Abstract
While acute activation of the novel endocannabinoid receptor GPR18 causes hypotension, there are no reports on GPR18 expression in the heart or its chronic modulation of cardiovascular function. In this study, after demonstrating GPR18 expression in the heart, we show that chronic (2 weeks) GPR18 activation with its agonist abnormal cannabidiol (abn-cbd; 100 µg/kg/day; i.p) produced hypotension, suppressed the cardiac sympathetic dominance, and improved left ventricular (LV) function (increased the contractility index dp/dtmax, and reduced LV end diastolic pressure, LVEDP) in conscious rats. Ex vivo studies revealed increased: (i) cardiac and plasma adiponectin (ADN) levels; (ii) vascular (aortic) endothelial nitric oxide synthase (eNOS) expression, (iii) vascular and serum nitric oxide (NO) levels; (iv) myocardial and plasma cyclic guanosine monophosphate (cGMP) levels; (v) phosphorylation of myocardial protein kinase B (Akt) and extracellular signal regulated kinase 1/2 (ERK1/2) along with reduced myocardial reactive oxygen species (ROS) in abn-cbd treated rats. These biochemical responses contributed to the hemodynamic responses and were GPR18-mediated because concurrent treatment with the competitive GPR18 antagonist (O-1918) abrogated the abn-cbd evoked hemodynamic and biochemical responses. The current findings present new evidence for a salutary cardiovascular role for GPR18, mediated, at least partly, via elevation in the levels of ADN.
Keywords: GPR18, abnormal cannabidiol, O-1918, left ventricular function, blood pressure, sympathovagal tone, adiponectin, cGMP
Introduction
There is growing interest in understanding the cardiovascular role of the endocannabinoid receptor GPR18, which is structurally distinct from the cannabinoid receptors 1 or 2 (CB1R/CB2R)1. GPR18 was originally described as the endothelial cannabinoid receptor because its activation by the endogenous cannabinoid, ananedamide (AEA) or by the synthetic cannabinoid abnormal cannabidiol (trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol, abn-cbd) caused vasodilation and hypotension2, 3. Abrogation of the latter responses by the structural analogue of abn-cbd, O-1918 [(−)-1.3-dimethoxy-2-(3-3,4-trans-p-menthadien-(1,8)-yl)-orcinol]2–4 lead to categorizing the receptor as the AEA or abn-cbd receptor. Thereafter, the abn-cbd receptor was identified as GPR18 along with abn-cbd and O-1918 as its agonist and antagonist, respectively5, and N- arachidonyl glycine (NAGly) as its endogenous ligand6.
While GPR18 is considered the abn-cbd receptor5, 7, failure of abn-cbd or NAGly to produce GPR18-dependent biochemical responses in some studies8, 9 fueled a debate on whether GPR18 is indeed the abn-cbd receptor. It is possible that the biochemical responses are tissue/cell type specific due to differences in the constitutive activity of GPR189. Importantly, many studies supported the role of GPR18 in regulating the cardiovascular function because NAGly caused relaxation of isolated rat mesenteric arteries10 and abn-cbd induced vasorelaxant and hypotensive responses that were abrogated by the GPR18 antagonist O-19182–4, 11, and are not dependent on CB1/CB2 receptors2, 3, 12. Further, while the endocannabinoid receptor GPR55 was originally considered the putative abn-cbd receptor1, 13, it is imperative to note that the abn-cbd evoked vasorelaxation and hypotension are not GPR55-dependent because these responses persisted in GPR55 knockout mice7, 14, and because the GPR55 agonist O-160214, 15 does not lower blood pressure (BP)15.
As a Gi/G0-protein coupled receptor, GPR18 mediates cyclic adenosine monophosphate (cAMP) reduction in vitro6, and activation of the PI3K/Akt and ERK1/2 pathways3, 5, 16. These findings may implicate endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) in the vasodilation and centrally mediated hypotensive responses, caused by GPR18 activation10, 16, because the PI3K/Akt-ERK1/2 pathway activates eNOS17. Notably, our recent findings highlighted a possible role for the antiinflammatory peptide, adiponectin (ADN) in the GPR18-mediated increase in NO and decrease in reactive oxygen species (ROS) levels in the brain stem11, 16. The latter findings are consistent with reported ADN-dependent increase in vascular eNOS-derived NO and decrease in ROS levels18. However, three fundamental issues need to be addressed to understand the cardiovascular role of GPR18. First, in the previous studies, the reported acute GPR18-mediated hypotensive response might have been confounded by the high abn-cbd doses and anesthesia2, 3. Second, it is not known if GPR18 is expressed in the heart and regulates cardiac function. Third, the molecular mechanisms implicated in the cardiovascular responses caused by chronic GPR18 activation need to be elucidated. In addition to gaining new insight into the role of GPR18 signaling in cardiovascular function, the findings of the present study might lead to clinically relevant information on the potential use of GPR18 agonists as promising new drugs for the treatment of cardiovascular anomalies.
The main objective of the present study was to investigate the effect of chronic GPR18 activation (abn-cbd) in the absence or presence of GPR18 blockade (O-1918) on BP and cardiac function and to elucidate the molecular mechanisms of the GPR18-mediated hemodynamic effects in conscious rats. To achieve these goals, hemodynamic measurements were conducted in conscious male rats treated with abn-cbd, O-1918 or their combination for 2 weeks. Blood and cardiovascular tissues, collected from the treatment and control groups at the conclusion of the study, were used for ex vivo biochemical studies.
Methods
Preparation of the rats
Male Wistar rats (250–300 g; Charles River Laboratories, Raleigh, NC) were used in the present study. Rats were housed 2 per cage in a room with a controlled environment at a constant temperature of 23 ± 1°C, 50% ± 10% humidity and a 12-hour light/dark cycle. Food (Prolab Rodent Chow, Prolab RMH 3000; Granville Milling, Creedmoor, NC) and water were provided ad libitum. All surgical, experimental and animal care procedures were conducted in accordance with, and approved by, the East Carolina University Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 2011).
Hemodynamic measurements
Measurements of mean arterial pressure (MAP), heart rate (HR), and left ventricular (LV) function were conducted in conscious unrestrained rats at the end of the 2-week treatment period. Arterial and LV catheterizations were done under sterile conditions and anesthesia using ketamine (9 mg/100 g) and xylazine (1 mg/100 g, i.p) as in our previous studies19–21. The rats were housed individually, and allowed 24 hrs. to recover from anesthesia. On the day of the experiment, the catheters were connected to Gould-Statham pressure transducers (Oxnard, CA) for hemodynamic measurements as in our previous studies19, 20. The measured LV function indices dp/dtmax, dp/dtmin and left ventricular end diastolic pressure (LVEDP), reflect LV compliance and intravascular volume and pressure19. MAP was computed as [1/3 (systolic pressure-diastolic pressure) + diastolic pressure] and HR was extracted from BP values. BP and HR were allowed to stabilize for at least for 30 min before collecting and analyzing hemodynamic data over 1 hr using ML870 (Power Lab 8/30) and Lab Chart 7 software (AD Instruments, Colorado Spring, CO) as in our previous studies19, 20.
Blood and tissues collection for biochemical studies
At the end of the in vivo measurements, blood was collected, centrifuged and stored at −80°C until use. Then animals were euthanized with a lethal dose of sodium pentobarbital (100 mg/kg), and the hearts and vascular tissues were removed, cleaned from blood, flash-frozen in 2-methylbutane on dry ice, and stored at −80°C until use19.
Frequency domain analysis
Frequency domain analysis, which determines the cardiac sympathetic and parasympathetic balance, was conducted, using software designed for rats (Nevrokard SA-BRS; Izola, Slovenia) as in our previous study22. Power of RR interval (RRI) spectral density oscillations were computed by 512-point fast Fourier transform and integrated over the specified low frequency (LF, 0.25–0.75 Hz) to high frequency (HF, 0.75–5.0 Hz) range. LF and HF values reflect sympathetic and parasympathetic dominance, respectively, while the RRILF/HF ratio is a measure of sympathovagal balance23. However, some studies indicate that LF represents the changes in both sympathetic and parasympathetic activities24 because the change in the total power will affect the LF and HF in the same direction. Notably, the calculation of LFRRI and HFRRI spectra in normalized units (n.u) will minimize this effect23.
