Abstract
BACKGROUND
Motivation for high-fat food is thought to contribute to excess caloric intake in obese individuals. A novel regulator of motivation for food may be Neuromedin U (NMU), a highly-conserved neuropeptide which influences food intake. Although these effects of NMU have primarily been attributed to signaling in the paraventricular nucleus of the hypothalamus (PVN), NMU has also been found in other brain regions involved in both feeding behavior and motivation. We investigate the effects of NMU on motivation for food and food intake, and identify the brain regions mediating these effects.
METHODS
The motivational state for a particular reinforcer (e.g., high-fat food) can be assessed using a progressive ratio schedule of reinforcement under which an increasing number of lever presses are required to obtain subsequent reinforcers. Here, we have employed a progressive ratio operant responding paradigm in combination with an assessment of cumulative food intake to evaluate the effects of NMU administration in rats, and identify the brain regions mediating these effects.
RESULTS
We found that peripheral administration of NMU decreases operant responding for high-fat food in rats. Evaluation of Fos-like immunoreactivity in response to peripheral NMU indicated the PVN and dorsal raphe nucleus (DRN) as sites of action for NMU. NMU infusion into either region mimics the effects of peripheral NMU on food intake and operant responding for food. NMU-containing projections from the lateral hypothalamus (LH) to the PVN and DRN were identified as an endogenous source of NMU.
CONCLUSIONS
These results identify the DRN as a site of action for NMU, demonstrate that the LH provides endogenous NMU to the PVN and DRN, and implicate NMU signaling in the PVN and DRN as a novel regulator of motivation for high-fat foods.
Introduction
Over one-third of the American population is obese, due in part to the overconsumption of high-fat foods (1). Recent research has suggested that obesogenic high-fat foods are overconsumed as a result of their reinforcing or motivating effects (2–4). However, the neuroanatomical and molecular mechanisms underlying the intake of high-fat foods remain poorly understood. Elucidating these mechanisms would identify key brain regions and proteins that alter consumption behavior and, ultimately, obesity.
A promising candidate in this regard is neuromedin U (NMU), an anorectic neuropeptide expressed in both the periphery and the central nervous system (CNS). Intraperitoneal (IP) and intracerebroventricular (ICV) administration of NMU decrease acute food intake and body weight in animal models (5–14). NMU expression is also upregulated in the brains of fasting animals (15), though the specific peripheral or central signaling pathways involved have yet to be identified. The paraventricular nucleus of the hypothalamus (PVN), a key region in the regulation of food intake, mediates some of the feeding effects of NMU (5, 9,11, 12, 16, 17). However, further understanding of the regions and pathways involved is essential to interpreting the behavioral effects of NMU. While the effects of NMU on food intake and body weight have been evaluated (8, 11, 18–21), little consideration has been given to the reinforcing properties of food. However, NMU has recently been shown to regulate the reinforcement value of alcohol (22), and signaling between NMU and its CNS receptor, NMUR2, regulates preference for obesogenic food (9). Although NMU and food preference have been linked, the ability of NMU-NMUR2 signaling to modulate food reinforcement remains unstudied, and the specific neuroanatomical regions mediating the effects of NMU are not fully understood.
Here we present behavioral data indicating that NMU regulates motivation for food. Peripheral NMU, administered with dimethylsulfoxide (DMSO) to promote brain access (23), decreases lever pressing for obesogenic food pellets on a progressive ratio schedule of reinforcement, a model of motivation. Furthermore, peripheral NMU induces changes in Fos-like immunoreactivity in both feeding and reinforcement-associated brain regions. We present neuroanatomical data linking NMU modulation of standard and high-fat food intake with specific brain regions, and show that food reinforcement is regulated by administration of NMU into the PVN and dorsal raphe nucleus (DRN). Finally, immunohistochemical studies demonstrate that these regions are endogenously innervated by NMU-positive projections from the lateral hypothalamus (LH), a region known regulate both high-fat food consumption and reinforcement (24–27).
Materials and Methods
Subjects
Male Sprague-Dawley rats (N=102; Harlan, Inc., Houston, TX) weighing 225–250 grams (at experiment start) were used for all experiments. Sample sizes were selected based on previous studies of NMUR2 and feeding (9) and NMU and Fos-like immunoreactivity (28). Separate cohorts of animals were used for analysis of: peripheral NMU and feeding, peripheral NMU and operant responding, Fos-like immunoreactivity (2 cohorts of 3 animals/group), viral tracing and NMU/NMUR2 localization, and central NMU administration experiments. All animals were randomly assigned to treatment or control groups, with baseline behavior balanced across groups; investigators were subsequently blinded to the treatment given to each animal, and behavioral and immunohistochemical data were stored and analyzed separately from treatment data. Colony environment was maintained at 71°F and 30–50% relative humidity, with lights-on between 06:00 and 18:00. Experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (29) and with the approval of the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
Feeding and Peripheral NMU Administration
Rats were separated into individual home cages for assessment of food intake. Feeding was assessed separately for standard diet (Teklad Mouse/Rat Diet 7912, Harlan, Inc., Houston, TX), containing 17% energy from fat, and high-fat diet (Open Source Diets formula D12451, Research Diets Inc., New Brunswick, NJ), containing 45% energy from fat. Rats (n=6/group) received an IP injection of NMU (0.3mg/kg; 046-39, Phoenix Pharmaceuticals, Inc., Burlingame, CA) in saline with 10% DMSO or vehicle alone (total volume 3mL), fifteen minutes prior to dark cycle start (17:45) on each test day. Food weight was measured immediately before dark cycle start, and monitored for three consecutive 24-hour periods (18:00-18:00).
Operant Conditioning and Peripheral NMU Administration
Between 13:00 and 17:00, rats were placed in standard rat operant chambers (Med Associates, Georgia, VT). Responding on the lever associated with food delivery resulted in delivery of a high-fat food pellet (45% energy from fat, 45mg, Bioserv F06162, Frenchtown, NJ). Rats were trained in half-hour sessions on a fixed ratio (FR) 1 schedule, where a single response on the active lever is needed to receive a pellet.
Once the percentage of responses on the active lever exceeded 85% for three consecutive days (30), animals were advanced to an FR3 and then an FR5 schedule, which require 3 and 5 correct responses for pellet delivery, respectively. Once animals have reached this criterion on the FR5, they move to a progressive ratio (PR) schedule, where earning each successive high-fat food pellet within the session requires a greater number of responses (1,2,3,6,9,12,15,20,25,32,40,50,62,77,95). Responses on the active, reinforced lever within a 60-minute test session were quantified. On test day, animals (n=11/group) received an IP injection of 0.3mg/kg NMU in DMSO (6), or vehicle alone fifteen minutes prior to testing (total volume 3mL).
