supported by a U.S. Department of Agriculture grant (2001-34359-10436).
We thank Jerry Suther at Suther Feeds Inc., Frankfort, Kansas, and Walid Ali, Amy Hansen, Xiaorong Shi, and Neil Wallace for technical assistance in the laboratory.
Footnotes
This paper is contribution no. 03-228-J from the Kansas Agricultural Experiment Station.
REFERENCES
- 1.Bach, S. J., T. A. McAllister, D. M. Veira, V. P. J. Gannon, and R. A. Holley. 2002. Effect of monensin on survival and growth of Escherichia coli O157:H7 in vitro. Can. Vet. J. 43:718-719. [PMC free article] [PubMed] [Google Scholar]
- 2.Bach, S. J., T. A. McAllister, D. M. Veira, V. P. J. Gannon, and R. A. Holley. 2003. Transmission and control of Escherichia coli O157:H7—a review. Can. J. Anim. Sci. 82:475-490. [Google Scholar]
- 3.Boyce, T. G., D. L. Swerdlow, and P. M. Griffin. 1995. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N. Engl. J. Med. 333:364-368. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchko, S. J., R. A. Holley, W. O. Olson, V. P. J. Gannon, and M. Veira. 2000. The effect of different grain diets on fecal shedding of Escherichia coli O157:H7 by steers. J. Food Prot. 63:1467-1474. [DOI] [PubMed] [Google Scholar]
- 6.Callaway, T. R., R. C. Anderson, T. S. Edrington, R. O. Elder, K. J. Genoese. K. M. Bischoff, T. L. Pools, Y. S. Jang, R. B. Harvey, and D. J. Nisbet. 2003. Preslaughter intervention strategies to reduce food-borne pathogens in food animals. J. Anim. Sci. 81(Suppl. 2):E17-E23. [Google Scholar]
- 7.Cray, W. C., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1999. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv. Exp. Biol. Med. 473:173-177. [DOI] [PubMed] [Google Scholar]
- 9.Diez-Gonzales, F., T. R. Calloway, M. G. Kizoulis, and J. B. Russell. 1998. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science 281:1666-1668. [DOI] [PubMed] [Google Scholar]
- 10.Diez-Gonzales, F., and J. B. Russell. 1997. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology 143:1175-1180. [DOI] [PubMed] [Google Scholar]
- 11.Edrington, T. S., T. R. Callaway, K. M. Bischoff, K. J. Genevese, R. O. Elder, R. C. Anderson, and D. J. Nisbet. 2003. Effect of feeding ionophores monensin and laidlomycin propionate and the antimicrobial bambermycin to sheep experimentally infected with E. coli O157:H7 and Salmonella typhimurium. J. Anim. Sci. 81:553-560. [DOI] [PubMed] [Google Scholar]
- 12.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodrich, R. D., J. E. Garrett, D. R. Ghast, M. A. Kirich, D. A. Larson, and J. C. Meiske. 1984. Influence of monensin on the performance of cattle. J. Anim. Sci. 58:1484-1498. [DOI] [PubMed] [Google Scholar]
- 14.Grauke, L. J., I. T. Kudva, J. W. Wong, C. W. Hunt, C. J. Williamson, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grauke, L. J., S. A. Wynia, H. Q. Sheng, J. W. Yoon, C. J. Williams, C. W. Hunt, and C. J. Hovde. 2003. Acid resistance of Escherichia coli O157:H7 from the gastrointestinal tract of cattle fed hay or grain. Vet. Microbiol. 95:211-225. [DOI] [PubMed] [Google Scholar]
- 16.Gregory, N. G., L. H. Jacobson, T. A. Nagle, R. W. Muirhead, and G. J. Leroux. 2000. Effect of preslaughter feeding system on weight loss, gut bacteria, and physico-chemical properties of digesta in cattle. N. Z. J. Agric. Res. 43:351-361. [Google Scholar]
