Abstract
AIMS
To classify trajectories of long term HbA1c values in patients after diagnosis of Type 2 diabetes and examine each trajectory's associations with subsequent microvascular and macrovascular events and mortality.
METHODS
Longitudinal follow-up of 28,016 patients newly diagnosed with Type 2 diabetes. Latent growth mixture modeling to identify ten-year HbA1c trajectories. Cox proportional hazards models to assess how HbA1c trajectories were associated with events (microvascular and macrovascular) and mortality.
RESULTS
We identified 5 HbA1c trajectories: “low stable” (82.5%), “moderate increasing late” (5.1%), “high decreasing early” (4.9%), “moderate peaking late” (4.1%) and “moderate peaking early” (3.3%). After adjusting for average HbA1c, compared to the low stable trajectory, all non-stable trajectories were associated with higher incidences of microvascular events (hazard ratio (HR) range, 1.28 (95% CI, 1.08-1.53) (high decreasing early) to 1.45 (95% CI, 1.20-1.75) (moderate peaking early). The high decreasing early trajectory was associated with an increased mortality risk (HR, 1.27 (95% CI, 1.03-1.58)). Trajectories were not associated with macrovascular events.
CONCLUSIONS
Non-stable HbA1c trajectories was associated with greater risk of microvascular events and mortality. These findings suggest a potential benefit of early diabetes detection, prioritizing good glycemic control, and maintaining control over time.
Keywords: epidemiology, legacy effect, microvascular disease, mortality, glycemic control
1. INTRODUCTION
Among patients with Type 2 diabetes mellitus, a primary strategy for reducing complications is controlling hyperglycemia. [1] Control of hyperglycemia often requires lifestyle changes and use of anti-glycemic medications. In clinical practice, medication treatment decisions are usually based on the most recent hemoglobin A1c (HbA1c) result with the goal of reaching prescribed HbA1c targets (e.g., HbA1c<53 mmol/mol, 7.0%). [1] Over time, sequential treatment decisions and other factors (e.g., baseline severity of disease, non-adherence to therapies, and changes in clinical standards) cumulatively shape a trajectory of HbA1c values in each patient that may have important, but currently poorly understood, long-term effects on diabetes outcomes.
While hyperglycemia is a well-established risk factor for diabetes complications, [2-5] evidence suggests that HbA1c trajectories may have important clinical implications. In the United Kingdom Prospective Diabetes Study (UKPDS), patients recently diagnosed with Type 2 diabetes mellitus who were randomized to receive intensive glycemic control had fewer microvascular events after about 10 years compared to those receiving standard control. [3, 4] Following the trial's conclusion, differences in glycemic control disappeared between the study arms, yet relative reductions in microvascular events persisted for 10 more years and additional reductions in myocardial infarction events and death emerged. [5] A similar pattern of glycemic control and long-term benefits was found in the post-trial follow-up of the Diabetes Complications and Control Trial (DCCT), Epidemiology of Diabetes Interventions and Complications (EDIC). [2]
These delayed or lagged effects have been called the legacy effect or metabolic memory and suggest that effects of hyperglycemia on diabetes outcomes may differ based on history of HbA1c control. However, to date, little is known about the HbA1c trajectories in patients diagnosed with Type 2 diabetes mellitus or how HbA1c trajectories affect diabetes-related outcomes, above and beyond the effects of average HbA1c values. The NIH-funded Diabetes & Aging Study supported this research to characterize ten-year HbA1c trajectories in patients with newly diagnosed Type 2 diabetes mellitus and examine associations of HbA1c trajectories with subsequent microvascular and macrovascular events and mortality.
2. SUBJECTS
The Kaiser Permanente Northern California Diabetes Registry (Registry) is a well-characterized, longitudinal cohort of all patients with diabetes mellitus cared for at Kaiser Permanente (Kaiser). [6, 7] The Registry identifies patients with diabetes mellitus through a validated algorithm based on outpatient visits, emergency department and hospitalizations; laboratory results; and pharmacy utilization data.[8] Compared to chart review, the Registry's algorithm has been found to be 99% sensitive at identifying patients with diabetes.[9] Institutional review board approval for the Diabetes & Aging Study was obtained from the Kaiser Foundation Research Institute and the University of Chicago.