Analysis of Fos-like immunoreactivity
To identify the central targets enabling NMU-mediated alterations in food intake and reinforcement, rats (n=6/group) were given IP NMU and Fos-like immunoreactivity was examined in CNS sites associated with feeding, reinforcement, and NMUR2 mRNA expression. Rats were injected with 0.3mg NMU/kg, in a saline and 10% DMSO solution, or an equal volume of vehicle (total volume 3mL), and euthanized two hours later. Euthanasia was performed between 13:00 and 15:30. Brains were extracted, cryoprotected and sliced into sections as previously described (9). Sections were analyzed for Fos expression as previously described (31).
Viral tracing and localization of NMUR2 and NMU
Regions demonstrating changes in Fos-like immunoreactivity were investigated to determine their potential to have reacted directly to the NMU treatment, and characterize endogenous sources of NMU for these regions. For viral tracing of signaling pathways, guide cannulae were implanted based on Kasper et al. 2016 (31). PVN coordinates were adjusted for a 10° outside angle and set at (A/P −0.18, M/L +0.15, D/V −0.82) from bregma; DRN coordinates were adjusted for a 30° outside angle, with internal cannula inserted at (A/P −0.71, M/L +0.32, D/V +0.83) from bregma.
Rats (n=3/group) received a guide cannula pointed to the lateral ventricle (A/P +0.14, M/L +0.23, D/V −0.54) from bregma. Additionally, each animal received an interstitial injection of 2µL of a replication-incompetent retrograde tracer, Rb-ΔG-B19-GFP into the PVN or the DRN, at above coordinates, at a rate of 0.2µL/30 seconds over a period of 5 minutes. Following injection, the needle remained in place for 3 minutes before removal. Incisions were stapled closed and post-operative care was administered following Benzon et al. 2014 (9). Animals were given 10 days to recover after surgery to allow for maximal viral expression (32, 33). Rats were then given 75µg colchicine in 1µl artificial cerebrospinal fluid (aCSF) ICV via the implanted guide cannula, to block axonal transport (34). Two days after colchicine administration, animals were euthanized and tissues were taken for immunohistochemistry as described above. Surgeries and euthanasia procedures were performed between 09:00 and 17:00.
Immunohistochemistry
Immunohistochemistry for NMUR2 was performed as previously published (9, 31) and validation of the specificity of the NMUR2 antibody was also previously published (9, 31). Briefly, sections of brain were washed 3X in 1X PBS, 5 minutes/wash, to remove residual sodium azide, and incubated in 1% SDS for 5 minutes for antigen unmasking. Sections (n=10–20/rat) were washed 3X in 1X PBS, 5 minutes/wash, and incubated for 1 hour in a blocking solution containing 3% normal donkey serum, 3% normal goat serum, and 0.3% Triton X-100 in 1X PBS. Primary antibodies against NMU (rabbit anti-NMU, 1672285, Thermo Scientific, Houston, TX; 1:100) and GFP (chicken anti-GFP, GFP-1020, Aves Labs, Tigard, OR; 1:1 000) were diluted in blocking solution and incubated on brain slices overnight (20 hours) at room temperature. Sections were washed 3X in 1X PBS, 5 minutes/wash, followed by secondary antibody application. Fluorescent secondary antibodies, Alexa Fluor 568 goat anti-rabbit (A-11011, Invitrogen, Carlsbad, CA) and Alexa Fluor 488 donkey anti-chicken (703-545-155, Jackson Immunoresearch, West Grove, PA) were used in 1X PBS at 1:200. For NMUR2 immunohistochemistry, no goat serum was used in the blocking, and the primary and secondary antibodies used were rabbit anti-NMUR2 (NBP1-02351, Novus Biologicals, Littleton, CO; 1:150) and Alexa Fluor 488 donkey anti-rabbit (NC0241229, Jackson Immunoresearch; 1:100) respectively. Slices were washed again, mounted, coverslipped and imaged as described above in “Analysis of Fos-like immunoreactivity.”
Feeding and Central NMU Administration
Having identified NMUR2-positive brain regions that showed altered Fos-like immunoreactivity following peripheral administration, we moved to central administration of the peptide to confirm that NMU-NMUR2 signaling in these regions mediates food intake and reinforcement. Following acclimation, rats received cannulae implantation surgery as described above in the “Viral Tracing and NMUR2 localization” section. In this instance, guide cannulae were implanted bilaterally targeting the PVN and unilaterally targeting the DRN at the previously stated coordinates. Brains in which one or more cannulae (n=6) or injections (n=10) were off-target were excluded from further analysis, and the corresponding animals’ data was not considered. Feeding assays were performed as above, with the exception of NMU treatment. Rather than receiving peripheral NMU, rats (n=8/group) received site-specific infusions of aCSF or 0.3 nmol NMU per cannula in aCSF (total volume 2µl/side, over five minutes), delivered via implanted guide cannula immediately prior to dark cycle start (18:00).
Operant conditioning and Central NMU Administration
Animals were trained to respond for high-fat food pellets in operant chambers, as described above in the “Operant Conditioning and Peripheral NMU Administration” section. After criterion was reached on PR responding (85% of responses on active lever) (30), animals received cannulation surgeries targeting the PVN and DRN, as described in the previous section. Following surgical recovery, an additional week of operant training was administered to confirm that all animals returned to criterion following surgery. As demonstrated with ICV-administered NMU prior to feeding by Wren et al. 2002 (11), a dose of 0.3 nmol NMU reduces food intake. PR testing was performed as described above, with interstitial infusions replacing intraperitoneal injections, and infusions immediately preceding testing. Cannulated rats (n=8/group) received 0.3 nmol NMU in aCSF, or vehicle (total volume 2µl/side, over five minutes) immediately prior to testing, followed by a 48-hour washout period prior to retesting. During this period, PR testing was performed to ensure the responsiveness of the animals had not been altered. Following the testing period, animals were euthanized as described above, and brains were examined to confirm cannula targeting.
Data Analysis
Feeding and operant conditioning data fit the assumptions of, and were analyzed using multiple-comparisons ANOVA tests, together with Sidak post-hoc analysis, to account for the multiple treatments and timepoints. Fos-like immunoreactivity data were analyzed using unpaired t-tests, to allow for bidirectional comparison between the independent treatment and control groups. Variance was analyzed using the Brown-Forsythe test or the F test of variances, for behavioral and Fos data, respectively.
Results
Dose selection of NMU
Peripheral doses were chosen based on Peier et al. 2011 (6), which indicated 0.3mg/kg NMU as the lowest dose producing the changes in core body temperature which accompany NMU’s anorectic effects. Interstitial doses of NMU were selected based on Wren et al. 2002 (11), which demonstrated a dose-effect of ICV NMU on feeding. Specifically, doses below 0.1 nmol do not produce a significant behavioral effect, and significant reductions in feeding are seen at 0.3 nmol and 1 nmol. To minimize animal usage, a single behaviorally relevant dose of 0.3 nmol was used.