Western blot analysis and biochemical studies
Heart and aortic tissues were homogenized on ice in lysis buffer (pH 7.5) containing 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM activated sodium orthovanadate, 1 µg/ml leupeptin and protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Samples were centrifuged at 4°C, and then protein content was determined in the supernatant by a standard Bio-Rad protein assay system (Bio-Rad Laboratories, Hercules, CA). SDS polyacrylamide gel (NuPAGE 4 to 12% Bis-Tris Gel, Invitrogen, Carlsbad, CA) was used to separate the proteins in each sample using MOPS NuPAGE running buffer. Proteins were transferred from the gels to nitrocellulose membranes using TransBlot SD transfer cell (Bio-Rad Laboratories) and were incubated at room temperature for 2 hrs. in Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE). Then membranes were incubated at 4°C overnight with a mixture of rabbit polyclonal anti-GPR18 antibody (1:500; Assay Biotech., Sunnyvale, CA) or rabbit polyclonal anti-ADN antibody (1:200; Abcam Inc., Cambridge, MA) and mouse monoclonal anti-GAPDH antibody (1:15000; Abcam Inc., Cambridge, MA) or mixture of rabbit polyclonal anti-eNOS (1:200; BD Biosciences, San Jose, CA) and mouse monoclonal anti-GAPDH antibody (1:15000; Abcam Inc., Cambridge, MA) and mouse monoclonal antiphospho-eNOS (pSer1177) antibody (1:200; BD Biosciences, San Jose, CA) or rabbit polyclonal anti-ERK1/2 antibody (1:500) and mouse monoclonal antiphospho-ERK1/2 (pTyr202/Y204) antibody (1:500) or rabbit polyclonal anti-Akt antibody (1:500) and mouse monoclonal antiphospho-Akt (pSer473) antibody (1:500). Antibodies for Akt, p-Akt, ERK1/2, and pERK1/2 were obtained from Cell Signaling Technology Inc. (Danvers, MA). After the end of incubation, membranes were washed and incubated for 60 min with mixture containing IRDye 680-conjugated goat anti-mouse and IR Dye 800-conjugated goat anti-rabbit (1:15000; LI-COR Biosciences). Bands were detected by Odyssey Infrared Imager and analyzed with Odyssey application software version 0.3 (LI-COR Biosciences). GPR18, ADN and eNOS expressions were quantified against GAPDH while myocardial p-ERK1/2, p-eNOS and p-Akt were quantified against the corresponding total protein. Protein expression and enzymatic activity were presented as percent of control (vehicle treated rats) as previously reported11.
Dual labeling immunofluorescence
Sections (20 µm) from naïve rats’ hearts (n = 5), fixed in 4% paraformaldehyde in PBS for 20 min, were washed with Tris-buffered saline (TBS) and incubated with blocking buffer for 2 hrs, and subsequently incubated for 48 hrs. with mixtures of rabbit polyclonal anti-GPR18 antibody (1:500; Assay Biotech., Sunnyvale, CA) and mouse myocyte specific protein alpha actin (1:200, Sigma-Aldrich; St. Louis, MO) in the presence or absence of the GPR18 antibody blocking peptide (1:200; Santa Cruz; Dallas, TX). A final 2 hrs. incubation in a mixture of fluorescein isothiocyanate-conjugated donkey anti-Rabbit (1:200; Abcam Inc., Cambridge, MA) and Cy3-conjugated donkey anti-mouse (1:200; Abcam Inc., Cambridge, MA) before the visualization, and acquisition of the fluorescent images by A Zeiss LSM 510 confocal microscope (Carl Zeiss Inc., Thornwood, New York) at the same brightness and contrast settings in accordance with established protocols in our lab11.
Immunohistochemistry
Six to eight sections from each heart (20 µm) were cut at −24°C with a microtome cryostat (HM 505 E; Microm International GmbH, Walldorf, Germany). The avidin-biotin complex method was used according to the manufacturer’s instruction (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). For immunohistochemical detection of ADN, the heart sections were incubated with rabbit anti-ADN primary antibody (1:200; Abcam Inc., Cambridge, MA). After rinsing with TBS, 3′-diaminobenzidine (in dH2O), the sections were examined under a microscope (Nikon Diaphot 300; Nikon, Tokyo, Japan) for the appearance of reddish brown staining. After dehydration the sections were sealed with Permount (Fisher Scientific Co., Pittsburgh, PA), observed under the microscope, and the images were processed and quantified in accordance with established protocols in our previous study25.
Measurement of nitrate/nitrite (NOx)
Serum, vascular (aortic) and myocardial nitrate/nitrite levels (index of NO) were measured calorimetrically by commercially available kit according to the manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI) and reported study11.
Measurement of myocardial and plasma cGMP
Plasma and myocardial cGMP levels were measured by a commercially available cGMP enzyme immunoassay (EIA) kit (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer’s instructions and reported study26.
Measurement of plasma adiponectin
ADN was measured in plasma using Rat Adiponectin EIA kit (B-Bridge International Inc., San Jose, CA) according to the manufacturer’s instructions and reported study27.
Measurement of myocardial ROS by DCFH-DA
Myocardial ROS level was measured by 2,7-dichlorodihydrofluorescin diacetate (DCFH-DA; Molecular Probe), which is oxidized by ROS and forms a fluorescent dicholorofluorescin (DCF) at excitation 485/emission 530 nm wavelengths as in our previous studies11, 19.
Experimental design and protocol
Experiment 1. Myocardial GPR18 expression
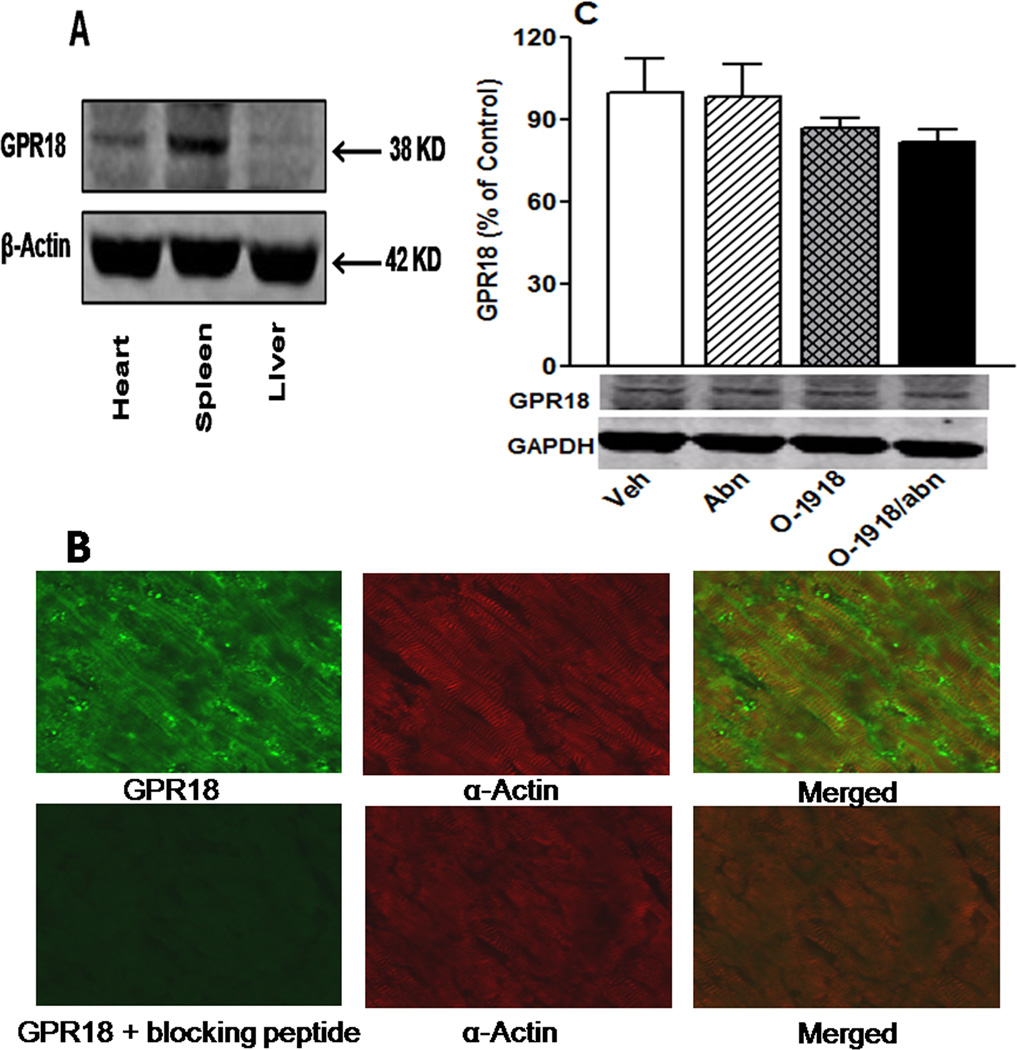
In the absence of any reports on cardiac GPR18 expression, we conducted Western blot studies on the hearts of control rats (n = 5) along with parallel studies in the spleen (rich in GPR18) and the liver (devoid of GPR18), as positive and negative controls, respectively28, to validate the findings. The specific anti-GPR18 antibody (Assay Biotech., Sunnyvale, CA) was quantified against GAPDH. Further, dual labeling immunofluorescence studies were conducted on myocardial sections in accordance with established protocols in our lab11 using rabbit anti-GPR18 antibody and the mouse myocyte specific protein alpha actin in the absence or presence of the GPR18 antibody blocking peptide.