This study focused on patients who were newly diagnosed with Type 2 diabetes mellitus during 1997-2001 and had recorded HbA1c values spanning 10 years (Fig. 1). We required that patients have Kaiser membership for at least two years prior to diagnosis, in order to minimize the likelihood that patients recently joined the health plan with preexisting diabetes, and survive for 10 years after diabetes diagnosis. We excluded those with known type 1 diabetes and those less than 30 years old at the time of diagnosis to minimize the inclusion of other forms of diabetes (N=32,006). We further excluded 2,854 patients who had anemia (hemoglobin <12 g/dl in women or <13 g/dl in men) or chronic kidney disease stage 4 or worse (estimated glomerular filtration rate (eGFR)<30 mL/min/1.73 m2) at the time of diagnosis because of the inaccuracy of HbA1c in assessing glycemic exposure.[10] We further excluded 306 patients missing HbA1c values for eight or more of the 10 years after diagnosis due to our statistical method (latent growth mixture modeling) relying on a minimum of three data points. We also excluded 830 patients with a ten-year average HbA1c <42 mmol/mol (<6.0%) who might not have glucose levels consistent with diagnostic criteria for diabetes mellitus. Our final sample included 28,016 patients in the HbA1c trajectory analysis. Of these, 92% (n=25,732) had complete data for all covariates (described below) and were included in the analyses of associations between HbA1c trajectories and outcomes of interest.
Figure 1. Study flow chart.
Flow chart of patients included in study.
3. MATERIALS AND METHODS
3.1 Time Frame for Analysis
For each patient, there were two exposure periods. The first exposure period was defined by the first 10 years after diabetes diagnosis. This exposure period was used to classify the type of HbA1c trajectory using latent growth mixture modeling. The second exposure period started at the end of the first exposure period, i.e., 10 years after diagnosis, and ending with the first occurrence of any outcome of interest or end of follow-up (December 31, 2013). Median follow-up time was 13.6 years (range, 10.1 to 17.0 years) after the diagnosis of diabetes. Loss-to-follow-up due to dis-enrollment from the health plan occurred in about 9% of the population prior to having a microvascular or macrovascular event.
3.2 HbA1c Data for Trajectories
HbA1c results were downloaded from electronic medical records (EMR) for the ten-year exposure period for each patient. All assays were conducted using high-performance liquid chromatography at the single, centralized laboratory serving Kaiser Permanente Northern California. During the study period, there were three time periods when the assay was updated; HbA1c values were adjusted before or after these periods, accordingly. We also standardized (z-score) HbA1c values across months to address variations in values due to the month of the year. To do this, we calculated the overall population's mean and standard deviation HbA1c and adjusted each month's HbA1c such that its mean and standard deviation was equal to the overall population's mean and standard deviation. If a patient had more than one HbA1c result in a given month, we calculated an average for that month; then, if a patient had an HbA1c result for more than one month within a year, we averaged monthly HbA1c values for that year. Thus, each patient had a maximum of one HbA1c measure per year during the ten-year period.
3.3 Outcomes
Outcomes of interest included any incident microvascular (end-stage renal disease, diabetic eye disease, and/or lower extremity amputation) or macrovascular (coronary artery disease, cerebrovascular disease, congestive heart failure, and/or vascular disease,) event, or mortality occurring after the first 10 years after diagnosis. Event ascertainment was based on a combination of outpatient, emergency department, or inpatient primary diagnostic codes (International Classification of Diseases, Ninth Revision) or procedure codes (Current Procedural Technology, Fourth edition) (Table 1). Mortality data were obtained from the California State Mortality File, Social Security Death Records, or Kaiser Administrative records.
Table 1.