- 17.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington State. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harmon, B. G., C. A. Brown, S. Tkalcic, P. O. E. Muller, A. Parks, A. V. Jain, T. Zhao, and M. P. Doyle. 1999. Fecal shedding and rumen growth of Escherichia coli O157:H7 in fasted calves. J. Food Prot. 62:574-579. [DOI] [PubMed] [Google Scholar]
- 20.Herriott, D. D., D. D. Hancock, E. D. Ebel, L. V. Carpenter, D. H. Rice, and T. E. Besser. 1998. Association of herd management factors with colonization of dairy cattle by Shiga toxin-positive Escherichia coli O157. J. Food Prot. 61:802-807. [DOI] [PubMed] [Google Scholar]
- 21.Hovde, C. J., P. R. Austin, K. A. Cloud, C. J. Williams, and C. W. Hunt. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan, D., and S. A. McEwen. 1998. Effect of duration of fasting and short-term high-roughage ration on the concentration of Escherichia coli biotype 1 in cattle feces. J. Food Prot. 61:531-534. [DOI] [PubMed] [Google Scholar]
- 23.Jordan, D., S. A. McEwen, A. M. Lammerding, W. B. McNab, and J. B. Wilson. 1999. Pre-slaughter control of Escherichia coli O157 in beef cattle: a simulation study. Prev. Vet. Med. 41:55-74. [DOI] [PubMed] [Google Scholar]
- 24.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudva, I. T., C. W. Hunt, C. J. Williams, U. M. Nance, and C. J. Hovde. 1997. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl. Environ. Microbiol. 63:3878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laven, R. A., A. Ashmore, and C. S. Stewart. 2003. Escherichia coli in the rumen and colon of slaughter cattle, with particular reference to E. coli O157. Vet. J. 165:78-83. [DOI] [PubMed] [Google Scholar]
- 27.McCuffey, R. K., L. F. Richardson, and J. I. D. Wilkinson. 2001. Ionophores for dairy cattle: current status and future outlook. J. Dairy Sci. 84(Suppl.):E194-E203. [Google Scholar]
- 28.Nagaraja, T. G. 1995. Ionophores and antibiotics in ruminants, p. 173-204. In R. J. Wallace and A. Chesson (ed.), Biotechnology in animal feeds and animal feeding. VCH Publishers, New York, N.Y.
- 29.Naylor, S., J. C. Low, T. E. Bresser, A. Mahajan, G. J. Gunn, M. C. Pearce, L. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newbold, C. J., R. J. Wallace, and N. D. Walker. 1993. The effect of tetronasin and monensin on fermentation, microbial numbers and the development of ionophore-resistant bacteria in the rumen. J. Appl. Bacteriol. 75:129-134. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen, M. A., T. L. Wickman, W. C. Cray, Jr., and T. A. Casey. 1999. Escherichia coli O157:H7 and the rumen environment, p. 39-49. In C. S. Stewart and H. J. Flynt (ed.), E. coli O157 in farm animals. CAB International, New York, N.Y.
- 32.Russell, J. B., F. Diez-Gonzalez, and G. N. Jarvis. 2000. Invited review: effects of diet shifts on Escherichia coli in cattle. J. Dairy Sci. 83:863-873. [DOI] [PubMed] [Google Scholar]
- 33.Russell, J. B., and H. J. Strobel. 1989. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 55:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SAS Institute. 1990. SAS user's guide: statistics, version 6, 3rd ed. SAS Institute, Cary, N.C.
- 35.Tkalcic, S., C. A. Brown, B. G. Harmon, A. V. Jain, E. P. O. Mueller, A. Parks, K. L. Jacobsen, S. A. Martin, T. Zhao, and M. P. Doyle. 2001. Effects of diet on rumen proliferation and fecal shedding of Escherichia coli O157:H7 in calves. J. Food Prot. 63:1630-1636. [DOI] [PubMed] [Google Scholar]