Behavioral effects of peripheral NMU administration
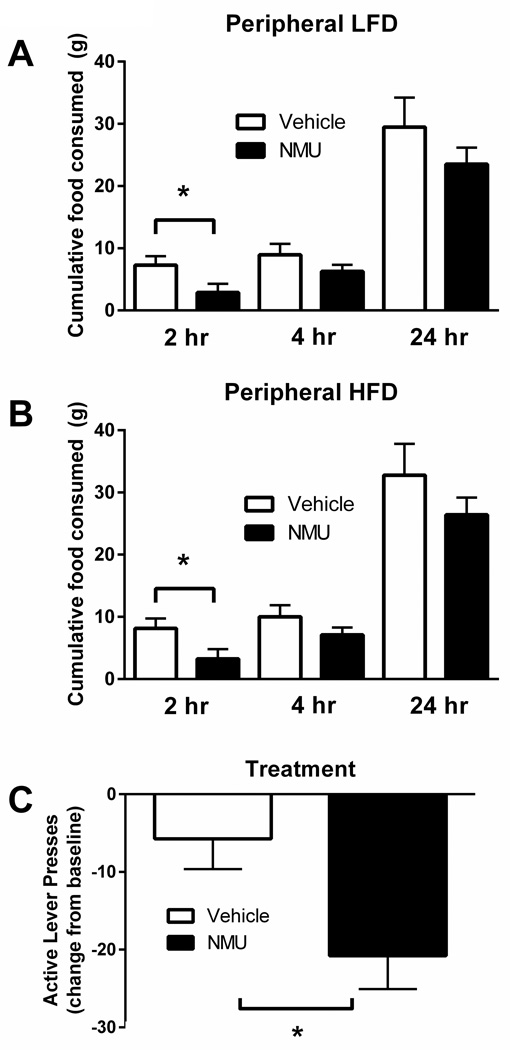
The effects of NMU signaling on total food consumption have been previously examined (5–14,18, 21, 35, 36). It has been shown that intra-PVN and ICV NMU regulate intake of a standard diet in rodents and that NMUR2 mediates preference for high-fat food (9). Here, we establish that IP administration of NMU significantly decreases consumption of both standard and high-fat diet (Figure 1). NMU (0.3mg/kg) significantly reduced standard diet intake compared to vehicle treatment at the 2-hour timepoint (p<0.05 by multiple-comparisons ANOVA, n=6/group, F=1.685 (n.s.); Figure 1A). A similar significant effect of peripheral NMU (0.3mg/kg) was observed upon high-fat diet intake vs. vehicle at the 2-hour timepoint (p<0.05 by multiple-comparisons ANOVA, n=6/group, F=2.188 (n.s.); Figure 1B). Additionally, there are no significant differences of NMU treatment on either diet after 2 hours, presumably due to the rapid breakdown of NMU (37). As previously described, NMU does not cause a taste aversion (10). Additional findings demonstrated that NMUR2 signaling in the PVN had no effect on sucrose preference or consumption (9), which also suggests that NMU does not cause a taste aversion. Based on our previous work indicating that NMUR2 regulates preference for a high-fat diet (9), we investigated the effects of NMU on motivated behavior. To study NMU as a mediator of motivation for food, we used operant conditioning on a PR schedule. The PR schedule specifically quantifies reinforcement efficacy. Therefore, increased levels of responding on this schedule are associated with increased motivation for the high-fat food reinforcer. We found that the number of lever presses for pellets of high-fat food was significantly decreased by peripheral NMU (0.3mg/kg) treatment (p<0.05 by unpaired t-test, n=11/group, F=1.294 (n.s.)), as compared to vehicle (Figure 1C). This suggests that NMU suppresses the motivation for high-fat food reinforcers.
Figure 1.
Peripheral administration of Neuromedin U (NMU) (0.3mg/kg, intraperitoneal) decreases food intake and motivation for both a low-fat diet (LFD) and a high-fat diet (HFD). (A) Animals treated with NMU consumed significantly less of a standard diet in the 2 hours following treatment (p<0.05 by Sidak’s multiple comparisons test). (B) Animals receiving NMU consumed significantly less of a high-fat diet in the 2 hours following treatment (p<0.05 by Sidak’s multiple comparisons test). (C) Peripheral treatment with NMU decreases responding for high-fat food pellets (p<0.05 by unpaired t-test). Error bars represent standard error of the mean.
Effects of NMU on Fos-like immunoreactivity
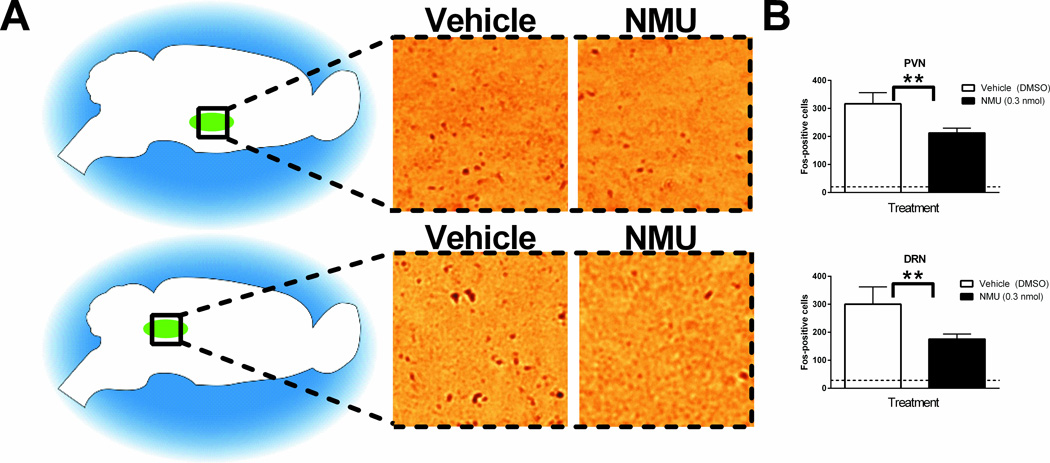
To identify candidate brain regions mediating the NMU-induced changes in behavior, we investigated changes in expression of Fos, an immediate early gene and indicator of neuronal activation (38), following peripheral NMU treatment (Figure 2). While most studies of CNS NMU-NMUR2 have focused on the PVN, we identified the DRN as responding to peripheral NMU administration. Both the PVN (Figure 2A, top) and DRN (Figure 2A, bottom) displayed significantly lower Fos-like immunoreactivity following peripheral NMU (0.3mg/kg) treatment, compared to vehicle (p<0.05 by unpaired t-test, n=6 animals/group, F=5.487 and 6.068, respectively (p<0.05)). Since the PVN and DRN displayed decreased Fos-like immunoreactivity over vehicle in response to peripheral NMU administration (Figure 2B), we further investigated these two brain regions as potential mediators of the effects of NMU on feeding and reinforcement. No significant treatment-dependent differences were noted in other feeding areas, such as the ventromedial hypothalamus (VMH) or regions associated with memory, such as the hippocampus (HC) (Supplementary Figure 1A, B). While significant increases in motor cortex (M1/M2) activity were found (Supplementary Figure 1C, p<0.05 by unpaired t-test), this has been previously observed and investigated (10, 39).
Figure 2.
Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus (PVN) and dorsal raphe nucleus (DRN) is significantly altered by peripheral Neuromedin U (NMU). (A) Representative images of vehicle-treated and NMU-treated PVN (top row) and DRN (bottom row). 20× magnification. (B) Significantly fewer Fos-positive cells are found in PVN and DRN of NMU-treated animals, as compared to vehicle-treated animals. (n=3/group, **p<0.01 by unpaired t-test). Dashed line indicates Fos-like immunoreactivity in naïve tissue. DMSO refers to 10% dimethylsulfoxide in saline. Error bars represent standard error of the mean.
Behavioral effects of centrally-administered NMU
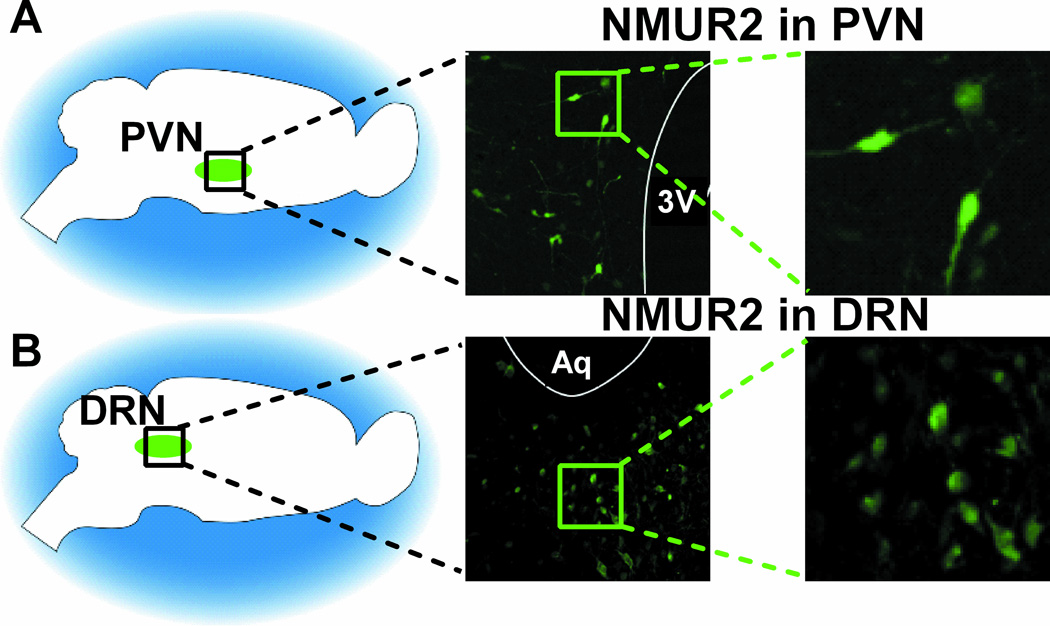
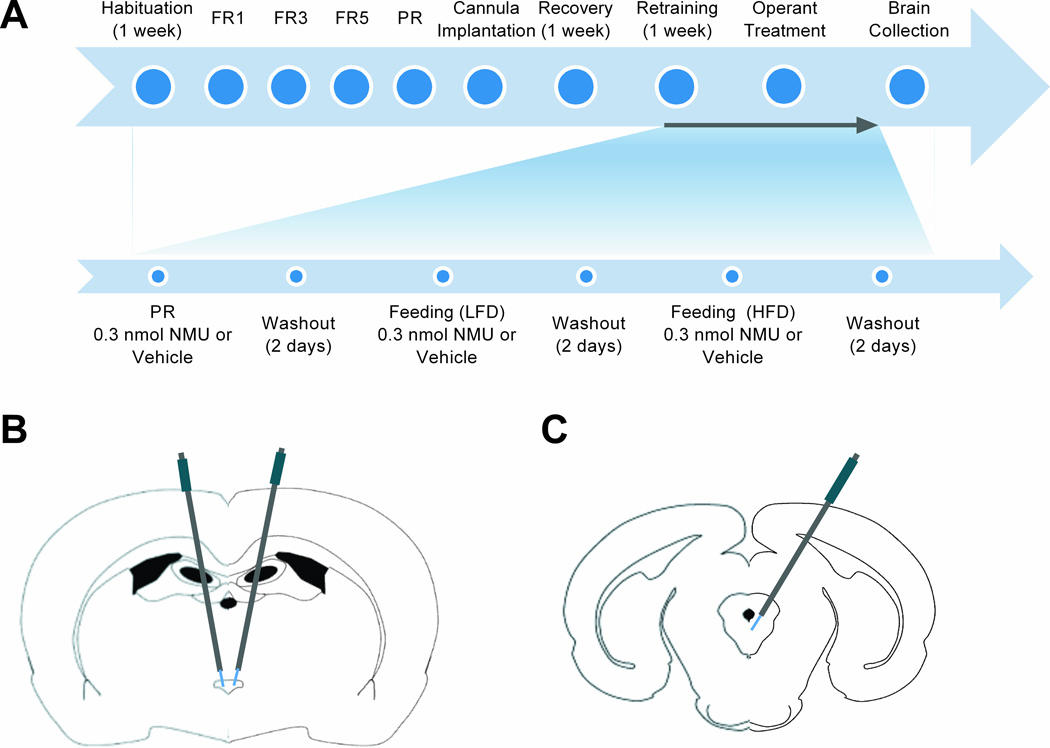
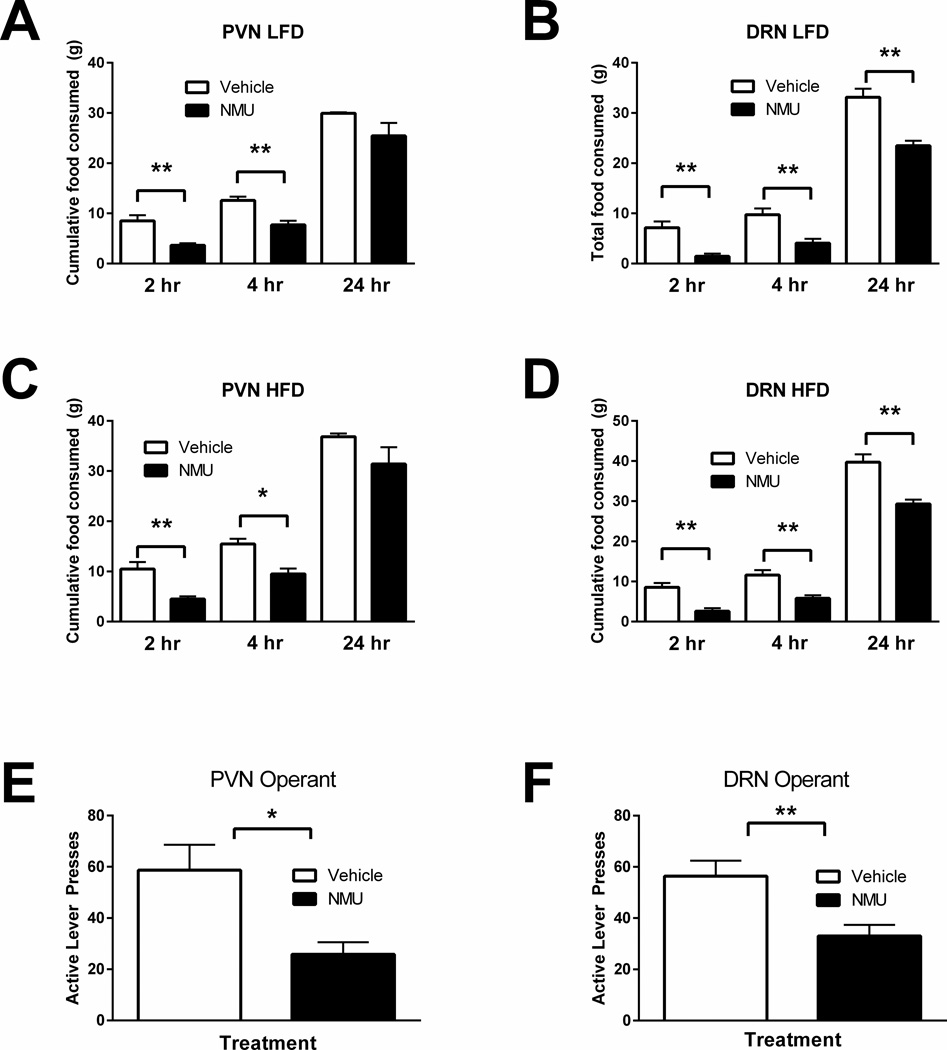
Since peripherally administered NMU produces significant effects on total food consumption and operant behavior and reduces Fos expression in brain regions associated with NMU-NMUR2 signaling, we sought to ascertain whether these brain regions specifically mediated the NMU-driven behavior. In agreement with the literature describing NMUR2 mRNA localization (39, 40), NMUR2 protein expression was identified in both the PVN (Figure 3A) and DRN (Figure 3B). Additional NMUR2 immunofluorescence was observed in several other regions known to express NMUR2 mRNA, including the prefrontal cortex, ventral tegmental area, and nucleus accumbens (data not shown). Following training, surgery, and recovery (Figure 4A), animals receiving either intra-PVN (Figure 4B) or intra-DRN (Figure 4C) infusions of NMU (0.3nmol/kg) were given access to food as described above and consumption of standard and high-fat diet was measured at intervals over a 24-hour period. Intra-PVN NMU decreased standard diet intake at 2 and 4 hours post-treatment (p<0.05 by multiple-comparisons ANOVA, n=5/group, F=0.9455(n.s.)) and high-fat diet intake at 2 and 4 hours post-treatment (p<0.05 and p<0.01, respectively, by multiple-comparisons ANOVA, n=5/group, F=0.7659 (n.s.)), versus aCSF (Figure 5A, C). Intra-DRN NMU decreased standard diet intake at 2, 4, and 24 hours post-treatment (p<0.01 by multiple-comparisons ANOVA, n=8/group, F=1.710 (n.s.)) and high-fat diet intake at 2, 4, and 24 hours post-treatment (p<0.01 by multiple-comparisons ANOVA, n=7/group, F=2.365 (n.s.)) as compared to vehicle baseline (Figure 5B, D). In correspondence with the activity patterns identified in the Fos expression experiment (Figure 2), NMU administration into either the PVN or DRN was sufficient to induce a significant decrease in intake of a standard or high-fat diet.