Data sources for outcomes
| Major Outcome Category | Data Source | ICD-9 CM Codes | CPT-codes |
|---|---|---|---|
| Microvascular events | |||
| End-stage renal disease (ESRD) | Inpatient or ESRD registry | 585.6 | |
| Diabetic eye disease | Outpatient | 362.02, 362.07, 369 | 67208, 67210, 67227, 67228 |
| Lower extremity amputation | Inpatient | 84.10-84.17, 84.18, 84.19 | 27598, 27880, 27881, 27882, 27884, 27886, 27888, 27889, 28800, 28805, 28820, 28825, 28810, 27290, 27295 |
| Macrovascular events | |||
| Coronary artery disease | Inpatient | 36.01, 36.02, 36.03, 36.04, 36.05, 36.06, 36.07, 36.08, 36.09, 36.10, 36.11, 36.12, 36.13, 36.14, 36.15, 36.16, 36.19, 410 | |
| Cerebrovascular disease | Inpatient | 38.11, 38.12, 431.x, 433.x, 434.x, 436.x. | 35301, 35390, 35501, 35506, 35507, 35508, 35509 |
| Congestive heart failure | Inpatient or emergency department | 402.01, 402.11, 402.91, 428 | |
| Vascular disease | Inpatient | 250.7, 39.50, 440.x, 441.x, 442.x, 443.9 | |
| Mortality | California State Mortality File, Social Security Death Records, or Kaiser Administrative records |
3.4 Other Variables
Models were adjusted for demographics (age at diabetes diagnosis, sex, and race/ethnicity), total and high-density lipoprotein cholesterol, body mass index (BMI), systolic and diastolic blood pressure, smoking status, hemoglobin, eGFR and Charlson comorbidity index. [11] Blood pressure values were recorded categorically in the Registry (see Table 2 comments for details). The lab values were based on the last recorded lab value during the last five years prior to the second exposure period. Similarly, the patient's medical history during the last five years prior to the second exposure period was used to calculate the Charlson comorbidity index. We included prevalent history of microvascular or macrovascular complications defined by the occurrence of any events during the entire first exposure period (first 10 years after diabetes diagnosis), because of the strong association between initial and subsequent complications.
Table 2.
Characteristics of patients with Type 2 diabetes mellitus 10 years after diabetes diagnosis classified by 10-year HbA1c trajectory
| Low stable | High decreasing early | Moderate increasing late | Moderate peaking late | Moderate peaking early | P Value | |
|---|---|---|---|---|---|---|
| N | 23,112 | 1370 | 1437 | 1320 | 935 | |
| Age at diagnosis, years | 57.7 | 51.9 | 47.8 | 48.9 | 49.8 | <0.001 |
| Female, % | 46.9 | 39.9 | 48.8 | 42.0 | 43.9 | <0.001 |
| Race/ethnicity, % | ||||||
| Non-Hispanic white | 52.9 | 39.3 | 38.3 | 38.6 | 36.5 | |
| Non-Hispanic black | 8.7 | 16.7 | 12.8 | 17.6 | 18.0 | |
| Hispanic | 11.6 | 16.1 | 21.5 | 20.1 | 18.6 | |
| Asian | 10.7 | 9.0 | 8.6 | 7.0 | 6.3 | |
| Filipino | 7.4 | 8.9 | 7.7 | 6.6 | 8.6 | <0.001 |
| Mixed | 6.3 | 5.9 | 6.3 | 6.0 | 7.2 | |
| Other | 1.1 | 1.7 | 2.3 | 1.0 | 2.6 | |
| Unknown | 1.2 | 2.3 | 2.5 | 3.0 | 2.4 | |
| BMI, kg/m2 | 31.1 | 32.2 | 32.4 | 32.4 | 33.4 | <0.001 |
| SBP category* | 2.3 | 2.4 | 2.5 | 2.5 | 2.5 | <0.001 |
| DBP category† | 1.2 | 1.4 | 1.6 | 1.5 | 1.5 | <0.001 |
| TC, mg/dL | 159.7 | 163.8 | 192.5 | 173.6 | 166.6 | <0.001 |
| HDL-C, mg/dL | 46.4 | 45.6 | 44.8 | 45.2 | 45.8 | <0.001 |
| Smoking status, % | ||||||
| Never | 47.6 | 44.8 | 50.3 | 47.4 | 46.3 | |
| Former | 40.9 | 35.5 | 32.4 | 32.0 | 32.9 | <0.001 |
| Current | 6.8 | 12.3 | 10.5 | 12.0 | 12.1 | |
| Unknown | 4.7 | 7.4 | 6.8 | 8.6 | 8.7 | |
| Hemoglobin, g/dL | 13.6 | 13.7 | 14.5 | 14.0 | 13.6 | <0.001 |
| eGFR, ml/min/1.73 m2 | 73.0 | 73.9 | 84.3 | 82.1 | 76.6 | <0.001 |