Experiment 2. Hemodynamic and sympathovagal effects of chronic GPR18 activation or blockade
This experiment was done to elucidate the cardiovascular effects of chronic GPR18 activation and/or blockade in conscious rats. To achieve this goal, 4 groups of rats (n = 8 per group) received the following daily i.p. injection(s) for 2 weeks: (i) the vehicle, mixture of dimethyl sulfoxide (DMSO) and PBS (1:3, pH 7.2); (ii) abn-cbd (100 µg/kg, i.p); (iii) O-1918 (100 µg/kg, i.p), or (iv) O-1918 30 min before abn-cbd (100 µg/kg, each). The selected systemic abn-cbd dose, which was based on preliminary findings in conscious rats (data not shown), constituted at least 1/10th the doses used in previous studies using anesthetized mice2, 3, 14. The selected dose (100 µg/kg) of the GPR18 antagonist (O-1918) was based on its ability to abrogate the effects of abn-cbd when both drugs were used in equal doses3, 5, 14. At the end of the 2-week treatment period, hemodynamic and spectral analysis studies were conducted in conscious state as described under methods.
Experiment 3. Ex vivo biochemical consequences of GPR18 activation or blockade
At the conclusion of the hemodynamic measurements under experiment 2, the rats were euthanized, and the tissues and blood were collected for conducting the following biochemical measurements: (i) myocardial GPR18 expression; (ii) plasma and vascular NOx levels; (iii) cardiac and plasma ADN and cGMP levels; (iv) myocardial NOx and ROS levels; (v) myocardial and vascular eNOS expression, and (vi) myocardial phosphorylated Akt, ERK1/2 and eNOS.
Drugs
Abn-cbd and O-1918 were purchased from Cayman Chemical (Ann Arbor, MI); each drug was dissolved in methylacetate and kept at −20°C. In order to prepare an aqueous solution of each drug, methyl acetate solvent is volatilized then each drug was dissolved in mixture of (DMSO: BPS, 1:3; pH 7.2). DMSO was purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analysis
Baseline values of the hemodynamics, ventricular functions as well as biochemical parameters were expressed as mean ± S.E.M. Statistical analysis was done using a one-way analysis of variance (ANOVA) followed by Tukey's Post-hoc test and Student’s t test, Prism 5.0 software (Graph Pad Software Inc., San Diego, CA) was used to perform statistical analysis and P < 0.05 was considered significant.
Results
Chronic GPR18 activation reduced BP and cardiac sympathetic dominance while improving the LV function
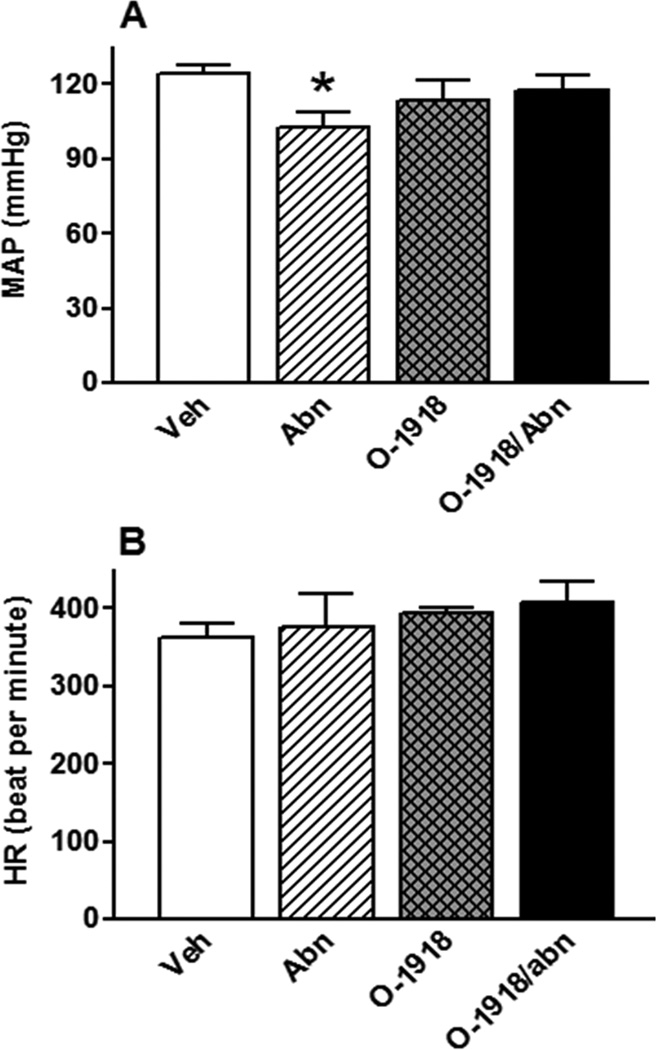
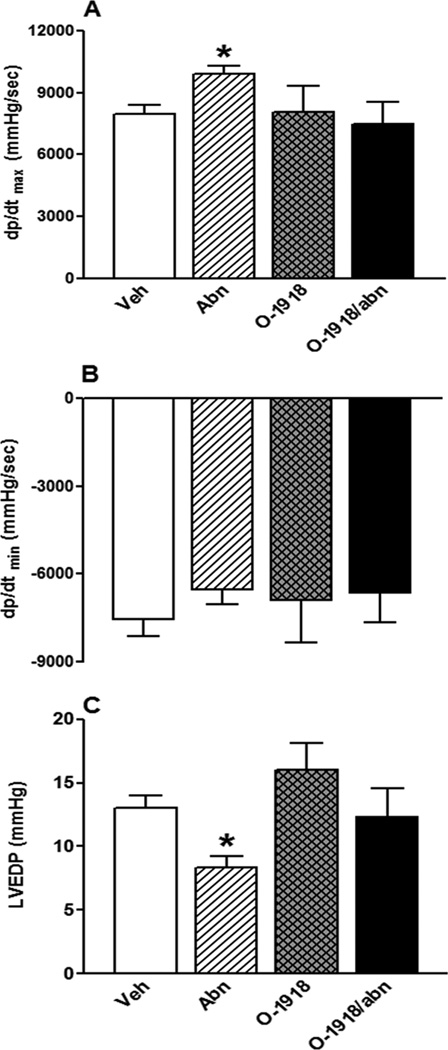
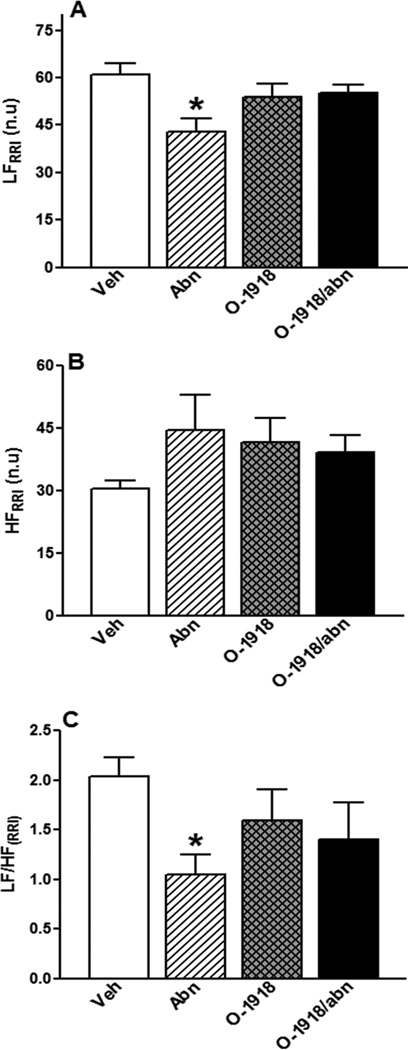
GPR18 activation (abn-cbd) significantly (P < 0.05) reduced BP (Fig. 1A), had no effect on HR (Fig. 1B), and improved LV function as demonstrated by the concomitant increase in LV contractility index, dp/dtmax, (Fig. 2A; P < 0.05) and the reduction in LVEDP (Fig. 2C; P < 0.05). The dp/dtmin, which reflects the LV performance during relaxation, did not significantly change by GPR18 activation (Fig. 2B). Frequency domain analysis revealed significant (P < 0.05) suppression of the sympathetic (LFRRI), but no change in the parasympathetic (HFRRI), component (lower LF/HFRRI ratio), which reflects a reduction in the sympathetic prevalence in abn-cbd treated rats (Fig. 3). While GPR18 blockade (O-1918) had no significant effect on the measured variables (Figs. 1 and 2), it abrogated the improvement in LV function (Figs. 2A and C) as well as the reductions in BP (Fig. 1A) and in the sympathetic prevalence (Fig. 3) caused by abn-cbd.