Figure 3.
Localization of Neuromedin U Receptor 2 (NMUR2) in the paraventricular nucleus of the hypothalamus (PVN) and dorsal raphe nucleus (DRN). (A) NMUR2 is expressed in the PVN. (B) NMUR2 is expressed in the DRN. 3V indicates third ventricle; Aq indicates cerebral aqueduct. 20× magnification.
Figure 4.
Timeline and targeting. (A) Timeline of experiments involving central Neuromedin U (NMU) administration. FR refers to fixed-ratio responding. PR refers to progressive-ratio responding. LFD and HFD refer to low-fat diet and high-fat diet, respectively. (B) Cannula targeting for the paraventricular nucleus of the hypothalamus (A/P −1.80 mm). (C) Cannula targeting for the dorsal raphe nucleus (A/P −7.10 mm).
Figure 5.
Administration of Neuromedin U (NMU) directly to the paraventricular nucleus of the hypothalamus (PVN) and dorsal raphe nucleus (DRN) decreases food intake and motivation for high-fat food. (A) NMU infused into the PVN reduces consumption of a low-fat diet (LFD) at 2 and 4 hours post-treatment (n=5), **p<0.01 by Sidak’s multiple comparisons test). (B) NMU infused into the DRN reduces consumption of a LFD at 2 hours, 4 hours, and 24 hours post-treatment (n=6, **p<0.01 by Sidak’s multiple comparisons test). (C) NMU infused into the PVN reduces consumption of a high-fat diet (HFD) at 2 and 4 hours post-treatment (n=5, *p<0.05, **p<0.01 by Sidak’s multiple comparisons test). (D) NMU infused into the DRN reduces consumption of a HFD at 2 hours, 4 hours, and 24 hours post-treatment (n=6, **p<0.01 by Sidak’s multiple comparisons test). (E) NMU infused into the PVN decreases motivated responding for high-fat food (n=6, *p<0.05 by unpaired t-test). (F) NMU infused into the DRN decreases motivated responding for high-fat food (n=8, **p<0.01 by unpaired t-test). Error bars represent standard error of the mean.
Mirroring our peripheral administration studies, animals trained to lever-press for high-fat food pellets were treated with intra-PVN or intra-DRN infusions of NMU (0.3nmol/kg) or aCSF immediately prior to testing sessions. NMU delivery into the PVN decreased PR responding for high-fat pellets, as compared to vehicle (Figure 5E; p<0.05 by unpaired t-test, n=8/group, F=4.415 (n.s)). Similarly, intra-DRN NMU administration caused a decrease in PR responding, significantly greater than that produced by vehicle treatment (p<0.05 by unpaired t-test, n=8/group, F=1.914 (n.s.)) (Figure 5F). This suggests that NMU acts directly via the PVN and DRN to regulate not only consumption of, but also motivation for high-fat food.