| Diabetic complications, % | ||||||
| Macrovascular disease | 22.8 | 21.6 | 15.0 | 18.6 | 19.9 | <0.001 |
| Microvascular disease | 6.0 | 13.3 | 7.2 | 11.0 | 15.0 | <0.001 |
| Charlson comorbidity index | 3.1 | 3.0 | 2.6 | 2.7 | 3.0 | <0.001 |
| 10 year HbA1c average, % | 7.2 | 8.7 | 9.2 | 9.5 | 9.4 | <0.001 |
| Year 1 HbA1c, % | 7.2 | 11.9 | 8.3 | 8.5 | 9.3 | <0.001 |
| Year 10 HbA1c, % | 7.2 | 8.0 | 11.4 | 8.9 | 8.3 | <0.001 |
Abbreviations: eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c, TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index
SI conversion factors: to convert TC and HDL-C to millimoles per liter, multiply by 0.259; HbA1c to proportion of total hemoglobin, multiply by 0.01
SBP category 1= ≤120 mmHg, 2=121-129 mmHg, 3=130-139 mmHg, 4=140-159 mmHg, 5=160-179 mmHg, 6= ≥180 mmHg
DBP category 1= ≤80 mmHg, 81-84 mmHg, 3=85-89 mmHg, 4=90-99 mmHg, 5=100-109 mmHg, 6= ≥110 mmHg
3.5 Statistical Analysis
We used latent growth mixture models to empirically determine if longitudinal HbA1c histories could be resolved into distinct trajectories. [12] A priori, we required that each trajectory subgroup contain a minimum of 1% of patients (n≥280) so that each subgroup would be large enough to have clinical relevance. We fit cubic-shape trajectories with sequentially increasing number of latent trajectory subgroups, assuming a constant variance-covariance structure (correlated random intercept, slope, quadratic, and cubic shape). We included the year of diabetes diagnosis as a fixed parameter in the latent growth mixture models. Model selection was based on the Lo-Mendell-Rubin fit index.[13] Based on our final model, we assigned patients to the trajectory for which they had the highest posterior probability. We qualitatively examined and named trajectories based on their visual appearance. All analyses were conducted using Mplus version 7.3.
We used Cox proportional hazards models to estimate associations between the identified HbA1c trajectories and subsequent outcomes among the 25,732 patients with complete covariate data. First, we fit an unadjusted time-to-event models using only the trajectory subgroup (Model 1). We then fit models with the trajectories and known risk factors (demographics, cardiovascular risk factors, comorbidity, history of microvascular or macrovascular complications, hemoglobin, and eGFR) (Model 2). Our final models (Model 3), adjusted for the average ten-year HbA1c value in addition to the factors included in Model 2. All analyses were completed using SAS version 9.4 (SAS Institute Inc.) and a two-sided p<.05 was considered statistically significant.
4. RESULTS
4.1 Ten-year HbA1c Trajectory Subgroups
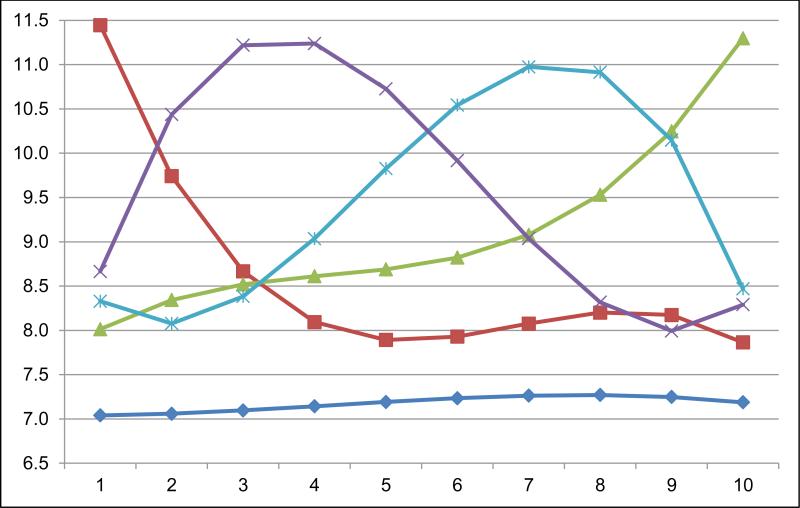
Five distinct ten-year HbA1c trajectories were identified among the 28,016 patients with newly diagnosed Type 2 diabetes mellitus (Fig. 2). The vast majority of patients (82.5%; n=23,112) had a low stable HbA1c (“low stable”) pattern. The other four trajectories had distinct shapes and were less common (<6% each): 4.9% (n=1370) had high initial HbA1c results which decreased to moderate HbA1c after four years (“high decreasing early”); 5.1% (n=1437) had moderate initial HbA1c results which increased gradually and then more rapidly later (“moderate increasing late”); 4.1% (n=1162) had moderate initial HbA1c results which increased to a high level and then decreased rapidly later (“moderate peaking late”); and 3.3% (n=935) had high moderate initial HbA1c results which increased to a high level early and then decreased gradually (“moderate peaking early”).