Figure 1.
Effect of 2-week treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination on mean arterial pressure (MAP; A) and heart rate (HR; B) in conscious male rats (n = 6–8). Values are mean ± SEM. *P < 0.05 versus control.
Figure 2.
Effect of 2-week treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination on left ventricular (LV) maximum contraction velocity (dp/dtmax; A); LV maximum relaxation velocity (dp/dtmin; B); and LV end diastolic pressure (LVEDP; C) in conscious male rats (n = 6–8). Values are mean ± SEM. *P < 0.05 versus control.
Figure 3.
(A) Low-frequency component of spectral analysis of RRI (0.25 to 0.75 Hz), index of cardiac sympathetic tone; (B) high-frequency component of the spectral analysis of RRI (0.75 to 3 Hz), index of cardiac vagal tone, and (C) LFRRI/HFRRI ratio as a measure of sympathovagal balance in conscious male rats (n = 6–8) treated with GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination for 2 weeks. Values are mean ± SEM. *P < 0.05 versus control.
GPR18 activation increased circulating levels of NO, cGMP and ADN as well as vascular eNOS/NO levels
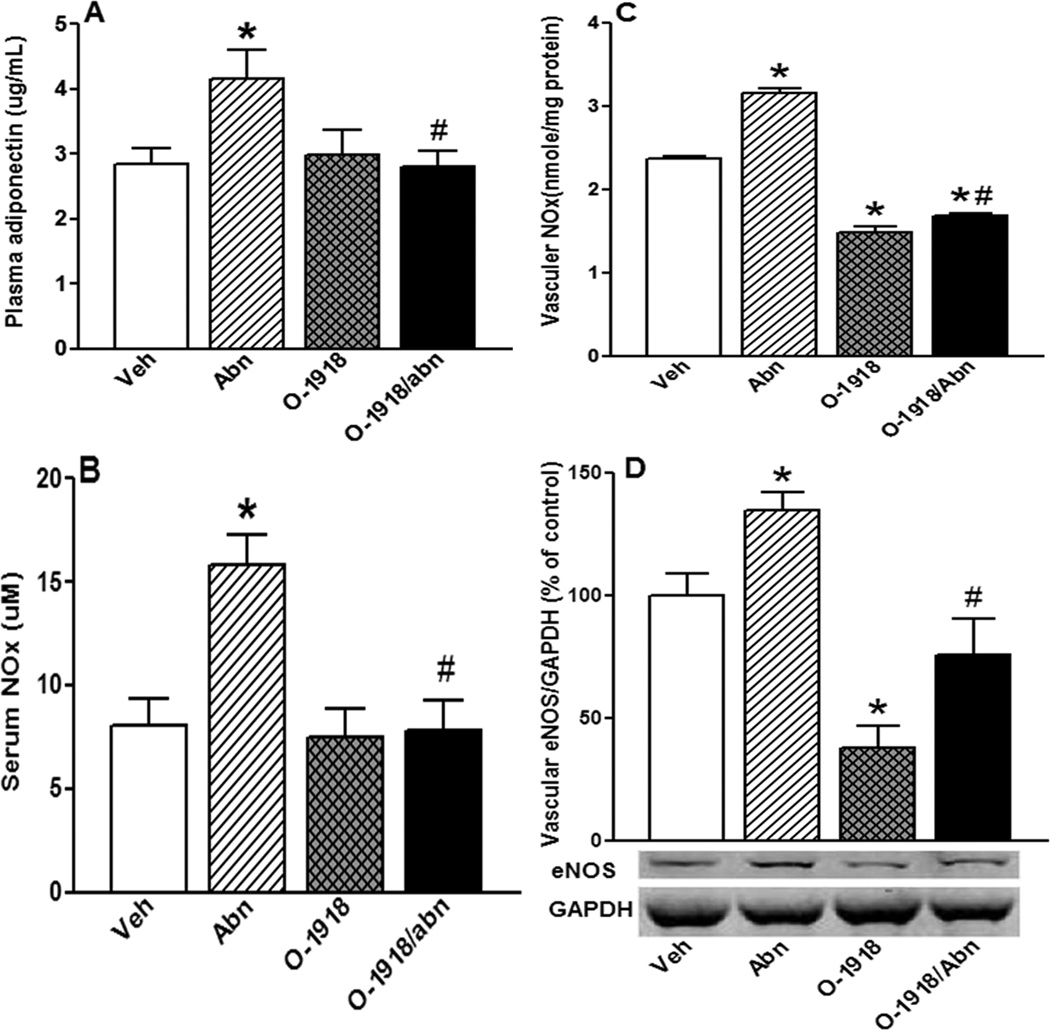
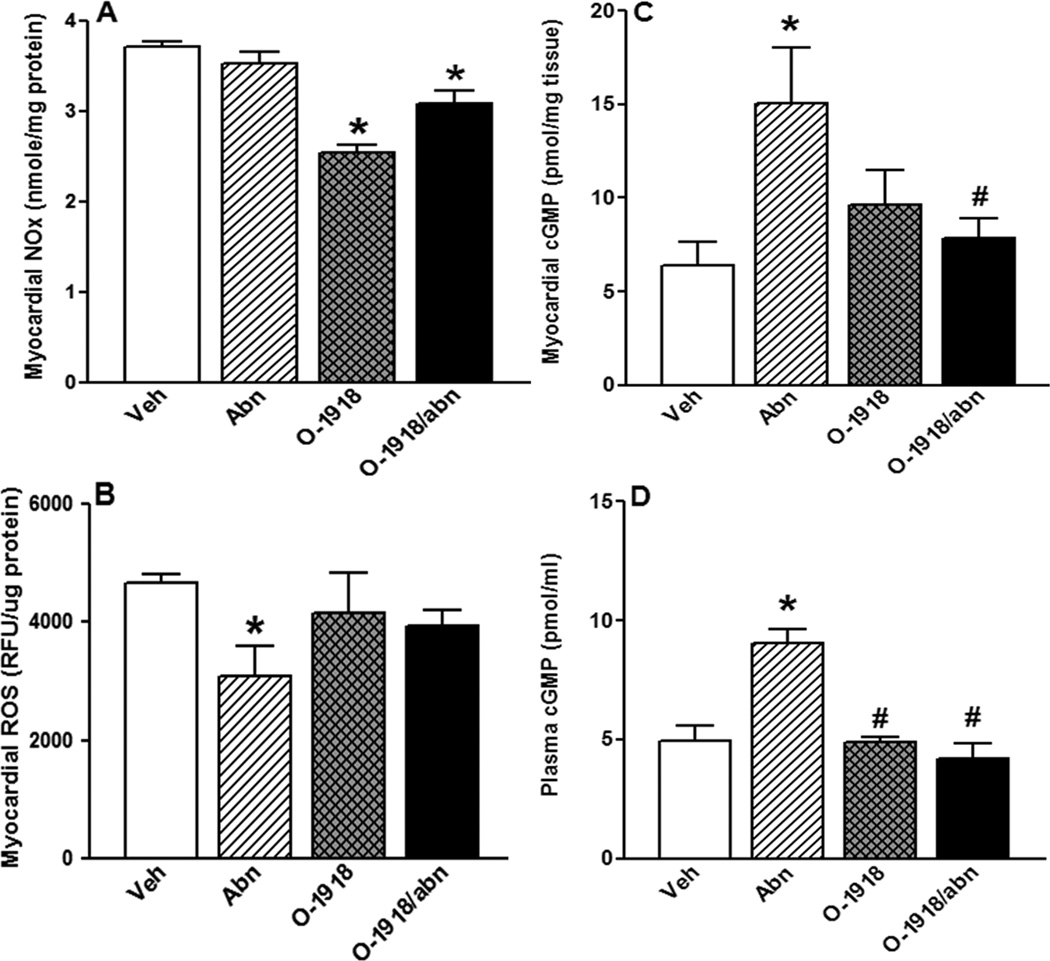
Chronic abn-cbd administration significantly (P < 0.05) increased circulating ADN (Fig. 5A), NO (Fig. 5B) and cGMP (Fig. 8D) levels along with a significant (P < 0.05) increase in vascular eNOS/NO levels (Figs. 5C and D). On the other hand, chronic GPR18 blockade (O-1918) alone significantly (P < 0.05) reduced vascular eNOS /NO levels (Figs. 5C and D) and abrogated the abn-cbd evoked increases in circulating ADN (Fig. 5A), NO (Fig. 5B) and cGMP (Fig. 8D) levels.