NMU-containing neurons in the LH project to the PVN and DRN
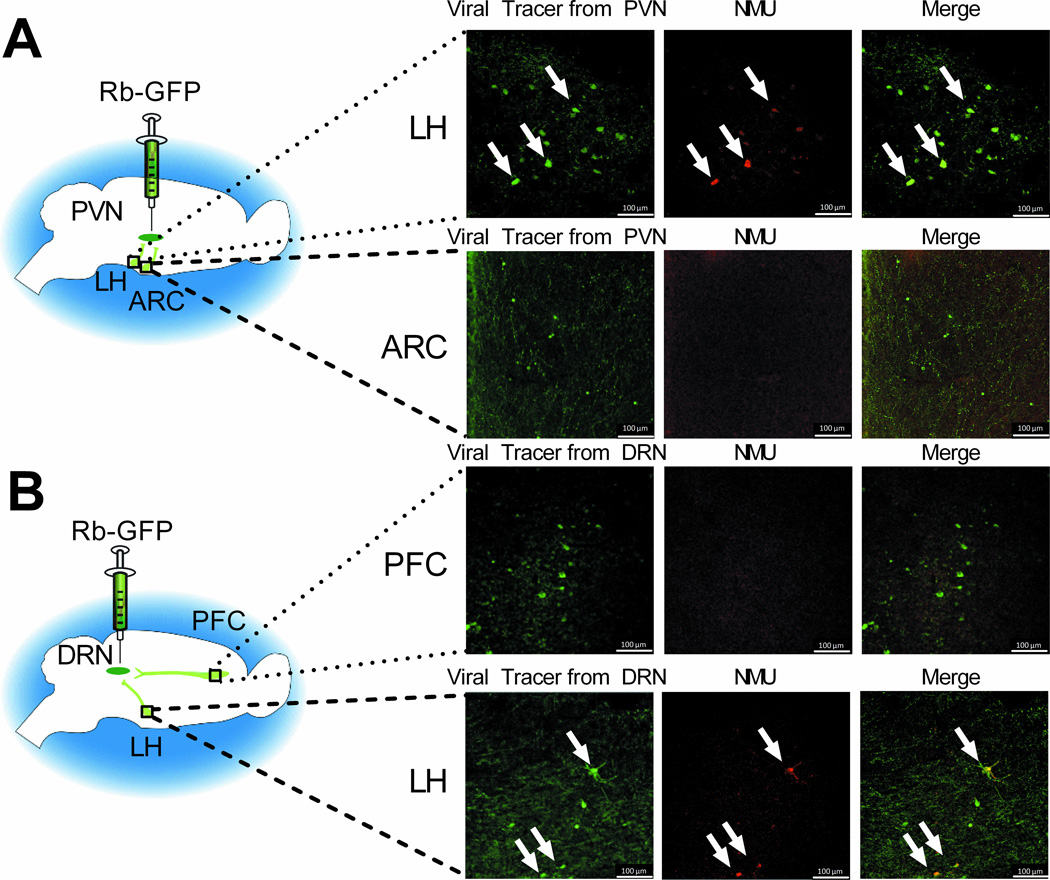
While NMU in the PVN and DRN is behaviorally relevant, the source of NMU-containing neurons that innervate these brain regions has not been established. To explore this neurocircuitry, animals were treated with a combination of ICV colchicine and an attenuated, replication-incompetent rabies virus, Rb-ΔG-B19-GFP, targeted at the PVN (Figure 6A) or DRN (Figure 6B). Rb-ΔG-B19-GFP serves as a retrograde tracer, infecting via the presynaptic terminal and being transported in a retrograde direction thus labeling the cell body of the projection neurons (32). Colchicine blocks axonal transport resulting in an accumulation of NMU in the cell body. This combination allows for visualization of afferent pathways producing NMU. Analysis focused on regions known to project to the PVN and DRN (41). Fluorescent immunohistochemistry was used to enhance the native GFP signal expressed by the attenuated rabies virus (Figure 6A and 6B, left column) and visualize NMU (Figure 6A and 6B, middle column) in the cell bodies of projection neurons. NMU immunoreactive cell bodies were observed in the LH, but not in the arcuate nucleus (ARC) or the prefrontal cortex (PFC) (Figure 6A and 6B). GFP-labeled cells were found in the LH and to a lesser extent, the ARC, following injection of the viral tracer into the PVN (Figure 6A, left column), and in the LH and PFC following injection of the viral tracer into the DRN (Figure 6B, left column). The data indicate that NMU-producing neurons projecting to the PVN (Figure 6A) and the DRN (Figure 6B, n=3 animals/group) primarily originate in the LH.
Figure 6.
Neuromedin U (NMU)-containing neurons in the lateral hypothalamus (LH) project to the paraventricular nucleus of the hypothalamus (PVN) and dorsal raphe nucleus (DRN). (A) Attenuated, replication-incompetent Rb-ΔG-B19-GFP is injected into the PVN, and traces to the LH and the arcuate nucleus (ARC). (B) Rb-ΔG-B19-GFP is injected into the DRN, and traces to the prefrontal cortex (PFC) and the LH. 20× magnification.
Discussion
One of the understudied aspects of obesity is the motivation for high-fat foods (2–4). Such foods are powerful drivers of obesity that contribute to, maintain, and promote overeating (42–46). This is due, in part, to the highly reinforcing properties of high-fat foods. Our work builds on previous data showing that NMUR2 signaling in the brain is capable of regulating preference for, and consumption of high-fat foods (9). Consistent with previously published data (6), we show that systemic NMU administration decreases standard food intake, and demonstrate its ability to reduce high-fat food intake. Systemic NMU was co-administered with DMSO to ensure that NMU would cross the blood brain barrier. This allowed us to evaluate the expression patterns of Fos, an immediate early gene marker of neuronal activity, and to identify potential anatomical targets in the brain for NMU binding. We identify increases in Fos-like immunoreactivity in the M1/M2 region of the motor cortex, a finding consistent with previously identified induction of locomotor activity by ICV NMU (39). Moreover, the PVN and DRN demonstrate decreases in Fos-like immunoreactivity following NMU administration, and importantly also express NMUR2 protein. Direct infusion of NMU into the PVN supports previous research indicating that hypothalamic NMU suppresses food intake (11), and extends these findings by demonstrating that PVN NMU signaling regulates both consumption of, and motivation for high-fat food. Importantly, a connection has been implied between PVN–mediated feeding effects and the nucleus accumbens, a key structure for the regulation of reinforcement behavior (47). Recent research has confirmed the existence of a pathway linking the PVN to the nucleus accumbens, and demonstrates that the pathway is capable of regulating social aspects of reinforcement (48), suggesting a potential downstream mechanism by which the PVN may regulate food reinforcement.
In addition, we have identified the DRN as a novel site of action for NMU-NMUR2 based regulation of food intake and motivation for food. The role of the DRN on feeding is not fully elucidated. However, its regulation of reinforcement behavior via serotonin signaling has been reported (49) and recent research demonstrates that food reinforcement activates serotonin neurons within the DRN (50). Additionally, PVN ghrelin signaling regulates appetite through DRN serotonin signaling (51). As ICV NMU has been demonstrated to alter serotonin expression in the brain, and the behavioral effects of NMU are regulated by serotonin receptor function (52), NMU may regulate food reinforcement via serotonin signaling downstream of the DRN.
Pathways linking the LH and PVN, as well as the LH and DRN, have been unexplored with regards to NMU regulation of reinforcement. The LH has, however, been investigated in both non-food (25, 53, 54), and food (55) reinforcement, and projections to the PVN and DRN (56, 57) have been characterized. The production of NMU by these projections, and the observed downstream regulation of feeding and food reinforcement by NMU release, is consistent with the alterations of NMU mRNA in response to energy balance (15). This suggests that endogenous regulation of the feeding and food reinforcement behaviors identified here may be driven by NMU-producing LH-PVN and LH-DRN neurons. There is also potential for alternative ligands driving endogenous NMUR2 signaling in the PVN; neuromedin S (NMS) has been shown to bind NMUR2 in the PVN (13), producing anorectic effects. However, NMS has not been identified in the DRN, or implicated in raphe-dependent regulation of feeding behavior.