Figure 2. Projected trajectories of HbA1c in a cohort of 28,016 patients with newly diagnosed Type 2 diabetes mellitus.
Projected 10 year HbA1c trajectories in newly diagnosed patients with Type 2 diabetes mellitus.
x-axis: Years since diagnosis of Type 2 diabetes
y-axis: HbA1c, %
Dark blue line with diamond: low stable (n=23,112)
Green line with triangle: moderate increasing late (n=1437)
Light blue line with asterisk: moderate peaking late (n=1320)
Purple line with x: moderate peaking early (n=935)
Red line with square: high decreasing early (n=1370)
Patients in the low stable trajectory were older, more likely non-Hispanic white, had lower eGFR, lower hemoglobin, and more comorbidities, but better cardiovascular risk factor profiles (e.g., lower BMI, less current smoking, lower total and higher HDL cholesterol, and lower blood pressure) (Table 2). The ten-year HbA1c average and HbA1c values at 1 and 10 years were lowest in the low stable subgroup.
4.2 Outcomes
Overall, there were 2,086 microvascular events during the follow-up period. Compared to the low stable subgroup, in unadjusted analyses (Model 1), we observed an increased risk of microvascular events in all other trajectory subgroups (Table 3). The increased risk of microvascular events remained statistically significant for all trajectory subgroups compared to the low stable subgroup, after adjustment for demographics, cardiovascular risk factors, history of microvascular/macrovascular events and comorbidity (Model 2). After additional adjustment for the 10 year mean HbA1c (Model 3), the risk of microvascular events was attenuated but still statistically significant for all trajectories.
Table 3.
Associations between 10-year HbA1c trajectory subgroups and diabetes-related outcomes after 10 years*.
| Outcome | Trajectory Subgroup | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Microvascular events | ||||
| Low stable | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| High decreasing early | 2.86 (2.45-3.34) | 2.06 (1.75-2.41) | 1.28 (1.08-1.53) | |
| Moderate increasing late | 2.69 (2.30-3.15) | 2.56 (2.16-3.02) | 1.36 (1.12-1.64) | |
| Moderate peaking late | 3.57 (3.07-4.16) | 3.12 (2.66-3.65) | 1.44 (1.18-1.75) | |
| Moderate peaking early | 4.12 (3.53-4.82) | 2.93 (2.49-3.45) | 1.45 (1.20-1.75) | |
| Macrovascular events | ||||
| Low stable | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| High decreasing early | 0.96 (0.86-1.08) | 1.21 (1.08-1.36) | 0.99 (0.87-1.12) | |
| Moderate increasing late | 0.78 (0.69-0.89) | 1.47 (1.29-1.68) | 1.10 (0.95-1.27) | |
| Moderate peaking late | 0.80 (0.70-0.91) | 1.27 (1.10-1.45) | 0.89 (0.76-1.04) | |
| Moderate peaking early | 0.99 (0.86-1.13) | 1.42 (1.24-1.63) | 1.02 (0.87-1.18) | |
| Mortality | ||||
| Low stable | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| High decreasing early | 1.03 (0.85-1.26) | 1.56 (1.28-1.90) | 1.27 (1.03-1.58) | |
| Moderate increasing late | 0.55 (0.42-0.72) | 1.31 (1.00-1.72) | 1.01 (0.76-1.35) | |
| Moderate peaking late | 0.75 (0.59-0.96) | 1.68 (1.30-2.16) | 1.20 (0.91-1.60) | |
| Moderate peaking early | 0.79 (0.61-1.03) | 1.43 (1.09-1.87) | 1.05 (0.79-1.41) |
Abbreviations: HbA1c, hemoglobin A1c
Model 1: unadjusted; Model 2: Adjusted for age, gender, race/ethnicity, body mass index, blood pressure, cholesterol, smoking status, hemoglobin, estimated glomerular filtration rate, history of microvascular and macrovascular complications, and comorbidity; Model 3: Model 2+ adjustment for 10 year mean HbA1c
During the follow-up period, there were 6,712 macrovascular events. In unadjusted analyses, the high decreasing early, moderate increasing late, and moderate peaking late trajectories were associated with lower risks of macrovascular events. After adjustment for demographics, cardiovascular risk factors, history of microvascular/macrovascular events and comorbidity (Model 2), all trajectory subgroups had a higher risk of macrovascular events compared to the low stable subgroup. However, these differences were no longer significant after adjusting for the 10 year mean HbA1c.