Figure 5.
Effect of 2-week treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination on the levels of plasma adiponectin (ADN; A); serum nitric oxide (NO; B); vascular (aortic) NO (C); and vascular eNOS expression (Western blot; D) in male rats (n = 6). Values are mean ± SEM. *P < 0.05 versus control, and #P < 0.05 versus abn-cbd.
Figure 8.
Effect of 2-week treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination on myocardial NO (A), myocardial ROS (B), myocardial cGMP (C) and plasma cGMP (D) levels in male rats (n = 8). Values are mean ± SEM. * P < 0.05 versus control and #P < 0.05 versus abn-cbd.
Myocardial GPR18 expression
Western blots revealed GPR18 expression in the heart and the findings were verified by incorporating positive (spleen) and negative (liver) controls (Fig. 4A). Immunofluorescence findings in the absence and presence of the GPR18 blocking peptide further verified the GPR18 expression in cardiac myocytes (Fig. 4B). Chronic (2 weeks) treatment with abn-cbd and/or O-1918 had no effect on myocardial GPR18 expression (Fig. 4C).
Figure 4.
(A) Expression of GPR18 (38 kDa) in the rat heart compared with expression in the spleen (positive controls) and liver (negative control) (tissues are obtained from naïve untreated male rats n = 5); (B) Dual-labeled immunofluorescence of naïve rat heart showing GPR18 expression in cardiomyocytes in presence or absence of the specific GPR18 antibody blocking peptide and (C) Western blot showing the effect of 2-weeks treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 or their combination (100 µg/kg/day, i.p, each) on myocardial GPR18 expression in male rats (n = 6). Protein expression was presented as percent of control. Values are mean ± SEM.
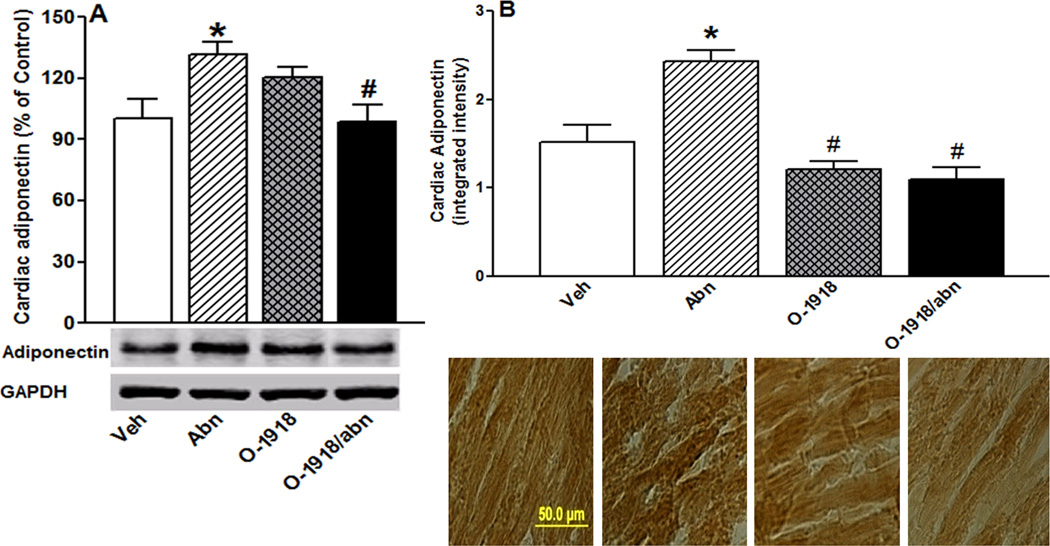
Chronic GPR18 activation increased cardiac ADN and cGMP levels and enhanced myocardial Akt and ERK1/2 phosphorylation
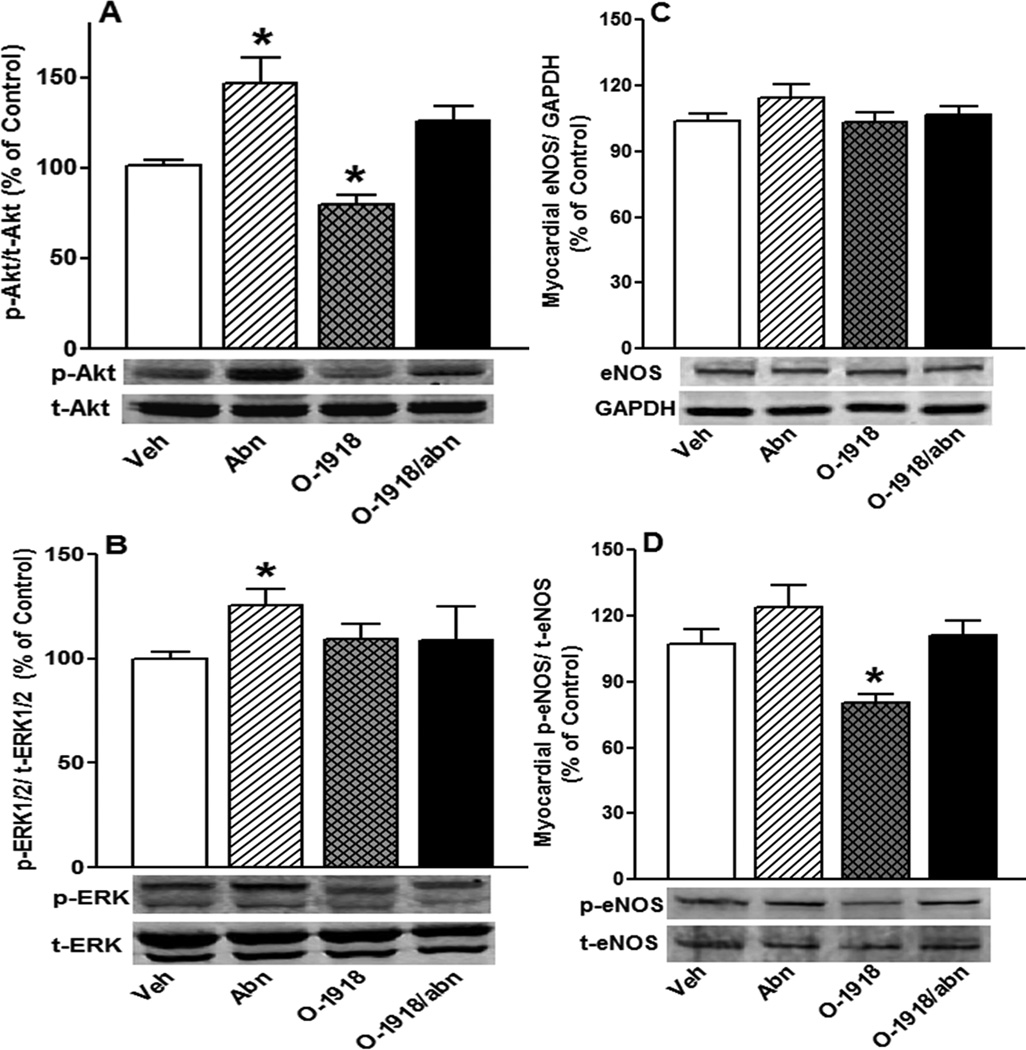
Chronic GPR18 activation (abn-cbd) significantly (P < 0.05) increased cardiac ADN (Fig. 6) levels and enhanced myocardial Akt and ERK1/2 phosphorylation (Figs. 7A and B). The heart of abn-cbd treated rats exhibited significantly (P < 0.05) higher cGMP level (Fig. 8C), but no change in eNOS expression/phosphorylation (Figs. 7C and D) or NO levels (Fig. 8A), compared to control values. Abn-cbd significantly (P < 0.05) reduced myocardial ROS level, an effect that was abrogated by O-1918 (Fig. 8B). Further, chronic GPR18 blockade (O-1918) caused significant (P < 0.05) reduction in myocardial NO (Fig. 8A), which paralleled significant (P < 0.05) reductions in myocardial Akt and eNOS phosphorylation (Figs. 7A and D), but had no effect on myocardial eNOS expression (Fig. 7C). Finally, O-1918 abrogated the abn-cbd evoked increases in cardiac ADN (Fig. 6) and cGMP (Fig. 8C) as well as myocardial ERK1/2 phosphorylation (Fig. 7B). These molecular responses paralleled the O-1918 abrogation of abn-cbd evoked hemodynamic effects (Figs. 1 and 2) in the same rats.