Taken together, these data indicate that NMU-NMUR2 signaling in the PVN and DRN assists in regulating consumption of, and motivation for high-fat food, a key element in the development of, or resistance to, obesity. These studies highlight the emerging role of NMU signaling in reinforcement, elucidate the neurocircuitry mediating its behavioral effects, and identify an endogenous source of the peptide. The combined result is the identification of specific NMU-NMUR2 signaling pathways as mediators of motivation for high-fat food. As motivated consumption of high-fat foods is a potent driver of obesity in both humans and animals (1–4), dysregulated NMU signaling pathways may underlie overconsumption of obesogenic food.
Supplementary Material
Acknowledgments
We thank Dr. Marcy Jordan for critically editing the manuscript. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK079638, R01DK106229), National Institute on Drug Abuse (R03DA033437, P30DA028821, and T32DA07287), and Clinical and Translational Science Award (UL1TR001439 and KL2TR001441) from the National Center for Advancing Translational Science.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Supplementary information is available at the International Journal of Obesity’s website.
References
- 1.Lichtenstein AH, Kennedy E, Barrier P, Danford D, Ernst ND, Grundy SM, et al. Dietary fat consumption and health. Nutrition reviews. 1998;56(5 Pt 2):S3–S19. doi: 10.1111/j.1753-4887.1998.tb01728.x. discussion S-28. [DOI] [PubMed] [Google Scholar]
- 2.Delaere F, Akaoka H, De Vadder F, Duchampt A, Mithieux G. Portal glucose influences the sensory, cortical and reward systems in rats. The European journal of neuroscience. 2013;38(10):3476–3486. doi: 10.1111/ejn.12354. [DOI] [PubMed] [Google Scholar]
- 3.Cansell C, Castel J, Denis RG, Rouch C, Delbes AS, Martinez S, et al. Dietary triglycerides act on mesolimbic structures to regulate the rewarding and motivational aspects of feeding. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity. 2009;17(4):640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 5.Semjonous NM, Smith KL, Parkinson JR, Gunner DJ, Liu YL, Murphy KG, et al. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. International journal of obesity. 2009;33(7):775–785. doi: 10.1038/ijo.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peier AM, Desai K, Hubert J, Du X, Yang L, Qian Y, et al. Effects of peripherally administered neuromedin U on energy and glucose homeostasis. Endocrinology. 2011;152(7):2644–2654. doi: 10.1210/en.2010-1463. [DOI] [PubMed] [Google Scholar]
- 7.Peier A, Kosinski J, Cox-York K, Qian Y, Desai K, Feng Y, et al. The antiobesity effects of centrally administered neuromedin U and neuromedin S are mediated predominantly by the neuromedin U receptor 2 (NMUR2) Endocrinology. 2009;150(7):3101–3109. doi: 10.1210/en.2008-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakazato M, Hanada R, Murakami N, Date Y, Mondal MS, Kojima M, et al. Central effects of neuromedin U in the regulation of energy homeostasis. Biochem Biophys Res Commun. 2000;277(1):191–194. doi: 10.1006/bbrc.2000.3669. [DOI] [PubMed] [Google Scholar]
- 9.Benzon CR, Johnson SB, McCue DL, Li D, Green TA, Hommel JD. Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neuroscience. 2014;258:270–279. doi: 10.1016/j.neuroscience.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, et al. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406(6791):70–74. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- 11.Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, et al. Hypothalamic actions of neuromedin U. Endocrinology. 2002;143(11):4227–4234. doi: 10.1210/en.2002-220308. [DOI] [PubMed] [Google Scholar]
- 12.Hanada R, Teranishi H, Pearson JT, Kurokawa M, Hosoda H, Fukushima N, et al. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nature medicine. 2004;10(10):1067–1073. doi: 10.1038/nm1106. [DOI] [PubMed] [Google Scholar]
- 13.Shousha S, Nakahara K, Sato M, Mori K, Miyazato M, Kangawa K, et al. Effect of neuromedin S on feeding regulation in the Japanese quail. Neuroscience letters. 2006;391(3):87–90. doi: 10.1016/j.neulet.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Shousha S, Nakahara K, Miyazato M, Kangawa K, Murakami N. Endogenous neuromedin U has anorectic effects in the Japanese quail. General and comparative endocrinology. 2005;140(3):156–163. doi: 10.1016/j.ygcen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Graham ES, Turnbull Y, Fotheringham P, Nilaweera K, Mercer JG, Morgan PJ, et al. Neuromedin U and Neuromedin U receptor-2 expression in the mouse and rat hypothalamus: effects of nutritional status. Journal of neurochemistry. 2003;87(5):1165–1173. doi: 10.1046/j.1471-4159.2003.02079.x. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki Y, Onaka T, Nakazato M, Saito J, Kanemoto K, Matsumoto T, et al. Centrally administered neuromedin U activates neurosecretion and induction of c-fos messenger ribonucleic acid in the paraventricular and supraoptic nuclei of rat. Endocrinology. 2002;143(11):4320–4329. doi: 10.1210/en.2002-220201. [DOI] [PubMed] [Google Scholar]
- 17.Brighton PJ, Szekeres PG, Willars GB. Neuromedin U and its receptors: structure, function, and physiological roles. Pharmacological reviews. 2004;56(2):231–248. doi: 10.1124/pr.56.2.3. [DOI] [PubMed] [Google Scholar]
- 18.Kamisoyama H, Honda K, Saneyasu T, Sugahara K, Hasegawa S. Central administration of neuromedin U suppresses food intake in chicks. Neuroscience letters. 2007;420(1):1–5. doi: 10.1016/j.neulet.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 19.Kojima M, Haruno R, Nakazato M, Date Y, Murakami N, Hanada R, et al. Purification and identification of neuromedin U as an endogenous ligand for an orphan receptor GPR66 (FM3) Biochem Biophys Res Commun. 2000;276(2):435–438. doi: 10.1006/bbrc.2000.3502. [DOI] [PubMed] [Google Scholar]
- 20.Miyazato M, Mori K, Ida T, Kojima M, Murakami N, Kangawa K. Identification and functional analysis of a novel ligand for G protein-coupled receptor, Neuromedin S. Regulatory peptides. 2008;145(1–3):37–41. doi: 10.1016/j.regpep.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Nakahara K, Katayama T, Maruyama K, Ida T, Mori K, Miyazato M, et al. Comparison of feeding suppression by the anorexigenic hormones neuromedin U and neuromedin S in rats. The Journal of endocrinology. 2010;207(2):185–193. doi: 10.1677/JOE-10-0081. [DOI] [PubMed] [Google Scholar]
- 22.Vallof D, Ulenius L, Egecioglu E, Engel JA, Jerlhag E. Central administration of the anorexigenic peptide neuromedin U decreases alcohol intake and attenuates alcohol-induced reward in rodents. Addiction biology. 2016 doi: 10.1111/adb.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brink JJ, Stein DG. Pemoline levels in brain: enhancement by dimethyl sulfoxide. Science. 1967;158(3807):1479–1480. doi: 10.1126/science.158.3807.1479. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Fardon R, Boutrel B. Orexin/hypocretin (Orx/Hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Frontiers in behavioral neuroscience. 2012;6:75. doi: 10.3389/fnbeh.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behavioural brain research. 1999;101(2):129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 26.Matzeu A, Zamora-Martinez ER, Martin-Fardon R. The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Frontiers in behavioral neuroscience. 2014;8:117. doi: 10.3389/fnbeh.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(7):2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, et al. Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology. 2004;177(1–2):1–14. doi: 10.1007/s00213-004-1918-3. [DOI] [PubMed] [Google Scholar]