During follow-up, there were 2,185 deaths. In unadjusted analyses, the moderate increasing late and moderate peaking late trajectories had lower risks of mortality than the low stable subgroup. These relationships reversed after adjustment for demographics, cardiovascular risk factors, history of microvascular/macrovascular events and comorbidity (Model 2), and there was also an increased risk of mortality associated with the high decreasing early and moderate peaking early subgroups. After adjusting for the 10 year mean HbA1c, we found that the high decreasing early trajectory was associated with a 27% increased mortality risk (HR 1.27, 95% 1.03-1.58) compared to the low stable subgroup, but other trajectories were not significantly associated with mortality.
5. DISCUSSION
Using a well characterized population of patients from an integrated healthcare system, we observed distinct patterns in the longitudinal changes in an individual's HbA1c and that these trajectories have important associations with clinical outcomes. The vast majority of Type 2 diabetes patients in this healthcare system had a low stable HbA1c trajectory during the first 10 years after diabetes diagnosis. Compared to this low stable HbA1c trajectory, other ten-year HbA1c trajectories were associated with higher risks of microvascular disease and mortality (but not macrovascular disease), independent of the ten-year HbA1c average. This suggests that stable, low HbA1c values may confer additional benefit above and beyond controlling modifiable cardiovascular risk factors or average glycemic control.
The differential findings for microvascular and macrovascular events are consistent with the temporal findings from the UKPDS. [3-5] In the UKPDS, the benefits of intensive glycemic control were present for microvascular events but not macrovascular events or mortality after 10 years. But, after an additional 10 years of follow-up, the benefits of intensive glycemic control emerged for macrovascular events and mortality. Those results suggest that the time scale for which hyperglycemia differs for the microvasculature and macrovasculature. Thus, the time scale of our study may be too short to discern changes in macrovascular events resulting from different HbA1c trajectories.
The nearly 30% increased risk of death associated with the high decreasing early trajectory deserves special attention. This particular trajectory had some of the highest initial HbA1c levels (e.g., HbA1c>97 mmol/mol, 11.0%) at the time of diagnosis. This may be a marker for a long period of undiagnosed diabetes resulting in more complete beta-cell secretory dysfunction (less endogenous insulin reserve) at diagnosis and greater risk for mortality. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, high baseline HbA1c levels (>69 mmol/mol, 8.5%,) were associated with a 64% increased mortality rate after 3 years. [14] Earlier detection of diabetes may prevent this particular trajectory and potentially reduce mortality. Also, analyzing ACCORD data using latent growth mixture models may provide novel explanations for the excess mortality observed in the intensive treatment arm.
Few previous studies have examined HbA1c trajectories in relation to health outcomes. However, prior studies have examined the impact of glycemic variability (i.e., within-person variation in glycemic levels), i.e., instability on a much shorter time scale. [15] Greater glycemic variability has been associated with increased risk of microvascular and macrovascular events and mortality in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial and small-medium cohort studies in Taiwan, Japan, Italy and Spain. [16-25] However, the value of reducing glycemic variability in clinical care is still widely debated. [26, 27] A major limitation of the previous research on the longer term glycemic variability may be that it summarizes complex, time-dependent phenomena into single measures (e.g., average standard deviation of HbA1c values over several years). In actuality, the linkage between glycemic variability and diabetic complications may be time-dependent, starting with hyperglycemia exposure, leading to a cascade of physiologic changes such as increasing oxidative stress, glycation of mitochondrial proteins, and formation of advanced glycation endproducts. These in turn may change the macro- and microvasculature leading to the milieu for vascular events and mortality over 10 to 20 years. [28, 29] Typically, glycemic variability measures ignore the time-dependent associations between hyperglycemia and outcomes, which we elucidated by using longitudinal trajectory analyses.