Figure 6.
Effect of 2-week treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination on cardiac adiponectin (ADN) levels measured by western blot analysis (A) and by immunohistochemical staining (B) in male rats (n = 6). Values are mean ± SEM. *P < 0.05 versus control.
Figure 7.
Western blot analyses showing the effect of 2-week treatment with the GPR18 agonist abn-cbd, its antagonist O-1918 (100 µg/kg/day, i.p, each) or their combination on the myocardial: p-Akt (A), p-ERK1/2 (B), eNOS expression (C) and p-eNOS (D). Data represent mean values of the ratio of p-Akt, p-ERK1/2 or p-eNOS normalized to the corresponding total protein while eNOS values are normalized to GAPDH in male rats (n = 8). Protein expression was presented as percent of control. Values are mean ± SEM. *P < 0.05 versus control.
Discussion
In the present study, chronic GPR18 activation (abn-cbd) reduced BP and cardiac sympathetic dominance along with improving LV function in conscious rats along with the following ex vivo biochemical effects: (i) elevated myocardial and circulating ADN and cGMP levels; (ii) enhanced vascular (aortic) eNOS/NO as well as serum NO levels; (iii) reduced myocardial oxidative stress, and activation of myocardial cell survival signaling molecules (Akt and ERK1/2). Equally important, when administered concurrently, the selective GPR18 antagonist O-1918 abrogated the biochemical and the cardiovascular effects of abn-cbd. Collectively, these findings identified molecular mechanisms that might explain the novel salutatory cardiovascular responses elicited by chronic GPR18 activation in conscious rats.
There is a growing interest in the role of the GPR18 agonist, abn-cbd, in cardiovascular regulation because it elicits hypotensive and vasodilator responses2–4. However, the acute abn-cbd evoked hypotension might have been confounded by anesthesia, which influences cardiovascular responses29, or by nonselective activation of other cannabinoid receptors such as GPR551, 13, 30 by the high abn-cbd doses (> 1 mg/kg) used in these studies2–4. Therefore, we addressed these limitations by studying the hemodynamic effects of a much lower dose of abn-cbd (100 µg/kg) in conscious rats. It is important to consider possible biased agonism for O-1918 at GPR18 because O-1918 increased calcium mobilization and mitogen activated protein kinase (MAPK) activity in GPR18-transfected HEK293 cells31. However, the possibility that these effects of O-1918 are tissue specific, reinforces the need for more studies to address this issue32.
The hypotensive response caused by chronic abn-cbd administration (Fig. 1A) or by central abn-cbd administration in our recent study11, and the abrogation of these effects by O-1918, implicates GPR18 in these responses. While, abn-cbd is an agonist at both GPR185 and GPR5513, 30, and activation of both receptors inhibits cAMP-dependent signaling in vitro33, the abn-cbd evoked vasodilation and hypotension are not GPR55-dependent because they persisted in GPR55 knockout mice7, 14. Interestingly, a lack of HR change (Fig. 1B), contrary to an expected reflex tachycardia to abn-cbd evoked hypotension, might be explained by the concurrent reduction in sympathetic prevalence (Fig. 3C). Notably, abn-cbd evoked bradycardia14 was observed following acute high abn-cbd doses and in the presence of anesthesia, which suppresses baroreflexes34.
The exact signaling mechanism implicated in the GPR18 mediated hypotension is still unknown. In reported studies, abn-cbd increased eNOS-derived NO in rabbit aortic endothelial cells35, and NAGly induced NO dependent vasorelaxation in isolated mesenteric arteries10. Further, in our recent study, nNOS-derived NO contributed to the sympathoinhibition and hypotension caused by central GPR18 activation in conscious rats16. Here, we show, for the first time, that the GPR18 (abn-cbd)-mediated hypotension (Fig. 1A) was associated with increases in vascular (aortic) eNOS/NO (Figs. 5C and D), and in circulating NO and cGMP (Figs. 5B and 8D) levels. The increase in the NO second messenger, cGMP (36), which is consistent with abn-cbd mediated increase in cGMP levels in endothelial cells4, supports NO involvement in GPR18 mediated hypotension. By contrast, other studies2–4, 12 ruled out NO involvement in abn-cbd evoked vasodilation, and suggested a role for the release of the endothelium derived hyperpolarizing factor (EDHF) in the observed vasodilation. Notably, the contribution of either NO or EDHF to vasorelaxtion is dependent on the size and location of the blood vessel as well as on the species used37.
It is important to comment on whether the increase in the circulating ADN (Fig. 5A) contributed to, or resulted from, the hypotensive response observed in abn-cbd treated rats. An elevated circulating level exists in patients with orthostatic hypotension and diabetes, compared with diabetes only38. Notably, this is an association finding in patients also exhibited worse kidney function, compared to patients with diabetes only38. On the other hand, other the present and findings support a causal role for ADN in abn-cbd evoked hypotension. First, GPR18 is detected in white adipose tissue, the major source of ADN39. Second, ADN improves vascular endothelial function and causes vasodilation by increasing eNOS-derived NO18, which is consistent with the association between the elevation in circulating ADN (Fig. 5A) and the increase in eNOS-derived NO (Fig. 5) and hypotension (Fig. 1A) in abn-cbd treated rats. The abrogation of these responses by concurrent treatment with O-1918 supports GPR18 mediation of the biochemical and hypotensive responses elicited by abn-cbd.
Whether these GPR18-mediated signaling events are functionally relevant in the heart has not been investigated. Results of the present study contribute to the understanding of the role of GPR18 in heart physiology by demonstrating and validating, for the first time, GPR18 expression in the heart (Fig. 4A), and by confirming the GPR18 location in cardiac myocytes (Fig. 4B). Functionally, chronic GPR18 activation increased the index for myocardial contractility, dp/dtmax (Fig. 2A), and reduced LVEDP (Fig. 2C). While the increase in dp/dtmax might involve GPR18-mediated Ca2+ influx4, 6, which increases myocardial contractility40, the reduced LVEDP (Fig. 2C) may be partly explained by the abn-cbd evoked hypotension (Fig. 1A) and by the increase in cardiac contractility (Fig. 2A).