- 29.(U.S.) IoLAR. Guide for the care and use of laboratory animals. 8th. Washington, D.C: National Academies Press; 2011. p. 246. [PubMed] [Google Scholar]
- 30.West EA, Forcelli PA, Murnen AT, McCue DL, Gale K, Malkova L. Transient inactivation of basolateral amygdala during selective satiation disrupts reinforcer devaluation in rats. Behavioral neuroscience. 2012;126(4):563–574. doi: 10.1037/a0029080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasper JM, McCue DL, Milton AJ, Szwed A, Sampson CM, Huang M, et al. Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U. Biological psychiatry. 2016 doi: 10.1016/j.biopsych.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickersham IR, Finke S, Conzelmann KK, Callaway EM. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nature methods. 2007;4(1):47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etessami R, Conzelmann KK, Fadai-Ghotbi B, Natelson B, Tsiang H, Ceccaldi PE. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J Gen Virol. 2000;81(Pt 9):2147–2153. doi: 10.1099/0022-1317-81-9-2147. [DOI] [PubMed] [Google Scholar]
- 34.Alexander K, Nikodemova M, Kucerova J, Strbak V. Colchicine treatment differently affects releasable thyrotropin-releasing hormone (TRH) pools in the hypothalamic paraventricular nucleus (PVN) and the median eminence (ME) Cellular and molecular neurobiology. 2005;25(3–4):681–695. doi: 10.1007/s10571-005-4008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, et al. Neuromedin U receptor 2-deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol. 2006;26(24):9352–9363. doi: 10.1128/MCB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jethwa PH, Small CJ, Smith KL, Seth A, Darch SJ, Abbott CR, et al. Neuromedin U has a physiological role in the regulation of food intake and partially mediates the effects of leptin. American journal of physiology Endocrinology and metabolism. 2005;289(2):E301–E305. doi: 10.1152/ajpendo.00404.2004. [DOI] [PubMed] [Google Scholar]
- 37.Neuner P, Peier AM, Talamo F, Ingallinella P, Lahm A, Barbato G, et al. Development of a neuromedin U-human serum albumin conjugate as a long-acting candidate for the treatment of obesity and diabetes. Comparison with the PEGylated peptide. Journal of peptide science : an official publication of the European Peptide Society. 2014;20(1):7–19. doi: 10.1002/psc.2582. [DOI] [PubMed] [Google Scholar]
- 38.Radulovic J, Kammermeier J, Spiess J. Relationship between fos production and classical fear conditioning: effects of novelty, latent inhibition, and unconditioned stimulus preexposure. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(18):7452–7461. doi: 10.1523/JNEUROSCI.18-18-07452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, et al. Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin-U following central administration in rats. Psychopharmacology. 2004;177(1–2):1–14. doi: 10.1007/s00213-004-1918-3. [DOI] [PubMed] [Google Scholar]
- 40.Guan XM, Yu H, Jiang Q, Van Der Ploeg LH, Liu Q. Distribution of neuromedin U receptor subtype 2 mRNA in the rat brain. Brain Res Gene Expr Patterns. 2001;1(1):1–4. doi: 10.1016/s1567-133x(00)00002-8. [DOI] [PubMed] [Google Scholar]
- 41.Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain research. 2003;963(1–2):57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- 42.Luo S, Romero A, Adam TC, Hu HH, Monterosso J, Page KA. Abdominal fat is associated with a greater brain reward response to high-calorie food cues in Hispanic women. Obesity. 2013;21(10):2029–2036. doi: 10.1002/oby.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, et al. Refined food addiction: a classic substance use disorder. Medical hypotheses. 2009;72(5):518–526. doi: 10.1016/j.mehy.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 44.Barson JR, Morganstern I, Leibowitz SF. Neurobiology of consummatory behavior: mechanisms underlying overeating and drug use. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2012;53(1):35–58. doi: 10.1093/ilar.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cerebral cortex (New York, NY : 1991) 2010;20(5):1082–1091. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- 46.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. The Journal of nutrition. 2009;139(3):629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajnal A, Mark GP, Rada PV, Lenard L, Hoebel BG. Norepinephrine microinjections in the hypothalamic paraventricular nucleus increase extracellular dopamine and decrease acetylcholine in the nucleus accumbens: relevance to feeding reinforcement. Journal of neurochemistry. 1997;68(2):667–674. doi: 10.1046/j.1471-4159.1997.68020667.x. [DOI] [PubMed] [Google Scholar]
- 48.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–1784. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo M, Zhou J, Liu Z. Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learning & memory (Cold Spring Harbor, NY) 2015;22(9):452–460. doi: 10.1101/lm.037317.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nature communications. 2016;7:10503. doi: 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wauson SE, Sarkodie K, Schuette LM, Currie PJ. Midbrain raphe 5-HT1A receptor activation alters the effects of ghrelin on appetite and performance in the elevated plus maze. Journal of psychopharmacology (Oxford, England) 2015;29(7):836–844. doi: 10.1177/0269881115581981. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka M, Telegdy G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behavioural brain research. 2014;259:196–199. doi: 10.1016/j.bbr.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Benaliouad F, Kapur S, Rompre PP. Blockade of 5-HT2a receptors reduces haloperidol-induced attenuation of reward. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(3):551–561. doi: 10.1038/sj.npp.1301136. [DOI] [PubMed] [Google Scholar]
- 54.Kallupi M, de Guglielmo G, Cannella N, Li HW, Calo G, Guerrini R, et al. Hypothalamic neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2013;226(2):347–355. doi: 10.1007/s00213-012-2910-y. [DOI] [PubMed] [Google Scholar]
- 55.Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, et al. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalewa J, Joshi A, McGinnity TM, Prasad G, Wong-Lin K, Holscher C. Neural circuit interactions between the dorsal raphe nucleus and the lateral hypothalamus: an experimental and computational study. PloS one. 2014;9(2):e88003. doi: 10.1371/journal.pone.0088003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirchgessner AL, Sclafani A, Nilaver G. Histochemical identification of a PVN-hindbrain feeding pathway. Physiology & behavior. 1988;42(6):529–543. doi: 10.1016/0031-9384(88)90154-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.