Our study had several limitations. The study was based on a population of members of a single healthcare delivery system located in northern California. Thus, the distribution of favorable patterns, like the low stable trajectory, compared to less favorable patterns may differ from populations with less access to care. That said, we believe the physiologic relationships between trajectories and outcomes to be widely generalizable. We chose to study a ten-year HbA1c trajectory because the UKPDS showed that there was about a 10 year delay between intensive glycemic control and decreases in microvascular events; however, one challenge of this long period is the potential for unmeasured confounding. Although our analyses included comorbidity as a covariate, residual confounding cannot be ruled out. Long-term studies such as this necessitated that we select patients newly diagnosed with Type 2 diabetes that survive and stay within Kaiser for at least 10 years. Also, while this study had an average 14-year follow-up period, it included a shorter follow-up time than the UKPDS or DCCT/EDIC. Longer follow-up will be necessary to determine if associations between trajectories and outcomes persist. Also, like all cohort studies, there was some loss to follow-up, in our case due to dis-enrollment from the health plan; however, it is unclear in which direction this would bias our findings. We also did not explore the independent impact of diabetes medications on health outcomes in this study, because we assumed that medications did not have independent effects on health outcomes above and beyond their effects on HbA1c..[30] In addition, we did not include diabetes medications as a covariate, since the relationship between HbA1c and diabetic medications is bidirectional (e.g., elevations in HbA1c may lead to more diabetic medications, and poor adherence to diabetic medications may lead to elevations in HbA1c). We were not able to examine associations between HbA1c trajectories and specific causes of mortality because we did not have cause-specific mortality data. Also, it remains unclear to what extent an individual's linkage to any given trajectory is a result of differential clinical attention or self-care versus the existence of different diabetes phenotypes (e.g., patients more susceptible to beta-cell dysfunction). Finally, we were unable to quantify the exposure to prolonged hyperglycemia prior to the diagnosis of diabetes. Thus we cannot verify that this prior exposure and the timing of diagnosis relative to the natural history of this metabolic disorder were similar across the trajectories.
Longitudinal data on HbA1c trajectories may complement individual HbA1c measures as informative for clinical decision-making. Because of the rapid uptake of electronic medical records in clinical practice, [32] it is now possible to plot a patient's HbA1c trajectory in real-time during clinic appointments. For individual patients, personal HbA1c trajectories may serve as concise reviews of their history with diabetes mellitus, inform clinicians about their risk for future complications. While some trajectories may not necessitate further intervention (e.g., high decreasing early trajectory), greater understanding of risk may motivate some patients to engage more seriously in diabetes self-management.[33] Also, health systems could use these trajectories to identify patients at high risk for complications and target these patients for aggressive glycemic management. This study provides an important lesson about the natural history of diabetes, and demonstrates how glycemic control at the very earliest stage after diagnosis has a long-term and serious impact on future health and survival.
In conclusion, relative to adults with newly diagnosed Type 2 diabetes that had low stable HbA1c trajectories for the first 10 years after diagnosis, those with non-stable HbA1c trajectories had increased microvascular events and mortality, independent of average HbA1c values. These findings demonstrate that 10-year HbA1c trajectory is a novel, independent risk factor for diabetes complications. Our analyses need to be replicated in other populations in order to determine the reproducibility of trajectory patterns and their associations.
ACKNOWLEDGEMENTS
There were no contributors to this manuscript, other than study authors.
FUNDING
Dr. Laiteerapong is supported by a NIDDK K23 DK092783. Dr. Huang is supported by a NIDDK K24 DK105340. Dr. Laiteerapong and Dr. Huang are members of the NIDDK Chicago Center for Diabetes Translation Research at the University of Chicago (P30 DK092949). Dr. Karter, Ms. Liu, and Mr. Moffet are members of the NIDDK Center for Diabetes Translational Research at Kaiser Permanente (P30 DK092924). This study was also supported by the NIDDK-funded Diabetes & Aging Study (R01DK081796).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None of the authors have any potential conflicts of interest, financial or otherwise.