The present findings suggest a pivotal role for increases in circulating (Fig. 5A) and cardiac (Fig. 6) ADN levels in the GPR18 (abn-cbd)-mediated improvement of cardiac function (Fig. 2A and C). This premise is initially supported by ADN synthesis in cardiomyocytes41. While ADN level is lower in heart than in plasma42, ADN functions in autocrine and paracrine manner to activate its myocardial ADN receptors, which are expressed in cardiomyocytes41, 42. This ADN action may result in ROS reduction43, at least partly, via an increase in tetrahydrobiopterin (BH4), a critical factor for eNOS coupling18, and subsequently improves cardiac function44. Consistent with these findings, abn-cbd treated rats exhibited elevations in plasma and cardiac ADN levels (Figs. 5A and 6), reduced myocardial ROS level (Fig. 8B) and improved cardiac function (Fig. 2A and C). We also observed enhancement in myocardial Akt and ERK1/2 phosphorylation in abn-cbd treated rats (Figs. 7A and B), which is consistent with a recently established mechanistic role for the activation of PI3K/Akt in favorable cellular effects of ADN45. It is also notable that the GPR18-medited activation of Akt and ERK1/2 agrees with our recent study and others3, 16. These signaling molecules exert vital cellular roles including cell survival46, inhibition of apoptosis47 and ROS reduction16, 48.
While a pivotal role for ADN in the GPR18-mediated improvement of myocardial redox status and function in our study is likely, we must consider the association between elevated circulating ADN levels and increased risk for mortality and cardiac morbidity49. It is worth noting, however, that other association-based clinical studies linked lower circulating ADN levels to cardiovascular anomalies in diabetes50. Therefore, further research is needed to mechanistically evaluate the role of circulating ADN levels in health and pathological conditions. The unavailability of selective ADN receptor blockers to causally link ADN to the GPR18-mediated cardiovascular responses is considered a limitation of our present study. While future studies in ADN receptor knockout mice are needed to address this limitation, O-1918 abrogation of the elevations in circulating and cardiac ADN (Figs. 5A and 6) as well as the improvement in cardiac function (Figs. 2A and C), caused by abn-cbd, support a role for ADN in the GPR18 (abn-cbd) mediated cardiac responses.
Finally, it is important to comment on the myocardial eNOS expression and phosphorylation and NO level in the hearts of abn-cbd treated rats. First, while myocardial eNOS expression was not affected by GPR18 activation or blockade (Fig. 7C), GPR18 blockade (O-1918) reduced myocardial eNOS phosphorylation and myocardial NO levels (Figs. 7D and 8A). While myocardial NO level was not altered in abn-cbd treated rats (Fig. 8A), there was a significant increase in its more stable second messenger cGMP and this increase was abrogated by O-1918 (Fig. 8C). O-1918 effect on cardiac eNOS might result, at least partly, from a reduction in myocardial Akt phosphorylation (Fig. 7A) because p-Akt increases NO levels via increased eNOS phosphorylation17. By contrast, vascular (aortic) eNOS expression increased and decreased, respectively, by GPR18 activation (abn-cbd) and blockade (O-1918) (Fig. 5D). The differences in eNOS expression and phosphorylation and in NO and cGMP levels in the heart and in blood vessels might highlight tissue specificity to GPR18 activation. Future studies are warranted to investigate this possibility.
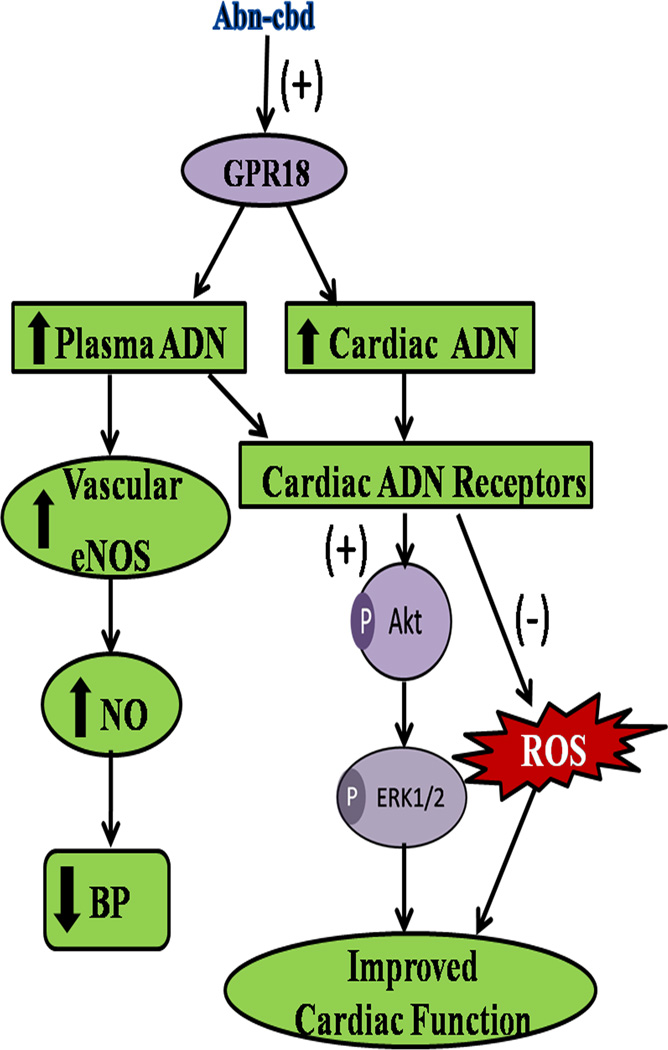
In conclusion, the current findings present new evidence for a salutary cardiovascular role for GPR18 in conscious rats mediated, at least partly, via elevations in myocardial and circulating levels of ADN, cGMP, activation of cell survival Akt and ERK1/2 phosphorylation and myocardial ROS reduction (see Fig. 9). Our conclusions are supported by the ability of GPR18 blockade to abrogate the indicated biochemical and cardiovascular functional responses. Finally, the present study highlights GPR18 as a viable molecular target for developing new antihypertensive therapeutics that simultaneously improve cardiac function.
Figure 9.
Suggested mechanisms for chronic GPR18-mediated favorable hemodynamic and cardio-protective effects in conscious rats. The sequence of events is based on the findings that chronic (2 weeks) GPR18 activation (abn-cbd): (i) reduced BP and sympathetic prevalence (lower LFRRI/HFRRI ratio) (Figs. 1 and 3), and improved cardiac function (Fig. 2) in conscious rats; (ii) increased plasma and cardiac adiponectin (ADN) (Figs. 5A and 6) and enhanced vascular eNOS expression along with elevated vascular and plasma NO levels (Fig. 5); (iii) reduced myocardial reactive oxygen species (ROS) level (Fig. 8B); (iv) enhanced Akt and ERK1/2 phosphorylation (Figs. 7A and B). These abn-cbd evoked biochemical and cardiovascular responses were abrogated by O-1918, the selective GPR18 antagonist (see text for details).
Acknowledgments
The authors appreciate the technical assistance provided by Ms. Kui Sun.
The study was Supported by a scholarship provided by the Egyptian Government (Scholarships Missions Program, Ministry of Higher Education) to Asmaa Matouk, and partially by NIH RO1 AA14441-09 (A. A).
Footnotes
The authors report no conflicts of interest.
Author contributions:
A.I.M., A.T., M.A.E., G.H.H. and A.A.A. conception and design of the research; A.I.M. conducted experiments; A.I.M. analyzed the data; A.I.M. prepared the figures; A.I.M. and A.A.A. interpreted results of the experiments; A.I.M. drafted the manuscript; A.I.M. and A.A.A. edited and revised the manuscript; A.I.M., A.T., M.A.E., G.H.H. and A.A.A. approved the final version of the manuscript.