REFERENCES
- 1.American Diabetes Association Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Ferrara A, Liu JY, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care. 2004;42(2):110–5. doi: 10.1097/01.mlr.0000109023.64650.73. [DOI] [PubMed] [Google Scholar]
- 7.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 8.Moffet HH, Adler N, Schillinger D, Ahmed AT, Laraia B, Selby JV, et al. Cohort Profile: The Diabetes Study of Northern California (DISTANCE)--objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol. 2009;38(1):38–47. doi: 10.1093/ije/dyn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karter AJ, Schillinger D, Adams AS, Moffet HH, Liu J, Adler NE, et al. Elevated Rates of Diabetes in Pacific Islanders and Asian Subgroups: The Diabetes Study of Northern California (DISTANCE). Diabetes Care. 2012 doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–23. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–9. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 13.Tofighi D, Enders CK. Identifying the correct number of classes in a growth mixture model. In: Hancock GR, editor. Mixture models in latent variable research. Information Age; Greenwich, CT: 2007. pp. 317–41. [Google Scholar]
- 14.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33(5):983–90. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceriello A, Ihnat MA. 'Glycaemic variability': a new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010;27(8):862–7. doi: 10.1111/j.1464-5491.2010.02967.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma WY, Li HY, Pei D, Hsia TL, Lu KC, Tsai LY, et al. Variability in hemoglobin A1c predicts all-cause mortality in patients with type 2 diabetes. J Diabetes Complications. 2012;26(4):296–300. doi: 10.1016/j.jdiacomp.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Takao T, Matsuyama Y, Yanagisawa H, Kikuchi M, Kawazu S. Association between HbA1c variability and mortality in patients with type 2 diabetes. J Diabetes Complications. 2014;28(4):494–9. doi: 10.1016/j.jdiacomp.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23(1):45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Gimeno-Orna JA, Castro-Alonso FJ, Boned-Juliani B, Lou-Arnal LM. Fasting plasma glucose variability as a risk factor of retinopathy in Type 2 diabetic patients. J Diabetes Complications. 2003;17(2):78–81. doi: 10.1016/s1056-8727(02)00197-6. [DOI] [PubMed] [Google Scholar]
- 20.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37(8):2359–65. doi: 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Lee YS, et al. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012;55(12):3163–72. doi: 10.1007/s00125-012-2700-4. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Segade S, Rodriguez J, Garcia Lopez JM, Casanueva FF, Camina F. Intrapersonal HbA(1c) variability and the risk of progression of nephropathy in patients with Type 2 diabetes. Diabet Med. 2012;29(12):1562–6. doi: 10.1111/j.1464-5491.2012.03767.x. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara A, Kawai K, Motohashi S, Saito K, Kodama S, Yachi Y, et al. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012;55(8):2128–31. doi: 10.1007/s00125-012-2572-7. [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, Yang CP, Li CI, Liu CS, Chen CC, Lin WY, et al. Visit-to-visit variability of fasting plasma glucose as predictor of ischemic stroke: competing risk analysis in a national cohort of Taiwan Diabetes Study. BMC Med. 2014;12:165. doi: 10.1186/s12916-014-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monnier L, Colette C. Glycemic variability: can we bridge the divide between controversies? Diabetes Care. 2011;34(4):1058–9. doi: 10.2337/dc11-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceriello A, Kilpatrick ES. Glycemic variability: both sides of the story. Diabetes Care. 2013;36(Suppl 2):S272–5. doi: 10.2337/dcS13-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceriello A. Hypothesis: the “metabolic memory”, the new challenge of diabetes. Diabetes Res Clin Pract. 2009;86(Suppl 1):S2–6. doi: 10.1016/S0168-8227(09)70002-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Chen B, Tang L. Metabolic memory: mechanisms and implications for diabetic retinopathy. Diabetes Res Clin Pract. 2012;96(3):286–93. doi: 10.1016/j.diabres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285–7. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 31.McEwen LN, Karter AJ, Curb JD, Marrero DG, Crosson JC, Herman WH. Temporal trends in recording of diabetes on death certificates: results from Translating Research Into Action for Diabetes (TRIAD). Diabetes Care. 2011;34(7):1529–33. doi: 10.2337/dc10-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao C-J, Hing E. Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001-2013. National Center for Health Statistics; Hyattsville, MD: 2014. NCHS data brief, no 143. [PubMed] [Google Scholar]
- 33.Welschen LM, Bot SD, Kostense PJ, Dekker JM, Timmermans DR, van der Weijden T, et al. Effects of cardiovascular disease risk communication for patients with type 2 diabetes on risk perception in a randomized controlled trial: the @RISK study. Diabetes Care. 2012;35(12):2485–92. doi: 10.2337/dc11-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]