References
- 1.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010 Dec;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999 Nov 23;96(24):14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003 Mar;63(3):699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 4.Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+- dependent K+ current. J Biol Chem. 2003 Nov 14;278(46):46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- 5.McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, Bradshaw HB. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010 Mar;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006 Sep 1;347(3):827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell MD, Hu SS, Viswanathan S, Bradshaw H, Kelly ME, Straiker A. A GPR18-based signalling system regulates IOP in murine eye. Br J Pharmacol. 2013 Jun;169(4):834–843. doi: 10.1111/bph.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu VB, Puhl HL, 3rd, Ikeda SR. N-Arachidonyl glycine does not activate G protein-coupled receptor 18 signaling via canonical pathways. Mol Pharmacol. 2012 Jan;83(1):267–282. doi: 10.1124/mol.112.081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay DB, Joseph WR, Grimsey NL, Glass M. GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. Peer J. 2016 Mar;4:e1835. doi: 10.7717/peerj.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmar N, Ho WS. N-arachidonoyl glycine, an endogenous lipid that acts as a vasorelaxant via nitric oxide and large conductance calcium-activated potassium channels. Br J Pharmacol. 2010 Jun;160(3):594–603. doi: 10.1111/j.1476-5381.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penumarti A, Abdel-Rahman AA. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharmacol Exp Ther. 2014 Apr;349(1):29–38. doi: 10.1124/jpet.113.209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003 Apr;138(7):1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci. 2009 Mar;30(3):156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007 Nov;152(5):825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh SK, Hepburn CY, Keown O, Astrand A, Lindblom A, Ryberg E, Hjorth S, Leslie SJ, Greasley PJ, Wainwright CL. Pharmacological profiling of the hemodynamic effects of cannabinoid ligands: a combined in vitro and in vivo approach. Pharmacol Res Perspect. 2015 Jun;3(3):e00143. doi: 10.1002/prp2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penumarti A, Abdel-Rahman AA. Neuronal nitric oxide synthase-dependent elevation in adiponectin in the rostral ventrolateral medulla underlies g protein-coupled receptor 18-mediated hypotension in conscious rats. J Pharmacol Exp Ther. 2014 Oct;351(1):44–53. doi: 10.1124/jpet.114.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000 Aug;106(4):493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013 Jun 4;127(22):2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 19.El-Mas MM, Abdel-Rahman AA. Nongenomic effects of estrogen mediate the dose-related myocardial oxidative stress and dysfunction caused by acute ethanol in female rats. Am J Physiol Endocrinol Metab. 2013 Apr 1;306(7):E740–E747. doi: 10.1152/ajpendo.00465.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim BM, Fan M, Abdel-Rahman AA. Oxidative stress and autonomic dysregulation contribute to the acute time-dependent myocardial depressant effect of ethanol in conscious female rats. Alcohol Clin Exp Res. 2014 May;38(5):1205–1215. doi: 10.1111/acer.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGee M, Abdel-Rahman A. Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther. 2012 Aug;342:461–471. doi: 10.1124/jpet.112.194464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaltout HA, Abdel-Rahman AA. Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther. 2005 Sep;314(3):1328–1337. doi: 10.1124/jpet.105.086314. [DOI] [PubMed] [Google Scholar]
- 23.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043–1065. [PubMed] [Google Scholar]
- 24.Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol. 1989 Nov 1;14(5):1139–1148. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- 25.El-Mas MM, Zhang J, Abdel-Rahman AA. Upregulation of vascular inducible nitric oxide synthase mediates the hypotensive effect of ethanol in conscious female rats. J Appl Physiol (1985) 2006 Mar;100(3):1011–1018. doi: 10.1152/japplphysiol.01058.2005. [DOI] [PubMed] [Google Scholar]
- 26.Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009 Sep;81(3):595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichimura M, Kato S, Tsuneyama K, Matsutake S, Kamogawa M, Hirao E, Miyata A, Mori S, Yamaguchi N, Suruga K, Omagari K. Phycocyanin prevents hypertension and low serum adiponectin level in a rat model of metabolic syndrome. Nutr Res. 2013 May;33(5):397–405. doi: 10.1016/j.nutres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Gantz I, Muraoka A, Yang YK, Samuelson LC, Zimmerman EM, Cook H, Yamada T. Cloning and chromosomal localization of a gene (GPR18) encoding a novel seven transmembrane receptor highly expressed in spleen and testis. Genomics. 1997 Jun 15;42(3):462–466. doi: 10.1006/geno.1997.4752. [DOI] [PubMed] [Google Scholar]
- 29.Vatner SF. Effects of anesthesia on cardiovascular control mechanisms. Environ Health Perspect. 1978 Oct;26:193–206. doi: 10.1289/ehp.7826193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007 Dec;152(7):1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol. 2014 Aug;171(16):3908–3917. doi: 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajaraman G, Simcocks A, Hryciw DH, Hutchinson DS, McAinch AJ. G protein coupled receptor 18: A potential role for endocannabinoid signaling in metabolic dysfunction. Mol Nutr Food Res. 2016 Jan;60(1):92–102. doi: 10.1002/mnfr.201500449. [DOI] [PubMed] [Google Scholar]
- 33.Martin AL, Steurer MA, Aronstam RS. Constitutive Activity among Orphan Class-A G Protein Coupled Receptors. PLoS One. 2015;10(9):e0138463. doi: 10.1371/journal.pone.0138463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vatner SF, Franklin D, Braunwald E. Effects of anesthesia and sleep on circulatory response to carotid sinus nerve stimulation. Am J Physiol. 1971 May;220(5):1249–1255. doi: 10.1152/ajplegacy.1971.220.5.1249. [DOI] [PubMed] [Google Scholar]
- 35.McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor--independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007 Jun;321(3):930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- 36.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001 Jan;21(1):1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Aso Y, Wakabayashi S, Terasawa T, Naruse R, Hara K, Takebayashi K, Inukai T. Elevation of plasma high molecular weight adiponectin in patients with Type 2 diabetes and orthostatic hypotension: association with arterial stiffness and hypercoagulability. Diabet Med. 2012 Jan;29(1):80–87. doi: 10.1111/j.1464-5491.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- 39.Amisten S, Neville M, Hawkes R, Persaud SJ, Karpe F, Salehi A. An atlas of G-protein coupled receptor expression and function in human subcutaneous adipose tissue. Pharmacol Ther. 2015 Feb;146:61–93. doi: 10.1016/j.pharmthera.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Clements RT, Cordeiro B, Feng J, Bianchi C, Sellke FW. Rottlerin increases cardiac contractile performance and coronary perfusion through BKCa++ channel activation after cold cardioplegic arrest in isolated hearts. Circulation. 2011 Sep 13;124(11 Suppl):S55–S61. doi: 10.1161/CIRCULATIONAHA.110.012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005 Sep 26;579(23):5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 42.Fujioka D, Kawabata K, Saito Y, Kobayashi T, Nakamura T, Kodama Y, Takano H, Obata JE, Kitta Y, Umetani K, Kugiyama K. Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2409–H2416. doi: 10.1152/ajpheart.00987.2005. [DOI] [PubMed] [Google Scholar]
- 43.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007 Mar 20;115(11):1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 44.Dong F, Ren J. Adiponectin improves cardiomyocyte contractile function in db/db diabetic obese mice. Obesity (Silver Spring) 2009 Feb;17(2):262–268. doi: 10.1038/oby.2008.545. [DOI] [PubMed] [Google Scholar]
- 45.Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005 Jun 24;332(1):200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 46.Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001 Aug;12(8):397–408. [PubMed] [Google Scholar]
- 47.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000 Feb 15;101(6):660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YJ, Jeong HY, Kim YB, Won SY, Shim JH, Cho MK, Nam HS, Lee SH. Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human mesothelioma MSTO-211H cells. Food Chem Toxicol. 2012 Feb;50(2):116–123. doi: 10.1016/j.fct.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 49.Witberg G, Ayers CR, Turer AT, Lev E, Kornowski R, de Lemos J, Neeland IJ. Relation of Adiponectin to All-Cause Mortality, Cardiovascular Mortality, and Major Adverse Cardiovascular Events (from the Dallas Heart Study) Am J Cardiol. 2016 Feb 15;117(4):574–579. doi: 10.1016/j.amjcard.2015.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Z, Zhang R, Li J, Xu G. Effect of telmisartan on the expression of adiponectin receptors and nicotinamide adenine dinucleotide phosphate oxidase in the heart and aorta in type 2 diabetic rats. Cardiovasc Diabetol. 2012;11:94. doi: 10.1186/1475-2840-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]